Abstract
Near infrared photoimmunotherapy (NIR‐PIT) is a highly selective tumor treatment that employs an antibody‐photo‐absorber conjugate (APC) which is activated by near infrared light. Here, we describe the efficacy of endoscopic NIR‐PIT using the APC trastuzumab‐IR700DX (tra‐IR700) in the setting of human epidermal growth factor 2 positive (HER2 + ) gastric carcinoma with peritoneal disseminations. In this in vivo study, fluorescence endoscopy showed high tumor accumulation of tra‐IR700 within disseminated peritoneal implants. Mice with disseminated peritoneal gastric cancer were separated into 4 groups: (i) control (no treatment); (ii) tra‐IR700 i.v. only; (iii) NIR light only; and (iv) endoscopic NIR‐PIT. NIR light irradiation was carried out through a fiber optic diffuser under endoscopic guidance. In vivo bioluminescence images showed significantly greater therapeutic effect in the endoscopic NIR‐PIT group than that in the control groups (P < .01 vs other control groups). Histological analysis showed diffuse cancer cell death in NIR‐PIT‐treated tumors. In conclusion, NIR‐PIT with NIR light delivered via an endoscopic fiber optic diffuser is a promising method for the treatment of peritoneal dissemination of gastric cancer. Moreover, this technique could be readily used in other types of cancers with peritoneal dissemination provided that suitable antibodies could be found.
Keywords: endoscope, fiber optic diffuser, gastric cancer, monoclonal antibodies, near infrared photoimmunotherapy
1. INTRODUCTION
Recent advances in the molecular phenotyping of gastric cancer have helped to identify predictive biomarkers that can help direct therapy. For instance, approximately 20% of gastric cancers are human epidermal growth factor receptor (EGFR) type 2 (HER2)‐positive.1, 2 Therefore, trastuzumab, an anti‐HER2 monoclonal antibody (mAb), can be successfully used to treat HER2‐positive gastric cancer.1 However, its therapeutic efficacy is still limited in patients with peritoneal dissemination (PD) of gastric cancer. Although PD is frequent in gastric cancer, no effective therapies have been established. As a result, the prognosis for gastric cancer metastatic to the peritoneum is extremely poor, with a mean survival of only 4 months after diagnosis.3 New therapies are urgently needed to improve the outcomes of gastric cancer patients with PD.
Near infrared photoimmunotherapy (NIR‐PIT) is a newly developed cancer treatment that employs a highly targeted monoclonal antibody (mAb) conjugated to a photo‐absorber.4 Each antibody‐photoabsorber conjugate (APC) binds to the appropriate cell surface antigen. Then, NIR light is delivered to excite the photo‐activatable silica‐phthalocyanine dye, IRDye700DX (IR700), leading to lethal damage to the cell membrane in a highly selective manner. NIR‐PIT has been shown to be effective with a variety of different antibodies conjugated to the same IR700 dye.5, 6, 7, 8, 9, 10 The cell killing after NIR‐PIT is rapid and results in necrotic/immunogenic cell death.9, 11 A first‐in‐human clinical trial of NIR‐PIT in recurrent head and neck cancer patients employed an EGFR‐targeted APC, cetuximab‐IR700 (https://clinicaltrials.gov/ct2/show/NCT02422979); a phase 2 trial was recently completed and fast‐track designation was received from the US FDA for a phase 3 trial beginning in 2018. In the phase 1/2 trial, patients were injected with cextuximab‐IR700 conjugate, referred to as RM‐1929 in the study. Approximately 24 hours later, the tumor was exposed to NIR light by means of a laser at a wavelength of 690 nm, which is absorbed by IR700. Very few side effects were seen and many patients experienced dramatic reductions in tumor size. Formal clinical results are still pending.
The therapeutic effects of NIR‐PIT depend on access of the APC to the target tissue and the ability to deliver NIR light to that tissue. NIR light can be easily delivered to lesions in the mouth and skin by means of an external light source. However, in more deeply located tumors light is rapidly attenuated,12 so that it must be delivered by interstitial light fibers placed within the tumor. In some body cavities, such as the peritoneum and thorax, light theoretically could be delivered into the cavity and diffused so that wide surface areas are exposed. It is important to note that the NIR light therapy is not thermal or destructive by itself so that it can be delivered safely to normal tissue without toxicity.
A solution to delivering NIR light to a body cavity such as the peritoneum is to introduce light fibers with distal optical diffusers, using needles or endoscopes. After prior injection of the APC, peritoneal metastases could bind the agent and then NIR light administered via endoscopic optical fiber diffuser could deliver sufficient light to treat in a relatively minimally invasive way.
Near infrared photoimmunotherapy has been shown to be effective with a variety of different APC in animal models using superficial NIR light illumination.4, 6, 7, 8, 9, 10 Although the feasibility of optical diffuser light delivery has been tested,13 this technology has not been integrated into an endoscopically guided therapy. In this study, we investigate the efficacy of endoscopic NIR‐PIT using a fiber optic diffuser for treating gastric cancer with PD in a mouse model.
2. MATERIALS AND METHODS
2.1. Reagents
Water soluble, silica‐phthalocyanine derivative, IRDye 700DX NHS ester, was obtained from LI‐COR Biosciences (Lincoln, NE, USA). Trastuzumab, 95% humanized IgG1 mAb directed against HER2, was purchased from Genentech (South San Francisco, CA, USA). All other chemicals were of reagent grade.
2.2. Synthesis of IR700‐conjugated trastuzumab
Conjugation of dye with mAb was performed according to a previous report.4 In brief, trastuzumab (1.0 mg, 6.8 nmol) was incubated with IR700 NHS ester (60.2 μg, 30.8 nmol) in 0.1 mol/L Na2HPO4 (pH 8.6) at room temperature for 1 hour. The mixture was purified with a Sephadex G25 column (PD‐10; GE Healthcare, Piscataway, NJ, USA). The protein concentration was determined with a Coomassie Plus Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL, USA) by measuring the absorption at 595 nm with UV‐Vis (8453 Value System; Agilent Technologies, Santa Clara, CA, USA). The concentration of IR700 was measured by absorption at 689 nm with spectroscopy to confirm the number of fluorophore molecules conjugated to each mAb. The synthesis was controlled so that an average of 2 IR700 molecules was bound to a single antibody. We abbreviate IR700 conjugated to trastuzumab as tra‐IR700.
2.3. Cell culture
N87GFP cells stably expressing green fluorescence protein (GFP) were purchased from ANTI CANCER (San Diego, CA, USA). Luciferase‐expressing N87GFP cells were established by transducing the cells with RediFect Red‐FLuc‐Puromycin Lentiviral Particles (PerkinElmer, Waltham, MA, USA). Their high GFP expression and high luciferase expression was confirmed in the absence of a selection agent after 10 passages. We abbreviate this cell line as N87GFP‐luc. Cells were grown in RPMI 1640 (Life Technologies, Gaithersburg, MD, USA) supplemented with 10% FBS and 1% penicillin/streptomycin (Life Technologies) in tissue culture flasks in a humidified incubator at 37°C in an atmosphere of 95% air and 5% carbon dioxide.
2.4. Animal models
All in vivo procedures were conducted in compliance with the Guide for the Care and Use of Laboratory Animal Resources (1996), US National Research Council, and approved by the local Animal Care and Use Committee. Six to eight‐week old female homozygote athymic nude mice were purchased from Charles River (NCI‐Frederick, Frederick, MD, USA). During the procedure, mice were anesthetized with inhaled 3%‐5% isoflurane and/or via intraperitoneal injection of 1 mg of sodium pentobarbital (Nembutal Sodium Solution, Ovation Pharmaceuticals, Deerfield, IL, USA).
2.5. In vivo fluorescence endoscopy
Endoscopic devices modified for fluorescence imaging are shown in Figure 1A. A model BF TYPE P60 bronchoscope (Olympus Medical Systems, Tokyo, Japan), which has a 4.9‐mm distal end diameter, 5.0‐mm insertion tube diameter and 2.2‐mm channel diameter with a single biopsy channel, was inserted into the abdominal cavity through a small lower abdominal incision, and the abdominal cavity was inflated with air. The surface of the peritoneum was observed with white light and fluorescence imaging using a clinical endoscopic light source (CLV‐S190, Olympus Medical Systems) equipped with multi‐band excitation filter. Endoscopic images were obtained via a beam splitter, where the white light images were detected using a color‐charged‐coupled device (CCD) camera (FLIR Integrated Imaging Solutions, BC, Canada) and the fluorescence images were filtered by emission filters (507‐527 nm band‐pass for GFP and 696‐736 nm band‐pass for IR700) and detected with an (EM)‐CCD camera (HAMAMATSU PHOTONICS, Shizuoka, Japan). Both images were displayed side by side on a monitor. Real‐time images of both white light and fluorescence images were recorded. To compare fluorescence intensities during NIR‐PIT, the distance between the tumor and the endoscope head was maintained using the biopsy forceps (FB‐56D‐1, Olympus Medical Systems) as a guide. Camera gain, exposure time and binning for the fluorescence images were held constant throughout the study.
Figure 1.

Multi‐color fluorescence mini‐endoscopic system and near infrared photoimmunotherapy (NIR‐PIT) procedure. A, Multi‐color fluorescence imaging system is based on a clinically available fiber optic endoscope and light source. Excitation light is provided by multi‐band excitation filters. Endoscopic images were obtained via a beam splitter, where the white light images were detected using the color‐CCD camera and the fluorescence images were filtered by multi‐color emission filters and detected with an (EM)‐CCD camera. Both images are displayed side by side on the monitor. B, Cylindrical light diffuser with an NIR laser system was used. The cylindrical light diffuser was used through the endoscope. C, The endoscope was inserted into abdominal cavity through a small lower abdominal incision, and the abdominal cavity was inflated with air. Peritoneal cavity was exposed to NIR laser light using the optical diffuser under endoscopic guidance
2.6. In vivo near infrared photoimmunotherapy
To generate the disseminated peritoneal cancer mouse model, 10 million N87GFP‐luc cells with PBS (total 400 μL) were injected into the peritoneal cavity. Mice developed disseminated peritoneal cancer at 8 days after cell implantation and were randomized into 4 groups of at least 10 animals per group for the following treatments: (i) no treatment (control); (ii) 100 μg of tra‐IR700 i.v., with no NIR light exposure (tra‐IR700 i.v. only); (iii) NIR light exposure only, with NIR light administered at 100 J/cm on day 1 (NIR light only); and (iv) 100 μg of tra‐IR700 i.v., with NIR light administered at 100 J/cm on day 1 after tra‐IR700 injection (endoscopic NIR‐PIT).
Endoscopic NIR‐PIT devices used in this study are shown in Figure 1B. The endoscope was inserted into the abdominal cavity through a small lower abdominal incision, and the abdominal cavity was inflated with air. The peritoneal cavity was irradiated using a fiber optic diffuser (Cylindrical Light Diffuser model RD30; Medlight, Ecublens, Switzerland) inserted through the endoscope and coupled to an NIR laser which emits light at 685‐695 nm (BWF5‐690‐8‐600‐0.37; B&W TEK, Newark, DE, USA) (Figure 1C). The output power density in mW/cm was measured with an optical power meter (PM 100, Thorlabs, Newton, NJ, USA).
For in vivo bioluminescence imaging (BLI), D‐luciferin (15 mg/mL, 200 μL) was injected intraperitoneally and the mice were imaged (Photon Imager) for luciferase activity (photons/min). Regions of interest (ROIs) were set for the entire abdomen to quantify the light flux. The ROIs were also placed on adjacent non‐tumor regions as background. Average light flux of each ROI was calculated.
2.7. Histological analysis
Real‐time tumor biopsies were performed through the endoscope using biopsy forceps (FB‐56D‐1, Olympus, Tokyo, Japan) beginning 6 hours after NIR‐PIT. Mice were euthanized with an overdose of carbon dioxide after the biopsies. Biopsy specimens were fixed for at least 24 hours with 10% formalin. Paraffin‐embedded sections were stained with H&E. Post NIR‐PIT tissue microscopy (BX61; Olympus America, Melville, NY, USA) was then performed.
2.8. Statistical analysis
Data are expressed as means ± SEM from a minimum of 10 experiments. Statistical analyses were carried out using GraphPad Prism version 7 (GraphPad Software, La Jolla, CA, USA). For multiple comparisons, ANOVA followed by Tukey's correction for multiple comparisons was used. A P‐value of < .05 was considered statistically significant.
3. RESULTS
3.1. In vivo fluorescence endoscopy and histological analysis
The treatment and imaging regimen is shown in Figure 2A. Implanted intraperitoneal disseminated tumors were evaluated by white light and endoscopic fluorescence imaging (Figure 2B, C). Tumor‐targeted NIR‐PIT was achieved using the fiber optic diffuser under endoscopic guidance (Figure 2B and Video S1) Fluorescence endoscopy demonstrated multiple 1‐3 mm intraperitoneal disseminated nodules based on GFP fluorescence. After tra‐IR700 injection the implanted intraperitoneal disseminated tumors demonstrated IR700 fluorescence which co‐localized with GFP (Figure 2C; tra‐IR700 i.v. only and endoscopic NIR‐PIT group). In contrast, no IR700 fluorescence was observed in tumors without tra‐IR700 injection (Figure 2C; control and NIR light only group). These results demonstrate that intravenous injection of tra‐IR700 was able to bind to intraperitoneal implants with preserved fluorescence capabilities. In animals undergoing endoscopic NIR‐PIT, IR700 fluorescence signal in tumors diminished due to a combination of cytoreduction caused by NIR‐PIT and partial photo‐bleaching of IR700 (Figure 2C; endoscopic NIR‐PIT group).
Figure 2.
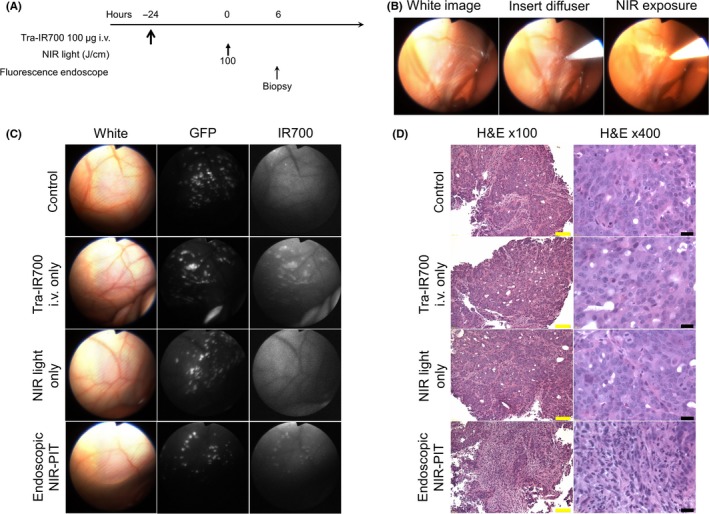
In vivo fluorescence real‐time endoscopic imaging and histological near infrared photoimmunotherapy (NIR‐PIT) effect. A, Treatment regimen is shown. Biopsy specimens were obtained 6 hours after NIR‐PIT. B, In vivo real‐time endoscopic intraperitoneal imaging of N87GFP‐luc tumor‐bearing mice. Peritoneal cavity was exposed to NIR light using an optical diffuser through the endoscope. C, In vivo fluorescence real‐time endoscopic intraperitoneal imaging of N87GFP‐luc tumor‐bearing mice. Tumors demonstrate GFP and IR700 fluorescence in mice administered tra‐IR700. In the absence of tra‐IR700 no IR700 fluorescence was seen. After NIR‐PIT, IR700 fluorescence decreased. D, Biopsy specimens stained with H&E demonstrate few scattered clusters of damaged tumor cells within a background of diffuse cellular necrosis and micro‐hemorrhage with infiltration of inflammatory cells consistent with acute granulation in endoscopic NIR‐PIT group, while no obvious damage was observed in control groups, including tra‐IR700 alone without NIR light and NIR light alone without tra‐IR700 groups. Yellow scale bars = 100 μm. Black scale bars = 20 μm
Real‐time tumor biopsies were performed with biopsy forceps 6 hours after NIR‐PIT (Video S2). H&E staining of NIR‐PIT‐treated N87GFP‐luc tumors revealed diffuse necrosis and micro‐hemorrhage, with scattered clusters of live but damaged tumor cells, while no obvious damage was observed in any of the control groups (Figure 2D).
3.2. In vivo endoscopic near infrared photoimmunotherapy
The treatment and imaging regimen of NIR‐PIT is shown in Figure 3A. IR700 fluorescence signal decreased immediately after NIR light exposure (Figure 3B). In contrast, no significant changes of GFP fluorescence signal were shown after NIR light exposure (Figure 3B). After treatment with NIR‐PIT the bioluminescence of tumors decreased (Figure 3C). Quantitative luciferase activity decreased significantly in the endoscopic NIR‐PIT group compared with the other control groups (P < .01) (Figure 3D). In contrast, luciferase activity of tumors in the control groups increased.
Figure 3.
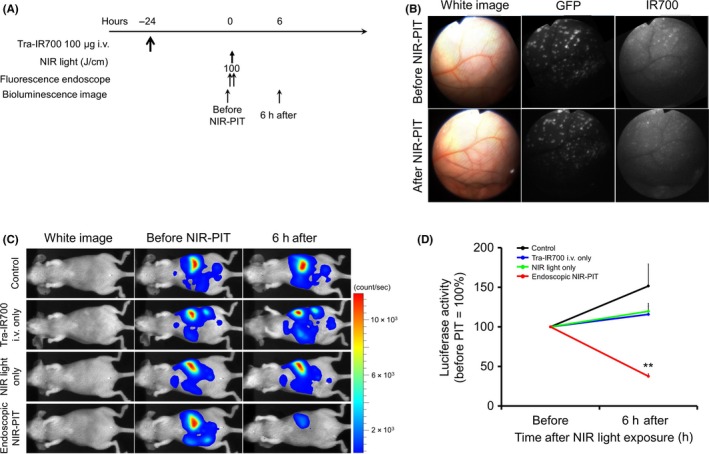
Endoscopic near infrared photoimmunotherapy (NIR‐PIT) and in vivo effect of NIR‐PIT in peritoneal N87GFP‐luc tumors. A, NIR‐PIT regimen. Endoscopic and bioluminescence images were obtained at each time point as indicated. B, In vivo fluorescence real‐time endoscopic intraperitoneal imaging of N87GFP‐luc tumor‐bearing mice. After NIR‐PIT IR700 fluorescence decreased. In contrast, no changes in GFP fluorescence were shown. C, In vivo bioluminescence imaging (BLI) of tumor‐bearing mice in response to NIR‐PIT. Before NIR‐PIT, tumors exhibited high signals on BLI. The tumors treated by NIR‐PIT showed a marked decrease in BLI. D, Quantitative luciferase activity (before NIR‐PIT is set to 100) showed a significant decrease in endoscopic NIR‐PIT groups compared with the 3 control groups (n ≥ 10, **P < .01 vs other groups, by Tukey's t test with ANOVA). Luciferase activity of tumor in other control groups showed an increase
4. DISCUSSION
Despite recent advances in chemotherapy and molecularly‐targeted therapies directed against advanced gastric cancer, the prognosis of patients with disseminated peritoneal gastric cancer remains poor.3 In this study, the therapeutic effect of NIR‐PIT is confirmed in a gastric cancer model with PD, as shown by the loss of luciferase activity immediately after endoscopic NIR‐PIT (Figure 3). Endoscopic NIR‐PIT results in acute cellular necrosis and microhemorrhage in the peritoneal implants (Figure 2). NIR‐PIT with intraperitoneal NIR exposure using a fiber optic diffuser under endoscope guidance successfully treated HER2‐positive disseminated peritoneal gastric cancers based on BLI and histologic evidence. This technique could be readily translated into clinical use for many types of peritoneally disseminated cancers and floating cancer cells arising from gastric, colon, ovarian and bladder cancers provided that a suitable antibody can be identified.
There are several preconditions for successful NIR‐PIT in vivo. First, therapeutic effects induced by NIR‐PIT depend on expression levels of the antigen on the target tumor as well as accessibility of the APC to the target.6, 9 In vivo fluorescence endoscopy clearly showed IR700 fluorescence within peritoneal implants 24 hours after intravenous injection of tra‐IR700 and IR700 signal co‐localized with GFP, while no IR700 fluorescence signal was observed in tumors without tra‐IR700 injection, as shown in Figure 2. These results demonstrate that intravenous injection of tra‐IR700 was able to penetrate into intraperitoneal implants via the vascular system. Thus, the conjugate tra‐IR700 proved to be an effective agent for treating an EGFR‐expressing tumor with NIR‐PIT. Another pre‐requisite for successful NIR‐PIT is the ability to deliver NIR light. In a clinical situation, peritoneal implants would have to be exposed to NIR light either during open surgery or via fiber optic laparoscope. The latter is less invasive and likely to lead to faster patient recovery. As shown in Figure 1, a fiber optic diffuser is introduced into the peritoneal cavity via an endoscope. Similarly, the fiber optic light diffuser could be placed within or near the tumor using laparoscopy, bronchoscopy or cystoscopy depending on the clinical situation. Such procedures could be performed more easily in human patients than in mouse models because an endoscopy or a laparoscopy can be conducted with better flexibility in greater peritoneal space in human patients. Therefore, peritoneally disseminated tumors in most of the peritoneal cavity could be covered with NIR‐PIT using laparoscopic procedures in human cancer patients.
Under fluorescence imaging, the conjugate tra‐IR700 achieved a sufficient target‐to‐background ratio (TBR) to be readily identified (Figure 2), indicating that it may be possible to use fluorescence imaging during surgical or endoscopic procedures to identify cancers that are amenable to NIR‐PIT. Therapeutic amounts of NIR light induce immediate cell membrane damage and death, which reduces the IR700 signal (Figures 2 and 3). Loss of IR700 fluorescence implies both cell death and photobleaching, a sign that NIR light has activated the APC on the target. Using endoscopic fluorescence imaging, the operator is provided with immediate feedback regarding the effectiveness of the initial treatment and information about untreated targets.
Near infrared photoimmunotherapy differs from conventional photodynamic therapy (PDT) in several aspects. PDT produces substantially more toxicity due to the non‐specificity of the photosensitizer which accumulates in tumor and non‐tumor tissue. Light activation causes on‐target and off‐target damage, resulting in dose limiting toxicities. Porphyrin photosensitizers used in PDT do not selectively target cancer at the cellular level.14, 15, 16 Precise control of laser irradiation during treatment is difficult to achieve, resulting in damage to surrounding healthy organs and/or blood vessels. Recently, flexible coaxial laser endoscopy, which localizes the laser illumination only to the selected tumor target, with minimal illumination of the surrounding tissue was reported on.17, 18 While this improves the safety of PDT there is still collateral damage. Moreover, after injection of modern porphyrin derivatives, the patient remains systemically photosensitive for over a week.19 In contrast, because a hydrophilic phthalocyanine‐based photosensitizer, IR700 is used in NIR‐PIT, the results are much more selective. No systemic photosensitivity is observed because the agent is only effective where it binds a sufficient number of target molecules on the cell membrane to cause damage.4 Because of the highly selective binding of APC to cancer cells compared with normal cells, NIR light delivery does not have to be as accurate as for PDT. Most PDT agents are activated by visible range light which penetrates only a few millimeters in tissue,20, 21 whereas the NIR light used in NIR‐PIT can penetrate up to 2 cm into tissue.12 Finally, because NIR‐PIT induces selective immunogenic cell death in cancer cells, it spares all the immune cells in the local tumor micro‐environment.11 Therefore, rapid and effective activation of anti‐cancer host immunity is induced by NIR‐PIT, whereas that effect is more muted in PDT.
This study has several practical limitations. First, many instances of PD of gastric cancers do not overexpress HER2,1, 2 and, therefore, this particular target may not be ideal for all disseminated peritoneal gastric cancer implants. However, NIR‐PIT has been proven to be effective against a variety of membrane antigens using the respective specific antibodies; therefore, it is likely that an appropriate APC or combination of multiple APC could be selected for treating several phenotypes of gastric cancers.6, 8, 22 Furthermore, HER2 expression of cancer cells is not homogeneous even in HER2‐positive tumors that might allow HER2‐negative population of cells to survive after NIR‐PIT. However, immunogenic cell death induced on HER2‐positive cells by NIR‐PIT could elicit host immune response even against additional antigens other than HER2 that would help to induce additional cytotoxicity against HER2‐negative cells. In the future it may be possible to routinely induce tumor cells to express the HER2 extracellular domain using adenoviral vectors, thus providing a target for NIR‐PIT on the surface of disseminated cancer cells that would otherwise not bind to a HER2 targeted antibody.23 This method that has the potential to induce expression of specific membrane antigens could make the tumor amenable to NIR‐PIT even when a cancer does not express any specific antigens. Another limitation of this study is that we could not treat mice repeatedly and could not observe the long‐term course in the mice because the endoscopic system used proved too invasive for mice. Clearly, repeated dosing of the APC with repeated NIR light exposure improves the therapeutic effect in EGFR‐targeted NIR‐PIT.24, 25 It would be desirable to extend these studies to multiple NIR‐PIT sessions and to monitor the long‐term course in appropriate large animal models.
In conclusion, endoscopic NIR‐PIT successfully treated PD of gastric cancer. Endoscopic NIR‐PIT could be a promising method for treating PD using fiber optic diffusers to deliver the activating light. In clinical settings, NIR light could be delivered in a similar manner using customized endoscopes to accommodate a number of anatomic sites throughout the body in which it is possible to insert endoscopic devices.
CONFLICT OF INTEREST
The authors have declared that no competing interest and no financial interest exists.
Supporting information
Nagaya T, Okuyama S, Ogata F, Maruoka Y, Choyke PL, Kobayashi H. Endoscopic near infrared photoimmunotherapy using a fiber optic diffuser for peritoneal dissemination of gastric cancer. Cancer Sci. 2018;109:1902–1908. https://doi.org/10.1111/cas.13621
Funding information
Intramural Research Program of the US National Institutes of Health, National Cancer Institute, Center for Cancer Research (ZIA BC011513).
REFERENCES
- 1. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet. 2010;376:687‐697. [DOI] [PubMed] [Google Scholar]
- 2. Park DI, Yun JW, Park JH, et al. HER‐2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci. 2006;51:1371‐1379. [DOI] [PubMed] [Google Scholar]
- 3. Thomassen I, van Gestel YR, van Ramshorst B, et al. Peritoneal carcinomatosis of gastric origin: a population‐based study on incidence, survival and risk factors. Int J Cancer. 2014;134:622‐628. [DOI] [PubMed] [Google Scholar]
- 4. Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell‐selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17:1685‐1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanaoka H, Nagaya T, Sato K, et al. Glypican‐3 targeted human heavy chain antibody as a drug carrier for hepatocellular carcinoma therapy. Mol Pharm. 2015;12:2151‐2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagaya T, Nakamura Y, Okuyama S, et al. Syngeneic mouse models of oral cancer are effectively targeted by anti‐CD44‐based NIR‐PIT. Mol Cancer Res. 2017;15:1667‐1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nagaya T, Nakamura Y, Okuyama S, et al. Near‐infrared photoimmunotherapy targeting prostate cancer with prostate‐specific membrane antigen (PSMA) Antibody. Mol Cancer Res. 2017;15:1153‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagaya T, Nakamura Y, Sato K, et al. Near infrared photoimmunotherapy with avelumab, an anti‐programmed death‐ligand 1 (PD‐L1) antibody. Oncotarget. 2017;8:8807‐8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagaya T, Nakamura Y, Sato K, Harada T, Choyke PL, Kobayashi H. Near infrared photoimmunotherapy of B‐cell lymphoma. Mol Oncol. 2016;10:1404‐1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nagaya T, Nakamura Y, Sato K, et al. Near infrared photoimmunotherapy with an anti‐mesothelin antibody. Oncotarget. 2016;7:23361‐23369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ogawa M, Tomita Y, Nakamura Y, et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget. 2017;8:10425‐10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henderson TA, Morries LD. Near‐infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr Dis Treat. 2015;11:2191‐2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shuhei O, Tadanob N, Kazuhide S, et al. Interstitial near‐infrared photoimmunotherapy: effective treatment areas and light doses needed for use with fiber optic diffusers. Oncotarget. 2018;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeLaney TF, Sindelar WF, Tochner Z, et al. Phase I study of debulking surgery and photodynamic therapy for disseminated intraperitoneal tumors. Int J Radiat Oncol Biol Phys. 1993;25:445‐457. [DOI] [PubMed] [Google Scholar]
- 15. Hino H, Murayama Y, Nakanishi M, Inoue K, Nakajima M, Otsuji E. 5‐Aminolevulinic acid‐mediated photodynamic therapy using light‐emitting diodes of different wavelengths in a mouse model of peritoneally disseminated gastric cancer. J Surg Res. 2013;185:119‐126. [DOI] [PubMed] [Google Scholar]
- 16. Kishi K, Yano M, Inoue M, et al. Talaporfin‐mediated photodynamic therapy for peritoneal metastasis of gastric cancer in an in vivo mouse model: drug distribution and efficacy studies. Int J Oncol. 2010;36:313‐320. [PubMed] [Google Scholar]
- 17. Hu Y, Masamune K. Flexible coaxial laser endoscope with arbitrarily selected spots in endoscopic view for photodynamic tumor therapy. Appl Opt. 2016;55:8433‐8440. [DOI] [PubMed] [Google Scholar]
- 18. Hu Y, Masamune K. Flexible laser endoscope for minimally invasive photodynamic diagnosis (PDD) and therapy (PDT) toward efficient tumor removal. Opt Express. 2017;25:16795‐16812. [DOI] [PubMed] [Google Scholar]
- 19. Acerbi F, Broggi M, Eoli M, et al. Fluorescein‐guided surgery for grade IV gliomas with a dedicated filter on the surgical microscope: preliminary results in 12 cases. Acta Neurochir (Wien). 2013;155:1277‐1286. [DOI] [PubMed] [Google Scholar]
- 20. Grant WE, Speight PM, Hopper C, Bown SG. Photodynamic therapy: an effective, but non‐selective treatment for superficial cancers of the oral cavity. Int J Cancer. 1997;71:937‐942. [DOI] [PubMed] [Google Scholar]
- 21. Kobayashi W, Liu Q, Nakagawa H, et al. Photodynamic therapy with mono‐L‐aspartyl chlorin e6 can cause necrosis of squamous cell carcinoma of tongue: experimental study on an animal model of nude mouse. Oral Oncol. 2006;42:46‐50. [DOI] [PubMed] [Google Scholar]
- 22. Shirasu N, Yamada H, Shibaguchi H, Kuroki M, Kuroki M. Potent and specific antitumor effect of CEA‐targeted photoimmunotherapy. Int J Cancer. 2014;135:2697‐2710. [DOI] [PubMed] [Google Scholar]
- 23. Ishida M, Kagawa S, Shimoyama K, et al. Trastuzumab‐based photoimmunotherapy integrated with viral HER2 transduction inhibits peritoneally disseminated HER2‐negative cancer. Mol Cancer Ther. 2016;15:402‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mitsunaga M, Nakajima T, Sano K, Choyke PL, Kobayashi H. Near‐infrared theranostic photoimmunotherapy (PIT): repeated exposure of light enhances the effect of immunoconjugate. Bioconjug Chem. 2012;23:604‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nagaya T, Sato K, Harada T, Nakamura Y, Choyke PL, Kobayashi H. Near infrared photoimmunotherapy targeting EGFR positive triple negative breast cancer: optimizing the conjugate‐light regimen. PLoS One. 2015;10:e0136829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


