Abstract
Efficacy of endoscopic screening for esophageal cancer is not sufficiently definitive and lacks randomized controlled trial evidence. The present study proved short‐term screening efficacy through describing and comparing disease stage distributions of intervention and control populations. Villages from Linzhou and Cixian were cluster randomly allocated to the intervention or to the control group and the target population of 52 729 and 43 068 individuals was 40‐69 years old, respectively, and the actual enrolled numbers were 18 316 and 21 178, respectively. TNM stage information and study‐defined stage information of esophageal cases from 2012 to 2016 were collected. Stage distributions were compared between the intervention and control groups in the total target population, as well as in the subgroup populations in terms of enrolment and before or after intervention. There were a total of 199 and 141 esophageal cancer cases in the intervention and control groups, respectively. For the target population, distributions of TNM stage were borderline significant between the two groups after intervention (P = .093). However, subgroup analysis of the enrolled population during the after‐intervention period had statistical significance for both TNM and study‐defined stage. Natural TNM stage distributions were approximately 32%, 41%, 24% and 3% for stages I to IV vs 71%, 19%, 7% and 3% in the intervention population. The natural study‐defined stage distributions from early, middle to advanced stages were approximately 18%, 49% and 33% vs 59%, 33% and 8%. Early‐stage esophageal cancer cases accounted for a higher proportion after endoscopy screening, and the efficacy in the target population depends on the intervention compliance.
Keywords: early detection of cancer, endoscopy, esophageal neoplasm, neoplasm staging, random allocation
Abbreviations
- CIS
carcinoma in situ
- EAC
esophageal adenocarcinoma
- EMR
endoscopic mucosal resection
- ESCC
esophageal squamous cell carcinoma
- ESD
endoscopic submucosal dissection
- mD
mild dysplasia
- MD
moderate dysplasia
- RCT
randomized controlled trial
- SD
severe dysplasia
1. INTRODUCTION
As the eighth most common cancer and the sixth most common cause of cancer death in the world, esophageal cancer has a severe disease burden with annually about 456 000 new cases and 400 000 deaths.1 As for China, which accounts for nearly 50% of esophageal cancer cases worldwide,2 estimation of new cases and deaths in 2013 were 277 000 and 206 000 respectively,3 and most cases are squamous cell carcinoma. As a result of limited knowledge and methods for etiology prevention, secondary prevention is the major approach to esophageal cancer prevention and control, especially for ESCC. In order to detect cancer early and improve prognosis, screening by endoscopy with iodine staining has been the major secondary prevention measure in China. Meanwhile, the efficacy of endoscopy screening has been confirmed by a long‐term follow‐up cohort study,4 but lack of randomization meant that the conclusion was not sufficiently definitive.
Cancer registry is an effective way to monitor cancer incidence, mortality and long‐term trend, as well as providing information on cancer distribution. With the gradual maturity of the cancer registry system in China, more and more sites have established the cancer registry, annually enriching cancer incidence and mortality information for real‐number estimation.5 However, quantitative increments of incidence and mortality information do not fully meet research requirements. If more treatment and diagnosis information was available in the cancer registry, there would be a better connection between clinical study and population‐based study. Unfortunately, in recent years, although cancer registry centers in some countries started to make the effort to add information such as pathological grade and stage,6 few papers referring to detailed and integrated clinical information in population‐based studies have been published worldwide. Large sample‐sized studies on clinical information are still hospital based,7 which may not always reflect actual disease distributions. Meanwhile, in population‐based studies, incidence and death are still the mainstream endpoints.
There is no doubt that stage is one of the most important pieces of clinical information, which guides subsequent treatment and predicts further prognosis. Therefore, we took stage as the endpoint in the present study, not only for providing stage distribution information of esophageal cases from the real world population, but also for evaluating the short‐term efficacy of endoscopy screening through comparing stage distributions between the cluster randomized intervention group and the control group. Moreover, through subgroup analysis, we determined the exact beneficial population and tried to eliminate volunteer bias.
2. MATERIALS AND METHODS
2.1. Study design and population
This was a multiple‐center cluster randomization ambispective cohort study. There were 54 villages from 2 towns in Linzhou and 64 villages from 8 towns in Cixian randomly equally allocated to the intervention or to the control group. The intervention group covered the target population of 52 729 villagers aged from 40 to 69 years, and the control group target population was 43 068. From January 2014 to June 2016, part of the intervention and control group target populations were voluntarily recruited to the enrolled population after completing the informed consent. The intervention and control group enrolled populations were 18 316 and 21 178 respectively (Figure 1). The study was approved by the ethics committee of Cancer Institute and Hospital, Chinese Academy of Medical Sciences.
Figure 1.

Flow chart of cluster randomization and population enrolment
2.2. Questionnaire survey and screening procedure
Both enrolled populations completed the same questionnaire survey on demographic information such as name, age, gender, education, income, smoking status (ever smoked regularly >6 months), alcohol use (any drinking of alcohol during the last 12 months), family history of cancer and so on. After the questionnaire survey, if without contraindications for endoscopic examination (eg, history of reaction to iodine or propofol), the intervention group underwent endoscopy screening. The entire endoscopic procedure was carried out under general anesthesia (20 mL of 1% propofol) with i.v. injection. All other detailed procedures such as examination steps, iodine staining,8 biopsy and so on, were consistent with the previous study;4 the same for histological diagnosis criteria.9 Dysplasia has been validated without doubt as a subsequent risk for carcinoma,10 so both carcinoma and dysplasia were the target of examination. If SD/CIS or early‐stage carcinoma was diagnosed, endoscopic therapy such as EMR and ESD was advised, with endoscopy review to be done 6‐12 months after therapy. Those with more advanced esophageal cancer were advised to treat with surgery, radiotherapy and chemotherapy. Those with mD and MD lesions were encouraged to participate in our endoscopy surveillance every 3 years and every 1 year, respectively, free of charge.
2.3. Incidence and stage information collection
According to local annual cancer registry reports, we collected all the target population esophageal cancer cases from January 2012 to June 2016, and connected the incidence and TNM stage information to the target population baseline information by matching a unique identity number. Both the cancer registry data from Linzhou and Cixian have been adopted by Cancer Incidence in Five Continents. Since recently published papers showed that prognostic implications for clinical categories (cTNM) were not equivalent to pathological categories (pTNM),7 we sacrificed part of the information integrity for quality and consistency, so that all TNM stages were pTNM. For cases without TNM stage information in the cancer registry, we went to all the local hospitals to find the medical records, pathological diagnosis and imaging diagnosis reports to add TNM information as much as possible. We then determined the TNM stage by following the criteria of the AJCC Cancer Staging Manual (7th edn).11 For those cases without sufficient information to determine TNM stage, we use the study‐defined criteria to determine stage. The criteria were as follows: (i) early stage, including stage I cases determined by TNM stage criteria, endoscopic therapy cases without detailed medical records; (ii) middle stage, including stages II and IIIa cases determined by TNM stage criteria, surgery therapy cases without detailed medical records (because the surgery was done in other cities or provinces), and cases suitable for surgery but not accepted because of other reasons (eg, economic reasons, surgery contraindication, patients chose radiotherapy or chemotherapy regardless of doctors’ recommendation); (iii) advanced stage, including stages IIIb, IIIc and IV cases determined by TNM stage criteria, cases that had radiotherapy or chemotherapy only but without any surgery records, cases for which doctors recommended radiotherapy or chemotherapy but this was not followed, and cases that left hospital without any treatment or reasons.
2.4. Statistical analysis
All statistical analyses were carried out through SAS 9.2 software (SAS Institute Inc., Cary, NC, USA). Type I error α was .05 and all tests were 2‐sided. Total numbers of target population esophageal cancer cases, cases with TNM stage and cases with study‐defined stage were summarized by group. Except for describing and comparing important population characteristics distributions, t tests and chi square tests were used to find the statistical significance between intervention and control groups in the enrolled population. After defining the before‐intervention period (2012‐2013) and the after‐intervention period (2014‐2016), distributions of TNM stage and study‐defined stage were compared in terms of period and whether or not there was enrolment between the two groups, to determine the actual endoscopy screening efficacy and the increments of early detection proportion in the real world.
3. RESULTS
3.1. Baseline information
From 2012 to 2016, there were a total of 199 and 141 esophageal cancer cases in the intervention and control group target populations, respectively (Figure 1). In the intervention group of 199 esophageal cancer cases, 101 (50.75%) cases had TNM stages and 168 (84.42%) cases had study‐defined stages. In the control group of 141 esophageal cancer cases, 68 (48.23%) cases had TNM stages and 117 (82.98%) cases had study‐defined stages. For the enrolled population, except for reflux esophagitis and age, the other important population characteristics (gender, education, smoking, alcohol use, family history of cancer, number of household members, income, height and weight) were all statistically significant between the intervention and control groups (Table 1). However, although statistically significant, the actual differences between smoking, alcohol use, height and weight were very small.
Table 1.
Comparison of baseline characteristics between intervention and control groups in the enrolled population
| N(%)/Mean ± SD | P‐value | ||
|---|---|---|---|
| Intervention group | Control group | ||
| Gender | |||
| Male | 7498 (40.94) | 9533 (45.01) | <.001 |
| Female | 10 818 (59.06) | 11 645 (54.99) | |
| Education | |||
| Primary school or under | 8761 (47.83) | 11 301 (53.36) | <.001 |
| Middle school or above | 9555 (52.17) | 9877 (46.64) | |
| Smoking history | |||
| No | 14 536 (79.36) | 16 677 (78.75) | <.001 |
| Yes | 3635 (19.85) | 4047 (19.11) | |
| Cessation | 145 (0.79) | 454 (2.14) | |
| Alcohol use | |||
| No | 16 321 (89.11) | 19 003 (89.73) | .045 |
| Yes | 1995 (10.89) | 2175 (10.27) | |
| Reflux esophagitis | |||
| No | 18 123 (98.95) | 20 928 (98.82) | .233 |
| Yes | 193 (1.05) | 250 (1.18) | |
| Family history of cancer | |||
| No | 11 400 (62.24) | 17 388 (82.10) | <.001 |
| Yes | 6916 (37.76) | 3790 (17.90) | |
| Age (y) | 55.05 ± 7.80 | 54.94 ± 8.10 | .166 |
| No. household members | 4.44 ± 1.91 | 4.32 ± 1.75 | <.001 |
| Income (¥) | 36 136.5 ± 26 699.2 | 43 395.8 ± 31 200.7 | <.001 |
| Height (cm) | 161.8 ± 7.60 | 163.2 ± 8.00 | <.001 |
| Weight (kg) | 65.76 ± 10.76 | 66.27 ± 10.22 | <.001 |
3.2. Comparison of stage distributions
For the total target population, proportions of TNM stage from I to IV were 43.56%, 34.65%, 20.79%, and 0.99% in the intervention group and 32.35%, 41.18%, 22.06%, and 4.41% in the control group, respectively (Table 2), without statistical significance (P = .277). As for study‐defined stage from early, middle to advanced, the proportions were 26.19%, 47.02%, and 26.79% in the intervention group and 18.80%, 48.72%, and 32.48% in the control group, respectively (Table 3), still without statistical significance (P = .296).
Table 2.
Comparison of TNM stage distributions between intervention and control groups
| Before‐intervention | After‐intervention | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention groupa | Control groupa | P‐value | Intervention groupa | Control groupa | P‐value | Intervention groupa | Control groupa | P‐ value | |
| Enrolled population | |||||||||
| Stage (TNM) | |||||||||
| I | 0 (0.00) | 5 (41.67) | >.999 | 22 (70.97) | 4 (23.53) | .006 | 22 (68.75) | 9 (31.03) | .022 |
| II | 1 (100.00) | 6 (50.00) | 6 (19.35) | 7 (41.18) | 7 (21.88) | 13 (44.83) | |||
| III | 0 (0.00) | 0 (0.00) | 2 (6.45) | 5 (29.41) | 2 (6.25) | 5 (17.24) | |||
| IV | 0 (0.00) | 1 (8.33) | 1 (3.23) | 1 (5.88) | 1 (3.13) | 2 (6.90) | |||
| Non‐enrolled population | |||||||||
| Stage (TNM) | |||||||||
| I | 10 (28.57) | 6 (33.33) | .522 | 12 (35.29) | 7 (33.33) | .782 | 22 (31.88) | 13 (33.33) | .735 |
| II | 18 (51.43) | 7 (38.89) | 10 (29.41) | 8 (38.10) | 28 (40.58) | 15 (38.46) | |||
| III | 7 (20.00) | 4 (22.22) | 12 (35.29) | 6 (28.57) | 19 (27.54) | 10 (25.64) | |||
| IV | 0 (0.00) | 1 (5.56) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (2.56) | |||
| Total target population | |||||||||
| Stage (TNM) | |||||||||
| I | 10 (27.78) | 11 (36.67) | .387 | 34 (52.31) | 11 (28.95) | .093 | 44 (43.56) | 22 (32.35) | .277 |
| II | 19 (52.78) | 13 (43.33) | 16 (24.62) | 15 (39.47) | 35 (34.65) | 28 (41.18) | |||
| III | 7 (19.44) | 4 (13.33) | 14 (21.54) | 11 (28.95) | 21 (20.79) | 15 (22.06) | |||
| IV | 0 (0.00) | 2 (6.67) | 1 (1.54) | 1 (2.63) | 1 (0.99) | 3 (4.41) | |||
Each cell contains number of cases and its column proportion, which is same in Table 3.
Table 3.
Comparison of study‐defined stage distributions between intervention and control groups
| Before‐intervention | After‐intervention | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Control group | P‐value | Intervention group | Control group | P‐value | Intervention group | Control group | P‐value | |
| Enrolled population | |||||||||
| Stage (study‐defined) | |||||||||
| Earlya | 0 (0.00) | 5 (26.32) | >.999 | 22 (59.46) | 4 (12.12) | <.001 | 22 (57.89) | 9 (17.31) | <.001 |
| Middleb | 1 (100.00) | 10 (52.63) | 12 (32.43) | 19 (57.58) | 13 (34.21) | 29 (55.77) | |||
| Advancedc | 0 (0.00) | 4 (21.05) | 3 (8.11) | 10 (30.30) | 3 (7.89) | 14 (26.92) | |||
| Non‐enrolled population | |||||||||
| Stage study‐defined) | |||||||||
| Earlya | 10 (20.83) | 6 (30.00) | .716 | 12 (14.63) | 7 (15.56) | .687 | 22 (16.92) | 13 (20.00) | .597 |
| Middleb | 25 (52.08) | 9 (45.00) | 41 (50.00) | 19 (42.22) | 66 (50.77) | 28 (43.08) | |||
| Advancedc | 13 (27.08) | 5 (25.00) | 29 (35.37) | 19 (42.22) | 42 (32.31) | 24 (36.92) | |||
| Total target population | |||||||||
| Stage (study‐defined) | |||||||||
| Earlya | 10 (20.41) | 11 (28.21) | .692 | 34 (28.57) | 11 (14.10) | .047 | 44 (26.19) | 22 (18.80) | .296 |
| Middleb | 26 (53.06) | 19 (48.72) | 53 (44.54) | 38 (48.72) | 79 (47.02) | 57 (48.72) | |||
| Advancedc | 13 (26.53) | 9 (23.08) | 32 (26.89) | 29 (37.18) | 45 (26.79) | 38 (32.48) | |||
Including stage I cases determined by TNM stage criteria, endoscopic therapy cases without detailed medical records.
Including stages II and IIIa cases determined by TNM stage criteria, surgery therapy cases without detailed medical records (because the surgery was carried out in other cities or provinces), and cases suitable for surgery but not accepted because of other reasons (e.g, economic reasons, surgery contraindication, patients chose radiotherapy or chemotherapy regardless of doctors’ recommendation).
Including stages IIIb, IIIc and IV cases determined by TNM stage criteria, cases having radiotherapy or chemotherapy only but without any surgery records, cases for which doctors recommended radiotherapy or chemotherapy but this was not followed, and cases that left hospital without any treatment or reasons.
In the subgroup analysis in which the population was divided into enrolled or non‐enrolled populations and time was divided into before‐intervention and after‐intervention, TNM stage distributions of intervention and control groups had statistical significance in the enrolled population during the after‐intervention (P = .006) and total periods only (P = .022), as well as borderline significance in total target population during the after‐intervention period (P = .093). As for study‐defined stage, distributions of the 2 groups were also statistically significant in the enrolled population during the after‐intervention (P < .001) and total periods (P < .001), as well as in the total target population during the after‐intervention period (P = .047). The few cases in the enrolled intervention group during the before‐intervention period did not allow a comparison to be made.
The above results showed that the stage distributions of the intervention group and the control group in the non‐enrolled population could be considered the same, and the control group in the enrolled population was without intervention, so we combined their stage distribution data to estimate the stage distribution proportions in the natural population. The estimations were about 32%, 41%, 24% and 3%, respectively, for stages I to IV vs 71%, 19%, 7% and 3% in the enrolled intervention group during the after‐intervention period (Figure 2). The estimation for study‐defined stage distributions were about 18%, 49% and 33% from early to advanced stage vs 59%, 33% and 8% in the enrolled intervention group during the after‐intervention period (Figure 3).
Figure 2.
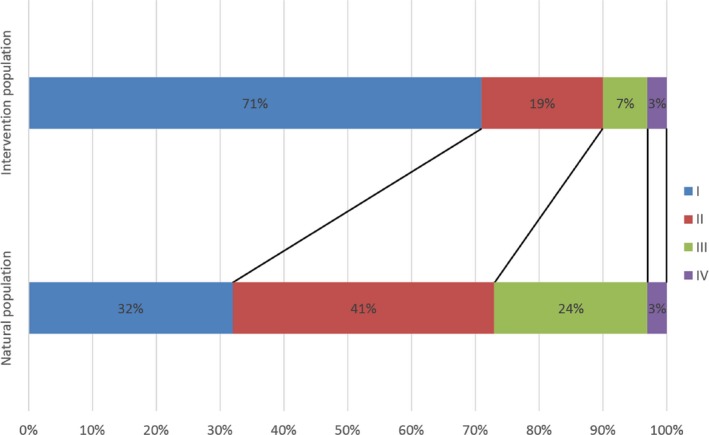
Comparison of estimated TNM stage distribution between natural and intervention populations
Figure 3.
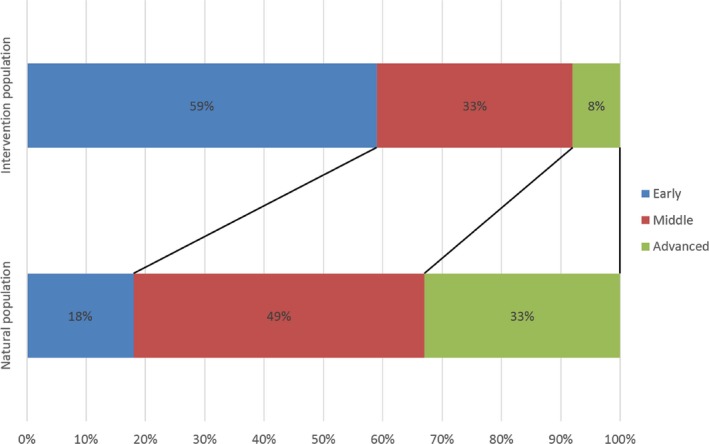
Comparison of estimated study‐defined stage distribution between natural and intervention populations
4. DISCUSSION
As an invasive early examination, endoscopy screening for esophageal cancer was generally considered controversial,12 but that depends on factors discussed below. For EAC and Barrett's esophagus, evidence from population‐based cohort studies have supported the decrease of mortality and better survival by endoscopy screening.13, 14 However, there is a lack of RCT to prove efficacy. Furthermore, cost‐effectiveness or cost‐utility models showed that current surveillance programs do more harm than good.15, 16 For ESCC and dysplasia, endoscopy screening has proven both a decrease in incidence and mortality through a population‐based cohort study,4 and health economics studies support screening in high‐risk areas in China.17, 18 However, with different health economics results, both pathological categories tend to support the effectiveness of endoscopy screening, but still need RCT to draw final conclusions. As individual randomization was not feasible, cluster randomization was used in this study. Disease stage was taken as the short‐term endpoint because follow up has just started and the mortality data are not currently available, as it is well associated with prognosis.
TNM stage distribution comparison in total target populations seemed to show only a borderline significant effect of endoscopy screening, but the trend of a higher proportion of early‐stage cases in the intervention group was obvious. Meanwhile, subgroup analysis shed light on where the real effect came from. In the subgroup analysis, statistical significance was seen in the enrolled population during the after‐intervention period for both TNM stage and study‐defined stage. Early diagnosed esophageal cancer cases accounted for a higher proportion in the intervention group than in the control group. This should mainly be because of many preclinical phase cases that were detected before symptoms appeared through endoscopy screening. We also expected that the screening intervention may at least bring the consciousness of esophageal cancer prevention to the non‐enrolled intervention group population although they were not enrolled. However, in terms of periods and whether enrolled or not, the other subgroups all showed no statistical significance between the 2 groups. This means that the screening effect was only reflected in the enrolled population and had no influence on the non‐enrolled population. Furthermore, the statistical significance of the enrolled population during the total period and the total target population during the after‐intervention period also mainly resulted from the enrolled population during the after‐intervention period. Hence, the viewpoint that the efficacy of screening intervention depends on population compliance. Going back to the total target populations results, it was the high proportion of the non‐enrolled population that diluted the total effect. Screening compliance in China is not always very high19 and is hard to improve, which restricts the expanding of intervention effects. Moreover, as endoscopy screening always costs much in the way of resources, risk stratification and more precise selection of intervention subjects should be another effective way to improve screening efficacy beyond the compliance improvement. Actually, this will not only increase the screening efficacy, but also leave a greater number of normal subjects without harm from endoscopy, and it is applicable for EAC to change the previous poor efficiency surveillance programs.
In our study, it is unavoidable to face the reality that the mere approximately 50% integrality of TNM stage information may lead to conclusions with obvious selection bias. The low integrality of TNM stage information resulted from the following. First, in these rural areas, there was always a fraction of esophageal cancer cases never going to hospital for any treatment or further clear diagnosis until death. Therefore, we couldn't get any medical records, let alone TNM stage information. Second, some cases would receive all their treatment outside the local hospitals. As the hospital information systems in China were not connected to each other, we could not get any medical records about those cases. However, for those having surgery therapy outside but with postoperative adjuvant radiotherapy or chemotherapy in local hospitals cases, although TNM stage information was not available, we at least recognized that their disease still allowed surgery therapy. So, too, the cases with only postoperative complications, recurrence or metastasis treatments in local hospitals. This is why we used the study‐defined stage to supplement the stage information. Moreover, there were also many unresectable local treated cases without exact records of distant organ or lymph node metastasis. Although, whether or not stage IV could not be determined, the advanced status of disease could be recognized after excluding other non‐disease related factors such as economic reasons, patients’ choice about treatments, surgery contraindication and so on. As for several cases leaving hospital without further treatment or reasons, as the medical records were not detailed, we tended to consider them as giving up treatment because of probable advanced disease status.
There was another confused result about the few esophageal cases in the enrolled intervention group population during the before‐intervention period that should be clarified here, no matter the TNM stage or study‐defined stage. For the reason of inevitable volunteer bias in the enrolled population, we had planned to show the screening effect through history control in the ambispective cohort study, expecting no statistical significance before intervention but inverse results after intervention to eliminate volunteer bias. However, the reality of the few esophageal cases in the enrolled intervention group population prevented the plan from proceeding. This may be because of inability to enrol as a result of death or unwillingness to enrol into the intervention group after treatment. Therefore, these were the actual real‐world results.
When comparing baseline information between enrolled intervention and control group populations, several meaningful differences deserve our attention. First is the gender imbalance, showing a higher percentage of females in the intervention group (59.06%) than in the control group (54.99%). As our enrollment was voluntary, we could see that the women's compliance for endoscopy screening should be higher than for men. Studies have shown that ESCC and EAC are more common in men than in women.1, 2, 20 So, the inverse situation indicated a greater need for compliance improvement in men. Besides, enrolled females in the 2 groups both accounted for more than half, which also showed the reality that women may be more willing or have greater opportunities to participate in some public programs. Second, the enrolled intervention group had higher education. This could be easily understood because a more highly educated population may have more health consciousness to participate in screening. As for family history of cancer and income, the difference should come from the information bias. As the questionnaire survey showed that the intervention group was in hospital whereas the control group was in the community, higher income and lower family history of cancer were reported in the control group due to conceal or modify unfavorable information. A previous study reported similar results.4 For other population characteristics such as smoking, alcohol use, height and weight, although statistically significant, the actual difference that may result from gender or education imbalance was very small, and would not strongly affect the comparability of the 2 groups. All in all, we think that the stage distributions between the 2 groups were generally comparable, even though the 2 populations voluntarily enrolled. Also, the cluster randomization should make the target populations comparable.
To our knowledge, this is the first population‐based study to show TNM stage distributions of esophageal cases worldwide. We indicated the proportions of esophageal cases in each stage, and compared differences between the intervention group and the control group in terms of enrolled or non‐enrolled populations and before‐intervention or after‐intervention periods. By taking the TNM and study‐defined stage, but not the previous incidence and mortality, as the endpoint, we again confirmed the efficacy of endoscopy screening. We finally extend the conclusion that screening has an early detection effect only in the enrolled population, and that screening efficacy in the total target population is determined by compliance. We suppose that the current increase in the proportion of early detection, which resulted from early detection of many preclinical phase cases, is just the beginning. As the aim of screening is not only to detect carcinoma, but also to detect precursor lesions, with close follow up and timely treatments for detected precursor lesions, just as the decrease of incidence and mortality,4 the subsequent diagnosed esophageal cases from the intervention group will also have a higher early detection proportion than the control group in the enrolled population. As a long‐term follow‐up cohort, it would be proven in our further study. Also, if such is the case, it will be powerful real‐world evidence to support the efficacy of endoscopy screening.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Science and Technology of the People's Republic of China [grant numbers 2016YFC0901400, 201502001].
Guan C‐T, Song G‐H, Li B‐Y, et al. Endoscopy screening effect on stage distributions of esophageal cancer: A cluster randomized cohort study in China. Cancer Sci. 2018;109:1995–2002. https://doi.org/10.1111/cas.13606
Funding information
Ministry of Science and Technology of the People's Republic of China (Grant/Award Numbers: ‘2016YFC0901400’, ‘201502001’).
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 2. Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381‐387. [DOI] [PubMed] [Google Scholar]
- 3. Chen W, Zheng R, Zhang S, Zeng H, Zou X, He J. Report of cancer incidence and mortality in China, 2013. China Cancer. 2017;26:1‐7. [Google Scholar]
- 4. Wei WQ, Chen ZF, He YT, et al. Long‐term follow‐up of a community assignment, one‐time endoscopic screening study of esophageal cancer in China. J Clin Oncol. 2015;33:1951‐1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115‐132. [DOI] [PubMed] [Google Scholar]
- 6. Kim E, Koroukian S, Thomas CR Jr. Conditional survival of esophageal cancer: an analysis from the SEER registry (1988‐2011). J Thorac Oncol. 2015;10:1490‐1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rice TW, Apperson‐Hansen C, DiPaola LM, et al. Worldwide esophageal cancer collaboration: clinical staging data. Dis Esophagus. 2016;29:707‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dawsey SM, Fleischer DE, Wang GQ, et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83:220‐231. [PubMed] [Google Scholar]
- 9. Dawsey SM, Lewin KJ, Liu FS, Wang GQ, Shen Q. Esophageal morphology from Linxian, China. Squamous histologic findings in 754 patients. Cancer. 1994;73:2027‐2037. [DOI] [PubMed] [Google Scholar]
- 10. Wang GQ, Abnet CC, Shen Q, et al. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rice TW, Rusch VW, Ishwaran H, Blackstone EH, Worldwide Esophageal Cancer Collaboration . Cancer of the esophagus and esophagogastric junction: data‐driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763‐3773. [DOI] [PubMed] [Google Scholar]
- 12. Voltaggio L, Cimino‐Mathews A, Bishop JA, et al. Current concepts in the diagnosis and pathobiology of intraepithelial neoplasia: a review by organ system. CA Cancer J Clin. 2016;66:408‐436. [DOI] [PubMed] [Google Scholar]
- 13. Corley DA, Levin TR, Habel LA, Weiss NS, Buffler PA. Surveillance and survival in Barrett's adenocarcinomas: a population‐based study. Gastroenterology. 2002;122:633‐640. [DOI] [PubMed] [Google Scholar]
- 14. Verbeek RE, Leenders M, Ten Kate FJ, et al. Surveillance of Barrett's esophagus and mortality from esophageal adenocarcinoma: a population‐based cohort study. Am J Gastroenterol. 2014;109:1215‐1222. [DOI] [PubMed] [Google Scholar]
- 15. Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N. Surveillance of Barrett's oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling. Health Technol Assess. 2006;10:1‐142, iii‐iv. [DOI] [PubMed] [Google Scholar]
- 16. Hirst NG, Gordon LG, Whiteman DC, Watson DI, Barendregt JJ. Is endoscopic surveillance for non‐dysplastic Barrett's esophagus cost‐effective? Review of economic evaluations. J Gastroenterol Hepatol. 2011;26:247‐254. [DOI] [PubMed] [Google Scholar]
- 17. Yang J, Wei WQ, Niu J, et al. Estimating the costs of esophageal cancer screening, early diagnosis and treatment in three high risk areas in China. Asian Pac J Cancer Prev. 2011;12:1245‐1250. [PubMed] [Google Scholar]
- 18. Yang J, Wei WQ, Niu J, Liu ZC, Yang CX, Qiao YL. Cost‐benefit analysis of esophageal cancer endoscopic screening in high‐risk areas of China. World J Gastroenterol. 2012;18:2493‐2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang M, Hao C, Zhao D, et al. Distribution of esophageal squamous cell cancer and precursor lesions in high‐risk areas, Linzhou in Henan province and Feicheng in Shandong province of China, 2005‐2009. Zhonghua yu fang yi xue za zhi [Chinese journal of preventive medicine]. 2015;49:677‐682. [PubMed] [Google Scholar]
- 20. Wheeler JB, Reed CE. Epidemiology of esophageal cancer. Surg Clin North Am. 2012;92:1077‐1087. [DOI] [PubMed] [Google Scholar]


