ABSTRACT
The aim of this study was to evaluate changes in hepatitis B surface antibody titers (anti-HBs) after booster vaccinations in children aged 5–15 y and to provide suitable immunization strategies. A total of 2208 children were initially enrolled in screening, and 559 children were finally included. The participants were divided into 2 groups according to their pre-booster anti-HBs levels: Group I, <10 mIU/ml and Group II, ≥10 mIU/ml. Group I was administered 3 doses of booster hepatitis B vaccine (0-1-6 months, 10 μg), and Group II was administered 1 dose of booster hepatitis B vaccine (10 μg). The antibody titer changes were examined at 4 time points: 1 month after dose 1 and dose 3, and 1 year and 5 years after dose 3. The protective seroconversion rates at those points were 95.65%, 99.67%, 97.59% and 91.05% (p < 0.001), respectively, in Group I, and 100.00%, 99.87%, 99.66% and 98.21% (χ2 = 6.04, p = 0.11), respectively, in Group II. The GMT in subjects aged 5–9 y were higher than that in subjects aged 10–15 y in both Group I and Group II at 1 month after dose 1, but no difference was observed at the other three time points. This study demonstrates that booster vaccination has a good medium-term effect. A booster dose for subjects with protective antibodies is not necessary but effective, and 3 doses of hepatitis B vaccination are recommended for those who have lost immunological memory. Receiving booster immunization at the age of 10-15 years may be more appropriate for individuals living in HBV high epidemic areas
KEYWORDS: Hepatitis B virus (HBV), vaccine, booster vaccination, children, follow-up, anti-HBs
Introduction
Hepatitis B virus (HBV) remains a worldwide public health problem. A report from WHO showed that 257 million persons, or 3.5% of the population, were living with chronic HBV infection in the world, and the African and Western Pacific regions accounted for 68% of those infected.1 China is a highly endemic area for HBV infection.2 The hepatitis B surface antigen (HBsAg) carrier rate in the general Chinese population aged 1 to 29 y was 2.6% according to a 2014 national seroepidemiological survey, indicating that more than 34 million people are chronically infected with HBV in China.3
Currently, there is no satisfactory treatment for chronic HBV infection and related diseases. Thus, hepatitis B vaccination is regarded as the most economical and effective measure for the prevention and control of hepatitis B infection.4 In 1992, the hepatitis B vaccine was incorporated into immunization planning management, and a free universal vaccination policy for infants was incorporated into the national immunization plan in 2002. A national serosurvey in 2014 showed that the HBsAg prevalence had decreased significantly in the population aged 1–29 y in China after decades of hepatitis B mass vaccination, particularly among children below 15 y of age.3
However, primary immunization may not be enough to protect children from HBV infection. It is widely accepted that HepB vaccination is ∼95% effective in preventing infection in infants/children and that infants and young children are at an increased risk of developing chronic infection.5-7 And the hepatitis B surface antibody titers (anti-HBs) in vaccines attenuate over time to below the protective level and even become undetectable.8,9 A 14-y follow-up study in China showed that 99% of individuals with 3 doses of the hepatitis B vaccine had seroprotective levels of anti-HBs after 1 year, but only 37% retained 10 mIU/ml of anti-HBs after 14 y.10 Research widely considers 10 mIU/ml of anti-HBs to be a seroprotective level; the risk of infection increases when antibody titers dip below 10 mIU/ml.11,12 Additionally, immunologic memory will decrease with time after basic immunization.13 Consequently, the need of revaccination in children has received wide attention.
In the present study, we included 2114 children who were negative for HBsAg and anti-HBc to explore the effect of booster immunization. Then, we divided these subjects into two groups according their anti-HB titers before the booster, and different procedures were administered. We examined the titers of anti-HBs in children with primary vaccination and evaluated the effect of revaccination over a 5-y follow-up period.
Results
Characteristics of the study subjects
A total of 2208 children between 5 to 15 y of age were initially enrolled in screening, and 559 children were finally included. Only subjects with two negative indices (HBsAg and anti-HBc) were included, and blood samples were collected at 1 month after dose 1 and dose 3, and 1 year and 5 years after dose 3. A total of 1649 individuals were excluded: 4 subjects were positive for HBsAg, 65 subjects were positive for anti-HBc, 25 subjects were positive for both HBsAg and anti-HBc, and 1555 subjects were lost to follow-up (Fig. 1).
Figure 1.
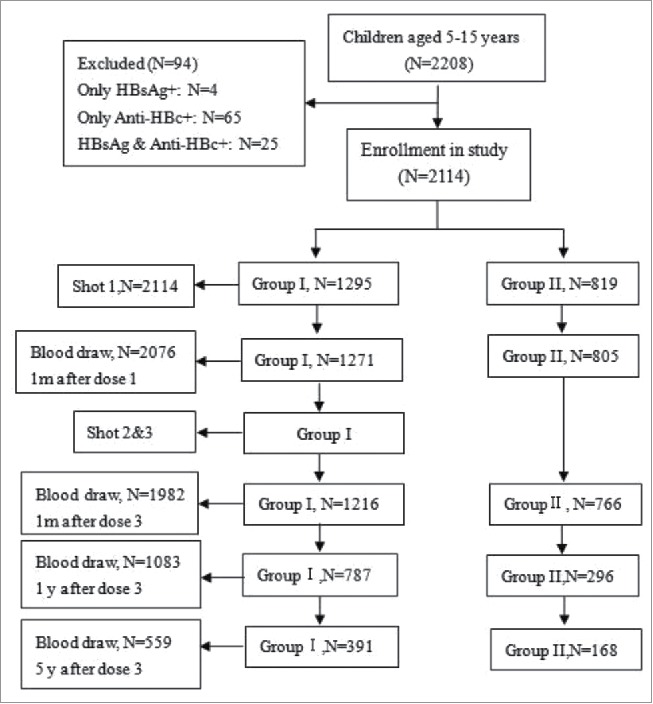
Study flow chart.
Children were divided into 2 groups based on whether they were in the cohort study. After five years of follow-up, 559 subjects could still be contacted, including 289 males and 270 females, with an average age of 8.48 ± 2.29 y. In addition, 1555 subjects were lost to follow-up, including 795 males and 760 females, with an average age of 11.08 ± 3.08 y. The age of those who were lost to follow-up was significant higher than the age of those in the followed-up cohort (p < 0.001), and no significant difference in gender was observed between the two groups (p = 0.816).
In the followed cohort, there were 391 and 168 individuals in Group I and Group II, respectively. The average age of children in Group I and Group II was 8.77 ± 2.26 and 7.83 ± 2.23 y, respectively (p < 0.001), and no statistically significant difference was observed in gender between Group I and Group II (p = 0.102). Details are shown in Table 1.
Table 1.
Age and sex distribution of the study subjects.
| Followed Cohort |
Lost to follow-up |
P value |
||||||
|---|---|---|---|---|---|---|---|---|
| aGroup | NO. | Age | Male(%) | NO. | Age | Male(%) | Age | Male |
| Group I | 391 | 8.77 ± 2.26 | 53.96 | 904 | 11.34 ± 2.91 | 53.10 | <0.001 | 0.774 |
| Group II | 168 | 7.83 ± 2.23 | 46.43 | 651 | 10.71 ± 3.26 | 48.40 | <0.001 | 0.651 |
| Total | 559 | 8.48 ± 2.29 | 51.70 | 1555 | 11.08 ± 3.08 | 51.13 | <0.001 | 0.816 |
| P I vs. II | <0.001 | 0.102 | <0.001 | 0.067 | ||||
anti-HBs levels prior to immune booster: Group I <10 mIU/ml; Group II ≥10 mIU/ml.
Antibody positive rates and GMT at 4 time points
At one month after dose 1, a total of 1984 subjects aged 5–15 y had protective antibodies, corresponding to a positive seroconversion rate of 95.57%. The positive rates in Group I and Group II were 95.65% and 100.00% (p < 0.001), respectively, with corresponding GMTs of 789.0 and 7317.2 mIU/ml (p < 0.001). In Group I, the positive rates of subjects aged 5–9 y and 10–15 y were 96.85% and 89.80% (p < 0.001), respectively, and the GMT of subjects aged 5–9 y was much higher than that in subjects aged 10–15 y (1697.4 vs. 435.7 mIU/ml). In Group II, the PSR of both age groups was 100.00%, and the GMT of subjects aged 5–9 y was significantly higher than that in subjects aged 10–15 y (8411.8 vs. 6336.0 mIU/ml).
At one month after dose 3, a total of 1977 subjects aged 5–15 y had protective antibodies, corresponding to a positive seroconversion rate of 99.75%. The positive rate in Group I with three booster doses was 4.02 percentage points higher than that at 1 month after dose 1 (99.67% vs. 95.65%, p < 0.001), and the GMT increased from 789.0 to 2464.0 mIU/ml (p < 0.001).The positive rates in Group I and Group II were 99.67% and 99.87% (p = 0.691), respectively, with corresponding GMTs of 2464.0 and 1779.1 mIU/ml (p < 0.001). The positive rates of subjects aged 5–9 y and 10–15 y were 100.00% and 99.42% (p = 0.217), respectively, in Group I, with corresponding GMTs of 2741.8 and 2273.1 mIU/ml (p = 0.911). The PSRs of subjects aged 5–9 y and 10–15 y were 99.74% and 100.00% (p = 1.000), respectively, in Group II, with corresponding GMTs of 1813.6 and 1744.8 mIU/ml (p = 0.141).
At one year after dose 3, 1063 subjects still maintained protective antibody levels, with a total anti-HBs PSR of 98.15%. No significant differences were observed in PSR between Group I and Group II (all p > 0.05), but the GMT of Group II was significantly higher than that in Group I (all p < 0.001). In addition, the PSR in subjects aged 5–9 y was significantly higher than that in 10–15 y in Group I (p = 0.016), but this difference was not significant in Group II (p = 0.287).
At five years after dose 3, the PSRs of Group I and Group II were 91.05% and 98.21%, respectively (p < 0.001). After stratifying by age, the PSR of subjects aged 5–9 y in Group I was significantly lower than that in Group II (p = 0.004), but this difference was not significant in subjects aged 10–15 y (88.30% vs. 94.74%, p = 0.673). The GMT in Group I was significantly lower than that in Group II at 5–9, 10–15 and 5–15 y (all p < 0.05). No significant differences were observed in GMT and PSR between 5–9 y and 10–15y in both Group I and Group II (all p > 0.05).
The pre-booster GMTs in Group I and Group II were 0.65 (95% CI: 0.57-0.73) and 63.3 (95% CI: 57.4-70.2) mIU/ml, respectively. At one month after dose 1 and dose 3 and 1 year and 5 years after dose 3, the anti-HBs positive rates were 95.65%, 99.67%, 97.59% and 91.05% in Group I (χ2 = 105.089, p < 0.001), respectively, and 100.00%, 99.87%, 99.66% and 98.21% in Group II (p = 0.001, Fisher's exact test), respectively. The GMTs in Group I were 789.0, 2464.0, 191.9, and 73.1 mIU/ml (F = 507.453, p < 0.001), respectively. After further multiple comparisons, we found that the GMTs at all 4 time points significantly differed from one another (all p < 0.001) in this group. The GMTs in Group II were 7317.2, 1779.1, 519.1, and 257.3 mIU/ml (F = 494.100, p < 0.001), respectively. After further multiple comparisons, we found that the GMTs decreased with time (all p < 0.001) in this group. The distributions of anti-HB positive rates and GMTs are shown in Table 2.
Table 2.
Anti-HBs PSR and GMTs in 2 age groups at 4 time points.
|
aGroup I |
aGroup II |
P value |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Age group | No. | PSR (%) | GMTs | No. | PSR (%) | GMTs | PSR (%) | GMTs | |
| 1 m after dose 1 | 5–9 | 555 | 96.85 | 1697.4 | 409 | 100.00 | 8411.8 | <0.001 | <0.001 |
| 10–15 | 716 | 89.80 | 435.7 | 396 | 100.00 | 6336.0 | <0.001 | <0.001 | |
| 5–15 | 1271 | 95.65 | 789.0 | 805 | 100.00 | 7317.2 | <0.001 | <0.001 | |
| P (5–9 vs. 10–15) | <0.001 | <0.001 | 1.000 | <0.001 | |||||
| 1 m after dose 3 | 5–9 | 523 | 100.00 | 2741.8 | 386 | 99.74 | 1813.6 | 0.425 b | <0.001 |
| 10–15 | 693 | 99.42 | 2273.1 | 380 | 100.00 | 1744.8 | 0.337 | 0.005 | |
| 5–15 | 1216 | 99.67 | 2464.0 | 766 | 99.87 | 1779.1 | 0.691 | <0.001 | |
| P (5–9 vs. 10–15) | 0.217 | 0.911 | 1.000 b | 0.141 | |||||
| 1 y after dose 3 | 5–9 | 461 | 98.65 | 204.3 | 211 | 100.00 | 529.5 | 0.221 | <0.001 |
| 10–15 | 326 | 96.01 | 175.6 | 85 | 98.82 | 494.1 | 0.349 | <0.001 | |
| 5–15 | 787 | 97.59 | 191.9 | 296 | 99.66 | 519.1 | 0.024 | <0.001 | |
| P (5–9 vs. 10–15) | 0.016 | 0.453 | 0.287b | 0.315 | |||||
| 5 y after dose 3 | 5–9 | 297 | 91.92 | 70.6 | 149 | 98.66 | 255.6 | 0.004 | <0.001 |
| 10–15 | 94 | 88.30 | 79.8 | 19 | 94.74 | 270.4 | 0.673 | 0.016 | |
| 5–15 | 391 | 91.05 | 73.1 | 168 | 98.21 | 257.3 | <0.001 | <0.001 | |
| P (5–9 vs. 10–15) | 0.284 | 0.513 | 0.304 b | 0.916 | |||||
| cCoefficient value | χ2 = 105.089 F = 507.453 | – F = 494.100 | |||||||
| P value | <0.001 | <0.001 | 0.001b | <0.001 | |||||
PSR: positive seroconversion rate; GMT: geometric mean titer.
anti-HBs levels prior to immune booster: Group I <10 mIU/ml; Group II ≥10 mIU/ml;
Fisher's exact test;
Coefficient value: the Pearson Chi-square test to compare the PSR between 4 time points; the One-way ANOVA Test to compare the GMT between 4 time points.
Discussion
Some studies have shown that anti-HBs positive seroconversion rates and GMTs of children with primary vaccination gradually decrease with increasing age.14,15 A study in Thailand16 showed that only 64% of individuals retained protective anti-HBs titers (≥10 mIU/ml) at 20 years after primary immunization, and an Italian17 study found that only 62% of participants still had anti-HBs ≥10 mIU/ml at 10 years after primary immunization. Moreover, a study in Gambia showed that the anti-HBc positive rate of children with primary vaccination in a high-HBV prevalence area reached 17.7%.18 These studies suggest that research on hepatitis B booster vaccination is crucial and urgent.
The results of this study showed that the booster vaccination in people without protective antibodies has a good medium-term response. In this study, a total of 391 children in Group I were contacted after five years for follow-up, and 91.05% still had protective anti-HB titers. The result of our study was higher than that in other reported results. Lu, S et al.19 found that 73.8% of participants had protective antibodies at five years after booster vaccination, and McMahon et al.20 found that 81.0% of participants had anti-HBs ≥10 mIU/ml after one year of follow-up. There are several factors, such as the different composition of the subjects and the different types of vaccines, which might explain this difference.
In this study, we found that Group II had a better response after booster vaccination. We can draw this conclusion based on the following results. First, the GMT of Group II at 1 month after dose 1 was much higher than that in Group I at 1 month after dose 3 (7317.2 vs. 2464.0 mIU/ml, p < 0.001), even though Group I received an additional two doses. Additionally, all GMT values in Group II were higher than those in Group I at 1 and 5 years after dose 3. This result demonstrated that subjects with high pre-booster GMTs had a better response in booster vaccination than subjects with low pre-booster GMTs, which is similar to some previous studies.21,22
Currently, booster vaccination of hepatitis B vaccines in people with protective antibodies is not recommended by the 2017 WHO position paper on HepB vaccines.23 However, this does not mean booster immunization is ineffective in people with protective antibodies. In our study, the GMT of Group II at 5 years after dose 3 was 257.3 mIU/ml, and it was significantly higher than that before the booster vaccination (257.3 vs. 54.6 mIU/ml, p < 0.001). We also found that, after more than 5 years, the PSR of Group II only decreased from 100.00% to 98.21%, because of one dose booster vaccination. If there is no booster vaccination, the PSR in Group II will have a larger decrease. A study reported by Feng Y et al.24 showed that the PSR in children with normal and high antibody responses decreased from 100% to 53.91% in 5 years. Therefore, we believe that for those who have protective antibodies, booster vaccination can increase their immune persistence.
It is generally considered that immunological memory will protect people from HBV infection even if the level of antibody is low or undetectable.25,26 In this present study, the PSR of Group I reached 95.65% at 1 month after dose 1, which indicates that the majority of subjects with anti-HBs <10 mIU/ml developed a rapid and robust anamnestic response. However, still 4.35% of the subjects had not received protective antibodies after one dose booster vaccination, which indicated that some subjects had lost their immunological memory. The results of this study were similar to some previous studies. With two additional doses, the PSR of Group I significantly increased from 95.65% to 99.67%, which demonstrates that most people who have lost immunological memory can receive protective antibodies through three doses of booster vaccination. The studies reported by Lu et al.19 and Salama et al.27 obtained the same result.
After stratifying by age, the anti-HBs GMT of the subjects aged 5–9 y was much higher than that of those aged 10–15 y at 1 month after dose 1 in both Group I and Group II, which indicates that subjects aged 5–9 y had a better immune response. This result is similar to previous studies.28-30 However, there were no significant differences observed between different age groups in both Group I and Group II at 1 month after dose 3, and 1 year and 5 years after dose 3, which suggests that there is no significant difference in the long-term immunization effect between two age groups. A meta-analysis directed by Jalal Poorolajal et al.31 suggested that the protection provided by three doses of the HB vaccine in infancy persists for at least two decades, and therefore, getting revaccination at 5–9 y and 10–15 y were both early enough to protect subjects from HBV infection. Although there is no difference in the efficacy of booster immunization between 5–10 y and 10–15 y, getting revaccination at 10–15 y is more appropriate because the total protective time provided by the vaccine will be longer.
There are some limitations to our study. First, we clustered subjects based on school enrollment, and some students were lost to follow-up because they graduated from their initial school and went on to attend a new school, which may explain why the average age of the group lost to follow-up was higher than that of the followed-up group. The high loss to follow-up may influence the reliability and the sample size of this study. Additionally, we failed to acquire the primary immunization responses of our subjects. Therefore, it was difficult to identify the cause of the negative responses to primary immunization or the reason for the disappearance of antibodies over time. Third, the results of the second dose are lacking because the parents or guardians of the subjects rejected the collection of blood samples twice in a short time. Another limitation of this study is that we did not include a cohort formed by subjects with anti-HBs < 10 mIU/ml but only received one dose.
In conclusion, this study showed that the booster vaccination has a good medium-term effect and that the subjects with high pre-booster GMTs had a better response to booster vaccination. A booster dose for subjects with protective antibodies is not necessary but effective. Most subjects with anti-HBs < 10 mIU/ml had an anamnestic response, but 3 doses of hepatitis B vaccination are recommended for those who have lost immunological memory. In addition, receiving booster immunization at the age of 10–15 may be more appropriate for individuals living in HBV high epidemic areas.
Materials and Methods
Study participants
The present study was conducted in Longquan County, Yuhuan County, and Kaihua County in Zhejiang province in 2009–2010 and aimed to evaluate the long-term efficacy of booster vaccination among children aged 5–15 y. First, we selected two towns in each country as research sites and clustered the subjects based on school enrollment. Then, we obtained the immunization information of all children by the child's immunization certificate kept by their parents or by review of the child's immunization card kept at the township hospital, and we selected children who meet our inclusion criteria. Third, we selected subjects whose blood tests for HBsAg and anti-HBc were negative. We randomly selected 2208 subjects, of whom 559 eligible subjects completed 5 years of follow-up. A flowchart of the participants enrolled in this study is summarized in Fig. 1. This study was approved by the Institutional Review Board of the Zhejiang Center for Disease Control and Prevention, and we obtained written informed consent from each participant.
The specific inclusion criteria were as follows:
-
(1)
Children born between January 1, 1993 and January 1, 2004 who had received a 3-dose series of the monovalent recombinant hepatitis B vaccine at 0, 1, and 6 months after birth.
-
(2)
Never received HepB booster vaccination after primary vaccination.
-
(3)
Willingness to participate in the follow-up study and to give a blood sample after vaccination.
-
(4)
No acute illness within the last 7 days, no fever within the past 3 days (armpit temperature ≥38℃), and no allergies or severe reactions to vaccination.
-
(5)
All information regarding the study was provided by the subject's parents or guardians, and signed consent was obtained.
-
(6)
No immune dysfunction.
-
(7)
Had not received any immune suppressive therapy (treated intravenously or orally with cortisone or chemotherapy)
-
(8)
Not at high risk of compromised immunity.
-
(9)
Had not received any kind of vaccination with 4 weeks.
Methods
After acquiring informed consent from the subjects or from their parents or guardians, 3-ml blood samples were collected from each subject and preserved for further testing. Booster vaccinations of the recombinant hepatitis B vaccine (lot number: 20090309(01-06), dosage: 10 μg HBsAg; produced by Dalianhanxin Biotechnology Co., Ltd.) were administered by intramuscular injection in the upper arm deltoid at months 0, 1, and 6 in Group I and at month 0 in Group II, according to the immunization procedures. At 1 month after dose 1 and dose 3, 3-ml blood samples were collected from each subject and preserved for testing. One and five years after the third dose, 2-ml blood samples were collected from the follow-up subjects and tested.
Lab testing
Sample processing
Frozen separated serum samples were sent to ADICON Clinical Laboratories. Inc. in Hangzhou for quantification of HBsAg, anti-HBc and anti-HBs by chemiluminescence immunoassay (CMIA).
Apparatus and reagents
An Architect-i2000 (Abbott, USA) was used for the chemiluminescence immunoassay. The reagent lot number for the HBsAg tests was 70318HN00 (Abbott Laboratories), and an HBsAg ≥ 0.05IU/ml was considered positive. The reagent 75684M100 (Abbott Laboratories) was used for the anti-HBs tests and an anti-HBs ≥ 10 mIU/ml was considered positive and able to provide protection against HBV infection. The commercial reagent 72448M100 (Abbott Laboratories) was used for the anti-HBc tests, and a S/CO ≥ 1 was considered positive.
Data analysis
The participants were divided into 2 groups according to their anti-HBs levels prior to the immune booster: Group I <10 mIU/ml and Group II ≥10 mIU/ml. We established a database using Epidata3.2 (Epidata; Norway and Denmark), and statistical analysis was performed using SPSS 18.0 and Excel 2003. One-way ANOVA test, T-test, Chi-square test, and Fisher's exact test were used to compare variables among the study groups. We used a two-tailed probability in the statistical tests, and p < 0.05 was considered statistically significant. Because we were unable to detect anti-HBs titers less than 0.01 mIU/ml, we assigned a value of 0.005 mIU/ml to these participants when calculating the GMT of anti-HBs, and samples with anti-HBs titers exceeding 15,000 mIU/ml were assigned a value of 15,000 mIU/ml when a long transformation was used for calculating GMT.
Funding Statement
This study was supported by the National Scientific and Technological Major Project of China (No. 2013ZX10004-904, NO. 2014ZX10004-008).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to the children and parents who volunteered to participate in this study.
References
- 1.Organization WH. Global hepatitis report 2017. 2017. [Google Scholar]
- 2.Liaw YF, Chu CM. Hepatitis B virus infection. Lancet (London, England). 2009;373(9663):582–592. doi: 10.1016/S0140-6736(09)60207-5. PMID:19217993. [DOI] [PubMed] [Google Scholar]
- 3.Cui F, Shen L, Li L, Wang H, Wang F, Bi S, Liu J, Zhang G, Wang F, Zheng H, et al.. Prevention of Chronic Hepatitis B after 3 Decades of Escalating Vaccination Policy, China. Emerg Infect Dis. 2017;23(5):765–772. doi: 10.3201/eid2305.161477. PMID:28418296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang MH, Hadzic D, Rouassant SH, Jonas M, Kohn IJ, Negro F, Roberts E, Sibal A, Asian Pan-Pacific Society for Pediatric Gastroenterology, Hepatology and Nutrition . Acute and chronic hepatitis: working group report of the Second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39(Suppl 2):S584–588. doi: 10.1097/00005176-200406002-00002. PMID:15184756. [DOI] [PubMed] [Google Scholar]
- 5.Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000–summary of a workshop. Gastroenterology. 2001;120(7):1828–1853. doi: 10.1053/gast.2001.24839. PMID:11375963. [DOI] [PubMed] [Google Scholar]
- 6.Beasley RP. Rocks along the road to the control of HBV and HCC. Ann Epidemiol. 2009;19(4):231–234. doi: 10.1016/j.annepidem.2009.01.017. PMID:19344859. [DOI] [PubMed] [Google Scholar]
- 7.Lu JJ, Cheng CC, Chou SM, Hor CB, Yang YC, Wang HL. Hepatitis B immunity in adolescents and necessity for boost vaccination: 23 years after nationwide hepatitis B virus vaccination program in Taiwan. Vaccine. 2009;27(47):6613. doi: 10.1016/j.vaccine.2009.08.007. PMID:19698812. [DOI] [PubMed] [Google Scholar]
- 8.Chen DS. Hepatitis B vaccination: The key towards elimination and eradication of hepatitis B. J Hepatol. 2009;50(4):805–816. doi: 10.1016/j.jhep.2009.01.002. PMID:19231008. [DOI] [PubMed] [Google Scholar]
- 9.Lu L, Chen YH, X L, JX H . Research of hepatitis B vaccine immune effect. Chinese Journal of Public Health Mangement. 2006;22(6):485–487. In Chinese. [Google Scholar]
- 10.Ma JC, Liu HB, Yl Zhang, et al.. Rural neonatal hepatitis b vaccine at 14 years after the immune effect evaluation. Chinese Immunization. 2002;8(4):181–184. In Chinese. [Google Scholar]
- 11.Hadler SC, Francis DP, Maynard JE, Thompson SE, Judson FN, Echenberg DF, Ostrow DG, O'Malley PM, Penley KA, Altman NL, et al.. Long-term immunogenicity and efficacy of hepatitis B vaccine in homosexual men. N Engl J Med. 1986;315(4):209–214. doi: 10.1056/NEJM198607243150401. PMID:2941687. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida T, Saito I. Hepatitis B booster vaccination for healthcare workers. Lancet (London, England). 2000;355(9213):1464. doi: 10.1016/S0140-6736(05)74663-8. PMID:10791552. [DOI] [PubMed] [Google Scholar]
- 13.Lao TT. Immune persistence after hepatitis B vaccination in infancy – Fact or fancy? Hum Vaccin. 2016;12(5):1172–1176. doi: 10.1080/21645515.2015.1130195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang LY, Hu CT, Ho TY, Lin HH. Geographic and ethnic variations of long-term efficacy and immunogenicity of hepatitis B vaccination in Hualien, a HBV hyperendemic area. Vaccine. 2006;24(20):4427–4432. doi: 10.1016/j.vaccine.2005.12.069. PMID:16574284. [DOI] [PubMed] [Google Scholar]
- 15.Lu SN, Chen CH, Chen TM, Lee PL, Wang JH, Tung HD, Hung CH, Lee CM, Changchien CS. Hepatitis B virus infection in adolescents in a rural township–15 years subsequent to mass hepatitis B vaccination in Taiwan. Vaccine. 2006;24(6):759–765. doi: 10.1016/j.vaccine.2005.08.062. PMID:16159687. [DOI] [PubMed] [Google Scholar]
- 16.Poovorawan Y, Chongsrisawat V, Theamboonlers A, Crasta PD, Messier M, Hardt K. Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response: a 20-year follow-up study in Thailand. Hum Vaccin Immunother. 2013;9(8):1679–1684. doi: 10.4161/hv.24844. PMID:23732904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spada E, Romano L, Tosti ME, Zuccaro O, Paladini S, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, et al.. Hepatitis B immunity in teenagers vaccinated as infants: an Italian 17-year follow-up study. Clin Microbiol Infect: The official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20(10):O680–686. doi: 10.1111/1469-0691.12591. [DOI] [PubMed] [Google Scholar]
- 18.van der Sande MA, Waight PA, Mendy M, Zaman S, Kaye S, Sam O, Kahn A, Jeffries D, Akum AA, Hall AJ, et al.. Long-term protection against HBV chronic carriage of Gambian adolescents vaccinated in infancy and immune response in HBV booster trial in adolescence. PloS one. 2007;2(8):e753. doi: 10.1371/journal.pone.0000753. PMID:17710152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu S, Ren J, Li Q, Jiang Z, Chen Y, Xu K, Ruan B, Yang S, Xie T, Yang L, et al.. Effects of hepatitis B vaccine boosters on anti-HBs-negative children after primary immunization. Hum Vaccin Immunother. 2017;13(4):903–908. doi: 10.1080/21645515.2016.1260794. PMID:27905821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Bulkow L, Fiore AE, Bell BP, Hennessy TW. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis. 2009;200(9):1390–1396. doi: 10.1086/606119. PMID:19785526. [DOI] [PubMed] [Google Scholar]
- 21.Jafarzadeh A, Zarei S, Shokri F. Low dose revaccination induces robust protective anti-HBs antibody response in the majority of healthy non-responder neonates. Vaccine. 2008;26(2):269–276. doi: 10.1016/j.vaccine.2007.10.044. PMID:18037544. [DOI] [PubMed] [Google Scholar]
- 22.Yao J, Ren J, Shen L, Chen Y, Liang X, Cui F, Li Q, Jiang Z, Wang F. The effects of booster vaccination of hepatitis B vaccine on anti-HBV surface antigen negative children 11–15 years after primary vaccination. Hum Vaccin. 2011;7(10):1055–1059. doi: 10.4161/hv.7.10.15990. PMID:21989290. [DOI] [PubMed] [Google Scholar]
- 23.Organization WH. Hepatitis B vaccines: WHO position paper – July 2017. Releve Epidemiologique Hebdomadaire. 2017;92(27):369. PMID:28685564. [PubMed] [Google Scholar]
- 24.Zanetti AR, Mariano A, Romanò L, D'Amelio R, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, Negrone FS, et al.. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet (London, England). 2005;366(9494):1379. doi: 10.1016/S0140-6736(05)67568-X. PMID:16226616. [DOI] [PubMed] [Google Scholar]
- 25.Mendy M, Peterson I, Hossin S, Peto T, Jobarteh ML, Jeng-Barry A, Sidibeh M, Jatta A, Moore SE, Hall AJ, et al.. Observational Study of Vaccine Efficacy 24 Years after the Start of Hepatitis B Vaccination in Two Gambian Villages: No Need for a Booster Dose. PloS one. 2013;8(3):e58029. doi: 10.1371/journal.pone.0058029. PMID:23533578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wenzel JJ, Jilg W. Loss of antibodies, but not of protection. Lancet Infectious Diseases. 2010;10(11):738. doi: 10.1016/S1473-3099(10)70217-6. PMID:21029981. [DOI] [PubMed] [Google Scholar]
- 27.Salama II, Sami SM, Salama SI, Rabah TM, El Etreby LA, Abdel Hamid AT, Elmosalami D, El Hariri H, Said ZN. Immune response to second vaccination series of hepatitis B virus among booster dose non-responders. Vaccine. 2016;34(16):1904–1908. doi: 10.1016/j.vaccine.2016.02.050. PMID:26930367. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Lv H, Gu H, Cui F, Wang F, Yao J, Xia S, Liang X. The effects of different dosage levels of hepatitis B vaccine as booster on anti-HBs-negative children 5–15 y after primary immunization; China, 2009–2010. Hum Vaccin Immunother. 2014;10(2):498–504. doi: 10.4161/hv.26936. PMID:24192508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su FH, Cheng SH, Li CY, Chen JD, Hsiao CY, Chien CC, Yang YC, Hung HH, Chu FY. Hepatitis B seroprevalence and anamnestic response amongst Taiwanese young adults with full vaccination in infancy, 20 years subsequent to national hepatitis B vaccination. Vaccine. 2007;25(47):8085–8090. doi: 10.1016/j.vaccine.2007.09.013. PMID:17920732. [DOI] [PubMed] [Google Scholar]
- 30.Chaves SS, Groeger J, Helgenberger L, Auerbach SB, Bialek SR, Hu DJ, Drobeniuc J. Improved anamnestic response among adolescents boosted with a higher dose of the hepatitis B vaccine. Vaccine. 2010;28(16):2860–2864. doi: 10.1016/j.vaccine.2010.01.059. PMID:20153793. [DOI] [PubMed] [Google Scholar]
- 31.Poorolajal J, Mahmoodi M, Majdzadeh R, Nasseri-Moghaddam S, Haghdoost A, Fotouhi A. Long-term protection provided by hepatitis B vaccine and need for booster dose: a meta-analysis. Vaccine. 2010;28(3):623–631. doi: 10.1016/j.vaccine.2009.10.068. PMID:19887132. [DOI] [PubMed] [Google Scholar]


