ABSTRACT
Africa historically has had the highest incidence of meningococcal disease with high endemic rates and periodic epidemics. The meningitis belt, a region of sub-Saharan Africa extending from Senegal to Ethiopia, has experienced large, devastating epidemics. However, dramatic shifts in the epidemiology of meningococcal disease have occurred recently. For instance, meningococcal capsular group A (NmA) epidemics in the meningitis belt have essentially been eliminated by use of conjugate vaccine. However, NmW epidemics have emerged and spread across the continent since 2000; NmX epidemics have occurred sporadically, and NmC recently emerged in Nigeria and Niger. Outside the meningitis belt, NmB predominates in North Africa, while NmW followed by NmB predominate in South Africa. Improved surveillance is necessary to address the challenges of this changing epidemiologic picture. A low-cost, multivalent conjugate vaccine covering NmA and the emergent and prevalent meningococcal capsular groups C, W, and X in the meningitis belt is a pressing need.
KEYWORDS: African meningitis belt, conjugate vaccine, Meningococcal, MenAfriVac, Neisseria meningitidis
Background
Africa has the highest burden of invasive meningococcal disease (IMD) globally with high endemic rates and periodic epidemics. Incidence rates of IMD are highly variable from year to year and between countries, which makes precise estimates of the overall burden in Africa challenging.1 In most global regions, meningococcal disease incidence rates are <5 cases per 100,000 persons. In contrast, nearly all African countries have high endemic rates of >10 cases per 100,000 and/or experience epidemics.2
Against this backdrop, there are ongoing drastic shifts in the epidemiology of meningococcal disease in Africa. In the meningitis belt, capsular group A meningococcal (NmA) epidemics have dramatically declined following the introduction of a highly effective monovalent group A meningococcal conjugate vaccine, MenAfriVac.3 On the other hand, NmW4,5 epidemics and smaller NmX6,7 outbreaks have persisted. Beginning in 2013, NmC emerged as a major cause of meningococcal disease in Nigeria and Niger and has the potential for further spread in the region.8,9 Outside the meningitis belt, there are reports of increased numbers of NmW cases and occasional reports of NmB.10-12 Close monitoring of these highly dynamic epidemiologic changes is essential for vaccine prevention efforts. Here, we review changes in the epidemiology of meningococcal disease across Africa and vaccine prevention strategies, with particular emphasis on the meningitis belt since the introduction of MenAfricVac in 2010.
Neisseria meningitidis
N. meningitidis, the gram-negative bacterium that causes IMD, is an exclusive human commensal that asymptomatically colonizes the pharynx. Carriage rates are generally highest in adolescents and young adults, with rates of around 5–15%.13 Asymptomatic pharyngeal carriers transmit meningococci through close contact at home, in dormitories, in schools or at mass gatherings.1 Occasionally, some meningococcal strains penetrate the mucosal barrier to cause invasive disease, such as meningitis, sepsis, pneumonia, or septic arthritis.13 The risk of developing invasive disease following colonization depends on the virulence of the infecting strain and a number of risk factors affecting individual susceptibility. Infants and individuals with immune deficiency conditions such as terminal complement deficiency and HIV infection are at increased risk of IMD.14,15 Meningococci are genetically highly diverse and possess a number of mechanisms for rapid, often unpredictable evolution that lead to emergence of new hypervirulent lineages or persistence of existing epidemic strains.16-18 The key virulence factor of invasive N. meningitidis is the polysaccharide capsule that is the primary target of most meningococcal vaccines. Out of 12 known capsular groups, six (A, B, C, W, X and Y) account for nearly all cases of IMD.19 Genetically, meningococci are classified based on multi-locus sequence typing (MLST) into sequence types (STs); closely related STs form a clonal complex (cc).20 More recently, meningococcal whole genome sequencing has enabled more precise classification of major hypervirulent clonal complexes into distinct sub-lineages.21-25
Meningococcal pharyngeal carriage is a pre-requisite to infection and asymptomatic carriers are the primary driving force for meningococcal transmission.14,26 Carriage surveys complement regular invasive disease surveillance by providing data on the extent of spread of known hypervirulent lineages and are an important indicator of herd protection from conjugate vaccines.14,26 Herd protection, the prevention of infection in unimmunized persons through reductions in pharyngeal carriage among vaccinated persons, has been shown to be a major benefit of conjugate vaccines for N. meningitidis, Haemophilus influenzae and Streptococcus pneumoniae.27-29 A seven-year longitudinal carriage study in northern Ghana30 found that meningococcal disease outbreaks were preceded by waves of colonization where the outbreak strain becomes predominant among carriers several weeks before the start of an epidemic.
Meningitis belt
A large part of sub-Saharan Africa extending from Senegal in the west to Ethiopia in the east comprises the meningitis belt (Figure 1), which experiences very high incidence rates of endemic IMD cases with periodic large epidemics.31-33 Incidence rates during epidemics have reached as high as 100–1,000 cases per 100,000, which are extraordinarily high rates for an invasive bacterial disease.2 The case fatality rate ranges from 6.6 to 10.0% and permanent sequelae including hearing loss and motor impairment affecting over 30% and 12% of survivors, respectively.34-36
Figure 1.
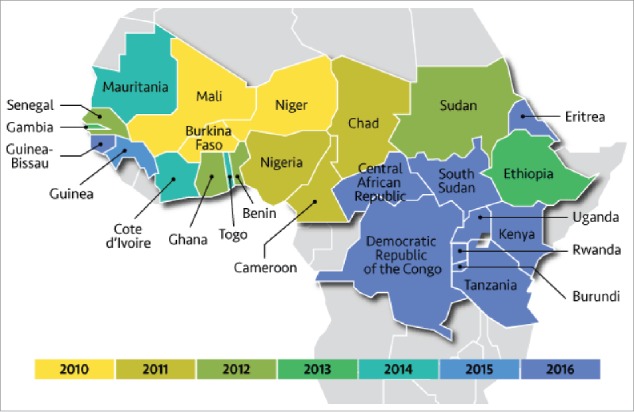
The meningitis belt of Africa. Map highlights 26 countries that make up the meningitis belt color coded by group A protein conjugate vaccine (MenAfriVac) roll out year. Reproduced with permission from www.path.org/menafrivac/meningitis-belt.php.
The meningitis belt was originally described in 1963 by Lapeyssonnie in a study of meningitis incidence data from Nigeria, Niger, Sudan and Chad.31 Lapeyssonnie and other researchers found meningitis epidemics were highest in regions roughly corresponding to areas with average annual rainfall of 300–1100 mm.31,37,38 Improved epidemiologic surveillance led to the inclusion of central African countries and the meningitis belt now consists of 26 countries.37 In the meningitis belt, meningococcal disease is highly seasonal with incidence increasing rapidly beginning in December and extending throughout the hot, dry months of January-April.39 The incidence drastically declines with the onset of the humid rainy season around May-June.38,39 In addition, factors such as dust, low relative humidity and co-circulating respiratory viruses, as well as close social contacts likely contribute to sustained meningococcal transmission and disease.37,40
Meningococcal epidemiology and capsular group distribution of the meningitis belt differs markedly from parts of northern and southern Africa. Within the last decade, the meningitis belt has experienced near elimination of NmA by a highly successful immunization campaign.33,41 Since 2000, this region has also witnessed the emergence of a NmW epidemics, occasional focal NmX outbreaks and most recently epidemics caused by a newly-emergent NmC strain.4,7,9 These ongoing dramatic shifts in the epidemiology of meningococcal disease in the meningitis belt have raised concerns that environmental and demographic changes could lead to geographic expansion of the meningitis belt and/or cause increases in the number of epidemics.37
Meningococcal surveillance in Africa
Measurement of meningococcal disease burden requires ongoing public health surveillance, which can be challenging in resource-poor countries. There has been substantial improvement in the availability of surveillance data in the meningitis belt over the last two decades through efforts spearheaded by the World Health Organization (WHO) inter-country support team.35,42 Weekly reports of suspected meningitis cases and serogroup data are now available from 20 countries. However, surveillance does not cover all countries at risk of epidemic meningococcal disease and there is large variation in confirmation rates between countries. In addition, more than 75% of reported meningitis cases lack laboratory confirmation of the infecting strain, which is crucial for determining vaccine preventability. For example, in 2016 only 22 of 831 (2.6%) suspected IMD cases in Nigeria had laboratory confirmation compared to 86% of 1,973 suspected cases in neighboring Niger. Timely epidemiologic and strain characterization data are essential for the proper allocation of scarce public health resources for treatment and vaccination efforts. Outside the meningitis belt, meningococcal surveillance data are sparse, usually gleaned from either single hospitals or geographically-limited surveillance studies. A notable exception is South Africa, where national surveillance data, laboratory confirmation, and strain characterization are available.
Capsular group A IMD
Historically, NmA was the predominant meningococcal group in the meningitis belt, accounting for over 90% of endemic and epidemic IMD. NmA epidemics and endemic cases were sustained by successive clonal waves of hypervirulent strains belonging to ST-5 clonal complex (cc5).22,43 Spread of hypervirulent cc5 strains from the Far East into Africa was facilitated by large annual Hajj gatherings in Mecca, Saudi Arabia.44 The last pandemic wave reached the meningitis belt in mid-1990s leading to the largest recorded meningococcal epidemic in 1996–1997 with over 250,000 cases and 50,000 deaths.33 The most recent large-scale NmA epidemic in 2009 resulted in an estimated 80,000 cases.33,35,45,46 The 1997 epidemic lineage persisted and evolved in the meningitis belt until the near elimination of NmA in 2010–2017 as a result of the introduction of MenAfriVac, which is covered in detail below. NmA epidemic strains from the 1980s, mid 1990s and 2003 onwards belonged to very closely related ST-5, ST-7 and ST-2859 that were all part of cc5. Extensive molecular and genomic studies demonstrated that cc5 strains differed by only small changes in outer membrane proteins and MLST genes.22,43,47 A recent genomic study of epidemic and asymptomatic carriage cc5 meningococcal strains from Chad demonstrated that even during epidemic spread, NmA may be heterogeneous with a number of closely related but genetically distinct co-circulating clones.25 These molecular studies highlight the dynamic nature of the meningococcal genome, which contributes to the changing epidemiology.
Control of NmA IMD through introduction of MenAfriVac
Meningococcal polysaccharide vaccines were the mainstay of meningococcal disease prevention in Africa for several decades. While polysaccharide vaccines are relatively inexpensive, they are ineffective in infants and do not confer long lasting immunity or induce herd protection.48 Polysaccharide vaccines covering capsular groups A and C and A, C, W, and Y continue to play a role in emergency epidemic response in Africa.9,42,49 However, these vaccination programs are reactive in nature and involve mobilization of vaccine in response to outbreaks and epidemics, which is a sub-optimal method for disease prevention.42 In addition, recent reports indicate shortages of polysaccharide vaccines during NmC epidemic in Nigeria and Niger presumably because a number of vaccine manufacturers are phasing out production of polysaccharide vaccines.9,50
In contrast to polysaccharide vaccines, polysaccharide-protein conjugate vaccines have proven to be highly effective in infants, confer long lasting immunity and protect unvaccinated persons through herd protection as classically demonstrated by NmC conjugate vaccine programs in the U.K.27,48 The devastating nature of NmA disease in Africa, and the immunologic limitations of polysaccharide vaccines led to the realization that development and utilization of NmA meningococcal conjugate vaccine could have a major public health impact in the meningitis belt.51,52
The Meningitis Vaccine Project (MVP) was formed as a collaboration between the World Health Organization, PATH (formerly known as the Program for Appropriate Technology in Health) and Bill & Melinda Gates Foundation with the goal of developing, testing and introducing a conjugate vaccine that would eliminate epidemic meningococcal disease in Africa.52 MVP, which was led by Dr. Marc LaForce, conducted a needs assessment with African public health officials who identified vaccine price as the most important consideration for sustainable vaccine programs in the continent.52 An innovative arrangement between MVP, the US Food and Drug Administration and a number of biotechnology companies allowed a protein-conjugate vaccine effective against NmA, (MenAfriVac) to be manufactured by the Serum Institute of India in Pune, India at a very low cost.52,53 Given the urgent need for an effective conjugate NmA vaccine, MenAfriVac was licensed through a fast track process that relied on excellent safety and immunogenicity profile, but no efficacy data.54,55
MenAfriVac was rolled out across the meningitis belt beginning in 2010.3 A key attribute of MenAfriVac was that at a cost of about US $0.50 per dose, it was substantially cheaper than comparable conjugate vaccines ($40-$80 per dose), thereby increasing the prospect of vaccine program sustainability even after donor funding may have waned.56 In addition, MenAfriVac became the first vaccine licensed by WHO as suitable for transport in limited temperature controlled, cold chain because it is stable for up to 4 days at 40º C ambient temperature.57 Reduced cold chain requirements led to substantial savings in program logistics and cost.
By 2016, more than 235 million persons aged 1–29 years in 16 countries had received a single dose of MenAfriVac.36,41 NmA cases dramatically declined in immunized districts through a combination of the direct effects of vaccination and herd protection from elimination of NmA carriage (Figure 2). Among nine countries in the meningitis belt, incidence of suspected meningitis cases declined 57%, with corresponding 59% reduction in epidemics and a remarkable 99% decline in confirmed NmA cases in 2010–2015.41 In 2016–2017, NmA made up only 0.8% of 2,897 confirmed IMD cases in the region (Figure 3A-B).35,36
Figure 2.
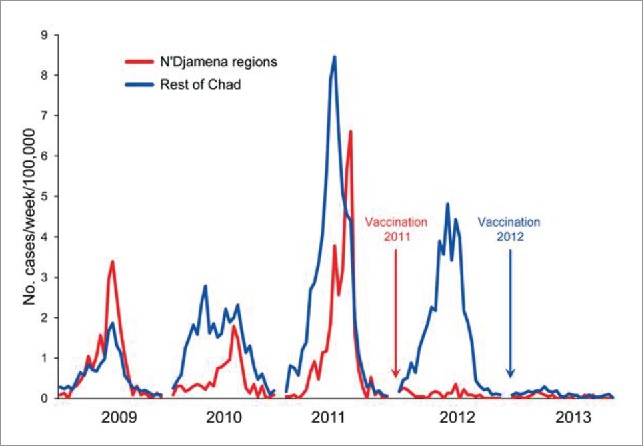
Reduction in incidence of all meningitis cases in Chad following introduction of MenAfriVac in 2011–2012. Reproduced from Gamougam et al.90
Figure 3.
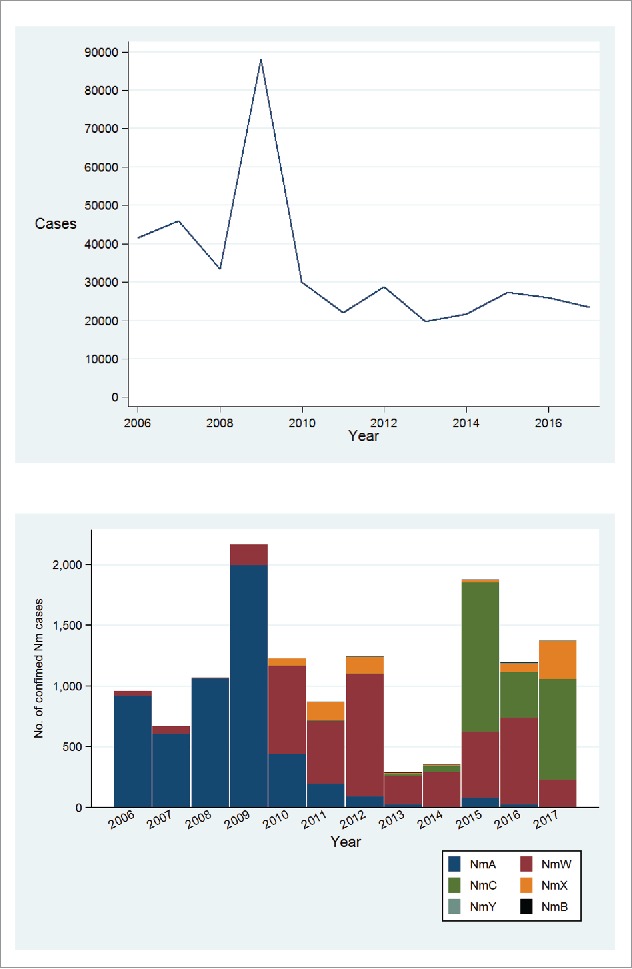
Total number of suspected meningitis cases (panel A) and capsular group distribution of confimed IMD cases (panel B) in the meningitis belt, 2006 to June 2017. Based on publically available data from the World Health Organization (www.who.int/csr/disease/meningococcal/epidemiological/en/).35 Data for 2006–2016 represent totals for 52 weeks (January to December) while data for 2017 are totals for first 26 weeks of the year when a vast majority of cases occur.
The evidence for herd protection is based on several carriage studies that were performed after MenAfriVac roll-out. As mentioned above, reductions in pharyngeal carriage is a major benefit of other conjugate vaccines and, therefore, there was keen interest in determining whether MenAfriVac provided herd protection in addition to direct protection. A large cross sectional survey in Burkina Faso found NmA in 0.39% of 20,325 persons before MenAfriVac with subsequent elimination of NmA carriage (0 out of 22,093 persons) in both vaccinated and unvaccinated individuals after introduction of Menafrivac.58 Likewise a carriage study of 13,396 persons in Chad found that NmA carriage declined from 0.7% to 0.2% following MenAfriVac.59 These data, when taken in the context of longitudinal studies30,60 indicate that MenAfriVac provides protection of unimmunized persons in areas in which the vaccine has been introduced.
Thus, MenAfriVac has led to the near elimination of NmA in the countries in which it has been introduced, perhaps one of the largest public health achievements of the past few decades. Long term prospect of elimination of NmA epidemics in Africa depends heavily on successful integration of conjugate A vaccine into routine immunization schedules of individual countries. In fact, it is predicted that without integration of MenAfriVac into routine immunization, NmA epidemics could return to Africa within 15 years.56 As a result, efforts are underway by international agencies to support integration of MenAfriVac into routine national immunization programs.36,61
One of the problems associated with the use of monovalent meningococcal conjugate vaccines such as MenAfriVac has been the persistence and/or emergence of capsular groups that are not covered by the vaccine. Despite the control of NmA disease, groups W and X have persisted as important public health concerns in the meningitis belt. In addition, IMD caused by NmC has recently emerged as a major public health threat in several countries in the region.
Capsular group W IMD
NmW caused only small case clusters in the 1980s and 1990s but became an important cause of IMD in Africa beginning in 2000–2001 following a NmW epidemic in Mecca, Saudi Arabia that spread globally.62 Since 2001, NmW has persisted as a cause of endemic IMD cases and occasional epidemics in the meningitis belt.62 Although NmW can cause large epidemics, incidence rates during epidemics tend to be lower, around 10–100 cases per 100,000 population compared to 100–1000 cases per 100,000 for NmA epidemics.63-65 Notable NmW epidemics were recorded in Burkina Faso in 2002 with over 12,000 cases and 1,400 deaths respectively and in Niger, 2010 with 1,188 cases.65,66 In 2012, a widespread NmW epidemic occurred in the meningitis belt with a focus in Burkina Faso (5,807 suspected cases) and Niger (1,149 cases) and extending to Nigeria, Benin, Ghana, Mali and Gambia.5,7,35,64,67,68 NmW remains a major cause of IMD in the meningitis belt accounting for 36.2% of 5,560 confirmed IMD cases in the region between January 2013 – June 2017 (Figure 3A-B).5,35,36
Genomic studies24,67,69 demonstrated that successive NmW epidemics were caused by a number of similar but distinct sub-lineages within a globally endemic hypervirulent ST-11 clonal complex (cc11). Some W cc11 strains such as those associated with Burkina Faso 2010–2012 epidemics are genetically highly similar, and may represent continued persistence and evolution of the Saudi Arabia outbreak strain (Hajj clone) while other cc11 strains may have been in circulation in Africa since the late 1990s.62,67,70 Since 2002, a small number of NmW cases associated with the ST-2881 lineage (cc175) were linked to endemic cases but not large epidemics.23,47 Genomic data from a longitudinal study of meningococcal carriage and disease demonstrated that cc175 strains were genetically similar to NmY strains and may have evolved through Y to W capsular switching, unrelated to cc11 lineage.23 In addition, only two out of seven ST-2881 sublineages were associated with invasive disease. These data are suggestive of varying pathogenic potential among different W ST-2881 sub-lineages.23 Therefore, a number of diverse NmW strains remain in circulation and continue to pose a threat of both endemic disease and epidemics in the meningitis belt.
Capsular group X IMD
NmX is a rare cause of IMD globally, representing less than 0.6% of total IMD in Spain,71 South Africa,11 Australia72 and the United States.73,74 However, NmX is an occasional cause of outbreaks and smaller epidemics in Africa.75,76 In 1990, a small NmX outbreak occurred in Niamey, Niger77 with an incidence of 6.6 cases per 100,000 population. NmX made up 3.9% of confirmed meningococcal cases in Niger from 1995–2000 where small outbreaks occurred in south-western parts of the country in 1997–1999.78 The incidence of NmX (13.7 per 100,000) in Niger was much lower than rates of 100–1000 per 100,000 reported during NmA epidemics. Niger experienced a larger NmX epidemic in 2006 with 581 confirmed cases and incidence of 28 cases per 100,000 population in Niamey.6 Epidemiologic and clinical characteristics, such as seasonality, age and sex distribution and case fatality rates were similar between NmX and NmA.78 A significant increase in NmX cases was seen in Togo and Burkina Faso from 2006–2010. Delrieu et al reported that 16% of 702 meningitis cases in Togo 2006–2009 and 7% of the 778 cases in Burkina Faso 2007-2010 belonged to NmX.7 A number of districts in Togo and Burkina Faso reported epidemics with the highest district cumulative incidence of 130/100,000, approaching rates seen during NmA epidemics.7 In 2006, an outbreak of NmX disease occurred among remote communities along the western Kenya/Uganda border region with 82 suspected cases in Kenya and a case fatality rate of 21%.79-81
NmX cases declined across the meningitis belt in 2013–2016. However, a recent increase in NmX has occurred with the proportion of cases increasing from 2.7% of 4150 confirmed cases in 2013–2016 to 22.0% of 1410 cases in 2017 (Figure 3B).35,36 The majority of NmX cases in 2017 were reported from Niger, Burkina Faso and Togo, where NmX outbreaks were previously reported. NmX strains in Niger, Togo and Benin belonged to ST-181 (cc181),6 were closely related to strains from Ghana (ST-751) but unrelated to strains from Kenya and Uganda (ST-5403).30,81 The increase in NmX is concerning given the lack of an effective vaccine and inadequate surveillance.
Capsular group C IMD
NmC was practically absent in the meningitis belt for the past four decades, with the exception of a few small isolated outbreaks in the late 1970s.33 However, NmC has emerged in recent years as a cause of meningococcal outbreaks and endemic cases in Nigeria and Niger. NmC was first reported in northwest Nigeria as a series of localized outbreaks in 2013–2014 with 1189 cases and 93 deaths.8 NmC subsequently spread more widely causing endemic cases and epidemics across north Nigeria and neighboring Niger in 2015–2017 (Figure 3B).9,35,36,82 Niger and Nigeria each experienced over nine thousand suspected cases and over five hundred deaths due to NmC epidemics in 2015 and 2017, respectively.9,35,36 NmC epidemics were characterized by very high attack rates in the range of 41.4-673 cases per 100,000 population, comparable to those seen during NmA epidemics.8,9,82 Since 2013 NmC cases have also been recorded in Burkina Faso, Ghana, Benin and Mali suggesting wide regional spread of an epidemic strain. Molecular epidemiologic and whole genome characterization studies showed that epidemic NmC strains belonged to a newly identified ST-10217 lineage. Interestingly, ST-10217 was unrelated to cc11 and other global hypervirulent NmC lineages.4,8 NmC emergence reinforces the need for sustained vigilance and a need for an effective conjugate vaccine against non-A meningococci in Africa. Enhanced disease surveillance is required to monitor the distribution of meningococcal strain types that will better inform prevention strategies and emergency preparedness.61
Vaccine prevention of non-A capsular groups in the meningitis belt
Persistence of serogroups W and X and the recent emergence of serogroup C epidemics in the meningitis belt underscores the need for effective vaccination strategies that will further reduce the public health burden of meningococcal disease in the region. Reactive vaccination strategies currently in place rely on the detection and timely reporting of suspected meningitis cases beyond a set threshold.83 Once an epidemic is confirmed, a request is made to the International Coordinating Group (ICG), which maintains regional vaccine stockpiles and provides such vaccines to national governments during epidemics. As such, the process of identifying the outbreak and then requesting vaccine from the ICG inevitably leads to delays in the overall public health response. The sudden emergence of NmC epidemics presented additional programmatic challenges to the control of meningococcal disease in affected countries.50,61 Emergency NmC vaccination campaigns deplete available vaccine stockpiles further limiting the extent and timeliness of vaccination responses.9
A multivalent, protein conjugate vaccine that is effective against serogroups A, C, W, and X would offer longer duration of protection than plain polysaccharide, would potentially protect unimmunized individuals through herd protection and could be used for routine infant immunization. Therefore, such a vaccine could substantially reduce the incidence of both A and non-A capsular groups, which is much more effective than a reactive strategy, and would reduce the need for costly emergency vaccination campaigns.
Nm epidemiology in Africa outside the meningitis belt
In other parts of Africa, systematic surveillance data are sparse but incidence rates are generally much lower than in the meningitis belt. In South Africa, incidence rates have fluctuated from 0.64 per 100,000 in 1999–2003, peaked above 1.5 in 2008 and subsequently declined to 0.36 per 100,000 in 2014.11,84 Large regional variations have occurred with the highest incidence in Gauteng Province and peak seasonal incidence in May-October.11 The predominant infecting strains have evolved over the last three decades. NmB strains belonging to global hypervirulent lineages cc32 and cc41/44 predominated in the late 1990s while NmW cc11 emerged in 2003 and became the leading cause of IMD in South Africa. Hypervirulent strains of NmY (cc23), NmA (cc5) and Nm C (cc11) have also persisted in South Africa in low numbers.11,84,85 In South Africa and other countries with high HIV prevalence, HIV infection is a strong risk factor for meningococcal disease affecting overall incidence rates of IMD.15
Limited surveillance data are available from other parts of southern Africa. A hospital-based study of over 51,000 consecutive CSF samples collected in 2000–2012 at a large hospital in Malawi estimated the incidence of bacterial meningitis to be 20 per 100,000 among children under 14.86 However, N. meningitidis accounted for less than 5% of bacterial meningitis cases. A prospective study of children under 15 years in rural Mozambique found incidence rates of IMD to be 11.6 per 100,000 in 1998–2008. NmW cc11 increased significantly in 2004–2008 and was found in 81% of 48 tested isolates.10 Likewise, case clusters of NmW cc11 and NmB have occurred in Madagascar.12,87
In northern Africa, a general shift from NmA to NmB as the predominant capsular group causing IMD has occurred and a reduced number of outbreaks have been reported.84,88 Laboratory surveillance in Egypt found N. meningitidis in 16% of confirmed meningitis cases with the majority being NmB (51% of 135) followed by NmA (35%), and NmW (4%). Strains from Egypt were genetically diverse, predominantly belonging to cc8 (NmB and NmA) and cc5 (NmA) lineages.89 Likewise, NmB is the most prevalent capsular group in Tunisia, Morocco and Algeria, followed by NmW.84 In summary, IMD incidence rates are much lower in northern and southern Africa compared to the meningitis belt, with NmW and NmB being the most prevalent capsular groups.
Future needs
The introduction of MenAfriVac and subsequent near elimination of NmA disease in Africa represents a monumental public health achievement. Sustaining the initial success of MenAfriVac will largely depend on the ability of countries to integrate administration of the vaccine at 9 months of age into routine immunization programs. So far, Sudan, Burkina Faso and Ghana have commenced integration and conducted mini catch-up campaigns in 2016.36 Furthermore, laboratory-based surveillance needs to be strengthened across the continent to both monitor the success of vaccines as well as identify persistence and emergence of other potentially vaccine-preventable strains.61,84
There is an urgent need for an affordable conjugate vaccine that covers all capsular groups relevant to the meningitis belt, particularly capsular groups C, W, and X (in addition to A). A vaccine that covers groups A, C, W, Y, and X is under development by the Serum Institute of India (www.seruminstitute.com/product_horizon.php) and may be available for clinical use by 2022.84 The future success of this vaccine will depend not only on its immunogenicity and efficacy but also on safety, cost, programmatic issues, the commitment of multiple stakeholders, and the ever-changing epidemiology of IMD on the African continent.
Conclusion
Elimination of NmA epidemics in Africa is a remarkable public health achievement. However, NmC, NmW, and NmX continue to cause epidemics in the meningitis belt. Concerted effort by multiple stakeholders is required to sustain the use of MenAfriVac, enhance surveillance, build diagnostic capacity, and introduce novel vaccines.
Disclosure of potential conflicts of interest
Dr. Harrison has served on a scientific advisory board for GSK.
Acknowledgment
We thank Jane Marsh for her thoughtful review of the manuscript.
References
- 1.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27 Suppl 2:B51–63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 2.Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, Eskola J, Fermon F, Klugman KP, Ramsay M, Sow S, et al.. Global epidemiology of invasive meningococcal disease. Popul Health Metr. 2013;11:17. doi: 10.1186/1478-7954-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Djingarey MH, Diomande FV, Barry R, Kandolo D, Shirehwa F, Lingani C, et al.. Introduction and rollout of a new group A Meningococcal conjugate Vaccine (PsA-TT) in African Meningitis Belt Countries, 2010–2014. Clin Infect Dis. 2015;61 Suppl 5:S434–41. doi: 10.1093/cid/civ551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kretz CB, Retchless AC, Sidikou F, Issaka B, Ousmane S, Schwartz S, Tate AH, Pana A, Njanpop-Lafourcade BM, Nzeyimana I, et al.. Whole-Genome characterization of epidemic Neisseria meningitidis Serogroup C and Resurgence of Serogroup W, Niger, 2015. Emerg Infect Dis. 2016;22:1762–8. doi: 10.3201/eid2210.160468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aku FY, Lessa FC, Asiedu-Bekoe F, Balagumyetime P, Ofosu W, Farrar J, Ouattara M, Vuong JT, Issah K, Opare J, et al.. Meningitis outbreak caused by vaccine-preventable bacterial Pathogens – Northern Ghana, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:806–10. doi: 10.15585/mmwr.mm6630a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boisier P, Nicolas P, Djibo S, Taha MK, Jeanne I, Mainassara HB, Tenebray B, Kairo KK, Giorgini D, Chanteau S. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin Infect Dis. 2007;44:657–63. doi: 10.1086/511646. [DOI] [PubMed] [Google Scholar]
- 7.Delrieu I, Yaro S, Tamekloe TA, Njanpop-Lafourcade BM, Tall H, Jaillard P, Ouedraogo MS, Badziklou K, Sanou O, Drabo A, et al.. Emergence of epidemic Neisseria meningitidis serogroup X meningitis in Togo and Burkina Faso. PLoS One. 2011;6:e19513. doi: 10.1371/journal.pone.0019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funk A, Uadiale K, Kamau C, Caugant DA, Ango U, Greig J. Sequential outbreaks due to a new strain of Neisseria meningitidis serogroup C in northern Nigeria, Edition 1. 2013–14. PLoS Curr. 2014;6. doi: 10.1371/currents.outbreaks.b50c2aaf1032b3ccade0fca0b63ee518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sidikou F, Zaneidou M, Alkassoum I, Schwartz S, Issaka B, Obama R, Lingani C, Tate A, Ake F, Sakande S, et al.. Emergence of epidemic Neisseria meningitidis serogroup C in Niger, 2015: an analysis of national surveillance data. Lancet Infect Dis. 2016;16:1288–94. doi: 10.1016/S1473-3099(16)30253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibarz-Pavon AB, Morais L, Sigauque B, Mandomando I, Bassat Q, Nhacolo A, et al.. Epidemiology, molecular characterization and antibiotic resistance of Neisseria meningitidis from patients </ = 15 years in Manhica, rural Mozambique. PLoS One. 2011;6:e19717. doi: 10.1371/journal.pone.0019717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Gottberg A, du Plessis M, Cohen C, Prentice E, Schrag S, de Gouveia L, Coulson G, de Jong G, Klugman K, Group for Enteric, Respiratory and Meningeal Disease Surveillance in South Africa . Emergence of endemic serogroup W135 meningococcal disease associated with a high mortality rate in South Africa. Clin Infect Dis. 2008;46:377–86. doi: 10.1086/525260. [DOI] [PubMed] [Google Scholar]
- 12.Rasoanandrasana S, Raberahona M, Milenkov M, Rakotomahefa Narison ML, Ranaivo Rabetokotany F, Rakotovao L, Randria MJ, Hong E, Paranhos-Baccalà G, Taha MK, et al.. Resurgence of Neisseria meningitidis serogroup W ST-11 (cc11) in Madagascar, 2015–2016. Int J Infect Dis. 2017;55:1–3. doi: 10.1016/j.ijid.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Stephens DS. Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitidis. Vaccine. 2009;27 Suppl 2:B71–7. doi: 10.1016/j.vaccine.2009.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369:2196–210. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- 15.Cohen C, Singh E, Wu HM, Martin S, de Gouveia L, Klugman KP, Meiring S, Govender N, von Gottberg A, Group for Enteric, Respiratory and Meningeal disease Surveillance in South Africa (GERMS-SA), et al.. Increased incidence of meningococcal disease in HIV-infected individuals associated with higher case-fatality ratios in South Africa. AIDS. 2010;24:1351–60. doi: 10.1097/QAD.0b013e32833a2520. [DOI] [PubMed] [Google Scholar]
- 16.Budroni S, Siena E, Dunning Hotopp JC, Seib KL, Serruto D, Nofroni C, Comanducci M, Riley DR, Daugherty SC, Angiuoli SV, et al.. Neisseria meningitidis is structured in clades associated with restriction modification systems that modulate homologous recombination. Proc Natl Acad Sci U S A. 2011;108:4494–9. doi: 10.1073/pnas.1019751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marri PR, Paniscus M, Weyand NJ, Rendon MA, Calton CM, Hernandez DR, Higashi DL, Sodergren E, Weinstock GM, Rounsley SD, et al.. Genome sequencing reveals widespread virulence gene exchange among human Neisseria species. PLoS One. 2010;5:e11835. doi: 10.1371/journal.pone.0011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swartley JS, Marfin AA, Edupuganti S, Liu LJ, Cieslak P, Perkins B, Wenger JD, Stephens DS. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci U S A. 1997;94:271–6. doi: 10.1073/pnas.94.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, Corton C, Care R, Poolman JT, Zollinger WD, et al.. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis. 2013;19:566–73. doi: 10.3201/eid1904.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant DA, et al.. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A. 1998;95:3140–5. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison OB, Schoen C, Retchless AC, Wang X, Jolley KA, Bray JE, Maiden MCJ. Neisseria genomics: current status and future perspectives. Pathog Dis. 2017;75(6). doi: 10.1093/femspd/ftx060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamelas A, Harris SR, Roltgen K, Dangy JP, Hauser J, Kingsley RA, Connor TR, Sie A, Hodgson A, Dougan G, et al.. Emergence of a new epidemic Neisseria meningitidis serogroup A Clone in the African meningitis belt: high-resolution picture of genomic changes that mediate immune evasion. MBio. 2014;5:e01974–14. doi: 10.1128/mBio.01974-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamelas A, Hauser J, Dangy J-P, Hamid A-WM, Röltgen K, Abdul Sater MR, et al.. Emergence and genomic diversification of a virulent serogroup W:ST-2881(CC175) Neisseria meningitidis clone in the African meningitis belt. Microbial Genomics. 2017;3(8):e000120. doi: 10.1099/mgen.0.000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mustapha MM, Marsh JW, Krauland MG, Fernandez JO, de Lemos APS, Dunning Hotopp JC, Wang X, Mayer LW, Lawrence JG, Hiller NL, et al.. Genomic Epidemiology of Hypervirulent Serogroup W, ST-11 Neisseria meningitidis. EBioMedicine. 2015;2:1447–55. doi: 10.1016/j.ebiom.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diallo K, Gamougam K, Daugla DM, Harrison OB, Bray JE, Caugant DA, Lucidarme J, Trotter CL, Hassan-King M, Stuart JM, et al.. Hierarchical genomic analysis of carried and invasive serogroup A Neisseria meningitidis during the 2011 epidemic in Chad. BMC Genomics. 2017;18:398. doi: 10.1186/s12864-017-3789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borrow R, Alarcon P, Carlos J, Caugant DA, Christensen H, Debbag R, De Wals P, Echániz-Aviles G, Findlow J, Head C, et al.. The Global Meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16:313–28. doi: 10.1080/14760584.2017.1258308. [DOI] [PubMed] [Google Scholar]
- 27.Maiden MC, Ibarz-Pavon AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, Walker AM, Evans MR, Kroll JS, Neal KR, et al.. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197:737–43. doi: 10.1086/527401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vetter V, Baxter R, Denizer G, Safadi MA, Silfverdal SA, Vyse A, Borrow R. Routinely vaccinating adolescents against meningococcus: targeting transmission & disease. Expert Rev Vaccines. 2016;15:641–58. doi: 10.1586/14760584.2016.1130628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Holtzman C, Harrison LH, Zansky SM, Rosen JB, Reingold A, Scherzinger K, et al.. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir Med. 2016;4:399–406. doi: 10.1016/S2213-2600(16)00052-7. [DOI] [PubMed] [Google Scholar]
- 30.Leimkugel J, Hodgson A, Forgor AA, Pfluger V, Dangy JP, Smith T, Achtman M, Gagneux S, Pluschke G. Clonal waves of Neisseria colonisation and disease in the African meningitis belt: eight- year longitudinal study in northern Ghana. PLoS Med. 2007;4:e101. doi: 10.1371/journal.pmed.0040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lapeyssonnie L. [Comparative epidemiologic study of meningococcic cerebrospinal meningitis in temperate regions and in the meningitis belt in Africa. Attempt at synthesis]. Med Trop (Mars). 1968;28:709–20. [PubMed] [Google Scholar]
- 32.Greenwood B. Editorial: 100 years of epidemic meningitis in West Africa – has anything changed?. Trop Med Int Health. 2006;11:773–80. doi: 10.1111/j.1365-3156.2006.01639.x. [DOI] [PubMed] [Google Scholar]
- 33.Mohammed I, Iliyasu G, Habib AG. Emergence and control of epidemic meningococcal meningitis in sub-Saharan Africa. Pathog Glob Health. 2017;111:1–6. doi: 10.1080/20477724.2016.1274068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jusot JF, Tohon Z, Yazi AA, Collard JM. Significant sequelae after bacterial meningitis in Niger: a cohort study. BMC Infect Dis. 2013;13:228. doi: 10.1186/1471-2334-13-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weekly feedback bulletin on cerebrospinal meningitis Meningitis weekly bulletin. http://www.who.int/csr/disease/meningococcal/epidemiological/en/index.html: World Health Organization, 2002–2017.
- 36.Epidemic meningitis control in countries of the African meningitis belt, 2016 Wkly Epidemiol Rec. 2017;92:145–54. [PubMed] [Google Scholar]
- 37.Agier L, Martiny N, Thiongane O, Mueller JE, Paireau J, Watkins ER, Irving TJ, Koutangni T, Broutin H. Towards understanding the epidemiology of Neisseria meningitidis in the African meningitis belt: a multi-disciplinary overview. Int J Infect Dis. 2017;54:103–12. doi: 10.1016/j.ijid.2016.10.032. [DOI] [PubMed] [Google Scholar]
- 38.Molesworth AM, Cuevas LE, Connor SJ, Morse AP, Thomson MC. Environmental risk and meningitis epidemics in Africa. Emerg Infect Dis. 2003;9:1287–93. doi: 10.3201/eid0910.030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paireau J, Chen A, Broutin H, Grenfell B, Basta NE. Seasonal dynamics of bacterial meningitis: a time-series analysis. Lancet Glob Health. 2016;4:e370–7. doi: 10.1016/S2214-109X(16)30064-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller JE, Gessner BD. A hypothetical explanatory model for meningococcal meningitis in the African meningitis belt. Int J Infect Dis. 2010;14:e553–9. doi: 10.1016/j.ijid.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 41.Trotter CL, Lingani C, Fernandez K, Cooper LV, Bita A, Tevi-Benissan C, Ronveaux O, Préziosi MP, Stuart JM. Impact of MenAfriVac in nine countries of the African meningitis belt, 2010–15: an analysis of surveillance data. Lancet Infect Dis. 2017;17:867–872. doi: 10.1016/S1473-3099(17)30301-8. [DOI] [PubMed] [Google Scholar]
- 42.Preparedness for outbreaks of meningococcal meningitis due to Neisseria meningitidis serogroup C in Africa: recommendations from a WHO expert consultation Wkly Epidemiol Rec. 2015;90:633–6. [PubMed] [Google Scholar]
- 43.Zhu P, van der Ende A, Falush D, Brieske N, Morelli G, Linz B, Popovic T, Schuurman IG, Adegbola RA, Zurth K, et al.. Fit genotypes and escape variants of subgroup III Neisseria meningitidis during three pandemics of epidemic meningitis. Proc Natl Acad Sci U S A. 2001;98:5234–9. doi: 10.1073/pnas.061386098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore PS, Reeves MW, Schwartz B, Gellin BG, Broome CV. Intercontinental spread of an epidemic group A Neisseria meningitidis strain. Lancet. 1989;2:260–3. doi: 10.1016/S0140-6736(89)90439-X. [DOI] [PubMed] [Google Scholar]
- 45.Meningitis in Chad, Niger and Nigeria: 2009 epidemic season Wkly Epidemiol Rec. 2010;85:47–63. [PubMed] [Google Scholar]
- 46.Lingani C, Bergeron-Caron C, Stuart JM, Fernandez K, Djingarey MH, Ronveaux O, Schnitzler JC, Perea WA. Meningococcal meningitis surveillance in the African Meningitis Belt, 2004–2013. Clin Infect Dis. 2015;61 Suppl 5:S410–5. doi: 10.1093/cid/civ597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicolas P, Norheim G, Garnotel E, Djibo S, Caugant DA. Molecular epidemiology of Neisseria meningitidis isolated in the African meningitis belt between 1988 and 2003 shows dominance of sequence type 5 (ST-5) and ST-11 complexes. J Clin Microbiol. 2005;43:5129–35. doi: 10.1128/JCM.43.10.5129-5135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohn AC, Harrison LH. Meningococcal vaccines: current issues and future strategies. Drugs. 2013;73:1147–55. doi: 10.1007/s40265-013-0079-2. [DOI] [PubMed] [Google Scholar]
- 49.Koumare B, Ouedraogo-Traore R, Sanou I, Yada AA, Sow I, Lusamba PS, Traoré E, Dabal M, Santamaria M, Hacen MM, et al.. The first large epidemic of meningococcal disease caused by serogroup W135, Burkina Faso, 2002. Vaccine. 2007;25 Suppl 1:A37–41. doi: 10.1016/j.vaccine.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 50.Maurice J. Vaccine shortage threatens spread of meningitis in Niger. Lancet. 2015;385:2241. doi: 10.1016/S0140-6736(15)61050-9. [DOI] [PubMed] [Google Scholar]
- 51.Sambo L, Chan M, Davis S, Lake A, Berkley S, Poonawalla C, Elias CJ. A vaccine meets its promise: success in controlling epidemic Meningitis in Sub-Saharan Africa. Clin Infect Dis. 2015;61 Suppl 5:S387–8. doi: 10.1093/cid/civ490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiffay K, Jodar L, Kieny MP, Socquet M, LaForce FM. The evolution of the Meningitis Vaccine Project. Clin Infect Dis. 2015;61 Suppl 5:S396–403. doi: 10.1093/cid/civ594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frasch CE, Kapre SV, Lee CH, Preaud JM. Technical development of a New Meningococcal Conjugate Vaccine. Clin Infect Dis. 2015;61 Suppl 5:S404–9. doi: 10.1093/cid/civ595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sow SO, Okoko BJ, Diallo A, Viviani S, Borrow R, Carlone G, Tapia M, Akinsola AK, Arduin P, Findlow H, et al.. Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans. N Engl J Med. 2011;364:2293–304. doi: 10.1056/NEJMoa1003812. [DOI] [PubMed] [Google Scholar]
- 55.Hirve S, Bavdekar A, Pandit A, Juvekar S, Patil M, Preziosi MP, Tang Y, Marchetti E, Martellet L, Findlow H, et al.. Immunogenicity and safety of a new meningococcal A conjugate vaccine in Indian children aged 2–10 years: a phase II/III double-blind randomized controlled trial. Vaccine. 2012;30:6456–60. doi: 10.1016/j.vaccine.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Colombini A, Trotter C, Madrid Y, Karachaliou A, Preziosi MP. Costs of Neisseria meningitidis Group a disease and economic impact of Vaccination in Burkina Faso. Clin Infect Dis. 2015;61 Suppl 5:S473–82. doi: 10.1093/cid/civ600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lydon P, Zipursky S, Tevi-Benissan C, Djingarey MH, Gbedonou P, Youssouf BO, Zaffran M. Economic benefits of keeping vaccines at ambient temperature during mass vaccination: the case of meningitis A vaccine in Chad. Bull World Health Organ. 2014;92:86–92. doi: 10.2471/BLT.13.123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kristiansen PA, Diomande F, Ba AK, Sanou I, Ouedraogo AS, Ouedraogo R, Sangaré L, Kandolo D, Aké F, Saga IM, et al.. Impact of the serogroup a meningococcal conjugate Vaccine, MenAfriVac, on carriage and herd immunity. Clin Infect Dis. 2013;56:354–563. [DOI] [PubMed] [Google Scholar]
- 59.MenAfriCar C. The diversity of Meningococcal carriage across the African Meningitis Belt and the impact of vaccination with a group A Meningococcal Conjugate Vaccine. J Infect Dis. 2015;212:1298–307. doi: 10.1093/infdis/jiv211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.MenAfriCar C. Household transmission of Neisseria meningitidis in the African meningitis belt: a longitudinal cohort study. Lancet Glob Health. 2016;4:e989–e95. doi: 10.1016/S2214-109X(16)30244-3. [DOI] [PubMed] [Google Scholar]
- 61.Obaro SK, Habib AG. Control of meningitis outbreaks in the African meningitis belt. Lancet Infect Dis. 2016;16:400–2. doi: 10.1016/S1473-3099(16)00121-3. [DOI] [PubMed] [Google Scholar]
- 62.Mustapha MM, Marsh JW, Harrison LH. Global epidemiology of capsular group W meningococcal disease (1970-2015): Multifocal emergence and persistence of hypervirulent sequence type (ST)-11 clonal complex. Vaccine. 2016;34:1515–23. doi: 10.1016/j.vaccine.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 63.MacNeil JR, Medah I, Koussoube D, Novak RT, Cohn AC, Diomande FV, Yelbeogo D, Kambou JL, Tarbangdo TF, Ouédraogo-Traoré R, et al.. Neisseria meningitidis serogroup W, Burkina Faso, 2012. Emerg Infect Dis. 2014;20:394–9. doi: 10.3201/eid2003.131407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hossain MJ, Roca A, Mackenzie GA, Jasseh M, Hossain MI, Muhammad S, Ahmed M, Chidiebere OD, Malick N, Bilquees SM, et al.. Serogroup W135 meningococcal disease, The Gambia, 2012. Emerg Infect Dis. 2013;19:1507–10. doi: 10.3201/eid1909.130077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Collard JM, Maman Z, Yacouba H, Djibo S, Nicolas P, Jusot JF, Rocourt J, Maitournam R. Increase in Neisseria meningitidis serogroup W135, Niger, 2010. Emerg Infect Dis. 2010;16:1496–8. doi: 10.3201/eid1609.100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Decosas J, Koama JB. Chronicle of an outbreak foretold: meningococcal meningitis W135 in Burkina Faso. Lancet Infect Dis. 2002;2:763–5. doi: 10.1016/S1473-3099(02)00455-3. [DOI] [PubMed] [Google Scholar]
- 67.Retchless AC, Hu F, Ouedraogo AS, Diarra S, Knipe K, Sheth M, Rowe LA, Sangaré L, Ky Ba A, Ouangraoua S, et al.. The establishment and diversification of epidemic-associated Serogroup W Meningococcus in the African Meningitis Belt, 1994 to 2012. mSphere. 2016;1(6). pii: e00201–16. doi: 10.1128/mSphere.00201-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meningococcal disease in countries of the African meningitis belt, 2012 – emerging needs and future perspectives Wkly Epidemiol Rec. 2013;88:129–36. [PubMed] [Google Scholar]
- 69.Lucidarme J, Hill DM, Bratcher HB, Gray SJ, du Plessis M, Tsang RS, Vazquez JA, Taha MK, Ceyhan M, Efron AM, et al.. Genomic resolution of an aggressive, widespread, diverse and expanding meningococcal serogroup B, C and W lineage. J Infect. 2015;71:544–552. doi: 10.1016/j.jinf.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mustapha MM, Marsh JW, Krauland MG, Fernandez JO, de Lemos AP, Dunning Hotopp JC, Wang X, Mayer LW, Lawrence JG, Hiller NL, et al.. Genomic investigation reveals highly Conserved, Mosaic, Recombination events associated with capsular switching among Invasive Neisseria meningitidis Serogroup W Sequence Type (ST)-11 Strains. Genome Biol Evol. 2016;8:2065–75. doi: 10.1093/gbe/evw122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.del Castillo CM, Vazquez JA, Romero J, Pascual A. Infections by Neisseria meningitidis serogroup X in Spain. Clin Microbiol Infect. 2003;9:964–5. doi: 10.1046/j.1469-0691.2003.00685.x. [DOI] [PubMed] [Google Scholar]
- 72.Tapsall J. Annual report of the Australian Meningococcal Surveillance Programme, 2007–Amended. Commun Dis Intell. 2009;33:1–9. [DOI] [PubMed] [Google Scholar]
- 73.Harrison LH, Shutt KA, Schmink SE, Marsh JW, Harcourt BH, Wang X, Whitney AM, Stephens DS, Cohn AA, Messonnier NE, et al.. Population structure and capsular switching of invasive Neisseria meningitidis isolates in the pre-meningococcal conjugate vaccine era–United States, 2000–2005. J Infect Dis. 2010;201:1208–24. doi: 10.1086/651505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X, Shutt KA, Vuong JT, Cohn A, MacNeil J, Schmink S, Plikaytis B, Messonnier NE, Harrison LH, Clark TA, et al.. Changes in the Population Structure of Invasive Neisseria meningitidis in the United States After Quadrivalent Meningococcal Conjugate Vaccine Licensure. J Infect Dis. 2015;211:1887–94. doi: 10.1093/infdis/jiu842. [DOI] [PubMed] [Google Scholar]
- 75.Xie O, Pollard AJ, Mueller JE, Norheim G. Emergence of serogroup X meningococcal disease in Africa: need for a vaccine. Vaccine. 2013;31:2852–61. doi: 10.1016/j.vaccine.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 76.Denis F, Rey JL, Amadou A, Saliou P, Prince-David M, M'Boup S, Cadox M, Mar ID, Etienne J. Emergence of meningococcal meningitis caused by W 135 subgroup in Africa. Lancet. 1982;2:1335–6. doi: 10.1016/S0140-6736(82)91533-1. [DOI] [PubMed] [Google Scholar]
- 77.Campagne G, Schuchat A, Djibo S, Ousseini A, Cisse L, Chippaux JP. Epidemiology of bacterial meningitis in Niamey, Niger, 1981–96. Bull World Health Organ. 1999;77:499–508. [PMC free article] [PubMed] [Google Scholar]
- 78.Djibo S, Nicolas P, Alonso JM, Djibo A, Couret D, Riou JY, Chippaux JP. Outbreaks of serogroup X meningococcal meningitis in Niger 1995–2000. Trop Med Int Health. 2003;8:1118–23. doi: 10.1046/j.1360-2276.2003.01126.x. [DOI] [PubMed] [Google Scholar]
- 79.Mutonga DM, Pimentel G, Muindi J, Nzioka C, Mutiso J, Klena JD, Morcos M, Ogaro T, Materu S, Tetteh C, et al.. Epidemiology and risk factors for serogroup X meningococcal meningitis during an outbreak in western Kenya, 2005–2006. Am J Trop Med Hyg. 2009;80:619–24. [PubMed] [Google Scholar]
- 80.Outbreak news Meningococcal disease, Uganda. Wkly Epidemiol Rec. 2007;82:33. [PubMed] [Google Scholar]
- 81.Materu S, Cox HS, Isaakidis P, Baruani B, Ogaro T, Caugant DA. Serogroup X in meningococcal disease, Western Kenya. Emerg Infect Dis. 2007;13:944–5. doi: 10.3201/eid1306.070042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chow J, Uadiale K, Bestman A, Kamau C, Caugant DA, Shehu A, Greig J. Invasive meningococcal meningitis Serogroup C Outbreak in Northwest Nigeria, 2015 – Third Consecutive outbreak of a new strain. PLoS Curr. 2016;8 pii: ecurrents.outbreaks.06d10b6b4e690917d8b0a04268906143 doi: 10.1371/currents.outbreaks.06d10b6b4e690917d8b0a04268906143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trotter CL, Cibrelus L, Fernandez K, Lingani C, Ronveaux O, Stuart JM. Response thresholds for epidemic meningitis in sub-Saharan Africa following the introduction of MenAfriVac(R). Vaccine. 2015;33:6212–7. doi: 10.1016/j.vaccine.2015.09.107. [DOI] [PubMed] [Google Scholar]
- 84.Borrow R, Caugant DA, Ceyhan M, Christensen H, Dinleyici EC, Findlow J, Glennie L, Von Gottberg A, Kechrid A, Vázquez Moreno J, et al.. Meningococcal disease in the Middle East and Africa: Findings and updates from the Global Meningococcal Initiative. J Infect. 2017;75:1–11. doi: 10.1016/j.jinf.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 85.Coulson GB, von Gottberg A, du Plessis M, Smith AM, de Gouveia L, Klugman KP, Group for Enteric, Respiratory and Meningeal Disease Surveillance in South Africa . Meningococcal disease in South Africa, 1999–2002. Emerg Infect Dis. 2007;13:273–81. doi: 10.3201/eid1302.051553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wall EC, Everett DB, Mukaka M, Bar-Zeev N, Feasey N, Jahn A, Moore M, van Oosterhout JJ, Pensalo P, Baguimira K, et al.. Bacterial meningitis in Malawian adults, adolescents, and children during the era of antiretroviral scale-up and Haemophilus influenzae type b vaccination, 2000–2012. Clin Infect Dis. 2014;58:e137–45. doi: 10.1093/cid/ciu057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Migliani R, Clouzeau J, Decousser JW, Ravelomanana N, Rasamoelisoa J, Rabijaona H, et al.. [Non-tubercular bacterial meningitis in children in Antananarivo, Madagascar]. Arch Pediatr. 2002;9:892–7. doi: 10.1016/S0929-693X(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 88.Ceyhan M, Anis S, Htun-Myint L, Pawinski R, Soriano-Gabarro M, Vyse A. Meningococcal disease in the Middle East and North Africa: an important public health consideration that requires further attention. Int J Infect Dis. 2012;16:e574–82. doi: 10.1016/j.ijid.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 89.Klena JD, Wasfy MO, Nada RA, Ahmed SF, Maksoud MA, Marfin A, Pimentel G. Characterization of Neisseria meningitidis isolates from Egypt using multilocus sequence typing. Trans R Soc Trop Med Hyg. 2012;106:309–14. doi: 10.1016/j.trstmh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 90.Gamougam K, Daugla DM, Toralta J, Ngadoua C, Fermon F, Page AL, Djingarey MH, Caugant DA, Manigart O, Trotter CL, et al.. Continuing effectiveness of serogroup A meningococcal conjugate vaccine, Chad, 2013. Emerg Infect Dis. 2015;21:115–8. doi: 10.3201/eid2101.140256. [DOI] [PMC free article] [PubMed] [Google Scholar]


