Abstract
Purpose
Repetitive strain to the optic nerve (ON) due to tethering in adduction has been recently proposed as an intraocular pressure-independent mechanism of optic neuropathy in primary open-angle glaucoma. Since strabismus may alter adduction, we investigated whether gaze-related ON straightening and associated globe translation differ in horizontal and vertical strabismus.
Methods
High-resolution orbital magnetic resonance imaging was obtained in 2-mm thick quasi-coronal planes using surface coils in 25 subjects (49 orbits) with esotropia (ET, 19 ± 3.6Δ SEM), 11 (15 orbits) with exotropia (XT, 33.7 ± 7.3Δ), 7 (12 orbits) with hypertropia (HT, 14.6 ± 3.2Δ), and 31 normal controls (62 orbits) in target-controlled central gaze, and in maximum attainable abduction and adduction. Area centroids were used to determine ON path sinuosity and globe positions.
Results
Adduction angles achieved in ET (30.6° ± 0.9°) and HT (27.2° ± 2.3°) did not significantly differ from normal (28.3° ± 0.7°), but significantly less adduction was achieved in XT (19.0° ± 2.5°, P = 0.005). ON sheath tethering in adduction occurred in ET and HT similarly to normal, but did not in XT. The globe translated significantly less than normal, nasally in adduction in XT and temporally in abduction in ET and HT (P < 0.02, for all). Globe retraction did not occur during abduction or adduction in any group.
Conclusions
Similar to normal subjects, the ON and sheath become tethered without globe retraction in ET and HT. In XT, adduction tethering does not occur, possibly due to limited adduction angle. Thus, therapeutic limitation of adduction could be considered as a possible treatment for ON sheath tethering.
Keywords: esotropia, exotropia, hypertropia, glaucoma, optic nerve
Optic nerve (ON) sheath tethering in adduction is a novel concept recently demonstrated by a magnetic resonance imaging (MRI).1 The human ON and its sheath remain sinuous in central gaze and abduction, but become straightened at a threshold adduction angle beyond which these structures exert progressive tractional force on the globe. While MRI has provided indirect evidence of the biomechanical effects of horizontal eye rotation on the globe, deformation of the ON head and peripapillary structures during horizontal duction has been directly visualized using spectral-domain optical coherence tomography (OCT).2–5 Adduction displaces the nasal peripapillary Bruch's membrane (ppBM) anteriorly and the temporal ppBM posteriorly, tilting the ON head temporally, with a reversal of this pattern in abduction.2,4 The deformation of the ON head and ppBM is greater in adduction than in abduction,2–5 with quantitative measures of deformation markedly increasing beyond an adduction threshold of 26°.4 This behavior supports the concept of initiation of ON sheath tethering at the adduction angle when length redundancy of these structures become exhausted, with progressively increasing deformational force occurring with additional adduction beyond this threshold at which ON and sheath are fully straightened. Beyond this threshold, ON and sheath path can increase only by tensile stretching.
Biomechanical studies predict mechanical stress (force/cross-sectional area) and strain (deformation caused by applied force) in the ON head and peripapillary region due to adduction. Our experimental study revealed that elastic stiffness of the ON sheath is much greater than that of peripapillary sclera, so that the much stiffer ON sheath can exert tractional force that mechanically loads the globe.6 The ON head deformation induced by ON sheath traction is predicted to exceed that caused by substantially elevated intraocular pressure (IOP).6–8 Moreover, finite element biomechanical analysis suggests that maximum stress and strain caused by the tethered ON sheath in adduction occur at the junction of the temporal scleral and ON sheath,6 which corresponds to the region of the peripapillary atrophy commonly observed in patients who have glaucoma.9–11 Most recently, MRI showed that adduction tethering is accompanied by significantly greater globe retraction in patients who have primary open angle glaucoma (POAG) without increased IOP, implying that abnormally high traction force is exerted in adduction in these patients.12 Based on these findings, repetitive ON sheath tethering over numerous adduction eye movements has been proposed as an IOP-independent mechanism of glaucomatous ON damage.
Binocular alignment is, by definition, abnormal in strabismic patients, although at present it has not been investigated if POAG is more common in any particular forms of strabismus. Since ON sheath tethering occurs at a threshold adduction angle, greater globe retraction during adduction would be anticipated in subjects having esotropia (ET), less in subjects having exotropia (XT), and perhaps little or no abnormality in vertical strabismus. In the present study, we investigated whether ON sheath tethering in adduction differs in subjects with horizontal and vertical strabismus, and whether globe retraction in adduction typical of POAG is more or less common in strabismic patients.
Methods
Subjects
This prospective study of ocular motility and strabismus was conducted according to a protocol approved by the Institutional Review Board for Protection of Human Subjects of the University of California, Los Angeles, and conforming to the tenets of the Declaration of Helsinki. Among recruited strabismic subjects, there were 25 with ET, 11 with XT, and 7 with hypertropia (HT) with adequate quality and number of image planes for analysis, compared with 31 normal controls recruited by advertising. Subjects gave written, informed consent prior to participation and underwent comprehensive eye examinations including corrected visual acuity, binocular alignment, ocular motility, IOP, and ocular anatomy.
Magnetic Resonance Imaging
High resolution T2-weighted fast spin echo MRI was performed in each subject using a dual-phased surface coil array (Medical Advances, Milwaukee, WI, USA) with a 1.5-T scanner (General Electric Signa; Medical Advances) as previously described in detail.13–15 Contiguous 2-mm thick quasi-coronal images were obtained perpendicular to the long axis of the orbit using a matrix of 256 × 256 pixels over an 8-cm field of view, giving 312 μm in-plane resolution. Axial images were also obtained at 2-mm thickness using a 256 × 256 matrix over a 10- to 11-cm field of view, giving 390 to 430 μm in-plane resolution (Fig. 1). Each subject fixated on an afocal, monocularly viewed target in central gaze, as well as in abduction at a distance of 2 cm. The eye in adduction was imaged during fixation of the abducting fellow eye, since the nasal bridge or surface coil occluded the nasally located targets.
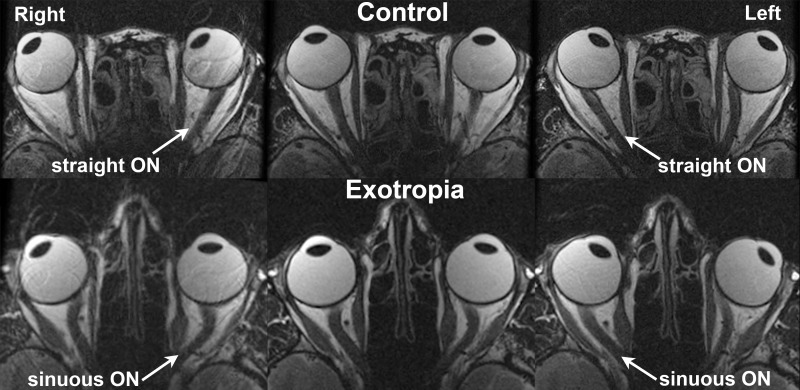
Figure 1.
Axial MRI in a normal control and a subject with exotropia, imaged in right (left column), central (middle column), and left (right column) gazes. While the ON straightened in adduction in the control, it remained sinuous in the exotropic subject.
Analysis
Digital images were quantified using the program ImageJ64 (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA). Left orbit images were reflected to the orientation of right orbits to permit uniform analysis. The most posterior image plane containing both globe and ON cross-sections was designated as plane 0 and image planes were numbered negatively posterior and positively anterior to the plane 0. The orbit, globe, and ON cross-sections were outlined manually for subsequent automated measurement of their area centroids. All analysis subsequent to manual outlining was fully automated using a custom spreadsheet (Microsoft Excel; Microsoft Corp., Redmond, WA, USA).
Using the area centroid of the ON in plane 0 in central gaze as a standard origin, displacements of the ON in plane 0 in eccentric gaze positions were used to measure ocular duction angles. The minimum ON length was measured as the straight-line distance connecting the ON area centroid of the most posterior plane with the ON area centroid of the most anterior image plane with a visible ON cross-section. Actual ON length was calculated by summing the lengths of ON area centroids in all contiguous image planes. To estimate ON sinuosity, the ratio of actual to minimum ON length was expressed as a percentage, which would always be at least 100%.
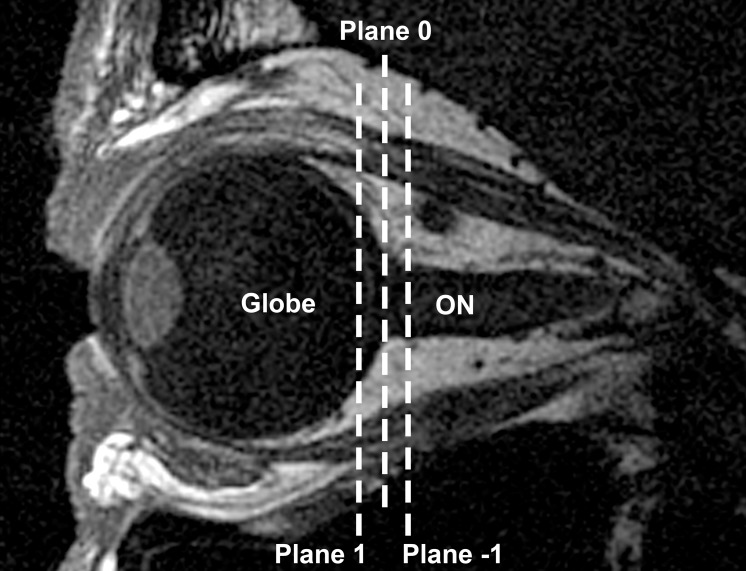
The approximately linear relationship between orbital cross-sectional area and local changes in orbital depth was exploited to measure globe translation and standardize plane 0 between gaze positions. Orbital cross-sectional areas in central gaze were measured in planes −1, 0, and +1 (Fig. 2) and a best-fit linear regression was calculated for area variation within those image planes. Then each orbital cross-sectional area of plane 0 in eccentric gaze was fit to that line to determine the relative anteroposterior locations of plane 0 in those gaze positions with respect to central gaze. Next, assuming each globe to be spherical, the three-dimensional (3D) center of the globe was determined to sub-pixel resolution as previously described in detail.16 Finally, after correcting for relative anteroposterior displacements of plane 0, anteroposterior, horizontal, and vertical translation of the globe center from central to eccentric gaze was calculated.
Figure 2.
Sagittal MRI of a normal control subject in central gaze. Cross-sectional areas in planes −1, 0, and 1 orthogonal to the long axis of the orbit were used to determine globe translation and the relative anteroposterior locations of plane 0 in eccentric gaze positions.
Statistical Analysis
Sinuosity of the ON in central gaze, abduction, and adduction, as well as globe translation in abduction and adduction, were compared among groups of strabismic subjects (ET, XT, and HT) and normal controls using the generalized estimating equation (GEE) method implemented in the software package used (SPSS ver. 24.0 for Mac; SPSS, Inc., Chicago, IL, USA). The GEE method is widely used in ophthalmic research to account for possible interocular correlations within subjects, treating both eyes as matched case-control data within same subject.17 The 0.05 level was considered statistically significant.
Results
Subject Characteristics
A total of 25 subjects (49 orbits) with ET, 11 subjects (15 orbits) with XT, 7 subjects (12 orbits) with HT, and 31 normal controls (62 orbits) were analyzed. For subjects with restrictive strabismus, only the affected eye was scanned. Clinical and demographic characteristics of subjects are listed in Table 1. For subjects with HT, both hyper- and hypotropic orbits were included. Age and sex distributions did not differ significantly among groups. Since axial MRI images were not available in all subjects, refractive error rather than axial globe length was analyzed. However, eight subjects (three in control, four in the ET and one in the HT group) had previous refractive surgery, so refractive error of these subjects may not correlate with axial length. While mean spherical equivalent refractive error in HT was significantly less myopic than control group (P = 0.016), mean differences in refraction did not exceed 1.5 diopters (D). No subject had ophthalmoscopic evidence of high myopia.
Table.
Clinical and Demographic Characteristics of Subjects
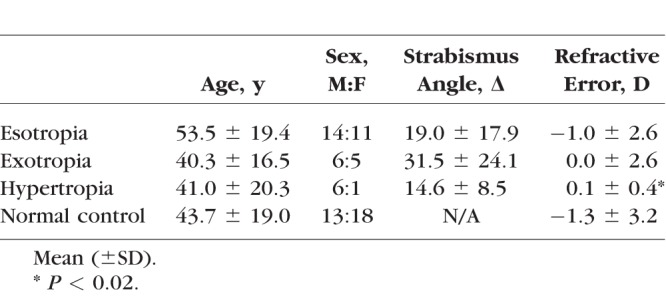
All strabismic subjects initially reported diplopia or complaints of binocular misalignment. Mean symptom duration was 8.9 ± 14.4 years (mean ± standard deviation) for subjects with ET, 11.8 ± 13.2 years for XT, and 7.6 ± 7.2 years for HT. In the ET group, 13 subjects had sagging eye syndrome18 (age-related divergence insufficiency esotropia), 8 had decompensated esophoria evolving presumably from childhood onset, 3 had abducens paresis, and 1 had spasm of the near reflex. In XT group, five subjects had restrictive XT that developed after previous strabismus, eyelid, or sinus surgery, five had decompensated intermittent XT of childhood onset, and one had progressive right medial rectus paresis. In the group with HT, six subjects had acquired superior oblique palsy and one had skew deviation.
Optic Nerve Sinuosity
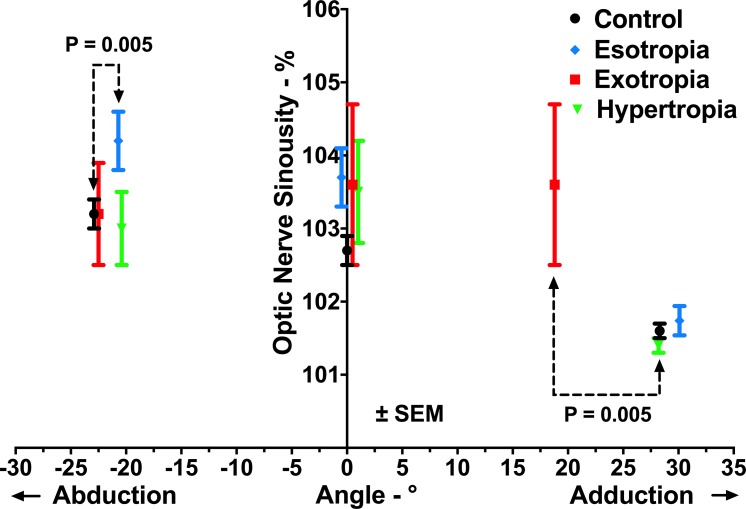
Subjects underwent MRI in central gaze and maximum attainable abduction and adduction. Adduction angles achieved in ET and HT were 30.6° ± 0.9° and 27.2° ± 2.3° (mean ± standard error of mean, SEM), not significantly different from normal (28.3° ± 0.7°, P = 0.11 and P = 0.72, respectively). Abduction angles achieved in XT and HT were 24.2° ± 1.1° and 21.4° ± 1.8°, also similar to normal at 22.9° ± 0.6° (P = 0.34, P = 0.36, respectively). However, adduction angle in XT and abduction angle in ET were 20.2° ± 0.7° and 19.0° ± 2.5°, both significantly subnormal (P = 0.005, for both). In all strabismic groups, small vertical ductions (range, −1.1° to 0.3°) were associated with horizontal duction, but in each group, this was similar to normal subjects (P > 0.3).
Sinuosity of the ON (Fig. 3) in each strabismic group was similar to normal in central gaze (P > 0.05, for all). In abduction, ON sinuosity was not significantly different from that in central gaze in any group (P > 0.1). In adduction, the ON became significantly straighter than in central gaze in control, ET, and HT subjects (P < 0.03, for all), but not in XT (P = 0.99). In the group with XT, ON sinuosity in both central gaze and adduction was 103.6%, implying the absence of ON sheath tethering in adduction.
Figure 3.
ON sinuosity in central gaze, abduction, and adduction in controls and strabismic subjects. Adduction angle achieved in XT and abduction angle in ET were significantly subnormal (P = 0.005 for both). Note that the ON was significantly straighter in adduction than in central gaze in all groups except in subjects with XT.
Since 5 of 11 subjects with XT had restrictive strabismus, a subgroup analysis of ON sinuosity compared restrictive versus nonrestrictive cases. The mean adduction angle of subjects with restrictive XT was insignificantly smaller than that of subjects with nonrestrictive XT (15.1° vs. 20.9°, respectively, P = 0.275). The ON was significantly more sinuous in all gaze positions in the group with restrictive XT (P < 0.01), but the ON did not straighten in adduction in either group.
Globe Translation
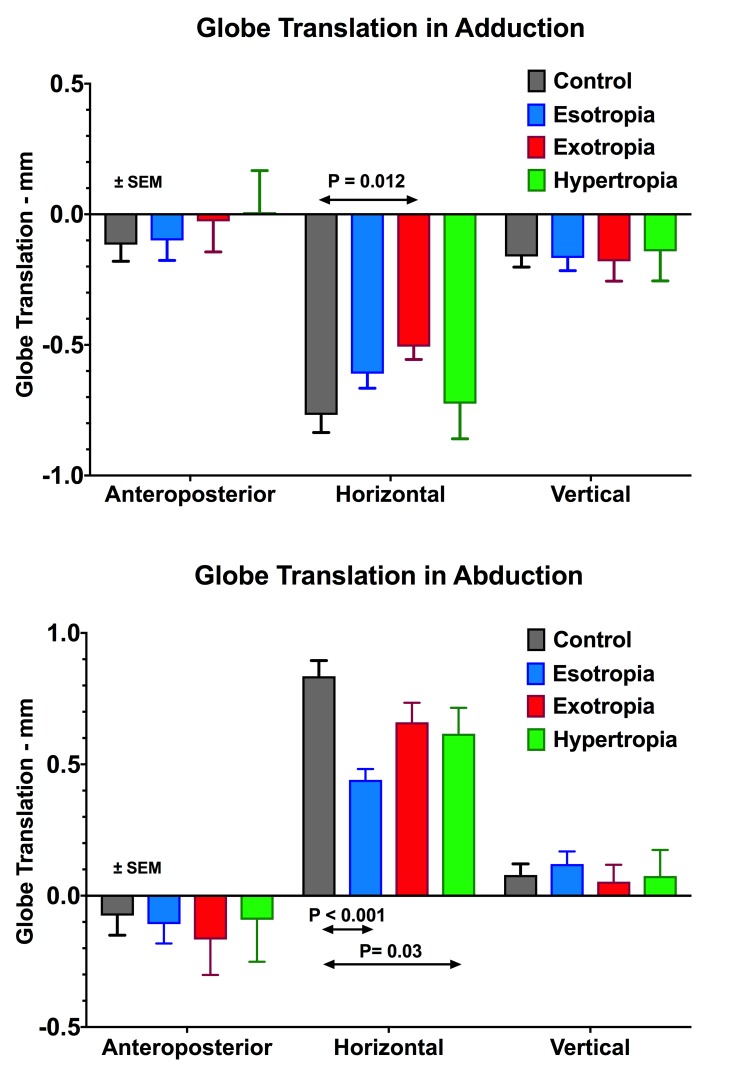
Globe translation in abduction and adduction relative to central gaze is compared between normal and strabismic subjects in Figure 4. Anterior, lateral, and superior globe translations are considered positive. In abduction, the globe translated laterally in all groups, but significantly less than the others in ET and HT (P < 0.001 and P = 0.03, respectively). In adduction, the globe translated medially in all groups, but significantly less in XT than in the others (P = 0.012). In all groups, the globe translated superiorly in abduction and inferiorly in adduction. In neither abduction nor adduction did anteroposterior globe translation differ significantly from normal in any strabismic group (P > 0.4).
Figure 4.
Three-dimensional globe translation during adduction and abduction. Anterior, lateral, and superior directions are defined as positive. None of the groups studied here demonstrated the significant posterior globe translation in adduction that is typical of POAG with normal IOP.
Discussion
MRI demonstrates that subjects with ET and HT manifest ON sheath tethering in adduction that is similar to orthotropic normal subjects, but tethering does not occur in XT. Adduction angles achieved in subjects with XT were significantly subnormal, perhaps less than the threshold angle required to cause ON straightening. Although the ON became tethered in ET and HT, this was not associated with globe retraction, similar to the behavior of normal subjects. The absence of significant posterior globe translation during adduction tethering in strabismic subjects presumably indicates that their ONs and sheaths were sufficiently pliable to stretch without retracting the globe.
High resolution, axial MRI in multiple gaze positions was first used to demonstrate ON sheath tethering in adduction by directly visualizing ON path straightening.1 However, in the current study, the ON did not become straightened in adduction in subjects with XT, but instead demonstrated similar sinuosity in both central gaze and adduction. While the earlier study qualitatively demonstrated in some strabismic subjects the configuration of the ON path using axial images, the current study used quasi-coronal images to reconstruct the intraorbital ON path in 3D and quantified ON sinuosity by comparing actual ON path length with minimum path length. It is plausible that the ON straightening evident in axial images in our earlier study was overestimated since the sinuosity of the ON perpendicular to the axial planes cannot be measured in axial images.
The current study adds to accumulating MRI evidence that ON straightening occurs at a threshold adduction angle between 22° and 26°.1 Moreover, a recent OCT study demonstrated that the ON head and peripapillary structures become progressively deformed with increasing ad- and abduction; however, significantly greater displacement of the ppBM and ON head tilt were observed beyond 26° adduction.4 In the current study, mean adduction angles achieved in subjects with restrictive and nonrestrictive XT were 15.1° and 20.2°, respectively, which were both below the reported threshold adduction angle. Strabismic limitation on maximum achievable adduction could explain why ON sheath tethering was not observed in either restrictive or nonrestrictive XT. Subnormal adduction can also account for the significantly less medial globe translation in adduction observed in eyes with XT.
Since initial positions of one or both eyes are generally more adducted than normal in esotropic subjects, greater tension on the ON during further adduction might have been expected to induce abnormally great globe retraction, similar to that recently demonstrated by MRI in patients with POAG without increased IOP.12 However, globe retraction was not observed here in esotropic subjects, suggesting that globe retraction in adduction may be specifically related to glaucoma, consistent (albeit not demonstrative) with a hypothesized causative role of ON sheath tethering in glaucomatous optic neuropathy. Although little is known about the mechanical properties of the ON sheath, recent biomechanical studies have suggested that it is much stiffer than the peripapillary sclera6–8 and thus might exert traction in adduction that could retract the globe. It is possible that the ON sheath could be particularly stiff in subjects who develop glaucoma, thus generating more traction on the globe when taut.
One might ask whether large adduction eye movements occur frequently enough in daily life to damage the ON. This is likely to be due to automatic and involuntary correlates of active behavior rather than conscious gaze shifts while sedentary. The adduction angle in the current study is only about half as large as the human horizontal oculomotor range of about ±55°.19 Large gaze shifts exceeding the adduction range of the current study commonly occur in daily life and involve the following stereotypic coordination of head and eye rotations. During a large angle gaze shift, a large, automatic saccade initially occurs in the direction of the gaze shift that carries the eye nearly to the limit of the oculomotor range, after which a slower head movement in the same direction automatically occurs associated with counter-rotational centering of the eye in the orbit due to activation of the vestibulo-ocular reflex.20,21 For example, the automatic saccade during coordinated eye-head gaze shift to a 50° target jump is as large as 40°.20 Not only are everyday saccades much larger than the static adduction studied here, but they are also very fast, and so presumably involve much higher muscle forces than occur during static target fixations. Therefore, the present study likely only demonstrates a lower bound on the ON sheath adduction tethering phenomenon that occurs numerous times each day during ambulation and other body motion.
The pathogenesis of POAG without elevated IOP is still poorly understood. Currently, treatment of POAG without elevated IOP remains exclusively focused on lowering an IOP22–24 that, before treatment, is already within the normal range. Although IOP reduction retards the progression of optic atrophy in some of these patients,22,25 there are frequent cases of progressive optic neuropathy in POAG despite therapeutic IOP reduction within or below the already normal range.23,26 Moreover, half of IOP-untreated patients having POAG without elevated IOP in a multicenter, clinical study did not progress over 5 to 7 years,27 strongly implying that the factors besides IOP contribute to the pathogenesis of this disease. If adduction tethering is truly an IOP-independent pathogenic factor in glaucomatous optic neuropathy, limiting adduction might avoid ON sheath tethering, similar to the situation observed for patients with XT. Adduction might be therapeutically limited by reducing the force of medial rectus (MR) muscle selectively in its field of action; such a limitation could be achieved by MR posterior scleral fixation28 or MR pulley fixation29,30 without creating binocular misalignment in primary gaze. However, these procedures should be considered only when the benefits would outweigh the potential of developing diplopia in eccentric gazes, and only after accumulation of additional supporting clinical evidence.
This study confirms and extends to strabismic subjects the novel concept of ON sheath tethering in adduction at and beyond a threshold angle. However, globe retraction during such tethering did not occur in strabismic subjects, supporting the specificity of this phenomenon to POAG.14 If further studies were to confirm the hypothesis that adduction tethering damages the ON in POAG, then limitation to adduction might become a therapeutic approach.
Acknowledgments
Supported by U.S. Public Health Service Grant EY008313 and an unrestricted grant from Research to Prevent Blindness (New York, NY, USA).
Disclosure: S.Y. Suh, None; R.A. Clark, Nevakar (C); J.L. Demer, Alcon Japan (R)
References
- 1. Demer JL. . Optic nerve sheath as a novel mechanical load on the globe in ocular duction. Invest Ophthalmol Vis Sci. 2016; 57: 1826– 1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sibony PA. . Gaze evoked deformations of the peripapillary retina in papilledema and ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2016; 57: 4979– 4987. [DOI] [PubMed] [Google Scholar]
- 3. Chang MY, Shin A, Park J,et al. . Deformation of optic nerve head and peripapillary tissues by horizontal duction. Am J Ophthalmol. 2017; 174: 85– 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suh SY, Le A, Shin A, Park J, Demer JL. . Progressive deformation of the optic nerve head and peripapillary structures by graded horizontal duction. Invest Ophthalmol Vis Sci. 2017; 58: 5015– 5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang X, Beotra MR, Tun TA,et al. . In vivo 3-dimensional strain mapping confirms large optic nerve head deformations following horizontal eye movements. Invest Ophthalmol Vis Sci. 2016; 57: 5825– 5833. [DOI] [PubMed] [Google Scholar]
- 6. Shin A, Yoo L, Park J, Demer JL. . Finite element biomechanics of optic nerve sheath traction in adduction. J Biomech Eng. 2017; 139: 101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang X, Rumpel H, Lim WE,et al. . Finite element analysis predicts large optic nerve head strains during horizontal eye movements. Invest Ophthalmol Vis Sci. 2016; 57: 2452– 2462. [DOI] [PubMed] [Google Scholar]
- 8. Wang X, Fisher LK, Milea D, Jonas JB, Girard MJ. . Predictions of optic nerve traction forces and peripapillary tissue stresses following horizontal eye movements. Invest Ophthalmol Vis Sci. 2017; 58: 2044– 2053. [DOI] [PubMed] [Google Scholar]
- 9. Jonas JB, Nguyen XN, Gusek GC, Naumann GO. . Parapapillary chorioretinal atrophy in normal and glaucoma eyes. I. Morphometric data. Invest Ophthalmol Vis Sci. 1989; 30: 908– 918. [PubMed] [Google Scholar]
- 10. Park KH, Tomita G, Liou SY, Kitazawa Y. . Correlation between peripapillary atrophy and optic nerve damage in normal-tension glaucoma. Ophthalmology. 1996; 103: 1899– 1906. [DOI] [PubMed] [Google Scholar]
- 11. Uchida H, Ugurlu S, Caprioli J. . Increasing peripapillary atrophy is associated with progressive glaucoma. Ophthalmology. 1998; 105: 1541– 1545. [DOI] [PubMed] [Google Scholar]
- 12. Demer JL, Clark RA, Suh SY,et al. . Magnetic resonance imaging of optic nerve traction during adduction in primary open-angle glaucoma with normal intraocular pressure. Invest Ophthalmol Vis Sci. 2017; 58: 4114– 4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Demer JL, Miller JM. . Magnetic resonance imaging of the functional anatomy of the superior oblique muscle. Invest Ophthalmol Vis Sci. 1995; 36: 906– 913. [PubMed] [Google Scholar]
- 14. Demer JL, Clark RA. . Magnetic resonance imaging of human extraocular muscles during static ocular counter-rolling. J Neurophysiol. 2005; 94: 3292– 3302. [DOI] [PubMed] [Google Scholar]
- 15. Demer JL, Dushyanth A. . T2-weighted fast spin-echo magnetic resonance imaging of extraocular muscles. J AAPOS. 2011; 15: 17– 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clark RA, Miller JM, Demer JL. . Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000; 41: 3787– 3797. [PubMed] [Google Scholar]
- 17. Fan Q, Teo YY, Saw SM. . Application of advanced statistics in ophthalmology. Invest Ophthalmol Vis Sci. 2011; 52: 6059– 6065. [DOI] [PubMed] [Google Scholar]
- 18. Chaudhuri Z, Demer JL. . Sagging eye syndrome: connective tissue involution as a cause of horizontal and vertical strabismus in older patients. JAMA Ophthalmol. 2013; 131: 619– 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guitton D. . Control of eye-head coordination during orienting gaze shifts. Trends Neurosci. 1992; 15: 174– 179. [DOI] [PubMed] [Google Scholar]
- 20. Proudlock FA, Gottlob I. . Physiology and pathology of eye-head coordination. Prog Retin Eye Res. 2007; 26: 486– 515. [DOI] [PubMed] [Google Scholar]
- 21. Laurutis VP, Robinson DA. . The vestibulo-ocular reflex during human saccadic eye movements. J Physiol. 1986; 373: 209– 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998; 126: 498– 505. [DOI] [PubMed] [Google Scholar]
- 23. Kim M, Kim DM, Park KH, Kim TW, Jeoung JW, Kim SH. . Intraocular pressure reduction with topical medications and progression of normal-tension glaucoma: a 12-year mean follow-up study. Acta Ophthalmol. 2013; 91: e270– e275. [DOI] [PubMed] [Google Scholar]
- 24. Song BJ, Caprioli J. . New directions in the treatment of normal tension glaucoma. Indian J Ophthalmol. 2014; 62: 529– 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998; 126: 487– 497. [DOI] [PubMed] [Google Scholar]
- 26. Jeong JH, Park KH, Jeoung JW, Kim DM. . Preperimetric normal tension glaucoma study: long-term clinical course and effect of therapeutic lowering of intraocular pressure. Acta Ophthalmol. 2014; 92: e185– e193. [DOI] [PubMed] [Google Scholar]
- 27. Anderson DR, Drance SM, Schulzer M; Collaborative Normal-Tension Glaucoma Study Group. . Natural history of normal-tension glaucoma. Ophthalmology. 2001; 108: 247– 253. [DOI] [PubMed] [Google Scholar]
- 28. Guyton DL. . The posterior fixation procedure: mechanism and indications. Int Ophthalmol Clin. 1985; 25: 79– 88. [DOI] [PubMed] [Google Scholar]
- 29. Clark RA, Ariyasu R, Demer JL. . Medial rectus pulley posterior fixation: a novel technique to augment recession. J AAPOS. 2004; 8: 451– 456. [DOI] [PubMed] [Google Scholar]
- 30. Clark RA, Ariyasu R, Demer JL. . Medial rectus pulley posterior fixation is as effective as scleral posterior fixation for acquired esotropia with a high AC/A ratio. Am J Ophthalmol. 2004; 137: 1026– 1033. [DOI] [PubMed] [Google Scholar]