ABSTRACT
Meningococcal disease continues to be a life threatening infection with high morbidity and mortality even in appropriately treated patients. Meningococcal vaccination plays a major role in the control of the disease; however, implementing vaccination remains problematic in the developing world. The objective of this review is to identify the challenges facing the use of meningococcal vaccines in the developing world in order to discuss the opportunities and available solutions to improve immunization in these countries. Inadequate epidemiologic information necessary to implement vaccination and financial challenges predominate. Multiple measures are needed to achieve the successful implementation of meningococcal conjugate vaccination programs that protect against circulating serogroups in developing countries including enhanced surveillance systems, financial support and aid through grants, product development partnerships that are the end result of effective collaboration and communication between different interdependent stakeholders to develop affordable vaccines, and demonstration of the cost-effectiveness of new meningococcal vaccines.
KEYWORDS: Meningococcus, vaccination, developing countries, challenges, opportunities, immunization schedule, prevention
Literature review methods
We conducted our literature search using PubMed and Medline databases. Search terms included “meningococcal disease”, “Neisseria meningitidis”, “invasive meningococcal disease”, “meningococcal serogroups”, and were combined with any of the following terms “immunization”, “vaccination”, “vaccine cost-effectiveness”, “outbreaks”, “challenges”, “opportunities”, “burden”, “surveillance”, “developing countries” and “developed countries”. The identified review and original articles were used to extract the data used in the current paper. Additional sources were identified within internal references of the initially retrieved papers and were used when found appropriate.
Background
Meningococcal disease constitutes a major public health problem, with the ability of Neisseria meningitidis to cause devastating infections including sepsis and meningitis.1 Invasive meningococcal disease (IMD) is difficult to diagnose and rapidly lethal with death occurring in as little as 24 hours.2,3 IMD is the most common infectious cause of childhood death in developed countries.4 Out of 12 serogroups, A, B, C, W, X, and Y are the major serogroups involved in IMD, with an estimated annual global number of cases reaching 1.2 million with 135,000 deaths, and a fatality rate of 11% leading to significant morbidity and mortality.1,5-10 Even in patients treated successfully, 11–19% end up with sequelae that cause long-term disability.11-13 Populations at increased risk for infection include those with impaired immune systems such as infants, young children and the immunocompromised as well as travelers to areas where N. meningitidis is hyperendemic or epidemic and microbiologists who are routinely exposed to isolates of N. meningitidis.14 Crowded populations such as dormitory students, military recruits,15 Hajj pilgrims,16 and oil refineries are also at risk.17 Carriage rates are low during infancy and peak at 19 years of age with young adults being the most important source of transmission to the community.18 The latter data on age group carriage rate mostly relies on studies conducted in developed countries, with limited information of meningococcal carriage available from developing countries.
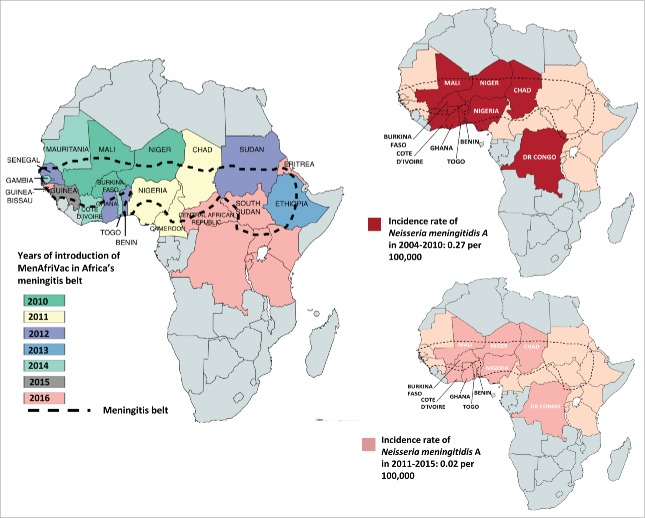
N. meningitidis can cause endemic, epidemic and pandemic infections that vary in incidence according to age group, with the highest incidence in infants and adolescents.19-21 In the United States (US), meningococcal infections are endemic, with an overall incidence of 0.2 cases per 100,000 persons in 201113; in the population aged above 65 years, the incidence is 0.3 cases per 100,000 persons whereas it is slightly higher in adolescents residing on campus (0.4 cases per 100,000 persons) and 10 times higher (2.6 cases per 100,000 persons) in children less than 2 years.13,22 Meningococcal infections due to serogroup A (MenA) had been associated with epidemics and pandemics in the African meningitis belt,24 which includes 25 countries and extends from Senegal in the west to Ethiopia in the east (Figure 1). These epidemics had been occurring since 1905, every 8 to 10 years, with an incidence rate that could reach 1 in 1000 of the total population and up to 1 in 100 for children less than 2 years of age.25-27 In the last 2 decades, the incidence rate of MenA decreased down to 0.27 per 100,000 in 2004–2010 and down to 0.02 per 100.000 in 2010–2015 after introduction of MenA conjugate vaccine.28,29 Outbreaks of meningococcal infections have occurred following the Hajj pilgrimage to Mecca, in Saudi Arabia.1
Figure 1.
Impact of MenAfriVac on the incidence of MenA in areas of the meningitis belt that introduced the vaccine. Starting in 2010, the meningococcal A conjugate vaccine was introduced progressively in the 26 countries in the African meningitis belt that included: Mauritania, Mali, Niger, Chad, Sudan, Eritrea, Ethiopia, South Sudan, Central African Republic, Cameroon, Nigeria, Togo, Benin, Ghana, Burkina Faso, Cote d'Ivoire, Guinea, Guinea-Bissau, Gambia, Senegal, Democratic Republic of Congo, Uganda, Rwanda, Burundi, Tanzania, Kenya. Only 10 countries were reporting data consistently to the WHO since 2004, these countries included Benin, Burkina Faso, Chad, Democratic Republic of Congo, Ghana, Côte d'Ivoire, Mali, Niger, Nigeria, Togo.28,29,38
Serogroups responsible for these endemic and epidemic infections are not distributed equally across countries. Serogroups B and C (MenB and MenC) predominate in Europe with serogroup W (MenW) emergence reported in the Northern European countries,30 and Serogroup Y (MenY) in Scandinavian and Central European countries.31 MenB, MenY, and MenC, to a lesser degree, predominate in the United States.32 In Canada, MenB and MenY are also the predominant serogroups, especially after the introduction of MenC conjugate vaccine into childhood immunization programs in the 2000s,33 with emergence of MenW and a recent increase in the percentage of MenW from 2.7% in 2012 to 18.8% in 2016.34 MenB and MenC predominate in South America,35 with a recent increase in the incidence rate of MenW in the countries in the South Cone in Latin America, especially Chile.36 MenA and MenW prevail during the Hajj season in Saudi Arabia.1,37 In Africa, while MenA was previously responsible for the large majority of endemic and epidemic disease in this continent, the mass vaccination against MenA left MenC and MenW as the most common circulating serogroups.38 Although MenB remains the most common serogroup in Australia, MenW and MenY have recently caused outbreaks.39,40 In the Middle East, MenA is the most common serogroup, but outbreaks of MenB, MenW and MenY are increasingly reported in some countries over the last 2 decades.41 MenA and MenB dominate the Asia-Pacific region with MenY emergence in Japan and Taiwan and MenW in Singapore.42 Moreover, serogroup distribution differs also according to age, with predominance of MenB in infants, MenC in adolescents, and MenB or MenY in older adults.9
Implementation of meningococcal immunization programs according to the most prevalent serogroups has contributed to a significant decrease in the endemic rates in the corresponding countries. In 1999, meningococcal C conjugate vaccine (MCV-C) was introduced in the United Kingdom (UK) to all children and young adults. Four years of experience revealed that the vaccine had a high effectiveness in reducing the incidence of IMD in vaccinated children, with a higher effectiveness of 97% in older children and adolescents as compared to 83% in young children. The vaccine effectiveness declined dramatically from 95% in the first year post vaccination to 31% in the fourth year post vaccination.43-45 However, there was a significant “herd immunity” where, in the 4 years following the implementation of vaccination, there was a 47% drop in disease rates in the non-vaccinated of all age groups.46 The latter herd effect was accomplished by means of a far-reaching catch-up vaccine program for 1 to 25 year olds,46 decreasing the carriage rate among the age groups that are responsible for the highest rates of carriage (adolescents and young adults),18 thus interrupting meningococcal disease transmission. More recently, IMD due to MenW has been increasing since 2009 in the UK. Genomic investigation has revealed that this is largely due to the spread of a hyper-virulent Wcc11 meningococcal strain resulting from a C to W capsular switching event that is also spreading globally.47,48
In Canada, MCV-C was introduced in the different provinces sequentially, starting from 2001 till 2007. Regardless of the number of primary doses given in infancy, the vaccine coverage of the groups at highest risk (infants and adolescents) led to the virtual elimination of MenC disease reaching unprecedented low incidence rates of 0.01 per 100,000 in 2011.49
Uruguay was previously the only country in the region of the Americas with a high incidence of IMD due to an elevated MenB endemic rate in 2001. The introduction of the Cuban outer membrane vesicle (OMV) vaccine led to a significant decrease in incidence in the following years.1 Despite this reduction in endemicity, MenB disease remains the predominant cause of IMD in Uruguay.50
Similarly, the overall effectiveness of the quadrivalent meningococcal A, C, W, and Y conjugate vaccine MenACWY-DT (diphtheria toxoid) for adolescents introduced in the US in 2005 showed an overall effectiveness of 79% (49%-91%) in the first year after vaccination but effectiveness waned to 61% (25%-79%), 3 to less than 8 years postvaccination.51 The latter estimates were essential in guiding the ACIP decision to recommend a booster dose at the age of 16 years, 5 years after the first dose.13 Furthermore, data showed that universal vaccination, implemented since 1970 by the military for all members entering all branches of service, was effective in significantly reducing the incidence of disease in the US military.52
The MenA conjugate vaccine was introduced sequentially in the African meningitis belt through mass vaccination of 1–29 year-olds, as part of the meningitis vaccine project (MVP), starting in 2010.38 Data from 10 countries (Benin, Burkina Faso, Chad, Democratic Republic of Congo, Ghana, Côte d’Ivoire, Mali, Niger, Nigeria, Togo) that were reporting consistently all suspected and confirmed cases of meningitis showed that vaccine introduction led to a 99% decrease in the incidence of confirmed MenA cases in those countries where MenAfriVac campaigns were completed (Figure 1).29 In addition, vaccine-induced herd immunity was demonstrated by the disappearance of MenA infections in both vaccinated and non-vaccinated populations.53,54 On the other hand, the number of confirmed N. meningitidis cases due to serogroups other than MenA increased substantially in African countries starting in 2010. After MenAfriVac introduction, multiple MenW outbreaks were recorded in Burkina Faso in 2012 and a major MenC outbreak was observed in Nigeria and Niger in 2015.38 In 2016, large-scale MenW outbreaks were recorded in Ghana, resulting in a large number of cases accompanied by a high case-fatality rate.38 Another MenW outbreak was reported in Togo and was confirmed to be caused by the same MenW clone responsible for the Burkina Faso 2002 epidemic.38
Moreover, the effectiveness of the four component MenB (4CMenB) vaccine offered to all infants in the UK after the first of July 2015 was estimated to be as high as 82.9% against all MenB IMD after 2 doses, with a 50% reduction rate in MenB cases in the vaccine-eligible cohort (37 cases), as compared to the prevaccine era (74 cases).55,56 Furthermore, multiple MenB outbreaks have been reported among university students between 2013 and 2017. In December 2013, the Food and Drug Administration (FDA) first authorized the use of 4CMenB for outbreak control in a U.S. university (Princeton University). The prevention of further MenB cases at Princeton and other universities demonstrated how primary prevention success can be achieved with aggressive and prompt vaccination campaigns.57 The 4CMenB vaccine administered was found to be immunogenic with 66% seropositivity against the outbreak strain and 86 to 100% against the reference strains.58 In Canada, a vaccination campaign with 4CMenB vaccine was introduced in Saguenay-Lac-Saint-Jean Region of Quebec in order to control Men B disease. The latter included subjects residing in this area or attending schools, aged between 2 months and 20 years. Out of the 43,740 individuals in this age group who received at least the first dose of the 4CMenB vaccine, no new cases of IMD were reported since the initiation of the vaccination campaign.59,60
Thus, there is mounting evidence that meningococcal vaccination plays a major role in the control of this fatal disease by decreasing the incidence rate of IMD and the nasopharyngeal carriage rate in vaccinated and unvaccinated subjects leading to herd immunity.61 However, the use of meningococcal vaccines remains low in the majority of developing countries due to limited resources and costly vaccines. Actually, cost-effectiveness is difficult to estimate in the setting of a difficult-to-establish burden of IMD, where basic surveillance data is lacking. Introducing meningococcal vaccines in such conditions is challenging. In this review, the main challenges and opportunities for meningococcal vaccination in developing countries will be discussed.
Challenges
In developed countries, implementing meningococcal immunization programs with adequate vaccine coverage has been effective in decreasing the burden of disease; however, achieving similar results in developing countries is hindered by several challenges. The World Bank classification of countries according to gross national income (GNI) per capita has been adopted in this review: Low and middle income countries are defined as those with a GNI per capita of $12,476 or less and high income countries as those with a higher GNI per capita.62 The former will be referred to as developing countries and the latter as developed countries in this review.
Lack of adequate epidemiology and surveillance data
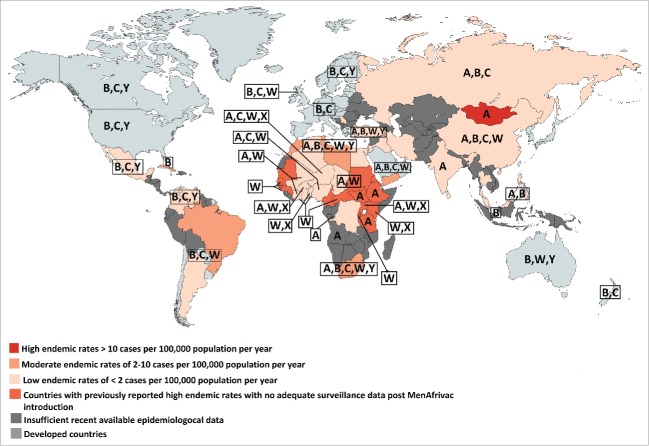
The epidemiology of meningococcal disease is continuously changing over time and varies according to age and geographical location, making continuous global and national surveillance necessary to detect the dominant circulating strains and implement interventions and modify immunization recommendations accordingly.12,13,56,63,64 Surveillance systems to detect locally prevalent serogroups are lacking in most developing countries and the epidemiology is not well studied.50 In fact, even in situations where the public health system is notified about such cases, there are multiple hurdles that affect the efficiency of reporting including the lack of obtaining CSF or blood cultures in many patients, the administration of antibiotics prior to obtaining these cultures, or simply lack of reporting. Advanced laboratory techniques that identify causative bacteria in these circumstances, such as PCR, are not always available or implemented. Figure 2 summarizes the epidemiology and burden of meningococcal disease in the developing world illustrating the predominant serogroups and showing large data gaps with many countries having insufficient available epidemiological data.1
Figure 2.
Map showing the endemic rates of IMD in developing countries and serogroups distribution in developed and developing countries. The most recent available data on incidence and serogroups distribution was used.1,35,40-42,65-83
No adequate surveillance data exists in the African region, except for the African meningitis belt, which until recently had the highest endemic rate of MenA IMD in the world.1,84 Between 2006 and 2010, serogroup X was responsible for outbreaks in Kenya, Niger, Togo, Uganda and Burkina Faso. Recently, epidemics of MenC meningitis started in Niger from late 2013 to 2015 and rapidly spread to Nigeria.23,85
In the Eastern Mediterranean Region (EMR), few countries have adequate data to allow a population-based estimate of the true incidence. A high endemic rate of meningococcal disease of more than 10 cases per 100,000 population was reported in Sudan, part of the African meningitis belt and considered by the WHO to be part of the EMR, with no recent available surveillance data post MenAfrivac introduction.1 MenA predominates in Sudan and outbreaks of MenW have been reported following the Hajj season.1 The most recent available data shows a moderate endemic rate of 2–10 cases per 100,000 population in Libya, Tunisia, Morocco, and Yemen.41 MenB predominates in Tunisia and Morocco, with no available data on the circulating serogroups for Libya and Yemen.86 A low endemic rate of less than 2 cases per 100,000 population has been reported in Egypt, Algeria, and Turkey.41 In Turkey, MenA and MenW were the most common serogroups detected in the recent years with the decrease in MenB prevalence.87 In Egypt, the most recent available data indicate a predominance of MenA and MenB.41 Algeria lacks data on meningococcal serogroups.41
In the developing countries of the Americas, surveillance data exists for Cuba, where there is a high prevalence of MenB,88 for Brazil where MenC predominates,42 and for Argentina, Chile, Colombia, Mexico and Venezuela where there is a lower incidence of IMD. MenB currently predominates in Colombia with increasing MenC and MenY cases being recorded in recent years.89 While MenB and MenC were the predominant serogoups in Chile and Argentina, major outbreaks of MenW have been reported recently.42,90,91 An increase in the proportion of cases due to MenY was reported in Venezuela in 2006, when it represented 50% of the identified isolates, with MenB and MenC accounting for 36% and 14% of the cases, respectively.72 The most recent data on Venezuela in 2010 describes a decrease in the proportion of cases due to MenY, where it represented 22% of the isolates identified.66 In Mexico, MenC has emerged in 2010 as the dominant strain, along with some cases of MenY and MenB.42 No data exists for the other countries in the region to allow an accurate estimate of the burden of the disease.1,72
In the Northern Asian region, surveillance data from the Russian Federation reported MenA and MenC predominance.65 In the South-East Asia region, data exists only for Korea, Thailand and India. Korea and Thailand experience low incidence rates of IMD, whereas India reported epidemics of MenA, the most recent in 2005.75 Data for other countries from the region are incomplete or unavailable.92
High endemic rates of IMD were recorded in Mongolia where several outbreaks of MenA were recorded in the 1990s. Other countries of the Western Pacific like China, Philippines, Singapore, Taiwan experience low rates of IMD, whereas inadequate data is available for other countries in this region.1
Therefore, there are significant gaps in population-based surveillance data that limit the description of IMD epidemiology and burden in most developing countries. The lack of definitive data showing the true burden of disease and the predominance of one or more serogroup responsible for IMD complicates the decision-making process when it comes to vaccine choice or prioritization.
Unfavorable vaccine prioritization
Since data about the burden of IMD and the predominantly prevalent serogroups is lacking from most developing countries, introduction of meningococcal vaccines in these countries is hindered by its competition with the introduction of other, more impactful, vaccines that target more prevalent infections associated with a significantly higher burden of disease. For example, pneumonia and gastroenteritis remain the major infectious disease killers of children under 5 years of age in developing countries.93,94 The WHO have prioritized the national introduction of pneumococcal conjugate vaccines and rotavirus vaccines, the major causes of pneumonia and gastroenteritis in children, respectively, even in the absence of local data supporting such introduction.95,96
No such etiological predominance is present in bacterial meningitis. Several bacteria such as S. pneumoniae and Haemophilus influenzae type b in addition to N. meningitidis can cause bacterial meningitis with variable predominance globally and historically.97 In formulating its recommendations, the WHO has prioritized the targeting of the first two organisms because of their higher prevalence and significantly larger burden of disease.98,99 This has left N. meningitidis with only a fraction, although sizeable, of the cases of bacterial meningitis in addition to other invasive meningococcal syndromes including meningococcemia and pneumonia in young children in the developing world. Thus, when vaccine-preventable infectious diseases in developing countries are considered, meningococcal infections fall down the ladder of priorities.
In contrast, IMD is one of the most common vaccine-preventable bacterial causes of childhood mortality in developed countries.4 In addition, these cases usually generate significant publicity and result in pressure on the health authorities in developed countries to “do something”. As a result, meningococcal vaccines have made it on the schedule of several developed countries.35,100 However, in developing countries, a child's death from meningococcal infection is hardly ever reported in the news and rarely results in any public outrage significant enough to influence vaccination policy or prioritization.92
Limitations inherent in meningococcal vaccines
Ideally, a cost-effective vaccine would require only a limited number of doses, would be effective in infancy, would cover most or all of the pathogenic serogroups, would be inexpensive, and would provide life-long immunity. Unfortunately, none of the available vaccines meets these criteria. Currently available vaccines for IMD include meningococcal polysaccharide vaccines (MPSV) or meningococcal polysaccharide-protein conjugated vaccines (MCV) with different combinations of serogroups A, C, W, and Y (Table 1). MCV use diphtheria toxoid (DT), its non-toxic diphtheria cross-reacting mutant CRM197 (CRM), or tetanus toxoid (TT) as carrier proteins.101,102 Additionally, two new MenB vaccines were recently licensed: the aforementioned 4CMenB vaccine and a two component factor H binding protein (MenB-FHbp) vaccine (Table 1).103 Serogroup A, C, W, and Y vaccines were developed using a fragment of the polysaccharide outer coating (capsule) of meningococcal bacteria to induce immunity. However, the same approach could not be used for MenB vaccine as the MenB polysaccharide capsule mimics many host glycoproteins. To overcome this challenge, MenB vaccines were developed using fragments of proteins present on the cell surface of MenB bacteria by novel techniques combining reverse genetics and membrane vesicle synthesis.104
Table 1.
| Type | Formulation | Serogroups | Trade name | Manufacturer | Carrier protein | Age group approved |
|---|---|---|---|---|---|---|
| Polysaccharide | MPSV-4 | A-C-W-Y | Menomune | Sanofi Pasteur | — | ≥ 2 y – 65 y |
| MPSV-4 | A-C-W-Y | Mencevax | GSK* | — | ≥ 2 y | |
| A-C-W | MencevaxACW | GSK* | — | ≥ 2 y | ||
| A-C-W | MenVax ACW | Bio-Manguinhos | — | ≥ 2 y | ||
| A-C | Men AC | Sanofi-Pasteur | — | ≥ 2 y | ||
| Conjugate | MenACWY-DT | A-C-W-Y | Menactra | Sanofi-Pasteur | DT | 9 mo – 55 y |
| MenACWY-CRM | A-C-W-Y | Menveo | Novartis | CRM197 | 2 mo – 55 y | |
| MenACWY-TT | A-C-W- Y | Nimenrix | GSK* | TT | ≥ 6 w | |
| Hib-MenCY-TT | C-Y | MenHibrix | GSK* | TT | 6 w -18 mo | |
| MCC-TT/Hib-TT | C | Menitorix | GSK* | TT | ≥ 6 w – 2 y | |
| MCC-CRM | C | Menjugate | GSK* | CRM197 | ≥ 6 w – 65 y | |
| MCC-CRM | C | Meningitec | Pfizer | CRM197 | ≥ 6 w – 65 y | |
| MCC-TT | C | NeisVac-C | Baxter Healthcare | TT | ≥ 8 w – 65 y | |
| PsA-TT | A | MenAfriVac | SIIL# | TT | 3 m – 29 y | |
| Protein | MenB-4C | B | Bexsero | Novartis | — | 2m-25 y |
| MenB-FHbp | B | Trumenba | Wyeth | — | 10–25 y |
GSK, GlaxoSmithKline; SIIL, Serum Institute of India Ltd, India.
MPSV have several disadvantages that make the use of MCV preferable. First, MPSV are not useful in younger children aged less than 2 years, who have the highest burden of disease, due to their low immunogenicity. Second, they are unable to provide long term immunity with recent data from a Cochrane review showing that immunity beyond the first year of vaccination is not well established.111 The WHO has concluded that the use of MPSV is not optimal in areas with high incidence rates and where outbreaks occur frequently like in the meningitis belt. For example, epidemics of IMD were still occurring in more than 90% of districts in Burkina Faso in 2006,112 despite the high vaccination coverage with MPSV in the preceding 5 years.113 Third, they induce hypo-responsiveness with cumulative doses, and fourth, and unlike conjugate vaccines, they do not decrease nasopharyngeal carriage and subsequently do not induce herd immunity and protection in the unvaccinated population.9,64,114
MCV on the other hand have distinct advantages over MPSV. In addition to being more immunogenic in infants under two years of age, they also induce immune memory,101 and prevent the acquisition of carriage among vaccines.115 However, although the duration of immunity provided by MCV is longer than MPSV, it is not life-long. For example, after a single immunogenic dose of MenACWY-CRM given for children 2–10 years of age and adolescents 11–18 years of age, there was a decline in human complement-based serum bactericidal assay (hSBA) titers up to 1 year, with stable titers up to 5 years post-vaccination. The decline in antibody levels was more pronounced for MenA and in younger age groups.116 Similarly, after 3 doses of MenACWY-CRM given at 2, 4, and 6 months of age in infants, there is waning immunity by 6 months after the third dose. Likewise, in toddlers, there is a decrease in antibody levels by 7 months after 2 doses received at 6–8 months and 12 months. For both age groups, the decline in antibody titers was also more pronounced for MenA.116 With this waning immunity, adolescents aged 16 through 21 years, known to be at higher risk for acquiring the disease, are not adequately protected. For this reason, the US Advisory Committee on Immunization Practices (ACIP) changed its recommendation to include 2 doses of MCV-4, the first at 11 years of age and the second booster dose 5 years later.35,117 The need to give booster doses represents another challenge for meningococcal vaccination, as it would significantly increase the cost in developing countries unable to afford even one dose of vaccine on their immunization schedule.
Another limitation facing MCV, similar to other vaccines, is the need for multiple dosing in infants and toddlers. For example, a 3-dose series and a 2-dose series of MenACWY-CRM are required for infants aged 2 to 6 months and for toddlers aged 7 to 23 months, respectively.116 This also applies to MenACWY-TT, where two primary dose series at 2 and 4 months and 1 booster dose at 12 months are needed.118 For MenACWY-DT, the number of doses needed is reduced to 2 doses at 9 and 12 months of age; however, it is ineffective under 9 months.119 This need for multiple dosing constitutes a significant financial burden on developing countries considering adding MCV on their immunization schedules.
Furthermore, MenB vaccines have several limitations making their use challenging in developing countries. First, while these vaccines cover for most of the circulating MenB strains, they are not able to provide immunity against all MenB circulating strains. Further ongoing studies are evaluating the extent of strain coverage. Second, multiple doses are required: for adolescents and young adults, MenB-FHbp is approved by FDA both as a 2-dose series administered at 0 and 6 months and as a 3-dose series administered at 0, 1–2 and 6 months,103 and 4CMenB is approved as a 2 dose-series, with a minimum interval-dose of 1 month.103 In Europe, Canada and Australia, 4CMenB vaccine has recently been licensed in infants aged more than 2 months as a minimum of two doses in different schedules depending on the age at which the vaccination is initiated.120 Third, data on MenB-FHbp showed a rapid decline in protective antibodies after vaccination and stabilization of the antibodies curve at 6 months after the third dose anticipating that the duration of protection will probably be short-lived.121 Data from a clinical trial on persistence of antibodies revealed that at 48 months post vaccination, more than 50% of subjects continued to have a hSBA above or equal to the lower limit of quantification against three of the four tested strains.121
Therefore, in the absence of long-term immunity provided by these vaccines and the multiple doses required to provide protection in infants, children, and adolescents, developing countries are faced with the task of devising immunization schedules that would target their most vulnerable populations in the absence of the robust surveillance systems that are needed in order to make these decisions.
Vaccine unaffordability
The cost of MCV makes them unaffordable for many of the developing countries. The different preparations of MCV-4 are sold in private practices for around $100 whereas the new MenB vaccines are sold for around $200 where available. Such prices put these vaccines beyond the reach of most of the populations of different developing countries. Therefore, even developing middle-income countries that may have adequate surveillance data are unable to afford introducing these vaccines. On the other hand, in some high-income countries,18 where monovalent MCV were effectively introduced, addition of available multivalent MCV or MenB vaccines has been hindered partially by the significant added cost.
Furthermore, the age distribution of meningococcal disease, with the highest incidence in infants and adolescents,1 would require a mass or “catch-up” vaccination campaign strategy in order to successfully control the disease. This entails a large initial financial commitment, which is considered another obstacle for vaccination in developing countries where resources are limited. In addition, the cost is further increased by the coexistence of all 5 major serogroups simultaneously in most countries, although in variable proportions, and their serogroup and age distribution is expected to unpredictably change with time. This leaves decision makers with two options: 1) use a monovalent vaccine that targets the predominant serogroup as was done in Western Europe and the African meningitis belt and target specific age groups; or 2) use a multivalent MCV that covers the majority of responsible serogroups for specific age groups.1,9,64 The first option would require fairly reliable surveillance data that is frequently lacking. The second option, although ideal from a scientific point of view, remains elusive. At this writing, at least two distinct vaccines would have to be used to protect against the five major serogroups, a MenACWY vaccine and a MenB vaccine. This would be a costly and unaffordable strategy for the majority of developing countries. Therefore, it is important to generate cost-effectiveness data in order to contribute to the decision-making process.
Limited cost-effectiveness data
Most of the cost-effectiveness data that is available comes from developed countries. Due to the short time of protection and the absence of herd immunity with MPSV-4, it was found to be two times less cost-effective than MCV-4.122,123 On the other hand, the cost-effectiveness of MCV in these countries was assessed in different studies.
In the 1990's, MenC was the main serogroup contributing to the disease burden in the UK with an estimated 1137 cases in people aged between 0–17 years and 72 deaths. The introduction of meningococcal serogroup C conjugate (MCV-C) vaccine in 1999 to the routine infant schedule with a “catch-up” dose for all children and adolescents younger than 18 years was successful in reducing the incidence of MenC disease by direct protection of vaccinated individuals and indirect effect by herd immunity.43 Shepard et al. used the UK's experience with routine MCV-C vaccination to predict the cost effectiveness of hypothetical routine MCV-4 vaccination of adolescents (1 dose at 11 years of age), toddlers (1 dose at 1 year of age), and infants (3 doses at 2, 4, and 6 months of age).124 They found that although MCV-4 is one of the most expensive vaccines on the market ($82 in the public sector, being 20–30% higher in the private sector), it would be cost effective for adolescents and toddlers, but much less cost-effective for infants.124
In the USA in June 2005, the ACIP recommended the routine use of MCV-4 for all children aged 11 years. Ortega-Sanchez et al. analyzed the cost effectiveness of routine MCV-4 child immunization at 11 years and a catch up vaccination for children and adolescents aged 11–17 years. It was shown that although routine and catch up vaccination would have a significant impact on disease burden, this would be 3 times more effective if the herd immunity effect was included and more cost effective if applied to US adolescents residing in areas where the incidence of meningococcal disease is above 1.57 cases per 100,000 population.122
In the Netherlands, after the occurrence of a MenC outbreak in 2000–2001, MCV-C was recommended by the National Immunization Program (NIP) at 14 months, accompanied by a catch up program for all children and adolescents aged between 1–18 years, due to the rapid decline of immunity in the year following vaccination in children vaccinated under the routine infant schedule.125 When the potential cost-effectiveness of this approach was evaluated with projected follow up for 9 years, seven additional cases of MenACWY disease would have been prevented; however, with the current epidemiology, this approach did not have the potential of being cost-effective like the single vaccination.126
De Wals et al evaluated the cost-effectiveness of switching from MCV-C to MCV-4 in the Canadian provinces.127 The replacement strategy, with a price difference of $15, was found not to be cost-effective in regions where the disease incidence is low (≤ 0.08/100,000 person-years) like in Quebec, the Maritimes and many other Canadian provinces and territories.127 Conversely, in provinces where the disease incidence is high (0.28/100,000 person-years) like in British Columbia or maybe Ontario, the incremental cost-effectiveness ratio would be more satisfactory, especially while taking in consideration the moderate to high effect of herd immunity.127
Since the introduction of MCV-C in Europe in 2002, MenB became predominant accounting for 60% of IMD in Italy and UK.128,129 Infants were mainly affected with subsequent high morbidity and mortality.130,131 In 2015, the UK was the first country to offer the 4CMenB vaccine for infants, as recommended by the Joint Committee on Vaccination and Immunization, as this is the most affected age group.132 A cost-effectiveness analysis on the use of the 4CMenB vaccine was carried out in Italy. The study showed that vaccination with 4 doses of 4CMenB, at 2, 4, 6 and 12 months of age and 1 booster dose at the age of 11 years could prevent 82.97 cases and 5.61 deaths in each birth cohort. This was considered cost-effective in the setting of underestimated incidence of the disease in Italy where surveillance system has several limitations.133 Furthermore, Christensen et al. assessed the cost-effectiveness of a catch-up 4CMenB vaccination in England for children above the routine age for meningococcal vaccination.134 The study revealed that the vaccine would still be cost-effective at catch up immunization of 1 year-old children, but at a lower vaccine price. This cost effectiveness decreased at 2 years of age and became not cost-effective at 3–4 years of age due to the decrease in disease incidence in older children.134
In developing countries, limited data exist on cost-effectiveness of meningococcal vaccines. In Brazil, where IMD is endemic, with progressive increase in the incidence rate of cases caused by MenC, accounting for around 68% of cases in 2008, MCV-C was introduced in 2010 into the routine immunization schedule of infants (three doses at 3, 5, and 12 months) with catch-up vaccination for children younger than 2 years of age. This childhood immunization schedule had the potential of being cost-effective, although some limited data in the epidemiology and in the estimation of indirect costs like deafness and neurological sequelae were not well evaluated.135
In West Africa, part of the meningitis belt where MenA and less frequently MenC disease occur in epidemics, a strategy of reactive emergency vaccination with MPSV A/C was introduced by the WHO in 1998; however, in such countries where effective surveillance systems do not exist, the detection of an outbreak would be delayed leading to increased morbidity and mortality. The cost-effectiveness of preventive immunization mass campaigns was compared to the emergency strategy through a theoretical modeling analysis. It found that the preventive vaccination strategy would be more cost effective as it would prevent 59% of the cases and save US$59 per case as compared to 49% of the cases and US$133, respectively in the reactive emergency vaccination strategy.136
After creation of the MVP in the African meningitis belt in 2001 in order to control epidemic meningitis in sub-Saharan Africa through introduction of MenAfriVac, the cost and potential savings from the vaccine introduction were analyzed. Results were impressive as catch-up campaign in 1 to 29 year olds would potentially save around $650 million by substituting for the more expensive and less immunogenic MPSV A/C.137 Additionally, a study published by Colombini et al. estimated the cost and savings of preventive immunization strategy with MenAfriVac as compared with reactive strategy over a 26-year period.138 This study showed that the preventive strategy has a positive economic impact and is more cost-saving as compared to the reactive strategy, mainly by decreasing the burden of disease and subsequently by decreasing the cost for the health system and the households.138
Cost-effectiveness analyses have become increasingly important in making decisions about vaccine implementation. Indeed, in the UK, a vaccine cannot be recommended by the in the routine immunization schedule unless it is proven to be cost-effective.42 However, although adopting a new vaccine without an economic evaluation would be challenging in most developing countries, the limited data on burden of disease in these countries compromises the feasibility of adequate cost-effectiveness analyses.
Technology transfer for local vaccine manufacture
The technological advances that led to the development of multivalent MCV and MenB vaccines were a result of years of research and development by different pharmaceutical companies. These profit-driven companies are unlikely to sell their products at or below cost to developing countries where these vaccines are most needed lest they compromise their financial investments. Donor organizations such as Global Alliance for Vaccines and Immunization (GAVI) are unlikely to fund commercially available meningococcal vaccines because of other priorities. Local manufacturers may be unavailable or lack the technological expertise required to manufacture these vaccines at a lower cost and are unlikely to make significant profits through local sales should they be mandated to supply these vaccines to local markets. As a result, technology transfer to developing countries and local production of vaccines has proven to be an effective strategy in increasing vaccine supply and availability. For example, Novartis Vaccines and Diagnostics and Brazilian Ezequiel Dias Foundation signed a strategic alliance in 2009, agreeing to supply meningococcal C conjugate vaccine for Brazil's National Immunization Program. Novartis Vaccines and Diagnostics manufactured the vaccine during the early part of the project while technology transfer to Ezequiel Dias Foundation was taking place. This partnership ensured Brazilian self-sufficiency in the production of meningococcal C conjugated vaccine.
Transportation and logistical challenges
As in the case of MVP's MenAfriVac, the absence of developed laboratories with expert personnel in the concerned meningitis belt countries necessitated the manufacturing of the vaccine abroad and its delivery to these countries. In this case, MenAfriVac was manufactured by the Serum Institute of India then transported to the countries of the meningitis belt. This is considered an additional challenge since it would necessitate the availability of transportation media with storage system, thus further increasing the cost of the vaccine. On the other hand, MCV similar to other vaccines except the MenAfriVac, require the maintenance of a cold chain, which in turn requires electricity, refrigerating equipment and ice pack production.42 In many developing countries, maximal cold chain capacity has already been reached due to other vaccines making the use of MCV problematic.
Crowding of the immunization schedule
Finally, saturation of the immunization schedule is an additional obstacle for administering meningococcal vaccines in developing countries. When a decision is made to include a new vaccine on a national immunization schedule, an effort is usually made to administer the vaccine along with other vaccines at an already scheduled visit. Having a full schedule would lead to additional visits to administer the meningococcal vaccine leading to more unaffordable expenses in resource-poor countries. This will be particularly apparent should an infant schedule become approved leading to significant crowding of the schedule in the first few months of life. More combined vaccines would help solving this problem by reducing multiple injections during a single visit, about which health care workers and parents tend to be concerned. Co-administration data, already available for most vaccines will have to be continuously updated.139
Opportunities
Improvement of surveillance systems
Identifying the epidemiology of meningococcal disease, which is unpredictable and dynamic, is the key challenge for most developing countries. Improved surveillance is necessary to implement vaccines covering the circulating strains and detect their impact on reducing the disease burden after their introduction. When national surveillance networks are not available, the actual burden of the disease is underestimated.
The WHO should strengthen the coordination of the global Invasive Bacterial Vaccine Preventable Diseases surveillance network (IBVPD) created in 2008 with the central public laboratories in developing countries responsible for communicable diseases surveillance. To this end, it will be important to build laboratory capacity of these laboratories by tapping into the expertise of partners at local academic institutions or at meningococcal units of some laboratories in developed countries that can act as reference or support laboratories. In addition, educational campaigns should be carried out that will reach out to health care providers, especially in remote areas, to emphasize the importance of notifying the surveillance units about cases, obtaining blood and CSF cultures in suspected cases of IMD, and to build the capacity of supporting laboratories in parallel. Moreover, a network of central or reference laboratories should be established, possibly involving several neighboring countries, where more advanced molecular testing such as the identification of the causative organism and the serogroup by real-time polymerase chain reaction (RT-PCR) and the identification of the molecular characteristics dominant strains by multi-locus sequence type or whole genome sequencing can be performed. For this to succeed, sufficient training should be provided at the local level in the isolation, preservation, DNA extraction, and shipping of isolates of interest. Once surveillance systems are in place, the public health response to outbreaks would be prompt through vaccination campaigns resulting in decreased death and reduced economic costs.140 Moreover, the value of a robust surveillance system cannot be underestimated in decisions regarding the implementation of a preventive vaccine strategy.
Finally, implementing an effective surveillance system for the detection and identification of public health problems has a considerable impact on the disease incidence. A study published by Somda et al. showed that the Integrated Disease Surveillance and Response (IDSR) system implemented by countries in the WHO African region can improve the cost-effectiveness of public health surveillance. Pre- and post- IDSR meningococcal meningitis surveillance data from Burkina Faso (1996–2002 and 2003–2007) were used to assess the impact of the IDSR system. IDSR was correlated with a median reduction of 2 weeks to peak of outbreaks and a reduction of 43 meningitis cases per 100,000. In fact, the cost-effectiveness was US$23 per case averted and US$98 per meningitis-related death averted.141 In 2014, the MenAfriNet program started and was designed to obtain high quality data and improve the regional capacity by standardizing tools, databases, training and laboratory personnel. This program started at Burkina Faso, Chad, Mali, Niger and Togo and has the ability to involve other countries in the same region.142
Manufacture of affordable vaccines through product development partnerships
Vaccines for low and middle-income countries need to be affordable, and this should be considered at the level of development, manufacturing and delivery of the vaccine. MenAfriVac, the outcome of the MVP lead by WHO-PATH, is a good model to be followed in future ventures. Although pharmaceutical companies contribute significantly to the advances in development of new vaccines, with every new vaccine costing in the range of $500–750 million,143 their main interest remains financial rather than humanitarian and they cannot be relied on to develop vaccines for non-lucrative markets. This was illustrated by the lack of Pharma interest in developing MenA conjugate vaccine to be used in Africa when at the same time they were busy developing MenC conjugate vaccines to be used in Europe where the burden of IMD was only a small fraction of that in Africa but where the financial rewards were significantly more. It was a simple business decision. In fact this apathy was one of the main reasons that led to the creation of the MVP in 2001 with support from the Bill & Melinda Gates Foundation (BMGF) with a single goal: to develop, test, license, and introduce an affordable MenA conjugate vaccine for Africa.142
The MVP identified the Serum Institute of India, Ltd (SIIL) as a partner in this product development partnership (PDP), due to its long experience in manufacturing several vaccines used globally. The journey to reach the successful end result 9 years later with a MenA conjugate vaccine sold at less than $0.50 per dose is described in detail.142 The success of the MVP and introduction of MenAfriVac, should be considered as a potential solution to make additional MCV affordable in the poor countries and eliminate the gap between the developed and developing countries. The task that the MVP faced was not easy. Difficulties in the long and complicated process came up at multiple levels including in the communication between the different interdependent partners. The scope of the stakeholders involved in the development of MenAfriVac in addition to the WHO, PATH, and the SIIL included the Center for Biologics Evaluation and Research (CBER), US Food and Drug Administration (FDA), SynCo Bio Partners, Aerial Laboratories, Illkirch-Graffenstaden, National Institute of Biological Standards and Control (NIBSC), US Centers for Disease Control and Prevention,32 UK Health Protection Agency (Public Health England), University of Cape Town, South Africa, and several MVP consultants, Ministry of Health/Burkina Faso, UNICEF, Global Alliance for Vaccines and Immunization, and the Norwegian Institute of Public Health/Oslo in addition to the public health officials of most countries in the African meningitis belt. Aligning and harmonizing the needs and demands of these various stakeholders was a momentous task that was successfully concluded during this PDP.142,144,145 The success of this project and the improved SIIL expertise in conjugation technology, earned after the development of MenAfriVac, opened the door for the future development of polyvalent conjugated pneumococcal and meningococcal vaccines.142
Funding strategies for vaccine PDP's
The successful manufacture of inexpensive vaccines by a developing country pharmaceutical company and their effective implementation significantly decreases the dependency on the goodwill of pharmaceutical companies based in developed countries. Whereas vaccine PDP's help overcome the significant financial hurdle faced by developing countries when aiming to implement otherwise expensive vaccines, a substantial amount of funding is still required to launch and successfully complete such a project. Again, taking MenAfriVac as an example, the BMGF provided the initial $6.25 million grant to set up the MVP by WHO and PATH. Subsequently, the SIIL invested an equal amount in upgrading its facilities in order to accommodate the large-scale development of the vaccine, and the MVP subsequently spent a further $37 million on the needed clinical trials before the vaccine was ready.142 Should non-governmental organizations (NGO's), philanthropists, the WHO, and other stakeholders develop a common interest to control IMD caused by other serogroups in developing countries, the process is now established and the model in place.
Finally, funding for future PDP's needs not necessarily come from philanthropists and NGO's, as welcome as that is. The governments of many developing countries have swollen military budgets but relatively modest healthcare budgets. Graft and financial waste is pervasive in many developing countries. Strong leadership and vision are needed to set the priorities straight and devise plans to control IMD, as well as other health burdens, and minimize its impact in different populations.
Conclusion
Developing countries continue to have the highest incidence of IMD and carry its highest burden.1 Vaccination had a significant role in decreasing disease incidence in the developed countries; however, implementing vaccination remains challenging in most developing countries. In the setting of dynamic changes in the epidemiology of IMD, effective surveillance and close monitoring of the disease are essential in order to implement updated vaccination strategies that adapt to the changing epidemiology and serogroup distribution. For developing countries with adequate surveillance data, financial challenges should be overcome by either adopting tiered-pricing system that provides the vaccine at a lower price in developing countries as compared to the developed, or by following the model of PDP that led to the development of MenAfriVac for polyvalent MCV. Additionally, demonstrating the financial burden of a vaccine-preventable disease and providing information about the cost effectiveness of the vaccine are instrumental in making final decisions regarding implementation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, Eskola J, Fermon F, Klugman KP, Ramsay M, Sow S, et al.. Global epidemiology of invasive meningococcal disease. Popul Health Metr. 2013;11(1):17. doi: 10.1186/1478-7954-11-17. PMID:24016339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine. 2012;30(Suppl 2):B3–9. doi: 10.1016/j.vaccine.2011.12.062. PMID:22607896. [DOI] [PubMed] [Google Scholar]
- 3.Thompson MJ, Ninis N, Perera R, Mayon-White R, Phillips C, Bailey L, Harnden A, Mant D, Levin M. Clinical recognition of meningococcal disease in children and adolescents. Lancet. 2006;367(9508):397–403. doi: 10.1016/S0140-6736(06)67932-4. PMID:16458763. [DOI] [PubMed] [Google Scholar]
- 4.Hart CA, Thomson AP. Meningococcal disease and its management in children. BMJ. 2006;333(7570):685–90. doi: 10.1136/bmj.38968.683958.AE. PMID:17008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol. 2012;799:1–20. doi: 10.1007/978-1-61779-346-2_1. PMID:21993636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Branco RG, Amoretti CF, Tasker RC. Meningococcal disease and meningitis. J Pediatr (Rio J). 2007;83(2 Suppl):S46–53. doi: 10.2223/JPED.1612. PMID:17486194. [DOI] [PubMed] [Google Scholar]
- 7.Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, Corton C, Care R, Poolman JT, Zollinger WD, et al.. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis. 2013;19(4):566–73. doi: 10.3201/eid1904.111799. PMID:23628376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelton SI. The global evolution of meningococcal epidemiology following the introduction of Meningococcal vaccines. J Adolesc Health. 2016;59(2 Suppl):S3–S11. doi: 10.1016/j.jadohealth.2016.04.012. PMID:27449148. [DOI] [PubMed] [Google Scholar]
- 9.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369(9580):2196–210. doi: 10.1016/S0140-6736(07)61016-2. PMID:17604802. [DOI] [PubMed] [Google Scholar]
- 10.van de Beek D. Progress and challenges in bacterial meningitis. Lancet. 2012;380(9854):1623–4. doi: 10.1016/S0140-6736(12)61808-X. PMID:23141602. [DOI] [PubMed] [Google Scholar]
- 11.Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(5):317–28. doi: 10.1016/S1473-3099(10)70048-7. PMID:20417414. [DOI] [PubMed] [Google Scholar]
- 12.Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, Pondo T, Arnold KE, Baumbach J, Bennett N, et al.. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: implications for prevention of meningococcal disease. Clin Infect Dis. 2010;50(2):184–91. doi: 10.1086/649209. PMID:20001736. [DOI] [PubMed] [Google Scholar]
- 13.Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE, Centers for Disease Control and Prevention (CDC) . Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR–2):1–28. PMID:23515099. [PubMed] [Google Scholar]
- 14.Bilukha O, Messonnier N, Fischer M. Use of meningococcal vaccines in the United States. Pediatr Infect Dis J. 2007;26(5):371–6. doi: 10.1097/01.inf.0000259996.95965.ef. PMID:17468644. [DOI] [PubMed] [Google Scholar]
- 15.Bruce MG, Rosenstein NE, Capparella JM, Shutt KA, Perkins BA, Collins M. Risk factors for meningococcal disease in college students. JAMA. 2001;286(6):688–93. doi: 10.1001/jama.286.6.688. PMID:11495618. [DOI] [PubMed] [Google Scholar]
- 16.Shibl A, Tufenkeji H, Khalil M, Memish Z, Meningococcal Leadership Forum Expert G . Consensus recommendation for meningococcal disease prevention in children and adolescents in the Middle East region. J Epidemiol Glob Health. 2012;2(1):23–30. doi: 10.1016/j.jegh.2012.02.002. PMID:23856395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liphaus BL, Cappeletti-Goncalves-Okai MI, Silva-Delemos AP, Gorla MC, Rodriguez-Fernandes M, Pacola MR, Fernandez-Collucci MÂ, Matsumoto-Shinkai IA, Takenori-Higa F, Ferreira-Catani C, et al.. Outbreak of Neisseria meningitidis C in a Brazilian oil refinery involving an adjacent community. Enferm Infecc Microbiol Clin. 2013;31(2):88–92. doi: 10.1016/j.eimc.2012.05.009. PMID:22943834. [DOI] [PubMed] [Google Scholar]
- 18.Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853–61. doi: 10.1016/S1473-3099(10)70251-6. PMID:21075057. [DOI] [PubMed] [Google Scholar]
- 19.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl 2):B51–63. doi: 10.1016/j.vaccine.2009.04.063. PMID:19477562. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease ControlPrevention Meningococcal disease. Surveillance. 2017. Available from: https://http://www.cdc.gov/meningococcal/surveillance/index.html. [Google Scholar]
- 21.Riou JY, Djibo S, Sangare L, Lombart JP, Fagot P, Chippaux JP, Guibourdenche M. A predictable comeback: the second pandemic of infections caused by Neisseria meningitidis serogroup A subgroup III in Africa, 1995. Bull World Health Organ. 1996;74(2):181–7. PMID:8706234. [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson SJ, Charlett A, Orr HJ, Barker RM, Neal KR, Taylor C, Monk PN, Evans MR, Stuart JM. Risk factors for meningococcal disease in university halls of residence. Epidemiol Infect. 2001;126(2):211–7. PMID:11349971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidikou F, Zaneidou M, Alkassoum I, Schwartz S, Issaka B, Obama R, Lingani C, Tate A, Ake F, Sakande S, et al.. Emergence of epidemic Neisseria meningitidis serogroup C in Niger, 2015: an analysis of national surveillance data. Lancet Infect Dis. 2016;16(11):1288–94. doi: 10.1016/S1473-3099(16)30253-5. PMID:27567107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guibourdenche M, Hoiby EA, Riou JY, Varaine F, Joguet C, Caugant DA. Epidemics of serogroup A Neisseria meningitidis of subgroup III in Africa, 1989–94. Epidemiol Infect. 1996;116(2):115–20. doi: 10.1017/S095026880005233X. PMID:8620901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatt KM, Bhatt SM, Mirza NB. Meningococcal meningitis. East Afr Med J. 1996;73(1):35–9. PMID:8625860. [PubMed] [Google Scholar]
- 26.Hart CA, Cuevas LE. Meningococcal disease in Africa. Ann Trop Med Parasitol. 1997;91(7):777–85. doi: 10.1080/00034983.1997.11813203. PMID:9625934. [DOI] [PubMed] [Google Scholar]
- 27.Chang Q, Tzeng YL, Stephens DS. Meningococcal disease: changes in epidemiology and prevention. Clin Epidemiol. 2012;4:237–45. doi: 10.2147/CLEP.S28410. PMID:23071402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lingani C, Bergeron-Caron C, Stuart JM, Fernandez K, Djingarey MH, Ronveaux O, Schnitzler JC, Perea WA. Meningococcal meningitis surveillance in the African Meningitis Belt, 2004–2013. Clin Infect Dis. 2015;61(Suppl 5):S410–5. doi: 10.1093/cid/civ597. PMID:26553668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trotter CL, Lingani C, Fernandez K, Cooper LV, Bita A, Tevi-Benissan C, Ronveaux O, Préziosi MP, Stuart JM. Impact of MenAfriVac in nine countries of the African meningitis belt, 2010–15: an analysis of surveillance data. Lancet Infect Dis. 2017;17(8):867–72. doi: 10.1016/S1473-3099(17)30301-8. PMID:28545721. [DOI] [PubMed] [Google Scholar]
- 30.Campbell H, Ladhani S. The importance of surveillance: Group W meningococcal disease outbreak response and control in England. Int Health. 2016;8(6):369–71. doi: 10.1093/inthealth/ihw037. PMID:27620924. [DOI] [PubMed] [Google Scholar]
- 31.Broker M, Emonet S, Fazio C, Jacobsson S, Koliou M, Kuusi M, Pace D, Paragi M, Pysik A, Simões MJ, et al.. Meningococcal serogroup Y disease in Europe: continuation of high importance in some European regions in 2013. Hum Vaccin Immunother. 2015;11(9):2281–6. doi: 10.1080/21645515.2015.1051276. PMID:26036710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CDC Active Bacterial Core Surveillance Report. Emerging Infections Program Network, Neisseria meningitidis 2015. [Google Scholar]
- 33.Tsang RSW, Deeks SL, Wong K, Marchand-Austin A, FB J. Invasive serogroup W Neisseria meningitidis (MenW) in Ontario, Canada shows potential clonal replacement during the period January 1, 2009 – June 30, 2016. Can Comm Dis Rep. 2016;42(12):263–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsang RSW, Hoang L, Tyrrell GJ, Horsman G, Van Caeseele P Jamieson F, Lefebvre B, Haldane D, Gad RR, German GJ, et al.. Increase in Neisseria meningitidis serogroup W invasive disease in Canada: 2009–2016. Can Commun Dis Rep. 2017;43(7/8):144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crum-Cianflone N, Sullivan E. Meningococcal vaccinations. Infect Dis Ther. 2016;5(2):89–112. doi: 10.1007/s40121-016-0107-0. PMID:27086142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abad R, Lopez EL, Debbag R, Vazquez JA. Serogroup W meningococcal disease: global spread and current affect on the Southern Cone in Latin America. Epidemiol Infect. 2014;142(12):2461–70. doi: 10.1017/S0950268814001149. PMID:24831052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aguilera JF, Perrocheau A, Meffre C, Hahne S, Group WW. Outbreak of serogroup W135 meningococcal disease after the Hajj pilgrimage, Europe, 2000. Emerg Infect Dis. 2002;8(8):761–7. doi: 10.3201/eid0805.010422. PMID:12141959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Epidemic meningitis control in countries of the African meningitis belt, 2016. Wkly Epidemiol Rec. 2017;92(13):145–54. PMID:28361528. [PubMed] [Google Scholar]
- 39.Lahra MM, for the National Neisseria Network A . Australian Meningococcal Surveillance Programme, 1 January to 31 March 2017. Commun Dis Intell Q Rep. 2017;41(2):E201. PMID:28899315. [PubMed] [Google Scholar]
- 40.Martin NV, Ong KS, Howden BP, Lahra MM, Lambert SB, Beard FH, Dowse GK, Saul N, Communicable Diseases Network Australia MenW Working Group . Rise in invasive serogroup W meningococcal disease in Australia 2013–2015. Commun Dis Intell Q Rep. 2016;40(4):E454–E9. PMID:28043219. [PubMed] [Google Scholar]
- 41.Ceyhan M, Anis S, Htun-Myint L, Pawinski R, Soriano-Gabarro M, Vyse A. Meningococcal disease in the Middle East and North Africa: an important public health consideration that requires further attention. Int J Infect Dis. 2012;16(8):e574–82. doi: 10.1016/j.ijid.2012.03.011. PMID:22647750. [DOI] [PubMed] [Google Scholar]
- 42.Borrow R, Alarcon P, Carlos J, Caugant DA, Christensen H, Debbag R, De Wals P, Echániz-Aviles G, Findlow J, Head C, et al.. The Global Meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16(4):313–28. doi: 10.1080/14760584.2017.1258308. PMID:27820969. [DOI] [PubMed] [Google Scholar]
- 43.Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364(9431):365–7. doi: 10.1016/S0140-6736(04)16725-1. PMID:15276396. [DOI] [PubMed] [Google Scholar]
- 44.Ramsay ME, Andrews N, Kaczmarski EB, Miller E. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet. 2001;357(9251):195–6. doi: 10.1016/S0140-6736(00)03594-7. PMID:11213098. [DOI] [PubMed] [Google Scholar]
- 45.Bose A, Coen P, Tully J, Viner R, Booy R. Effectiveness of meningococcal C conjugate vaccine in teenagers in England. Lancet. 2003;361(9358):675–6. doi: 10.1016/S0140-6736(03)12563-9. PMID:12606181. [DOI] [PubMed] [Google Scholar]
- 46.Campbell H, Andrews N, Borrow R, Trotter C, Miller E. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol. 2010;17(5):840–7. doi: 10.1128/CVI.00529-09. PMID:20219881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ladhani SN, Beebeejaun K, Lucidarme J, Campbell H, Gray S, Kaczmarski E, Ramsay ME, Borrow R. Increase in endemic Neisseria meningitidis capsular group W sequence type 11 complex associated with severe invasive disease in England and Wales. Clin Infect Dis. 2015;60(4):578–85. doi: 10.1093/cid/ciu881. PMID:25389259. [DOI] [PubMed] [Google Scholar]
- 48.Mustapha MM, Marsh JW, Krauland MG, Fernandez JO, de Lemos AP, Dunning Hotopp JC, Wang X, Mayer LW, Lawrence JG, Hiller NL, et al.. Genomic investigation reveals highly conserved, mosaic, recombination events associated with capsular switching among invasive Neisseria meningitidis Serogroup W Sequence Type (ST)-11 strains. Genome Biol Evol. 2016;8(6):2065–75. doi: 10.1093/gbe/evw122. PMID:27289093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Safadi MA, Bettinger JA, Maturana GM, Enwere G, Borrow R, Global Meningococcal I. Evolving meningococcal immunization strategies. Expert Rev Vaccines. 2015;14(4):505–17. doi: 10.1586/14760584.2015.979799. PMID:25494168. [DOI] [PubMed] [Google Scholar]
- 50.Safadi MA, O'Ryan M, Valenzuela Bravo MT, Brandileone MC, Gorla MC, de Lemos AP, Moreno G, Vazquez JA, López EL, Taha MK, et al.. The current situation of meningococcal disease in Latin America and updated Global Meningococcal Initiative (GMI) recommendations. Vaccine. 2015;33(48):6529–36. doi: 10.1016/j.vaccine.2015.10.055. PMID:26597036. [DOI] [PubMed] [Google Scholar]
- 51.Cohn AC, MacNeil JR, Harrison LH, Lynfield R, Reingold A, Schaffner W, Zell ER, Plikaytis B, Wang X, Messonnier NE, et al.. Effectiveness and duration of protection of one dose of a meningococcal conjugate vaccine. Pediatrics. 2017;139(2):2016–2193. doi: 10.1542/peds.2016-2193. PMID:28100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Broderick MP, Faix DJ, Hansen CJ, Blair PJ. Trends in meningococcal disease in the United States military, 1971–2010. Emerg Infect Dis. 2012;18(9):1430–7. doi: 10.3201/eid1809.120257. PMID:22932005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kshirsagar N, Mur N, Thatte U, Gogtay N, Viviani S, Preziosi MP, Elie C, Findlow H, Carlone G, Borrow R, et al.. Safety, immunogenicity, and antibody persistence of a new meningococcal group A conjugate vaccine in healthy Indian adults. Vaccine. 2007;25(Suppl 1):A101–7. doi: 10.1016/j.vaccine.2007.04.050. PMID:17532101. [DOI] [PubMed] [Google Scholar]
- 54.Kristiansen PA, Diomande F, Ba AK, Sanou I, Ouedraogo AS, Ouedraogo R, Sangaré L, Kandolo D, Aké F, Saga IM, et al.. Impact of the serogroup A meningococcal conjugate vaccine, MenAfriVac, on carriage and herd immunity. Clin Infect Dis. 2013;56(3):354–63. doi: 10.1093/cid/cis892. PMID:23087396. [DOI] [PubMed] [Google Scholar]
- 55.Parikh SR, Andrews NJ, Beebeejaun K, Campbell H, Ribeiro S, Ward C, White JM, Borrow R, Ramsay ME, Ladhani SN. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet. 2016;388(10061):2775–82. doi: 10.1016/S0140-6736(16)31921-3. PMID:28100432. [DOI] [PubMed] [Google Scholar]
- 56.Wang NY, Pollard AJ. The next chapter for group B meningococcal vaccines. Crit Rev Microbiol. 2017;44(1):1–17. doi: 10.1080/1040841X.2017.1329276. PMID:28557577. [DOI] [PubMed] [Google Scholar]
- 57.Grogan J, Roos K. Serogroup B meningococcus outbreaks, prevalence, and the case for standard vaccination. Curr Infect Dis Rep. 2017;19(9):30. doi: 10.1007/s11908-017-0587-4. PMID:28770496. [DOI] [PubMed] [Google Scholar]
- 58.Basta NE, Mahmoud AA, Wolfson J, Ploss A, Heller BL, Hanna S, Johnsen P, Izzo R, Grenfell BT, Findlow J, et al.. Immunogenicity of a Meningococcal B Vaccine during a University Outbreak. N Engl J Med. 2016;375(3):220–8. doi: 10.1056/NEJMoa1514866. PMID:27468058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuhdari P, Stefanati A, Lupi S, Valente N, Gabutti G. Meningococcal B vaccination: real-world experience and future perspectives. Pathog Glob Health. 2016;110(4–5):148–56. doi: 10.1080/20477724.2016.1195072. PMID:27309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Findlow J. Vaccines for the prevention of meningococcal capsular group B disease: what have we recently learned? Hum Vaccin Immunother. 2016;12(1):235–8. doi: 10.1080/21645515.2015.1091131. PMID:26619037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ali A, Jafri RZ, Messonnier N, Tevi-Benissan C, Durrheim D, Eskola J, Fermon F, Klugman KP, Ramsay M, Sow S, et al.. Global practices of meningococcal vaccine use and impact on invasive disease. Pathog Glob Health. 2014;108(1):11–20. doi: 10.1179/2047773214Y.0000000126. PMID:24548156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.World Bank Country and Lending Groups - Country Classification 2017. [Google Scholar]
- 63.Broutin H, Philippon S, Constantin de Magny G, Courel MF, Sultan B, Guegan JF. Comparative study of meningitis dynamics across nine African countries: a global perspective. Int J Health Geogr. 2007;6:29. doi: 10.1186/1476-072X-6-29. PMID:17623084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dbaibo G, Tatochenko V, Wutzler P. Issues in pediatric vaccine-preventable diseases in low- to middle-income countries. Hum Vaccin Immunother. 2016;12(9):2365–77. doi: 10.1080/21645515.2016.1181243. PMID:27322436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gabutti G, Stefanati A, Kuhdari P. Epidemiology of Neisseria meningitidis infections: case distribution by age and relevance of carriage. J Prev Med Hyg. 2015;56(3):E116–20. PMID:26788731. [PMC free article] [PubMed] [Google Scholar]
- 66.Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, Ladhani SN, McIntyre P, Ramsay ME, Sáfadi MA. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30(Suppl 2):B26–36. doi: 10.1016/j.vaccine.2011.12.032. PMID:22178525. [DOI] [PubMed] [Google Scholar]
- 67.Fukusumi M, Kamiya H, Takahashi H, Kanai M, Hachisu Y, Saitoh T, Ohnishi M, Oishi K, Sunagawa T. National surveillance for meningococcal disease in Japan, 1999–2014. Vaccine. 2016;34(34):4068–71. doi: 10.1016/j.vaccine.2016.06.018. PMID:27291085. [DOI] [PubMed] [Google Scholar]
- 68.Chiou CS, Liao JC, Liao TL, Li CC, Chou CY, Chang HL, Yao SM, Lee YS. Molecular epidemiology and emergence of worldwide epidemic clones of Neisseria meningitidis in Taiwan. BMC Infect Dis. 2006;6:25. doi: 10.1186/1471-2334-6-25. PMID:16478548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Invasive Meningococcal Disease - National Surveillance Report Australian Government Department of Health; 2017. p. 1–8. [Google Scholar]
- 70.World Health Organization – Emergencies preparedness, response – Meningococcal disease [4/10/17] Available from: http://www.who.int/entity/csr/don/archive/disease/meningococcal_disease/en/index.html.
- 71.Abstracts - The European Meningococcal Disease Society Austria. 2013. [Google Scholar]
- 72.Safadi MA, Cintra OA. Epidemiology of meningococcal disease in Latin America: current situation and opportunities for prevention. Neurol Res. 2010;32(3):263–71. doi: 10.1179/016164110X12644252260754. PMID:20406604. [DOI] [PubMed] [Google Scholar]
- 73.Perez AE, Dickinson FO, Rodriguez M. Community acquired bacterial meningitis in Cuba: a follow up of a decade. BMC Infect Dis. 2010;10:130. doi: 10.1186/1471-2334-10-130. PMID:20500858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mendsaikhan J, Watt JP, Mansoor O, Suvdmaa N, Edmond K, Litt DJ, Nymadawa P, Baoping Y, Altantsetseg D, Slack M. Childhood bacterial meningitis in Ulaanbaatar, Mongolia, 2002–2004. Clin Infect Dis. 2009;48(Suppl 2):S141–6. doi: 10.1086/596493. PMID:19191628. [DOI] [PubMed] [Google Scholar]
- 75.Sinclair D, Preziosi MP, Jacob John T, Greenwood B. The epidemiology of meningococcal disease in India. Trop Med Int Health. 2010;15(12):1421–35. doi: 10.1111/j.1365-3156.2010.02660.x. PMID:21054695. [DOI] [PubMed] [Google Scholar]
- 76.World Health Organization Regional Office for the Eastern Mediterranea (EMRO) – Country profiles 2008. Available from: http://www.emro.who.int/emrinfo.
- 77.Yemen Ministry of Health Annual statistical health report 2009. 2009. Available from: http://www.mophp-ye.org/arabic/docs/Report2009.pdf.
- 78.Afifi S, Wasfy MO, Azab MA, Youssef FG, Pimentel G, Graham TW, Mansour H, Elsayed N, Earhart K, Hajjeh R, et al.. Laboratory-based surveillance of patients with bacterial meningitis in Egypt (1998–2004). Eur J Clin Microbiol Infect Dis. 2007;26(5):331–40. doi: 10.1007/s10096-007-0280-x. PMID:17404766. [DOI] [PubMed] [Google Scholar]
- 79.Ceyhan M, Yildirim I, Balmer P, Borrow R, Dikici B, Turgut M, Kurt N, Aydogan A, Ecevit C, Anlar Y, et al.. A prospective study of etiology of childhood acute bacterial meningitis, Turkey. Emerg Infect Dis. 2008;14(7):1089–96. doi: 10.3201/eid1407.070938. PMID:18598630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ceyhan M, Gurler N, Ozsurekci Y, Keser M, Aycan AE, Gurbuz V, Salman N, Camcioglu Y, Dinleyici EC, Ozkan S, et al.. Meningitis caused by Neisseria Meningitidis, Hemophilus Influenzae Type B and Streptococcus Pneumoniae during 2005–2012 in Turkey. A multicenter prospective surveillance study. Hum Vaccin Immunother. 2014;10(9):2706–12. doi: 10.4161/hv.29678. PMID:25483487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Borrow R, Lee JS, Vazquez JA, Enwere G, Taha MK, Kamiya H, Kim HM, Jo DS, Global Meningococcal Initiative . Meningococcal disease in the Asia-Pacific region: findings and recommendations from the Global Meningococcal Initiative. Vaccine. 2016;34(48):5855–62. doi: 10.1016/j.vaccine.2016.10.022. PMID:27780631. [DOI] [PubMed] [Google Scholar]
- 82.Bahrain Ministry of Health Health statistics 2007. Available from: http://www.moh.gov.bh/PDF/Publications/Statistics/hs2007/pdf/CH07-publi-chealth_2007.pdf.
- 83.World Health Organization Control of epidemic meningococcal disease. WHO practical guidelines. 2nd ed. Available from: http://www.who.int/csr/resources/publications/meningitis/whoemcbac983.pdf.
- 84.Greenwood B. Editorial: 100 years of epidemic meningitis in West Africa – has anything changed? Trop Med Int Health. 2006;11(6):773–80. doi: 10.1111/j.1365-3156.2006.01639.x. PMID:16771997. [DOI] [PubMed] [Google Scholar]
- 85.Mohammed I, Iliyasu G, Habib AG. Emergence and control of epidemic meningococcal meningitis in sub-Saharan Africa. Pathog Glob Health. 2017;111(1):1–6. doi: 10.1080/20477724.2016.1274068. PMID:28081671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Borrow R, Caugant DA, Ceyhan M, Christensen H, Dinleyici EC, Findlow J, Glennie L, Von Gottberg A Kechrid A, Vázquez Moreno J, et al.. Meningococcal disease in the Middle East and Africa: Findings and updates from the Global Meningococcal Initiative. J Infect. 2017;75(1):1–11. doi: 10.1016/j.jinf.2017.04.007. PMID:28455205. [DOI] [PubMed] [Google Scholar]
- 87.Ceyhan M, Ozsurekci Y, Gurler N, Karadag Oncel E, Camcioglu Y, Salman N, Celik M, Emiroglu MK, Akin F, Tezer H, et al.. Bacterial agents causing meningitis during 2013–2014 in Turkey: A multi-center hospital-based prospective surveillance study. Hum Vaccin Immunother. 2016;12(11):2940–5. doi: 10.1080/21645515.2016.1209278. PMID:27454468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dickinson FO, Perez AE. Bacterial meningitis in children and adolescents: an observational study based on the national surveillance system. BMC Infect Dis. 2005;5:103. doi: 10.1186/1471-2334-5-103. PMID:16288649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Velez-van-Meerbeke A, Medina-Silva N, Besada-Lombana, Mojica-Madero JA. Epidemiology of meningococcal disease in Colombia. Colombia: Neuroscience (NEUROS) Research Group; 2015. doi: 10.1016/j.infect.2016.02.004. [DOI] [Google Scholar]
- 90.Safadi MA, Berezin EN, Arlant LH. Meningococcal disease: epidemiology and early effects of immunization programs. J Pediatric Infect Dis Soc. 2014;3(2):91–3. doi: 10.1093/jpids/piu027. PMID:26625360. [DOI] [PubMed] [Google Scholar]
- 91.Safadi MA, de los Monteros LE, Lopez EL, Saez-Llorens X, Lemos AP, Moreno-Espinosa S, Ayala SG, Torres JP, de Moraes JC, Vázquez JA. The current situation of meningococcal disease in Latin America and recommendations for a new case definition from the Global Meningococcal Initiative. Expert Rev Vaccines. 2013;12(8):903–15. doi: 10.1586/14760584.2013.814879. PMID:23909747. [DOI] [PubMed] [Google Scholar]
- 92.Vyse A, Wolter JM, Chen J, Ng T, Soriano-Gabarro M. Meningococcal disease in Asia: an under-recognized public health burden. Epidemiol Infect. 2011;139(7):967–85. doi: 10.1017/S0950268811000574. PMID:21492496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–40. doi: 10.1016/S0140-6736(14)61698-6. PMID:25280870. [DOI] [PubMed] [Google Scholar]
- 94.Benet T, Picot VS, Awasthi S, Pandey N, Bavdekar A, Kawade A, Robinson A, Rakoto-Andrianarivelo M, Sylla M, Diallo S, et al.. Severity of Pneumonia in under 5-year-old children from developing countries: a multicenter, prospective, observational study. Am J Trop Med Hyg. 2017;97(1):68–76. doi: 10.4269/ajtmh.16-0733. PMID:28719310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Glass RI, Bresee JS, Turcios R, Fischer TK, Parashar UD, Steele AD. Rotavirus vaccines: targeting the developing world. J Infect Dis. 2005;192(Suppl 1):S160–6. doi: 10.1086/431504. PMID:16088799. [DOI] [PubMed] [Google Scholar]
- 96.Esposito S, Principi N. Pneumococcal vaccines and the prevention of community-acquired pneumonia. Pulm Pharmacol Ther. 2015;32:124–9. doi: 10.1016/j.pupt.2014.02.003. PMID:24607597. [DOI] [PubMed] [Google Scholar]
- 97.Castelblanco RL, Lee M, Hasbun R. Epidemiology of bacterial meningitis in the USA from 1997 to 2010: a population-based observational study. Lancet Infect Dis. 2014;14(9):813–9. doi: 10.1016/S1473-3099(14)70805-9. PMID:25104307. [DOI] [PubMed] [Google Scholar]
- 98.Pneumococcal conjugate vaccine for childhood immunization–WHO position paper. Wkly Epidemiol Rec. 2007;82(12):93–104. PMID:17380597. [PubMed] [Google Scholar]
- 99.Haemophilus influenzae type b (Hib) Vaccination Position Paper – July 2013. Wkly Epidemiol Rec. 2013;88(39):413–26. PMID:24143842. [PubMed] [Google Scholar]
- 100.Yezli S, Bin Saeed AA, Assiri AM, Alhakeem RF, Yunus MA, Turkistani AM, Booy R, Alotaibi BM. Prevention of meningococcal disease during the Hajj and Umrah mass gatherings: past and current measures and future prospects. Int J Infect Dis. 2016;47:71–8. doi: 10.1016/j.ijid.2015.12.010. PMID:26707071. [DOI] [PubMed] [Google Scholar]
- 101.Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9(3):213–20. doi: 10.1038/nri2494. PMID:19214194. [DOI] [PubMed] [Google Scholar]
- 102.Broker M, Cooper B, Detora LM, Stoddard JJ. Critical appraisal of a quadrivalent CRM(197) conjugate vaccine against meningococcal serogroups A, C W-135 and Y (Menveo) in the context of treatment and prevention of invasive disease. Infect Drug Resist. 2011;4:137–47. doi: 10.2147/IDR.S12716. PMID:21904459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Patton ME, Stephens D, Moore K, MacNeil JR. Updated recommendations for use of MenB-FHbp Serogroup B Meningococcal Vaccine – advisory committee on immunization practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(19):509–13. doi: 10.15585/mmwr.mm6619a6. PMID:28520709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tan LK, Carlone GM, Borrow R. Advances in the development of vaccines against Neisseria meningitidis. N Engl J Med. 2010;362(16):1511–20. doi: 10.1056/NEJMra0906357. PMID:20410516. [DOI] [PubMed] [Google Scholar]
- 105.Meningococcal Polysaccharide Vaccine Bivalent AC or Trivalent ACW 2005. [Google Scholar]
- 106.Meningococcal Vaccines: Market & Supply Update 2015. 1–9] Available from: https://http://www.unicef.org/supply/files/Meningitis_Vaccine_Market_Supply_Update.pdf.
- 107.European Medicines Agency Nimenrix: summary of product characteristics. GlaskoSmithKline; 2017. p. 1–65. [Google Scholar]
- 108.NeisVac-C® (meningococcal group C-TT conjugate vaccine, adsorbed) Product Monograph Pfizer Canada Inc.; 2015. p. 1–23. [Google Scholar]
- 109.Product Information - Meningitec - Meningococcal Serogroup C Conjugate Vaccine Pfizer Australia Pty Ltd; 2011. p. 1–9. [Google Scholar]
- 110.Menjugate®Liquid - Product Monograph GSK Canada. 2015:1–22. [Google Scholar]
- 111.Patel M, Lee CK. Polysaccharide vaccines for preventing serogroup A meningococcal meningitis. Cochrane Database Syst Rev. 2005;(1):CD001093. doi: 10.1002/14651858.CD001093.pub2. PMID:15674874. [DOI] [PubMed] [Google Scholar]
- 112.World Health Organization – Meningococcal disease in the African Meningitis Belt epidemic season 2006. 2006 [6/10/17]. Available from: http://www.who.int/csr/don/2006_03_21/en/.
- 113.The use of polysaccharide trivalent ACW vaccine for the control of epidemic meningococcal disease outbreaks in countries of the African meningitis belt. Switzerland: World Health Organization; 2003. [Google Scholar]
- 114.Poland GA. Prevention of meningococcal disease: current use of polysaccharide and conjugate vaccines. Clin Infect Dis. 2010;50(Suppl 2):S45–53. doi: 10.1086/648964. PMID:20144016. [DOI] [PubMed] [Google Scholar]
- 115.Khatami A, Pollard AJ. The epidemiology of meningococcal disease and the impact of vaccines. Expert Rev Vaccines. 2010;9(3):285–98. doi: 10.1586/erv.10.3. PMID:20218857. [DOI] [PubMed] [Google Scholar]
- 116.Baxter R, Keshavan P, Welsch JA, Han L, Smolenov I. Persistence of the immune response after MenACWY-CRM vaccination and response to a booster dose, in adolescents, children and infants. Hum Vaccin Immunother. 2016;12(5):1300–10. doi: 10.1080/21645515.2015.1136040. PMID:26829877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Erlich KS, Congeni BL. Importance of circulating antibodies in protection against meningococcal disease. Hum Vaccin Immunother. 2012;8(8):1029–35. doi: 10.4161/hv.20473. PMID:22854672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Merino Arribas JM, Carmona Martinez A, Horn M, Perez Porcuna XM, Otero Reigada MD, Mares Bermudez J, Centeno Malfaz F, Miranda M, Mendez M, Garcia Cabezas MA, et al.. Safety and immunogenicity of the quadrivalent Meningococcal Serogroups A, C, W and Y tetanus toxoid conjugate vaccine coadministered with routine childhood vaccines in European Infants: an open, randomized trial. Pediatr Infect Dis J. 2017;36(4):e98–e107. doi: 10.1097/INF.0000000000001484. PMID:28002359. [DOI] [PubMed] [Google Scholar]
- 119.Zahlanie YC, Hammadi MM, Ghanem ST, Dbaibo GS. Review of meningococcal vaccines with updates on immunization in adults. Hum Vaccin Immunother. 2014;10(4):995–1007. PMID:24500529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.European Centre for Disease PreventionControl Expert opinion on the introduction of the meningococcal B (4CMenB) vaccine in the EU/EEA. Stockholm: ECDC; 2017. [Google Scholar]
- 121.MacNeil JR, Rubin L, Folaranmi T, Ortega-Sanchez IR, Patel M, Martin Stacey W. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the advisory committee on immunization practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(41):1171–6. PMID:26492381. [DOI] [PubMed] [Google Scholar]
- 122.Ortega-Sanchez IR, Meltzer MI, Shepard C, Zell E, Messonnier ML, Bilukha O, Zhang X, Stephens DS, Messonnier NE, Active Bacterial Core Surveillance Team . Economics of an adolescent meningococcal conjugate vaccination catch-up campaign in the United States. Clin Infect Dis. 2008;46(1):1–13. doi: 10.1086/524041. PMID:18171206. [DOI] [PubMed] [Google Scholar]
- 123.Scott RD, 2nd Meltzer MI, Erickson LJ, De Wals P Rosenstein NE. Vaccinating first-year college students living in dormitories for Meningococcal disease: an economic analysis. Am J Prev Med. 2002;23(2):98–105. PMID:12121797. [DOI] [PubMed] [Google Scholar]
- 124.Shepard CW, Ortega-Sanchez IR, Scott RD, 2nd Rosenstein NE, Team AB. Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics. 2005;115(5):1220–32. doi: 10.1542/peds.2004-2514. PMID:15867028. [DOI] [PubMed] [Google Scholar]
- 125.De Voer RM, Mollema L, Schepp RM, de Greeff SC, PG vG . Immunity against Neisseria meningitides serogroup C in the Dutch population before and after introduction of the meningococcal C conjugate vaccine. PLoS One. 2010;5(e12144). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hepkema H, Pouwels KB, van der Ende A, Westra TA, Postma MJ. Meningococcal serogroup A, C, W(1)(3)(5) and Y conjugated vaccine: a cost-effectiveness analysis in the Netherlands. PLoS One. 2013;8(5):e65036. doi: 10.1371/journal.pone.0065036. PMID:23741448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.De Wals P, Zhou Z. Cost-effectiveness comparison of monovalent C versus quadrivalent ACWY meningococcal conjugate vaccination in Canada. Pediatr Infect Dis J. 2017;36(7):e203–e7. doi: 10.1097/INF.0000000000001512. PMID:28027288. [DOI] [PubMed] [Google Scholar]
- 128.European Centre for Disease PreventionControl: Surveillance of invasive bacterial diseases in Europe 2008/2009 Stockholm 2011. 1–136. [Google Scholar]
- 129.Ladhani SN, Ramsay M, Borrow R, Riordan A, Watson JM, Pollard AJ. Enter B and W: two new meningococcal vaccine programmes launched. Arch Dis Child. 2016;101(1):91–5. doi: 10.1136/archdischild-2015-308928. PMID:26672098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gasparini R, Panatto D. Meningococcal glycoconjugate vaccines. Hum Vaccin. 2011;7(2):170–82. doi: 10.4161/hv.7.2.13717. PMID:21178398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Panatto D, Amicizia D, Lai PL, Gasparini R. Neisseria meningitidis B vaccines. Expert Rev Vaccines. 2011;10(9):1337–51. doi: 10.1586/erv.11.103. PMID:21919622. [DOI] [PubMed] [Google Scholar]
- 132.JCVI position statement on use of Bexsero® meningococcal B vaccine in the UK. 2014. [Google Scholar]
- 133.Gasparini R, Landa P, Amicizia D, Icardi G, Ricciardi W, de Waure C, Tanfani E, Bonanni P, Lucioni C, Testi A, et al.. Vaccinating Italian infants with a new multicomponent vaccine (Bexsero(R)) against meningococcal B disease: a cost-effectiveness analysis. Hum Vaccin Immunother. 2016;12(8):2148–61. doi: 10.1080/21645515.2016.1160177. PMID:27163398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Christensen H, Trotter CL. Modelling the cost-effectiveness of catch-up ‘MenB’ (Bexsero) vaccination in England. Vaccine. 2017;35(2):208–11. doi: 10.1016/j.vaccine.2016.11.076. PMID:27923519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.de Soarez PC, Sartori AM, de Andrade Lagoa Nobrega L, Itria A, Novaes HM. Cost-effectiveness analysis of a universal infant immunization program with meningococcal C conjugate vaccine in Brazil. Value Health. 2011;14(8):1019–27. doi: 10.1016/j.jval.2011.05.045. PMID:22152170. [DOI] [PubMed] [Google Scholar]
- 136.Parent du Chatelet I, Gessner BD, da Silva A. Comparison of cost-effectiveness of preventive and reactive mass immunization campaigns against meningococcal meningitis in West Africa: a theoretical modeling analysis. Vaccine. 2001;19(25–26):3420–31. doi: 10.1016/S0264-410X(01)00066-4. PMID:11348706. [DOI] [PubMed] [Google Scholar]
- 137.LaForce M. Introduction of a group A meningococcal conjugate vaccine in sub-Saharan Africa: Cost/savings analysis. MVP unpublished data. 2008. [Google Scholar]
- 138.Colombini A, Trotter C, Madrid Y, Karachaliou A, Preziosi MP. Costs of Neisseria meningitidis Group A disease and economic impact of vaccination in Burkina Faso. Clin Infect Dis. 2015;61(Suppl 5):S473–82. doi: 10.1093/cid/civ600. PMID:26553677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Assaf-Casals A, Dbaibo G. Meningococcal quadrivalent tetanus toxoid conjugate vaccine (MenACWY-TT, Nimenrix): a review of its immunogenicity, safety, co-administration, and antibody persistence. Hum Vaccin Immunother. 2016;12(7):1825–37. doi: 10.1080/21645515.2016.1143157. PMID:26900984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Greenwood B, Chiarot E, MacLennan CA, O'Ryan M. Can we defeat meningococcal disease in low and middle income countries? Vaccine. 2012;30(Suppl 2):B63–6. doi: 10.1016/j.vaccine.2011.12.063. PMID:22607901. [DOI] [PubMed] [Google Scholar]
- 141.Somda ZC, Perry HN, Messonnier NR, Djingarey MH, Ki SO, Meltzer MI. Modeling the cost-effectiveness of the integrated disease surveillance and response (IDSR) system: meningitis in Burkina Faso. PLoS One. 2010;5(9). doi: 10.1371/journal.pone.0013044. PMID:20927386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kulkarni PS, Socquet M, Jadhav SS, Kapre SV, LaForce FM, Poonawalla CS. Challenges and opportunities while developing a Group A meningococcal conjugate vaccine within a product development partnership: a manufacturer's perspective from the Serum Institute of India. Clin Infect Dis. 2015;61(Suppl 5):S483–8. doi: 10.1093/cid/civ500. PMID:26553678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dougla R, Samant V. The vaccine industry In: Plotkin S, Orenstein W, Offit P , editors. Vaccines. 6th ed. Scotland: Saunders Elsevier; 2012. p. 33–43. [Google Scholar]
- 144.Meningitis Vaccine Project Revolutionary meningitis vaccine breaks another barrier: first to gain approval to travel outside cold chain. Atlanta (GA): Meningitis Vaccine Project; 2012. [Google Scholar]
- 145.Zipursky S, Djingarey MH, Lodjo JC, Olodo L, Tiendrebeogo S, Ronveaux O. Benefits of using vaccines out of the cold chain: delivering meningitis A vaccine in a controlled temperature chain during the mass immunization campaign in Benin. Vaccine. 2014;32(13):1431–5. doi: 10.1016/j.vaccine.2014.01.038. PMID:24559895. [DOI] [PMC free article] [PubMed] [Google Scholar]