ABSTRACT
Neutralizing antibodies (NTAbs) is a major criterion for evaluation the immunogenicity of many vaccines, for example, poliovirus and EV71 vaccine. Here, we firstly discovered that polyclonal antibodies induced by inactivated CVA16 vaccine and lived CVA16 virus have poor ability to neutralize circulating CVA16 strains in vitro. However, the passive transfer of poorly neutralizing polyclonal antibodies can protect suckling mice from lethally challenged with circulating strains in vivo. In addition, the obvious dose response was found between the titer of antibodies and the survival rate. Interestingly, poorly neutralizing polyclonal antibodies against circulating CVA16 strains, have good ability to neutralize prototype strain G10 in vitro. Between G10 and circulating CVA16 strains, there are total 47 variant sites in capsid, which are near the interface of VP1, VP2, and VP3, and close to 2-fold axis. Based on the structure of CVA16, the obvious structural changes were observed in residue 213 of VP1 GH loop, residue 139 of VP2 EF loop, and residues 59, 182 and 183 of VP3 GH loop. What we found may provide a new sight for the development of CVA16 vaccine.
KEYWORDS: coxsackievirus A16, hand foot and mouth disease, neutralizing epitopes, structure, vaccine
Introduction
Hand, Foot, and Mouth Disease (HFMD) is a common infectious disease. It usually affects infants and children under 5 years old.1 HFMD epidemics have occurred in western pacific region and caused major threats to public health. Enterovirus 71 (EV-A71) and Coxsackievirus A16 (CVA16) are the major etiological agents of HFMD in mainland China revealed by national HFMD surveillance data collected since 2008.2 Prophylactic EV-A71 vaccines developed by three companies (Beijing Vigoo Biological, Sinovac Biotech Co. Ltd, Institute of Medical Biology) have all shown good efficacy in phase III clinical trials, which will greatly decrease the EV-A71-associated HFMD incidences in the future.3,4,5 Unfortunately, these vaccines have no protection against CVA16-associated HFMD. Therefore, several vaccine companies and academic institutions launched projects to develop CVA16 monovalent or bivalent vaccine.6 But there are many challenges in CVA16 vaccine development, and it would be a long way to go before clinical trial.7
The prototype of CVA16 is G10 strain, which was firstly isolated in South Africa in 1954. The CVA16 is a single-stranded RNA (ssRNA) virus with nonenveloped icosahedral capsid. CVA16 is belonged to Picornavirus. The capsid of CVA16 comprises 60 copies of viral proteins (VP1-4). VP1, -2, and -3 are arranged with pseudo T = 3 symmetry on the outside of the capsid, while their N-terminal extensions and VP4 are in the interior and may interact with the RNA genome. The VP1 GH loop and VP3 GH loop, which was the immunodominant epitope, might be involved in interactions with virus receptor SCARB2.8 In addition, the EF loop of VP2 was also a neutralizing epitope.9
Humoral immunity is an essential component for protection against lethal infection. Therefore, the ability to stimulate production of neutralizing antibodies (NTAbs) is a major criterion for evaluation the immunogenicity of many vaccines. Cytopathogenic effect assay was widely used to test the NTAb titer in vitro.10,11 Moreover, the virus challenge and protection was also important assay in vivo for evaluating efficacy of vaccine.12 However, some study, for example, showed that anti-WNV (West Nile Virus, WNV) antibodies with poor in vitro neutralizing abilities can mediate in vivo protection in mice.13
In the study, we firstly discovered that antibodies induced by inactivated CVA16 vaccine and lived CVA16 virus have poor ability to neutralize circulating CVA16 strains in vitro. However, the passive transfer of poorly neutralizing polyclonal antibodies can protect suckling mice from lethally challenged with circulating strains in vivo. Interestingly, poorly neutralizing polyclonal antibodies against circulating CVA16 strains, have good ability to neutralize prototype strain G10. What we found may provide a new sight for the development of CVA16 vaccine.
Results
Antibodies induced by lived virus against circulating CVA16 strains
The rats were immunized with 10 live CVA16 strains separately and the cross neutralizing protection of serum samples was evaluated between G10 and circulating CVA16 trains. The result showed that all anti-rat sera could neutralize G10 with high titer, varying from 1:69.3 (95% CI: 1:39.7–1:101.8) to 1:210.7 (95% CI: 1:131.5–1:300.5). For the other circulating CVA16 strains, these polyclonal antibodies can not neutralize them in titer 1:8 (Table 1). In addition, Vero and RD cell were both used in these neutralization tests and similar results were obtained.
Table 1.
The cross neutralizing titer of live virus immunized sera.a
| Neutralizing Strains |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti Sera | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | G10* |
| Anti-S1 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | 69.3(39.7–101.8) |
| Anti-S2 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | 210.7(131.5–300.5) |
| Anti-S3 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | 146.8(96.7–203.3) |
| Anti-S4 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | 151.2(96.3–214.2) |
| Anti-S5 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | 164.9(117.0–217.6) |
| Anti-S6 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | 188.8(141.4–279.3) |
| Anti-S7 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | 143.8(80.5–218.1) |
| Anti-S8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | 112.5(68.1–101.4) |
| Anti-S9 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | 156.8(116.1–201.8) |
| Anti-S10 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | 122.7(76.6–174.8) |
Antibodies induced by inactivated vaccine against circulating CVA16 strains
In mice experiment, the polyclonal antibodies induced by inactivated vaccine can neutralize S1 strain with titer below 1:8. In contrast, The polyclonal antibodies can neutralize G10 with titer 1:43.2 (95% CI: 1:24.6–1:62.6), which is significantly higher than S1 strain (Fig. 1a).
Figure 1.
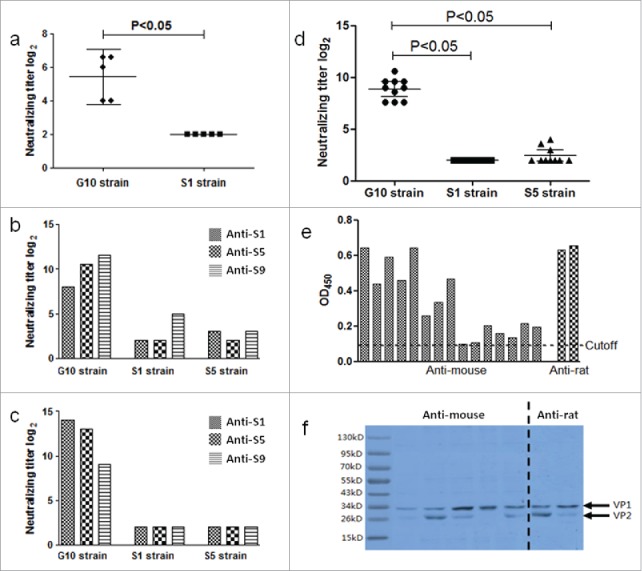
(a) Using the G10 and circulating strains (S1 strain) to test the neutralizing titer of antibodies induced by inactivated CAV16 vaccine produced by S-1 strain in mice. (b) Using the G10 and circulating strains (S1 strain and S5 strain) to test the neutralizing titer of antibodies induced by inactivated CAV16 vaccine produced by S1, S5 and S9 strain in goats. (c) Using the G10 and circulating strains (S1 strain and S5 strain) to test the neutralizing titer of antibodies induced by inactivated CAV16 vaccine produced by S1, S5 and S9 strain in rabbit. (d) Using the G10 and circulating strains (S1 strain and S5 strain) to test the neutralizing titer of antibodies in 10 convalescent sera from CVA16 infected patients. (e) Using ELISA to test the anti-CVA16 antibodies exist sera. The sera were diluted in 1:1000. (f) Using the WB to test anti-CVA16 antibodies exist in the sera.
In goat experiment, 3 serum samples, from goats immunized with 3 formalin inactivated CVA16 vaccines respectively, could neutralize S-1 and S-5 strains with titer below 1:32. In contrast, the 3 serum samples could neutralize G10 with titers varying from 1:256 to 1:3072 (Fig. 1b).
In rabbit experiment, 3 serum samples, from rabbits immunized with 3 formalin inactivated CVA16 vaccines, could neutralize S-1 and S-5 strains with titer below 1:8. In contrast, the 3 serum samples could neutralize G10 with titers varying from 1:512 to 1:16384. (Fig. 1c).
Human convalescent sera against circulating CVA16 strains
Ten convalescent serum samples from patients infected with CVA16 were collected. The neutralizing titers of human serum samples against S5 and G10 were 1:5.5 (95% CI: 1:3.9–1:6.8) and 1:467.2 (95% CI: 1:313.5–1:645.7), respectively. All serum samples failed to neutralize S1 strain. The neutralizing titers against G10 is significantly higher than against circulating strains (Fig. 1d).
Anti-CVA16 existing in immunized serum samples
The result of ELISA showed that the titer of anti-CVA16 in mice sera were more than 1:1000, and the titers of anti-CVA16 in rat sera were 1:1000 to 1:10000 (Fig. 1e). The western blotting proved that the immunized sera could bind to VP1 and/or VP2 of CVA16 (Fig. 1f). These results confirmed that anti-CVA16 were existed in the immunized sera.
Maternal antibodies protects suckling mice from challenge with circulating strains in vivo
To investigate whether the polyclonal antibodies could protect CVA16 infection in vivo, suckling mice were used in this study. In high dose group (0.5 μg/0.5ml/mouse), the neutralizing titers of maternal mouse against G10 were 1:17.5 (95% CI: 7.8–25.1), 1:12.8 (95% CI: 4.6–19.0), 1:14.7 (95% CI: 5.8–21.7) at indicated 3 time points, respectively. The survival rate of suckling mice was 100%. In middle dose group (0.125 μg/0.5 ml/mouse), the neutralizing titers of maternal mice were 1:7.0 (95% CI: 3.6–9.6), 1:6.0 (95% CI: 3.5–7.8), 1:5.0 (95% CI: 3.4–7.2) at indicated 3 time points. The survival rate of suckling mice was 60%. In the low dose group (0.031 μg/0.5ml/mouse), all the suckling mice died within 10 days and the neutralizing titers of maternal mice were below 1:4 (Fig. 2). These results indicated that the maternal antibodies elicited by CVA16 vaccine with poor neutralizing activity in cell culture assay could protect suckling mice from challenge with circulating CVA16 strains in vivo.
Figure 2.
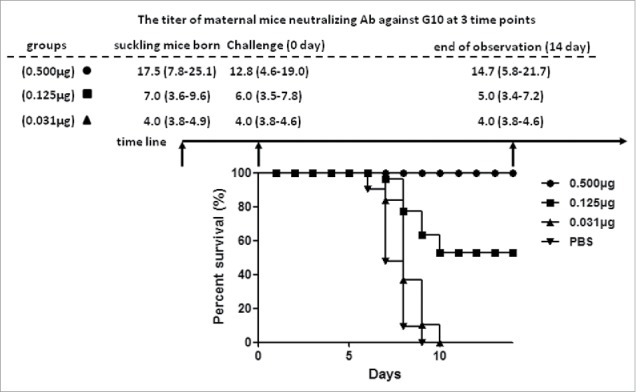
Maternal transfer antibody protects suckling mice from challenged with circulating recombinant strain in vivo. The neutralizing titer against G10 of maternal mice was tested in 3 time point: suckling mice born, challenge and end of observation. The • indicates high dose group (0.500 μg); the ▪indicates middle dose group (0.125 μg); the ▴ indicates low dose group (0.031 μg); the ▾ indicates PBS group.
Passive protects suckling mice from challenge with circulating strains in vivo
The serial diluted immunized sera, which were from mice, goat, rat, and human, were mixed with CVA16 virus, respectively. Neonatal BALB/c mice were challenged intraperitoneally with mixture. As shown in Fig. 3a-3d, anti-CVA16 antibodies can protect suckling mice from lethal challenge. In addition, the obvious dose response was found between the titer of antibodies and the survival rate. The ED50 were 9.6, 6.3, 12.6 and 4.9 for mice, goat, rat, and human antibodies, respectively.
Figure 3.
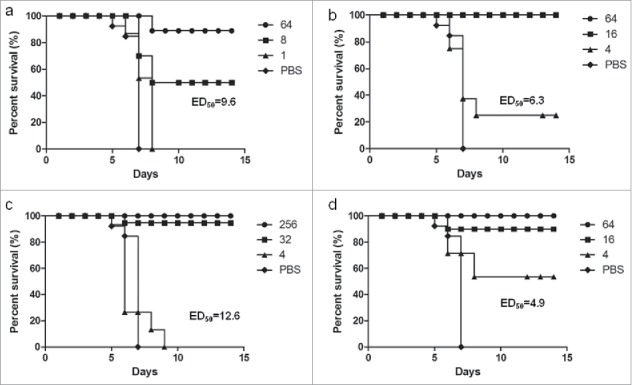
Passive protects suckling mice from challenge with circulating strains in vivo. The sera were from mice (a), goat (b), rat (c), and human (d). The ED50 were 9.6, 6.3, 12.6 and 4.9 for mice, goat, rat, and human antibodies, respectively.
Structural variations between G10 and circulating recombinant CVA16 strains
The neutralizing ability of polyclonal antibodies to G10 and circulating CVA16 strains was different, which probably rose from structural differences on the surface of the viruses. Based on the protein sequence alignment, there are 47 variant sites in P1 region between G10 and circulating stains, including 24 variant sites in VP1 region, 12 sites in VP2, 13 sites in VP3. These different sites were distributed in 4 functional regions: VP1 GH loop, VP2 EF loop, VP3 GH loop and VP1 N terminal (Fig. 4).
Figure 4.
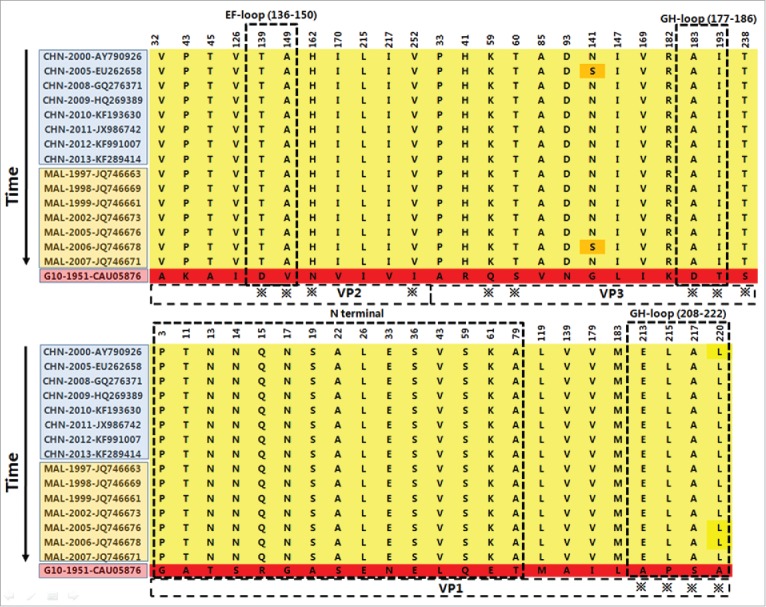
The variant amino acid sites in the structural protein of P1 region between G10 and circulating recombinant strains from 1997 to 2013 in Asia. VP1 GH loop (208–222), VP2 EF loop (136–150), VP3 GH loop (177–186) and VP1 N terminal (1–80) are the important functional region for CVA16. The ※ indicates the sites on the surface of virus. CHN indicates mainland China; MAL indicates Malaysia.
These variant sites were mainly distributed at the interface of VP1, VP2 and VP3 (Fig. 5a), which were far away from 3-fold axis (Fig. 5b) and 5-fold axis (Fig. 5c), but were close to 2-fold axis (Fig. 5d). It worth to note, the GH loop residues 213, 215, 217 of VP1, together with residues 149, 252 of VP2, were close to the 2-fold axis (Fig. 5d).
Figure 5.
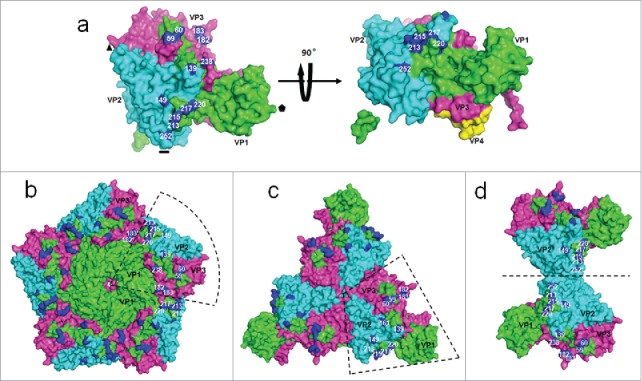
The distribution of surface variant sites between G10 and circulating recombinant strains. (a) Asymmetric units of capsid are viewed from top and side. The indicates 5-fold axis; the ▴ indicates 3-fold axis, the indicates 2-fold axis. Capsid proteins are shown in green, cyan, and magenta for VP1, VP2 and VP3. The variant amino acids sites are shown in blue. These variant sites are mainly distributed in the interface of VP1, VP2 and VP3. (a) The view of distribution of variant sites in 5-fold axis. (c) The view of distribution of variant sites in 3-fold axis. (d) The view of distribution of variant sites in 2-fold axis. 182′ indicates the residue 182 of an adjacent protomer.
To further examine the structural variation between G10 and circulating strains, the structure of G10 was remodeled using homology modeling based on the published crystal structure of CVA16 (PDB: 5C4W). The obvious changes were observed in residue 213 of VP1 GH loop, residue 139 of VP2 EF loop, and 59,182,183 of VP3 GH loop (Fig. 6). Among these variant sites, the residues side chains of the length and/or charge were changed significantly.
Figure 6.
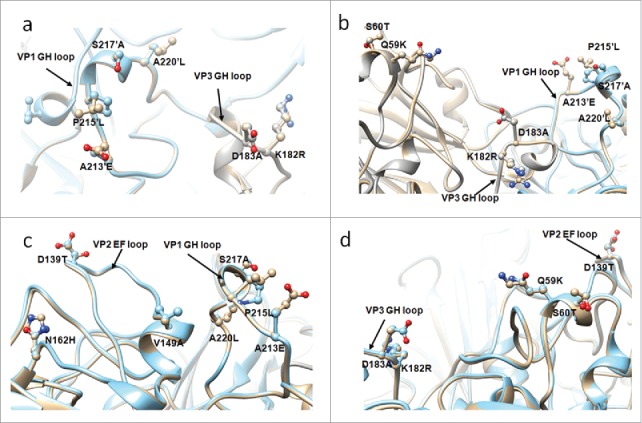
The adjacent variant sites are displayed in the structure. (a) VP1 GH loop and VP3 GH loop, (b) VP1 GH loop, VP3 GH loop and residue 59, 60 of VP3, (c) VP1 GH loop and VP2 EF loop, (d) VP2 EF loop and residue 59, 60 of VP3. The structure of circulating recombinant strain colored in golden, G10 colored in cyan. 220′ indicates the residue 220 of an adjacent protomer.
In addition, the VP3 GH loop is close to the VP1 GH loop of an adjacent protomer and likely forms a single antigenic site.8 In particular, the residue 213 of VP1 and the residue 183 of VP3, which the side chains of the length and/or charge were changed significantly, are close to each other (Fig. 6a). These variants may change the interaction between VP1 GH loop and VP3 GH loop. Furthermore, VP3 GH loop and the residue 59, 60, together with the VP1 GH loop of adjacent protomer, formed a cavity and residues 182 and 183 of VP3 GH loop were located at the bottom of the cavity, while residues 213–220 of VP1 GH loop and 59, 60 of VP3 surrounded them (Fig. 6b). In one protomer, the VP2 EF loop is close to VP1 GH loop (Fig. 6c) and residues 59, 60 of VP3 (Fig. 6d).
Discussion
NTAbs is a major criterion for evaluation the immunogenicity of EV71 and poliovirus vaccine. According to our previous studies on EV-A71,14,15 anti-CVA16 antibodies were induced by live CVA16 strains in rat. Based on cell culture neutralization assay, the neutralizing titer of antibodies was tested. Unlike to the EV71, the result showed that sera have poor neutralizing activities to circulating CVA16 strains. Moreover, anti-CVA16 antibodies were induced by formalin inactivated CVA16 strains in different animals, including mice, goat and rabbit, also have poor neutralizing activities against circulating CVA16 strains. However, the results of ELISA and WB proved the high titer of anti-CVA16 antibodies were existed in sera.
More interestingly, the results from convalescent serum samples from CVA16 infected patients were consistent, which led to the assumption that the anti-CVA16 neutralizing antibodies could not protect CVA16-infection. However, in our follow-up study on anti-CVA16 seropositive population, no cases with CVA16-caused disease were found (data unpublished). Moreover, there was no report on CVA16 re-infection caused diseases in people according to the epidemiologic data. This might indicated that anti-CVA16 antibodies, although with poor neutralizing activity in cell culture, could prevent CVA16-caused diseases in vivo. In our study, the results indicated that the maternal antibodies elicited by CVA16 vaccine with poor neutralizing activity in cell culture assay could protect suckling mice from challenge with circulating CVA16 strains in vivo. Moreover, the results of passive protection proved further that the anti-CVA16 antibodies can protect suckling mice from lethal challenge. In addition, the obvious dose response was found between the titer of antibodies and the survival rate in maternal and passive proteciton.
Poorly neutralizing antibodies have been reported on flaviviruses,16,17 alphaviruses,18 and rhabdoviruses.19 Although the mechanism of protection in animals remained uncharacterized, complement C1q and activating Fcγ receptors might contribute to in vivo protection.20 However, relevant studies in enterovirus, such as Polio and EV-A71 have been not reported. We firstly discovered and proved that anti-CVA16 antibodies, though with poor neutralizing activity in cell culture, could prevent CVA16-caused diseases in vivo. The mechanism in CVA16 need to be studied further.
The neutralizing ability of antibodies to G10 and circulating strains was different, which presumably rose from structural differences on the surface of these viruses. Between G10 and circulating CVA16 strain, there are total 47 variant sites, which are near the interface of VP1, VP2, and VP3, and close to 2-fold axis. It is thought that the 2-fold axis is the channel for poliovirus to release RNA.21 Compared to 80S-like structure of EV-A71, the CVA16 135S structure has larger 2-fold axis channel, which may facilitate the movement of VP1 N terminus to exit the capsid at the 2-fold axis channel.8
The variant sites were distributed within 4 functional regions, including VP1 GH loop, VP2 EF loop, VP3 GH loop and VP1 N terminal. The VP1 GH loop is an “adaptor-sensor” for cellular receptor attachment,22 which is the important neutralizing epitope of CVA16 and EV-A71.23,24 The VP1 and VP3 GH loops both undergo large conformation changes during uncoating, and this area might be involved in SCARB2 binding.8 The EF loop of VP2 is observed as an immunodominant epitope in CVA16 and EV-A71.9The VP1 N terminus of picornavirus, although in the interior of capsid, may take part in the membrane-association-catalysed process in 2-fold axis.25,26
Salt bridge, charge interactions and hydrogen bond formed by side chain play important roles in antigen-antibody interactions.27,28 Between G10 and circulating strains, the obvious side chain and charge changes were observed in residue 213 of VP1 GH loop, residue 139 of VP2 EF loop, and 59,182,183 of VP3 GH loop. These changed residues in immunodominant epitope may disturb the binding for neutralizing antibody.
The CVA16 are common in Chinese Mainland.29 Recently, inactive and recombinant CVA16 vaccine was developed. Both of the vaccines have faced similar challenges, including how to screen the vaccine candidate strains and how to evaluate immunogenicity of vaccine. We firstly discovered that antibodies induced by inactivated CVA16 vaccine and lived CVA16 virus have poor ability to neutralize circulating CVA16 strains in vitro. However, the poorly neutralizing polyclonal antibodies can protect suckling mice from lethally challenged with circulating strains in vivo. Our studies indicated that the prototype, G10 strain, might be used to detect the neutralizing antibodies titer before proper circulating CVA16 strain screened. What we found may provide new sight for development of CVA16 vaccine.
Materials and methods
Ethics statement
All animal experiments were carried out in accordance with the guidelines of the National Institutes for Food and Drug Control Committee for the Care and Use of Laboratory Animals and were approved by the National Institutes for Food and Drug Control Laboratory Animal Management Ethics Committee. Written informed consent was obtained from the donor for use of the human samples. Ethics committee approval was obtained from the Ethic Committee of Jiangsu Provincial Center of Disease Control and Prevention. All experimental protocols were approved by the National Institutes for Food and Drug Control.
Virus strains isolate and culture
CVA16 virus used in this study included prototype strain G10 and circulating strains. The circulating CVA16 virus were isolated from HFMD patients infected with CVA16. The patients were from 9 regions including south, middle and north of China, 2008 to 2011. Confluent cell cultures were seeded in microplate wells and inoculated with 100 μl of maintenance medium and 50 μl of pharyngeal swab samples. The cell culture were then incubated at 37°Cin 5% CO2 and observed for 7 days to check for cytopathic effects. A blind passage was performed once if no cytopathic effect was observed by the end of the observation period. These isolates were stored at −80°C.
Preparation of inactivated CVA16 vaccines
Inactivated CVA16 vaccine was prepared from Vero cells infected with CVA16. Briefly, lysate of CVA16-infected cells was treated with formaldehyde (1:4000) to inactivate the virus; the inactivated virus was subsequently purified by sucrose gradient ultracentrifugation. The final CVA16 vaccine was prepared by mixed purified CVA16 bulk with an equal volume of aluminum adjuvant.
Lived CVA16 virus induced NTAb in mice
Ten live CAV16 strains, including S1 to S10 strain, were used to evaluate cross protection of serum samples between G10 and circulating CVA16 viruses. Female BALB/c mice aged 6–8 weeks were divided 10 groups (n = 8). Each group was immunized different live CVA16 virus by intramuscularly injection and boosted with the same dose at interval of 2 weeks. Two weeks after last immunization, serum samples were prepared and used to analyze neutralization. Eleven strains, including G10 and S1 to S10 were used to test the neutralizing titer for serum samples.
Inactivated CVA16 vaccine induced NTAb in mice, goat and rabbit
Female BALB/c mice aged 6–8 weeks were used for the immunogenicity studies. Five mice were immunized with inactivated CAV16 vaccine produced by S-1 strain (1.0 μg/0.5ml/mouse) and boosted with the same dose at interval of 2 weeks. Two weeks after last immunization, serum samples were prepared and used to analyze neutralization. The G10, S1 strains were used to test the neutralizing titer for serum samples.
In goat experiment, inactivated CVA16 vaccine were diluted to 15 μg/ml. Using 3 vaccines, produced by S1, S5 and S9 strain, 3 goats were immunized with 2ml intramuscularly, respectively. Three goats were boosted 3 times with the same dose at an interval of two weeks. Two weeks after last immunization, serum samples were prepared and used to analyze neutralization. The G10, S1 and S5 strains were used to test the neutralizing titer for serum samples.
Likely, in rabbit experiment, inactivated CVA16 vaccine were diluted to 15 μg/ml. Using same 3 vaccines, produced by S1, S5 and S9 strains, 3 rabbits were immunized into the back with 2ml by multiple sites subcutaneous injection, respectively. The rabbits were boosted 3 times with the same dose at an interval of two weeks. Two weeks after last immunization, serum samples were prepared and used to analyze neutralization. The G10, S1 and S5 strains were used to test the neutralizing titer for serum samples.
The NTAb of convalescent sera from CVA16 infected human
Ten convalescent sera from CVA16 infected human were collected. The G10, S1 and S5 strains were used to test the neutralizing titer for the 10 convalescent sera.
Maternal immunization and virus challenge in vivo
Fifteen female BALB/c mice aged 6–8 weeks were divided into three groups and immunized CVA16 vaccine with three doses (0.5 μg/0.5ml/mouse, 0.125 μg/0.5ml/mouse, and 0.031 μg/0.5ml/mouse) by intraperitoneal injection. PBS was used as a negative control. Immunized female mice were allowed to mate 2 weeks after 2nd booster injection. On the 7th postnatal day, CVA16 strain was administered intracranially to 20 newborn suckling mice at 20 times the median lethal dose (LD50). The suckling mice were then observed for 14 days, recording death rate. The anti-CVA16 titer against G10 in maternal mice were tested in 3 time points: suckling mice born, challenge (0 day) and end of observation (14 day). Three independent experiments were performed. The virus strain, CVA16-CLS used for challenge was mice-adapted strain prepared by Sinovac Biotech Co. Ltd
Passive immunization and virus challenge in vivo
The serial diluted immunized sera (1:4, 1:8, 1:16, 1:64), which were from mice, goat, rat, and human, were inactivated at 56°C for 30 minutes and then mixed with CVA16 virus in 37°C for 1 hour. Twenty 7 day old BALB/c mice were injected intraperitoneally with each mixture. The suckling mice were then observed for 14 days, recording death rate. The ED50 were calculated.
Virus neutralizing assay
Cytopathogenic effect assay was performed as described to evaluate neutralizing antibody (NTAb) titers. Blood samples were inactivated at 56°C for 30 minutes, serially diluted from 1:4, and mixed with equal volumes of 100 median tissue culture infective doses (TCID50) of virus. After incubation at 37°C for 2 hours, RD or Vero suspension was added to the mixture. The cultures were then incubated in CO2 incubators for 7 days at 35°C. Cytopathogenic effect was observed under a light microscope.
Antibody measurement Enzyme-linked immunosorbent assay (ELISA)
Anti-CVA16 IgG titers were determined by endpoint titer ELISA. Briefly, The 96-well microtiter plates were coated with bicarbonate coating buffer (pH 9.6) containing 1 mg/ml of CVA16 antigen produced by S-1 strain. The coated plate was then incubated with 100 μl/well of serial diluted serum sample at 37°C for 1h, and followed by incubating HRP-conjugated secondary antibody at 37°C for 30 min. A total of 100 ml of tetramethylbenzidine dihydrochloride (TMB) was added for incubation for 15 min at room temperature. Finally, the absorbance at 450 nm was recorded using an ELISA plate reader. Endpoint titer was reported as the highest serum dilution that had an absorbance ≥ 0.105 OD unit above the blank.
Western blotting analysis
Western blotting was performed as follow: inactivated CVA16 produced by S-1 strain was separated on 12% polyacrylamide gels and transferred onto PVDF membranes; membranes were then detected with the anti-CVA16 mice sera or rabbit sera and HRP-conjugated anti-mice or anti-rabbit IgG antibody.
Structural analysis and homology modeling
Based on the published crystal structure of CVA16 (PDB ID: 5C4W), the variant amino acids between G10 and recombinant strains were analyzed by PyMOL (version:1.8.4.0). A three-dimensional structural model of G10 was generated by homology modeling with the crystal structure of CVA16 (PDB ID: 5C4W) as the template, using the SWISS-MODEL (http://swissmodel.expasy.org/). The UCSF Chimera (1.10.2) was used to make comparison of the structures.
Abbreviations
- CVA16
Coxsackievirus A 16
- EVA71
Enterovirus A 71
- HFMD
- NTAbs
Neutralizing Antibodies
Supplementary Material
Funding Statement
This work was supported by the Major Special Projects Funding Program (No.2016ZX09101120).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Thanks Jiangsu Provincial Center of Disease Control and Prevention for collecting samples.
References
- 1.Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis. 2010;10:778–90. doi: 10.1016/S1473-3099(10)70194-8. PMID:20961813. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Feng Z, Yang Y, Self S, Gao Y, Longini IM, Wakefield J, Zhang J, Wang L, Chen X, et al.. Hand, foot, and mouth disease in China: patterns of spread and transmissibility. Epidemiology. 2011;22:781–92. doi: 10.1097/EDE.0b013e318231d67a. PMID:21968769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, Zhang YT, Yao X, Chu K, Chen QH, et al.. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2013;381:2024–32. doi: 10.1016/S0140-6736(13)61049-1. PMID:23726161. [DOI] [PubMed] [Google Scholar]
- 4.Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, Tan X, Wang L, Mao Q, Wu J, et al.. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med. 2014;370:818–28. doi: 10.1056/NEJMoa1304923. PMID:24571754. [DOI] [PubMed] [Google Scholar]
- 5.Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, Zhang Y, Li Y, Mao Q, Wang J, et al.. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. 2014;370:829–37. doi: 10.1056/NEJMoa1303224. PMID:24571755. [DOI] [PubMed] [Google Scholar]
- 6.Liu Q, Yan K, Feng Y, Huang X, Ku Z, Cai Y, Liu F, Shi J, Huang Z. A virus-like particle vaccine for coxsackievirus A16 potently elicits neutralizing antibodies that protect mice against lethal challenge. Vaccine. 2012;30:6642–8. doi: 10.1016/j.vaccine.2012.08.071. PMID:22959985. [DOI] [PubMed] [Google Scholar]
- 7.Mao Q, Wang Y, Yao X, Bian L, Wu X, Xu M, Liang Z. Coxsackievirus A16: epidemiology, diagnosis, and vaccine. Hum Vaccin Immunother. 2014;10:360–7. doi: 10.4161/hv.27087. PMID:24231751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren J, Wang X, Zhu L, Hu Z, Gao Q, Yang P, Li X, Wang J, Shen X, Fry EE, et al.. Structures of coxsackievirus A16 capsids with native antigenicity: Implications for particle expansion, receptor binding, and immunogenicity. J Virol. 2015;89:10500–11. doi: 10.1128/JVI.01102-15. PMID:26269176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu L, He D, Yang L, Li Z, Ye X, Yu H, zhao H, Li S, Yuan L, Qian H, et al.. A Broadly cross-protective vaccine presenting the neighboring epitopes within the VP1 GH Loop and VP2 EF Loop of enterovirus 71. Sci Rep. 2015;5:12973. doi: 10.1038/srep12973. PMID:26243660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang Z, Wang J. EV71 vaccine, an invaluable gift for children. Clin Transl Immunol. 2014;3:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Resik S, Tejeda A, Fonseca M, Sein C, Hung LH, Martinez Y, Diaz M, Okayasu H, Sutter RW. Decay of Sabin inactivated poliovirus vaccine (IPV)-boosted poliovirus antibodies. Trials Vaccinol. 2015;4:71–74. PMID:27066157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan Q, Wu L, Chen L, Qin Y, Pan Z, Chen M. Vesicular stomatitis virus-based vaccines expressing EV71 virus-like particles elicit strong immune responses and protect newborn mice from lethal challenges. Vaccine. 2016;34:4196–4204. PMID:27373596. [DOI] [PubMed] [Google Scholar]
- 13.Vogt MR, Dowd KA, Engle M, Tesh RB, Johnson S, Pierson TC, Diamond MS. Poorly neutralizing cross-reactive antibodies against the fusion loop of West Nile virus envelope protein protect in vivo via Fcgamma receptor and complement-dependent effector mechanisms. J Virol. 2011;85:11567–80. PMID:21917960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bek EJ, Hussain KM, Phuektes P, Kok CC, Gao Q, Cai F, Gao Z, McMinn PC. Formalin-inactivated vaccine provokes cross-protective immunity in a mouse model of human enterovirus 71 infection. Vaccine. 2011;29:4829–38. PMID:21550375. [DOI] [PubMed] [Google Scholar]
- 15.Mao Q, Li N, Yu X, Yao X, Li F, Lu F, Zhuang H, Liang Z, Wang J. Antigenicity, animal protective effect and genetic characteristics of candidate vaccine strains of enterovirus 71. Arch Virol. 2012;157:37–41. PMID:21984267. [DOI] [PubMed] [Google Scholar]
- 16.Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi BE, Olshevsky U, Fremont DH, et al.. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J Virol. 2006;80:12149–59. PMID:17035317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufman BM, Summers PL, Dubois DR, Cohen WH, Gentry MK, Timchak RL, Burke DS, Eckels KH. Monoclonal antibodies for dengue virus prM glycoprotein protect mice against lethal dengue infection. Am J Trop Med Hyg. 1989;41:576–80. PMID:2817214. [DOI] [PubMed] [Google Scholar]
- 18.Nakanaga K, Yamanouchi K, Fujiwara K. Protective effect of monoclonal antibodies on lethal mouse hepatitis virus infection in mice. J Virol. 1986;59:168–171. PMID:3012115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefrancois L. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J Virol. 1984;51:208–14. PMID:6328040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogt MR, Dowd KA, Engle M, Tesh RB, Johnson S, Pierson TC, Diamond MS. Poorly neutralizing cross-reactive antibodies against the fusion loop of West Nile virus envelope protein protect in vivo via Fcgamma receptor and complement-dependent effector mechanisms. J Virol. 2011;85:11567–80. PMID:21917960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bostina M, Levy H, Filman DJ, Hogle JM. Poliovirus RNA is released from the capsid near a two fold symmetry axis. J Virol. 2011;85:776–783. PMID:20980499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Peng W, Ren J, Hu Z, Xu J, Lou Z, Li X, Yin W, Shen X, Porta C, et al.. A sensor-adaptor mechanism for enterovirus uncoating from structures of EV71. Nat Struct Mol Biol. 2012;19:424–9. PMID:22388738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi J, Huang X, Liu Q, Huang Z. Identification of conserved neutralizing linear epitopes within the VP1 protein of coxsackievirus A16. Vaccine. 2013;31:2130–2136. PMID:23499595. [DOI] [PubMed] [Google Scholar]
- 24.Foo DG, Alonso S, Phoon MC, Ramachandran NP, Chow VT, Poh CL. Identification of neutralizing linear epitopes from the VP1 capsid protein of Enterovirus 71 using synthetic peptides. Virus Res. 2007;125:61–8. PMID:17222936. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Yafal AG, Lee YM, Hogle J, Chow M. Poliovirus neutralization by antibodies to internal epitopes of VP4 and VP1 results from reversible exposure of these sequences at physiological temperature. J Virol. 1994;68:3965–3970. PMID:7514682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin J, Lee LY, Roivainen M, Filman DJ, Hogle JM, Belnap DM. Structure of the Fab-labeled “breathing” state of native poliovirus. J Virol. 2012;86:5959–62. PMID:22398295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novotny J, Sharp K. Electrostatic fields in antibodies and antibody/antigen complexes. Prog Biophys Mol Biol. 1992;58:203–224. PMID:1509093. [DOI] [PubMed] [Google Scholar]
- 28.Yokota A, Tsumoto K, Shiroishi M, Nakanishi T, Kondo H, Kumagai I. Contribution of asparagine residues to the stabilization of a proteinaceous antigen-antibody complex, HyHEL-10-hen egg white lysozyme. J Biol Chem. 2010;285:7686–96. PMID:20038580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He SZ, Chen MY, Xu XR, Yan Q, Niu JJ, Wu WH, Su XS, Ge SX, Zhang SY, Xia NS. Epidemics and aetiology of hand, foot and mouth disease in Xiamen, China, from 2008 to 2015. Epidemiol Infect. 2017;145:1865–1874. PMID:28367766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


