ABSTRACT
The significant increase in the incidence rates and ongoing outbreaks of serogroup C meningococcal (MenC) disease, associated with the sequence type-103 complex, motivated the incorporation of the meningococcal C conjugate (MCC) vaccine in the routine immunization program in the State of Bahia, Brazil in early 2010, targeting children younger than 5 years of age. In its capital, Salvador, the program also included a catch-up campaign for individuals 10–24 years of age. We performed an observational, ecological study, analyzing data collected from 2007 to 2015, to compare the impact of these two immunization strategies on meningococcal disease incidence and mortality rates.
In Salvador, following the vaccination program, a dramatic early impact on MenC disease and mortality rates could be observed, with significant reductions in incidence rates of MenC disease in all age groups, including individuals that were too old to have been vaccinated, indicating the presence of herd protection. Compared to the pre-vaccine period, a virtual disappearance of MenC disease was observed in 2015. However, in the state of Bahia (excluding the city of Salvador), no herd protection could be observed, with significant impact only among vaccine-eligible children within 5 years of introduction of the MCC vaccination program.
These results highlight the importance of catch-up campaigns, including adolescents and young adults, to induce herd protection compared to immunization strategies restricted to infants and young children. This information is crucial for identifying optimal immunization policies and future strategies, focused on adolescents, to optimize the impact of MCC vaccination programs.
KEYWORDS: Meningococcal disease, Neisseria meningitidis, meningococcal conjugate vaccines, surveillance, adolescent, herd protection
Introduction
Meningococcal disease (MD) is a leading cause of morbidity and mortality worldwide,1 affecting 0.5–1.2 million people, each year. Even in treated patients, the case-fatality rate (CFR) of MD often exceeds 10%, with 50,000 to 135,000 deaths occurring annually.2,3 In addition, a range of long term sequelae (including paralysis, hearing loss, mental impairment, amputations, and seizures) may affect up to 20% of survivors.4 Meningitis and septicemia are the most common clinical presentations of the disease.3,5 Although MD affects individuals of all ages, the highest rates of disease are usually found among children aged <5 years, especially in infants. Peaks in incidence are also seen in adolescents as well as in elderly in some countries.4-6 The incidence rates and serogroup distribution of MD varies by country and over time. However, 6 of the 12 recognized serogroups (A, B, C, W, X and Y) are known to cause virtually all cases of the disease worldwide.4-6
Vaccination is considered the best control strategy for prevention of meningococcal disease. Even though meningococcal polysaccharide vaccines have played a pivotal role in disease prevention, controlling outbreaks and epidemics, they have several limitations. They do not generate an adequate immune response against serogroup C in young children, even in patients over 2 years of age the protection offered is of limited duration. Furthermore, they are capable of inducing hypo-responsiveness after subsequent doses. Meningococcal conjugate vaccines, in contrast, offer a number of advantages over polysaccharide formulations, inducing the production of high antibody levels, including in young infants, higher antibody avidity and increased serum bactericidal activity. They can also prevent acquisition of meningococci nasopharyngeal carriage among vaccinees, which proved to be of paramount importance in the success of the immunization programs with meningococcal C conjugate (MCC) vaccines.7 The United Kingdom was the first country to implement a national MCC immunization program in 1999, through routine infant immunization and a large catch-up campaign including all children, adolescents and young adults, resulting in significant and sustained disease reduction, with the induction of herd protection.8,9 More recently, the introduction of MenAfriVac, a meningococcal group A conjugate vaccine, through mass immunization campaigns of 1–29-year-olds in the “meningitis belt” countries in Africa, also has had a dramatic effect on the incidence of meningitis cases.10
In Brazil, MD has been a major public health concern over time, providing opportunities for several vaccination programs, with important lessons learned from these experiences for an effective and successful control of the disease. In the 1970s, the spread of a serogroup A epidemic strain, with incidence rates as high as 180 cases/100,000 population in determined regions, provided the first major experience in the world with polysaccharide A and C vaccines on a large scale, resulting in the successful control of the epidemic. During the 1980s and 1990s, serogroup B meningococcal (MenB) disease became prevalent in Brazil, with practically no cases of serogroup A reported. Epidemics attributed to MenB in several locations around the country were observed, motivating a reactive vaccination campaign in the early 1990s with the Cuban serogroup B outer membrane vesicle vaccine targeting almost 3 million children in the State of Sao Paulo, with limited impact, if any, on the disease rates and a lack of effectiveness in young children.11,12 From 2002 onward, a significant rise in the number and proportion of cases due to serogroup C, associated with the ST-103 complex, was registered, with several outbreaks affecting different regions across the country.11,12
During 2007–2009, the incidence rate of MenC disease increased substantially in the state of Bahia. In response, the state government initiated in 2010 an immunization campaign with the meningococcal C conjugate (MCC) vaccine, with a two-dose primary immunization series (at 2 and 4 months) followed by a booster dose at 12–15 months. Children between 1 and 5 years of age were eligible to receive one dose of MCC vaccine. In the capital, Salvador, a different strategy was implemented extending the vaccination to the population aged 10–24 years-old.13 Furthermore, in late 2010, the Brazilian Ministry of Health introduced the MCC vaccine for children <2 years-old into the routine schedule of the national immunization program.12-14 The MCC schedule for infants included two doses at either 2 and 4 months or 3 and 5 months followed by a booster at 12–15 months of age. Unlike Salvador, the MCC vaccination program in the rest of the country was implemented without a catch-up campaign.12-14
The provision of a surveillance system to estimate disease burden of MD plays a pivotal role in the development and implementation of the disease control and prevention strategies by monitoring changes in the epidemiology of MD across the time, such as disease rates, mortality rates, serogroup replacement, and detecting outbreaks. The aims of the present study were to analyze and compare the effects of two different vaccination strategies against MenC disease in the epidemiology of MD, one in the State of Bahia (excluding its capital, Salvador), that targeted only children younger than 5 years of age and the other in the city of Salvador, the only place in Brazil where the MCC vaccination program was implemented with an extended catch-up campaign, including adolescents and young adults from 10–24 years.
Results
From 2007 to 2015, a total of 1,264 cases of MD and 325 deaths were reported in the state of Bahia, of which 517 cases of MD and 128 deaths were reported in the city of Salvador. Males accounted for 58.1% of all cases and 57.5% of deaths.
Serogroup information was available for 715 cases of MD (56%). In the State of Bahia, a lower proportion of confirmed cases with serogroup information was observed compared to Salvador (47.5% vs 69.1%). Among the 715 cases of MD in the State of Bahia with serogroup information, 320 (44.7%) were from Salvador and 395 (55.3%) from the State of Bahia (without Salvador), with a clear predominance of serogroup C, responsible for 85.3% and 89.6% of the identified cases, respectively, in the city of Salvador and in the State of Bahia (without Salvador), followed by serogroup B (9.5% and 7.1%) and serogroup W (5.2% and 3.3%). No trends in the proportion of confirmed cases without serogroup information was observed over time during the study period.
In Salvador, a rapid and significant increase in MenC disease incidence rates was observed during the pre-vaccination period, from 0.49/100,000 in 2007 to a peak of 4.0/100,000 population in 2010 (Fig. 1). The highest incidence rates of MenC disease in the pre-vaccine period were observed in the 0–4 years age group (5.45/100,000 population), followed by the 5–9 years (4.06/100,000 population), 10–19 years (2.94/100,000 population), 20–39 years (1.92/100,000 population) and individuals > 40 years (0.96/100,000 population) (Table 1). In the State of Bahia (without Salvador) incidence rates of MenC disease also increased significantly, from 0.16/100,000 population in 2007 to 0.54/100,000 population in 2010. A similar pattern was observed in the age group distribution, with the highest incidence rates in the 0–4 years age group and a decreasing trend toward older age groups (Table 1).
Figure 1.
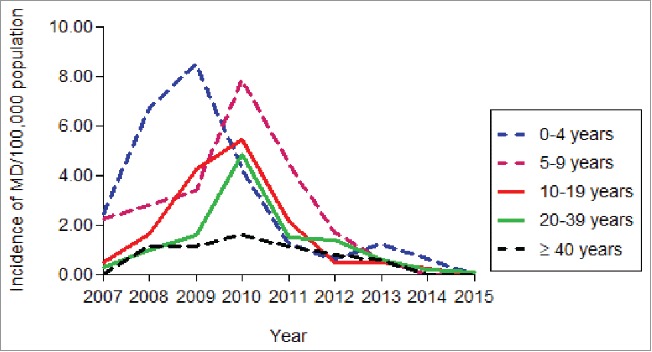
Incidence rates of serogroup C meningococcal disease, by age group, in the city of Salvador, 2007 – 2015.
Table 1.
Incidence rates of MenC disease by age group in the city of Salvador and in the state of Bahia (without Salvador), Brazil, in pre- and post-vaccine periods.
| Salvador |
Bahia (without Salvador) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age group (years) | Pre-vaccine period Rateα | Post-vaccine period Rateα | RR* | (95% CI)† | p value | Pre-vaccine period Rateα | Post-vaccine period Rateα | RR* | (95% CI)† | p value |
| 0-4 | 5.45 | 0.73 | 0.11 | (0.04-0.28) | <0.0001 | 0.78 | 0.24 | 0.30 | (0.15-0.63) | 0.001 |
| 5-9 | 3.91 | 1.11 | 0.28 | (0.13-0.58) | 0.006 | 0.48 | 0.27 | 0.57 | (0.21-1.13) | 0.116 |
| 10-19 | 3.05 | 0.70 | 0.23 | (0.12-0.40) | 0.006 | 0.55 | 0.42 | 0.76 | (0.51-1.14) | 0.203 |
| 20-39 | 1.92 | 0.78 | 0.39 | (0.27-0.58) | 0.001 | 0.23 | 0.19 | 0.84 | (0.53-1.34) | 0.480 |
| ≥ 40 | 1.01 | 0.45 | 0.44 | (0.25-0.76) | 0.004 | 0.15 | 0.11 | 0.76 | (0.41-1.40) | 0.389 |
| Total | 2.13 | 0.67 | 0.31 | (0.24-0.39) | <0.0001 | 0.33 | 0.22 | 0.67 | (0.53-0.85) | 0.001 |
Relative risk;
Confidence interval;
Average annual incidence rate per 100,000 population.
After the introduction of MCC vaccination in 2010, a significant decrease in incidence rates of MenC disease was observed in the city of Salvador. Comparing the post-vaccination period with the pre-vaccination period we observed a significant reduction of 69% (p < 0.0001) in overall MenC disease rates in Salvador (Table 1). In the first year after the MCC vaccination campaign, a 58% reduction in the incidence rates of MenC disease was already observed, decreasing from 4.0/100,000 population in 2010 to 1.67/100,000 population in 2011 (Fig. 1), followed by a reduction of 75% (1.0/100,000 population) in 2012, 86.2% (0.55/100,000 population) in 2013, 92.9% (0.14/100,000 population) in 2014 and 98% (0.03/100,000 population) in 2015. Table 1 clearly shows that reductions in incidence rates observed in the City of Salvador were significant in all age groups, including individuals aged ≥ 40 years, an age group that was not eligible to receive the vaccine. A significant reduction in MenC mortality rates of 57% (p = 0.005), from 0.46/100,000 population in the pre-vaccine period to 0.20/100,0000 population in the post-vaccine period, was also observed in Salvador (Table 2).
Table 2.
Mortality rates of Meningococcal disease by serogroup in the city of Salvador and in the state of Bahia (without Salvador), Brazil, in pre- and post-vaccine periods.
| Meningococcal disease |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Salvador |
Bahia (without Salvador) |
||||||||||
| Deaths | Mortalityπ | RR* | 95% CI† | p-value | Deaths | Mortalityπ | RR* | 95% CI† | p-value | ||
| All cases | |||||||||||
| Pre-vaccine periodα | 83 | 0.78 | ref | 109 | 0.25 | ref | |||||
| Post-vaccine periodβ | 45 | 0.34 | 0.43 | (0.30-0.62) | <0.0001 | 88 | 0.16 | 0.64 | (0.48-0.85) | 0.002 | |
| MenC | |||||||||||
| Pre-vaccine period | 50 | 0.46 | ref | 27 | 0.06 | ref | |||||
| Post-vaccine period | 27 | 0.20 | 0.43 | (0.27-0.68) | 0.005 | 33 | 0.06 | 0.97 | (0.58-1.62) | 0.9351 | |
| MenW | |||||||||||
| Pre-vaccine period | 0 | 0.00 | ref | 1 | 0.002 | ref | |||||
| Post-vaccine period | 6 | 0.04 | — | — | 0.25 | 6 | 0.01 | 4.8 | (3.10-7.71) | <0.0001 | |
| MenB | |||||||||||
| Pre-vaccine period | 4 | 0.04 | ref | 1 | 0.002 | ref | |||||
| Post-vaccine period | 2 | 0.00 | 0.00 | (0.00-39.00) | 0.25 | 4 | 0.007 | 3.2 | (2.02-5.23) | <0.0001 | |
| Unidentified Serogroups | |||||||||||
| Pre-vaccine period | 29 | 0.26 | ref | 80 | 0.18 | ref | |||||
| Post-vaccine period | 10 | 0.07 | 0.29 | (0.29-1.50) | 0.050 | 44 | 0.08 | 0.45 | (0.18-1.03) | 0.021 | |
Relative risk;
Confidence interval;
2007-2010;
2011-2015;
Average annual mortality rate per 100,000 population.
The analysis of the impact on MenC disease rates in the State of Bahia (excluding the city of Salvador), where the vaccination program only targeted children < 5 years, showed that in the post-vaccination period a significant reduction of 33% (p = 0.001) in overall MenC disease rates was observed (Table 1). In the first year after the MCC vaccination campaign, a reduction of only 9% in the incidence of MenC disease was observed, decreasing from 0.55/100,000 population in 2010 to 0.50/100,000 population in 2011 (Fig. 2), followed by a reduction of 58% (0.23/100,000 population) in 2012, 65.4% (0.19/100,000 population) in 2013, 65.4% (0.19/100,000 population) in 2014 and 88% (0.06/100,000 population) in 2015. However, performing the analysis stratifying the impact on the different age groups, we could observe that reductions of MenC disease rates in the post-vaccination period reached statistical significance only for children 0–4 years, the age group that was targeted by the vaccination program, with no significant impact noted in other age groups (Table 1). Mortality rates of MenC disease in the post-vaccine period were identical to the pre-vaccine period in the State of Bahia (without Salvador) (Table 2).
Figure 2.
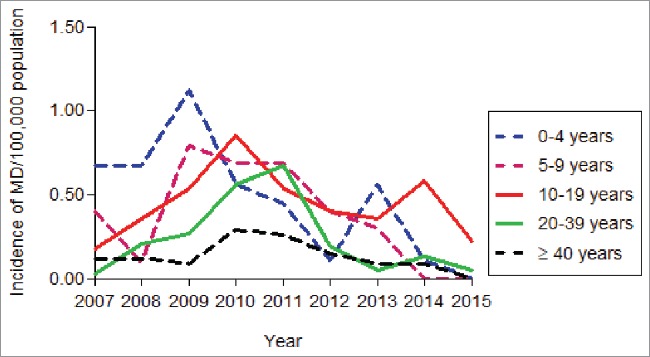
Incidence rates of serogroup C meningococcal disease, by age group, in the state of Bahia (without Salvador), 2007–2015.
According to the descriptive data, no significant difference was observed in MenB disease rates in Salvador and also in the State of Bahia (without Salvador) in the post-vaccination period compared to the pre-vaccination period, although a decreasing incidence trend was observed (Table 3). Few cases of MenW were identified from 2011 in both regions, with increased incidence rates during the post-vaccine period in the state of Bahia (without Salvador).
Table 3.
Incidence rates of meningococcal disease by serogroup in the city of Salvador and in the state of Bahia (without Salvador), Brazil, in pre- and post-vaccine periods, 2007–2015.
| Salvador |
Bahia (without Salvador) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age group (years) | Pre-vaccine period Rateα | Post-vaccine period Rateα | RR* | (95% CI)† | p value | Pre-vaccine period Rateα | Post-vaccine period Rateα | RR* | (95% CI)† | p value |
| 0-4 | 5.45 | 0.73 | 0.11 | (0.04-0.28) | <0.0001 | 0.78 | 0.24 | 0.30 | (0.15-0.63) | 0.001 |
| 5-9 | 3.91 | 1.11 | 0.28 | (0.13-0.58) | 0.006 | 0.48 | 0.27 | 0.57 | (0.21-1.13) | 0.116 |
| 10-19 | 3.05 | 0.70 | 0.23 | (0.12-0.40) | 0.006 | 0.55 | 0.42 | 0.76 | (0.51-1.14) | 0.203 |
| 20-39 | 1.92 | 0.78 | 0.39 | (0.27-0.58) | 0.001 | 0.23 | 0.19 | 0.84 | (0.53-1.34) | 0.480 |
| ≥ 40 | 1.01 | 0.45 | 0.44 | (0.25-0.76) | 0.004 | 0.15 | 0.11 | 0.76 | (0.41-1.40) | 0.389 |
Relative risk;
Confidence interval;
2007-2010;
2011-2015;
Average annual incidence rate per 100,000 population.
Discussion
Two different vaccination strategies were implemented to control MD in the State of Bahia, one is its capital, Salvador, with routine immunization of infants and a “one-time” catch-up campaign including children aged < 5 years and individuals 10–24 years of age and the other, in the rest of the state, with routine immunization of infants and a limited catch-up campaign only including children aged < 5 years. The significant number of cases associated with high case fatality rates reported among adolescents and young adults in the City of Salvador provided the rational for the decision to also include these age groups in the vaccination strategy implemented. These different approaches provided a unique opportunity to compare the effects of these vaccination strategies on MD burden.
Our results showed that in Salvador, after the vaccination program, a dramatic early impact on MenC disease and mortality rates could be observed, with significant reductions in incidence rates of MenC disease observed in all age groups, including individuals that were too old to have been vaccinated, indicating the presence of herd protection. Decreases in incidence rates were already observed in the first year after the implementation of the vaccination program, when a 58% reduction was demonstrated in overall MenC rates and were sustained for at least 5 years after implementation. Compared to the pre-vaccine period, a virtual disappearance of Men C disease was observed in 2015.
Overall, the effects of the vaccination program in Salvador were similar to those observed in UK and several other European countries, Australia and Canada, that also introduced MCC vaccination with catch-up campaigns targeting adolescents and young adults.15-19 The success of the MCC vaccination program in these countries was attributed to the combined efficacy of the vaccine not only preventing disease but also preventing acquisition of carriage.8,20 A striking feature of these MCC vaccination programs, which included catch-up campaigns achieving high coverage among adolescents and young adults, has been the additional decrease in disease incidence in unvaccinated individuals as a result of herd protection.21 Immunization of adolescents and young adults (the age groups usually responsible for the highest rates of colonization) in these catch-up campaigns prevents the acquisition of MenC carriage in the vaccinated age group, interrupting transmission of the organism in the community.8,20,21
In Salvador, an 80% coverage was achieved among young adolescents 10–14 years and a 67% coverage among older adolescents 15–19 years. The importance of achieving high coverage rates among adolescents and young adults in catch-up campaigns to obtain herd effects is highlighted by the recent example of France, where MCC vaccination coverage rates in these age groups were too low, limiting the impact on disease rates in unvaccinated age groups.22
Comparing to Salvador, the effects of the vaccination program in the state of Bahia (excluding Salvador), however, were limited. Despite the dramatic decrease in the incidence rates of MD among children < 5 years, the age group that was vaccinated, no significant impact was observed in other age groups within 5 years of introduction of the MCC vaccination program. In Spain, where the MCC vaccination program initially targeted only children up to the age of six years, without immunizing adolescents and young adults, herd effects were also limited, with no apparent impact on unvaccinated children.18 Interestingly, other studies that evaluated the impact of the MCC vaccination program in Brazil, where vaccination was restricted to infants and toddlers, without catch-up in adolescents and young adults, found similar results to those observed in the state of Bahia, excluding the city of Salvador. In a preliminary analysis of the early impact of the routine infant MCC vaccination program in Brazil, the authors could observe significant decreases in incidence rates only among age groups that were vaccinated.23 More recently, other studies that analyzed the effects of the MCC vaccination after three24 and four25 years of its introduction into the Brazilian NIP, also concluded that the impact on disease rates in Brazil could be observed only among vaccine-eligible age groups.
Moving forward with the MCC vaccination program in Brazil, to achieve the same impact that was observed in the city of Salvador, a key strategy to maximize control of MD and further reduce serogroup C disease rates and interrupt meningococcal transmission, beyond the levels observed so far, would be to induce herd protection in populations where it is currently lacking.
Despite the lack of reliable baseline data collected prior to vaccine introduction in Brazil, a study performed 4 years after the implementation of the MCC vaccination program in Salvador showed that MenC carriage rates among a representative sample of adolescents were very low.26 However, in a similar study performed in adolescents during the post-vaccination period in the state of Sao Paulo, where the immunization program was restricted to infants and toddlers, the authors found an unusually high dominance of serogroup C, associated with the virulent clone belonging to the ST-103 complex, almost eight times higher than the prevalence rate of MenC carriage found in Salvador.27 Interestingly, comparing to data from European and North-American countries, where carriage is most frequent in young adults,28 these trials in Brazil demonstrated that carriage rates were highest at younger ages.
Taking all this evidence into account the Ministry of Health in Brazil decided to change the National Immunization Program, including a limited phased catch-up campaign with the MCC vaccine during adolescence.29 In the next four years, two cohorts will be vaccinated each year, starting in 2017 with adolescents 12–13 years of age, followed by 11–12 years of age in 2018, 10–11 years of age in 2019 and 9–10 years of age in 2020. After this phased catch-up campaign, a booster dose of MCC vaccine will be routinely recommended at 9 years of age together with the HPV vaccine, to optimize the coverage of these two vaccines. The routine adolescent vaccination, inducing high levels of serum bactericidal antibodies, will be of paramount importance not only to provide direct protection against disease, since there is evidence suggesting that infant/toddler vaccination is not sufficient for long-term protection,29-30 but also to maintain the herd protection that is expected to occur after the phased catch-up campaign.31-33
This is an observational, ecological study, and thus subject to potential limitations. First, the burden of the disease may have been underestimated since we are analyzing secondary data that are not updated and underreporting is a common event. Second, rates were analyzed without redistribution of cases of unidentified serogroups; therefore serogroup-specific incidence was probably underestimated as well. A further limitation is that we may have different characteristics, in terms of density and carriage rates, in the two populations (from Salvador, an urban metropolis; and in the rest of the State, where urban and rural areas are present), that might have influenced the evaluated outcomes. However, all these potential limitations should not detract from the demonstration of the importance of catch-up campaigns, as already shown with meningococcal vaccination programs elsewhere.9-10
Finally, although there is no clear evidence to date that MCC vaccination may lead to vaccine-driven serogroup replacement,34-36 continuous surveillance is mandatory after implementation of mass immunization programs. Our results showed that MenB disease rates remained stable during the study period, without significant differences between pre- and post-vaccine periods, which is in line with the experience reported in other countries that introduced the MCC vaccine in mass immunization programs.9,37–41 Although limited to few cases, we observed a rise in the incidence and mortality rates of serogroup W disease in the post-vaccination period. The emergence of the MenW hypervirulent ST-11 clonal complex, which is associated with high case-fatality rates and a propensity to cause outbreaks and epidemics, has also been observed in the southern region in Brazil, Argentina, Chile, Australia, UK as well as other European countries42-48 requiring close monitoring and continuous surveillance.
In summary, the evidence gathered during this study provides robust evidence on the importance of achieving herd protection with MCC vaccination programs in order to substantially reduce morbidity and mortality rates due to serogroup C MD. It is important to emphasize that this is the first evidence in the literature showing the ability of MCC vaccines to provide herd protection in a place with increased incidence of MenC disease associated with the spread of the hypervirulent clone belonging to the ST-103 complex, highly unrelated to ST-11 complex strains. This information is vital for a better understanding of the MD epidemiology in Brazil and to anticipate the potential impact of different immunization strategies with MCC vaccines on disease burden. Continuous surveillance is required to provide information for evaluating meningococcal conjugate immunization schedules and to guide future vaccination strategies.
Patients and methods
Study design and population
This is an observational, ecological study conducted in the state of Bahia, located in the northeast region of Brazil. We investigated the incidence and distribution patterns of the confirmed cases of MD occurred before (2007-2010) and after (2011-2015) the immunization campaign with MCC vaccine.
The MCC vaccination was implemented in the routine infant immunization program in the State of Bahia (14,016,906 inhabitants in 2010) in February 2010. The recommended schedule was 2 doses, at 3 and 5 months of age, followed by a booster dose at 12–15 months. A limited catch-up vaccination was performed, only including children between one and five years, that received one dose of the vaccine.
In the capital of the State, Salvador (population of 2,675,656 inhabitants in 2010), the vaccination program also targeted children aged < 5years, but included an extended catch-up, targeting individuals from 10–24 years of age performed in three phases, between May and August 2010. Individuals from 10–14 years were the first group vaccinated, followed by the group from 15–19 years and the group from 20–24 years. Children from 5–9 years were not vaccinated.
Both MenC-TT (Neisvac-C®, Baxter Vaccines) and MenC-CRM (Menjugate®, Novartis Vaccines) vaccines were used during the campaign. Initially, in February 2010, MenC-TT was the only vaccine used in the State of Bahia, replaced by the use of MenC-CRM vaccine, once MCC vaccination was implemented in the whole country, from August 2010.
Coverage rates were calculated using the information system of the National Immunization Program (PNI, http://tabnet.datasus.gov.br/cgi/deftohtm.exe?pni/cnv/cpniuf.def). Coverage rates with at least one dose of the vaccine in 2011, the first year after the implementation of the vaccination program, for children < 5 years of age were estimated at 93% both in Salvador and in the State of Bahia. For the age groups included in the catch-up campaign in Salvador, coverage reached 80% for the group from 10–14 years, 67% for the group 15–19 years and 40% for the group 20–24 years at the end of 2010.
Data source
Information was based on the national Notifiable Diseases Information System (SINAN) sponsored by the Informatics Department of the National Health System (DATASUS), Health Ministry, Brazil.49
In Brazil, the notification of cases of MD is mandatory. A confirmed case is defined by the presence of at least one of the following: isolation of Neisseria meningitidis from a normally sterile site, detection of bacterial DNA by polymerase chain reaction (PCR), antigen detection, clinical-epidemiological criteria (case of close contact with a laboratory confirmed case), gram-staining, or clinical criteria (patient with suggestive symptoms and petechial or purpuric rash).50 In this study, all confirmed cases for meningococcal meningitis, meningococcemia and/or meningococcal meningitis plus meningococcemia were considered.
Data analysis
The incidence of MD was assessed year by year, comparing aggregated data for the pre- and post-vaccination period according to sex, age group (0-4, 5–9, 10–19, 20–39 and ≥40 years) and serogroup of Neisseria meningitidis. The incidence and mortality rates were calculated by serogroup and time period. Rates were not adjusted for the proportion of confirmed MD cases with unidentified serogroup. The annual number of deaths of MD was assessed for 2007–2015 and analyzed by serogroup and sex. Death was recorded as the outcome reported at the DATASUS database. We obtained population estimates for the city of Salvador and for the State of Bahia from the Brazilian Institute of Geography and Statistics (IBGE) and used the 2010 national census.51
For the morbidity and mortality rates, we calculated serogroup-specific relative risk (RR) to compare pre-vaccine to post-vaccine periods with the respective confidence intervals (95% CI). The significance level was set at p≤0.05. Statistical analysis was performed using STATA version 12 (STATA Corp., Texas, USA).
Disclosure of potential conflicts of interest
M.A.P.S. has received grants to support research projects and consultancy fee from GSK, Pfizer and Sanofi Pasteur; the other authors have nothing to disclose.
References
- 1.Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. New England Journal of Medicine. 2001;344(18):1378–1388. doi: 10.1056/NEJM200105033441807. PMID:11333996 [DOI] [PubMed] [Google Scholar]
- 2.Rouphael NG, Stephens DS. Neisseria meningitidis: Biology, microbiology, and epidemiology. Methods Mol Biol. 2012;799:1–20. doi: 10.1007/978-1-61779-346-2_1. PMID:21993636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369(9580):2196–1210. doi: 10.1016/S0140-6736(07)61016-2. PMID:17604802 [DOI] [PubMed] [Google Scholar]
- 4.Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE. Prevention and control of meningococcal disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Atlanta (GA): National Center for Immunization and Respiratory Diseases. 2013;MMWR 62:1–22. [PubMed] [Google Scholar]
- 5.Albu C, Brusin S, Dias JG, Ciancio B, Bacci S, Bancroft E, Carrillo-Santisteve P, Czumbel I, Danielsson N, Derrough T, et al.. Annual epidemiological report 2014 – Vaccine-preventable diseases – invasive bacterial diseases. 2014. Stockholm: European Centre for Disease Prevention and Control; 2015; 13–20. [Google Scholar]
- 6.Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, Corton C, Care R, Poolman JT, Zollinger WD, et al.. Description and nomenclature of Neisseria meningitidis capsule locus. Emerging Infectious Diseases. 2013;19(4):566–573. doi: 10.3201/eid1904.111799. PMID:23628376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granoff DM, Pelton S, Harrison LH, Meningococcal vaccines In: Vaccines. Plotkin SA, Orenstein WA, Offit PA (Eds). Sixth Edition. WB Saunders, Philadelphia, PA, USA, 2013; p 388–418. [Google Scholar]
- 8.Maiden MC, Ibarz-Pavón AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, Walker AM, Evans MR, Kroll JS, Neal KR, et al.. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197(5):737–743. doi: 10.1086/527401. PMID:18271745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maiden MC, MacLennan JM. Fifteen Years of Protection by Meningococcal C Conjugate Vaccines: Lessons From Disease Surveillance. Clin Infect Dis. 2014;59(9):1222–1224. doi: 10.1093/cid/ciu599. PMID:25069870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trotter CL, Lingani C, Fernandez K, Cooper LV, Bita A, Tevi-Benissan C, Ronveaux O, Préziosi MP, Stuart J, et al.. Impact of MenAfriVac in nine countries of the African meningitis belt, 2010–15: an analysis of surveillance data. Lancet Infect Dis 2017. 17:867–872. doi: 10.1016/S1473-3099(17)30301-8. [DOI] [PubMed] [Google Scholar]
- 11.Sáfadi MAP, Cintra OAL. Epidemiology of meningococcal disease in Latin America: current situation and opportunities for prevention. Neurol Res. 2010;32(3):263–271. doi: 10.1179/016164110X12644252260754. PMID:20406604 [DOI] [PubMed] [Google Scholar]
- 12.Safadi MAP, Berezin EN, Oselka GW. A critical appraisal of the recommendations for the use of meningococcal conjugate vaccines. Jornal de Pediatria. 2012;88(3):195–202. PMID:22622596 [DOI] [PubMed] [Google Scholar]
- 13.Cardoso CW, Ribeiro GS, Reis MG, Flannery B, Reis JN. Effectiveness of meningococcal C conjugate vaccine in Salvador, Brazil: a case-control study. Plos One. 2015;10(4):e0123734. doi: 10.1371/journal.pone.0123734. PMID:25874777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safadi MAP, McIntosh EDG. Epidemiology and prevention of meningococcal disease: A critical appraisal of vaccine policies. Expert Review of Vaccines. 2011;10(12):1717–1730. doi: 10.1586/erv.11.159. PMID:22085175 [DOI] [PubMed] [Google Scholar]
- 15.De Wals P, Deceuninck G, Boulianne N, De Serres G. Effectiveness of a mass immunization campaign using serogroup C meningococcal conjugate vaccine. Jama. 2004;292(20):2491–2494. doi: 10.1001/jama.292.20.2491. PMID:15562128 [DOI] [PubMed] [Google Scholar]
- 16.Khatami A, Pollard AJ. The epidemiology of meningococcal disease and the impact of vaccines. Expert Rev Vaccines. 2010;9(3):285–298. doi: 10.1586/erv.10.3. PMID:20218857 [DOI] [PubMed] [Google Scholar]
- 17.Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 2001;20(Suppl 1):S58–S67. PII: S0264-410X(01)00299-7. doi: 10.1016/S0264-410X(01)00299-7. PMID:11587814 [DOI] [PubMed] [Google Scholar]
- 18.Larrauri A, Cano R, García M, Mateo SD. Impact and effectiveness of meningococcal C conjugate vaccine following its introduction in Spain. Vaccine. 2005;23(32):4097–4100. doi: 10.1016/j.vaccine.2005.03.045. PMID:15908059 [DOI] [PubMed] [Google Scholar]
- 19.Lahra MM, Enriquez RP. Australian Meningococcal Surveillance Programme Annual Report, 2013. Sydney: Australian Government Department of Health; 2014. Australian Meningococcal Surveillance Programme publication. [Google Scholar]
- 20.Ramsay ME, Andrews NJ, Trotter CL, Kaczmarski EB, Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: Database analysis. BMJ. 2003;26(7385):365–366. doi: 10.1136/bmj.326.7385.365. PMID:12586669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borrow R, Alarcón P, Carlos J, Caugant DA, Christensen H, Debbag R, De Wals P, Echániz-Aviles G, Findlow J, Head C, et al.. The Global Meningococcal Initiative: Global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Review of Vaccines. 2016;16(4):313–328. doi: 10.1080/14760584.2017.1258308. PMID:27820969 [DOI] [PubMed] [Google Scholar]
- 22.Parent: du Chatelet I, Deghmane AE, Antona D, Hong E, Fonteneau L, Taha MK, Lévy-Bruhl D. Characteristics and changes in invasive meningococcal disease epidemiology in France, 2006–2015. J Infect. 2017;74(6):564–574. doi: 10.1016/j.jinf.2017.02.011. PMID:28279715 [DOI] [PubMed] [Google Scholar]
- 23.Safadi MAP, Berezin EN, Arlant LHF. Meningococcal Disease: Epidemiology and Early Effects of Immunization Programs. J Pediatric Infect Dis Soc. 2014;3(2):91–93. PMID:26625360 [DOI] [PubMed] [Google Scholar]
- 24.Moraes C, Moraes JC, Silva GD, Duarte EC. Evaluation of the impact of serogroup C meningococcal disease vaccination program in Brazil and its regions: A population-based study, 2001–2013. Mem Inst Oswaldo Cruz. 2017;112(4):237–246. doi: 10.1590/0074-02760160173. PMID:28327788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrade AL, Minamisava R, Tomichet L, Lemos AP, Gorla MC, De Cunto Brandileone MC, Domingues CMS, De Moraes C, Policena G, Bierrenbach AL. Impact of Meningococcal C Conjugate Vaccination Four Years After Introduction of Routine Childhood Immunization in Brazil. Vaccine. 2017;35(16):2025–2033. doi: 10.1016/j.vaccine.2017.03.010. PMID:28318769 [DOI] [PubMed] [Google Scholar]
- 26.De Moraes JC, Kemp B, De Lemos APS, Gorla MCO, Marques EGL, Ferreira MC, Sacchi C, Carvalhanas TRMP, Ribeiro AF, Ferreira CM, et al.. Prevalence, risk factors and molecular characteristics of meningococcal carriage among Brazilian adolescents. Pediatr Infect Dis J. 2015;34(11):1197–1202. doi: 10.1097/INF.0000000000000853. PMID:26222063 [DOI] [PubMed] [Google Scholar]
- 27.Nunes AM, Ribeiro GS, Ferreira ÍE, Moura AR, Felzemburgh RD, De Lemos AP, Reis MG, De Moraes JC, Campos LC. Meningococcal Carriage among Adolescents after Mass Meningococcal C Conjugate Vaccination Campaigns in Salvador, Brazil. Plos One. 2016;11(11):e0166475. doi: 10.1371/journal.pone.0166475. PMID:27861618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: A systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853–861. doi: 10.1016/S1473-3099(10)70251-6. PMID:21075057 [DOI] [PubMed] [Google Scholar]
- 29.Brazil Ministry of Health Technical Report on changes in the National Immunization Program for the year of 2017. Brasília (DF); 2016 Oct 13 [accessed 2017 July 22].http://www.portalarquivos.saude.gov.br/images/pdf/2016/outubro/20/Nota-Informativa-311-Calendario-Nacional-de-Vacinacao-2017.pdf.
- 30.Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364(9431):365–367. doi: 10.1016/S0140-6736(04)16725-1. PMID:15276396 [DOI] [PubMed] [Google Scholar]
- 31.Vetter V, Baxter R, Denizer G, Sáfadi MAP, Silfverdal SA, Vyse A, Borrow R. Routinely vaccinating adolescents against meningococcus: targeting transmission & disease. Expert Review of Vaccines. 2016;15(5):641–658. doi: 10.1586/14760584.2016.1130628. PMID:26651380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Safadi MAP, Bettinger JA, Maturana GM, Enwere G, Borrow R. Global Meningococcal Initiative. Evolving meningococcal immunization strategies. Expert Review of Vaccines. 2015;14(4):505–517. doi: 10.1586/14760584.2015.979799. PMID:25494168 [DOI] [PubMed] [Google Scholar]
- 33.Findlow H, Borrow R. What Would be the Best Schedule for Prevention of Meningococcal Disease in All Ages? The UK Experience. Paediatric drugs. 2016;18(2):83–87. doi: 10.1007/s40272-016-0169-1. PMID:26913860 [DOI] [PubMed] [Google Scholar]
- 34.Kinlin LM, Jamieson F, Brown EM, Brown S, Rawte P, Dolman S, Drews SJ, Fisman DN. Rapid identification of herd effects with the introduction of serogroup C meningococcal conjugate vaccine in Ontario, Canada, 2000–2006. Vaccine. 2009;27(11):1735–1740. doi: 10.1016/j.vaccine.2009.01.026. PMID:19186206 [DOI] [PubMed] [Google Scholar]
- 35.Poore KD, Bauch CT. The impact of aggregating serogroups in dynamic models of Neisseria meningitidis transmission. BMC Infectious Diseases. 2015;15:300. doi: 10.1186/s12879-015-1015-8. PMID:26223223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibarz‐Pavón AB, Maclennan J, Andrews NJ, Gray SJ, Urwin R, Clarke SC, Walker AM, Evans MR, Kroll JS, Neal KR, et al.. Changes in serogroup and genotype prevalence among carried meningococci in the United Kingdom during vaccine implementation. J Infect Dis. 2011;04(7):1046–1053. doi: 10.1093/infdis/jir466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borrow R, Abad R, Trotter C, Van Der Klis FR, Vazquez JA. Effectiveness of meningococcal serogroup C vaccine programmes. Vaccine. 2013; 31(41):4477–4486. doi: 10.1016/j.vaccine.2013.07.083. PMID:23933336 [DOI] [PubMed] [Google Scholar]
- 38.Ladhani SN, Flood JS, Ramsay ME, Campbell H, Gray SJ, Kaczmarski EB, Mallard RH, Guiver M, Newbold LS, Borrow R. Invasive meningococcal disease in England and Wales: implications for the introduction of new vaccines. Vaccine. 2012;30(24):3710–3716. doi: 10.1016/j.vaccine.2012.03.011. PMID:22429756 [DOI] [PubMed] [Google Scholar]
- 39.Bettinger JA, Scheifele DW, Le Saux N, Halperin SA, Vaudry W, Tsang R. The Impact of Childhood Meningococcal Serogroup C Conjugate Vaccine Programs in Canada. Pediatric Infectious Disease Journal. 2009;28(3):220–224. doi: 10.1097/INF.0b013e31819040e7. PMID:19209096 [DOI] [PubMed] [Google Scholar]
- 40.Sadarangani M, Scheifele DW, Halperin SA, Vaudry W, Le Saux N, Tsang R, Bettinger JA. The impact of the meningococcal serogroup C conjugate vaccine in Canada between 2002 and 2012. Clin Infect Dis. 2014;59(9):1208–1215. doi: 10.1093/cid/ciu597. PMID:25069868 [DOI] [PubMed] [Google Scholar]
- 41.Bijlsma MW, Brouwer MC, Spanjaard L, Van De Beek D, Van Der Ende A. A decade of herd protection after introduction of meningococcal serogroup C conjugate vaccination. Clin Infect Dis. 2014;59(9):1216–1221. doi: 10.1093/cid/ciu601. PMID:25069869 [DOI] [PubMed] [Google Scholar]
- 42.Mustapha MM, Marsh JW, Harrison LH. Global epidemiology of capsular group W meningococcal disease (1970-2015): Multifocal emergence and persistence of hypervirulent sequence type (ST)-11 clonal complex. Vaccine. 2016;34(13):1515–1523. doi: 10.1016/j.vaccine.2016.02.014. PMID:26876439 [DOI] [PubMed] [Google Scholar]
- 43.Ladhani SN, Beebeejaun K, Lucidarme J, Campbell H, Gray S, Kaczmarski E, Ramsay ME, Borrow R. Increase in endemic Neisseria meningitidis capsular group W sequence type 11 complex associated with severe invasive disease in England and Wales. Clin Infect Dis. 2015;60(4):578–585. doi: 10.1093/cid/ciu881. PMID:25389259 [DOI] [PubMed] [Google Scholar]
- 44.Australian Government Department of Health Invasive meningococcal disease National Surveillance Report, with a focus on MenW. Sydney: National Notifiable Diseases Surveillance System; 2017 Aug 28. [accessed 2017May]. http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-meningococcal-W.htm. [Google Scholar]
- 45.Lucidarme J, Scott KJ, Ure R, Smith A, Lindsay D, Stenmark B, Jacobsson S, Fredlund H, Cameron JC, Smith-Palmer A, et al.. An international invasive meningococcal disease outbreak due to a novel and rapidly expanding serogroup W strain, Scotland and Sweden, July to August 2015. Euro Surveill. 2016;21(45):pii = 30395. doi: 10.2807/1560-7917.ES.2016.21.45.30395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Safadi MAP, O'Ryan M, Bravo MTV, Brandileone MC, Gorla MC, De Lemos AP, Moreno G, Vazquez JA, López EL, Taha MK, et al.. The current situation of meningococcal disease in Latin America and updated Global Meningococcal Initiative (GMI) recommendations. Vaccine. 2015;33(48):6529–6536. doi: 10.1016/j.vaccine.2015.10.055. PMID:26597036 [DOI] [PubMed] [Google Scholar]
- 47.Weidlich L, Baethgen LF, Mayer LW, Moraes C, Klein CC, Nunes LS, Rios SS, Kmetzsch CI, Rossetti ML, Zaha A. High prevalence of Neisseria meningitidis hypervirulent lineages and emergence of W135:P1.5,2:ST-11 clone in Southern Brazil. Journal of Infection. 2008;57(4):324–331. doi: 10.1016/j.jinf.2008.07.014. PMID:18814914 [DOI] [PubMed] [Google Scholar]
- 48.Lucidarme J, Hill DMC, Bratcher HB, Gray SJ, Du Plessis M, Tsang RSW, Vazquez JA, Taha MK, Ceyhan M, Efron AM, et al.. Genomic resolution of an aggressive, widespread, diverse and expanding meningococcal serogroup B, C and W lineage. Journal of Infection. 2015;71(5):544–552. doi: 10.1016/j.jinf.2015.07.007. PMID:26226598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brazil Ministry of Health Datasus. Brasília (DF): Health Surveillance; 2008. [accessed 2017January29] www2.datasus.gov.br/DATASUS/index.php?area=02.
- 50.Brazil Ministry of Health Guia de Vigilância em Saúde. Brasília (DF): Health Surveillance; 2016. [accessed 2017January31]. http://portalarquivos.saude.gov.br/images/pdf/2016/agosto/25/GVS-online.pdf.
- 51.Brazil Ministry of Health Censos (1980, 1991, 2000 e 2010), contagem (1996) e projeções intercensitárias (1981 a 2012), segundo faixa etária, sexo e situação de domicílio. Brasília (DF): Health Surveillance; 2012 [accessed 2017April02]. www2.datasus.gov.br/DATASUS/index.php?area=0206&id=6942&VObj=http://tabnet.datasus.gov.br/cgi/deftohtm.exe?ibge/cnv/pop.


