ABSTRACT
Background: Meningococcal disease (MD) is a major cause of meningitis and sepsis worldwide, with a high case fatality rate and frequent sequelae. Neisseria meningitidis serogroups A, B, C, W, X and Y are responsible for most of these life-threatening infections, and its unpredictable epidemiology can cause outbreaks in communities, with significant health, social and economic impact. Currently, serogroup B is the main cause of MD in Europe and North America and one of the most prevalent serogroups in Latin America. Mass vaccination strategies using polysaccharide vaccines have been deployed since the 1970s and the use of conjugate vaccines has controlled endemic and epidemic disease caused by serogroups A, C, W and Y and more recently serogroup B using geographically-specific outer membrane vesicle based vaccines. Two novel protein-based vaccines are a significant addition to our armamentarium against N. meningitidis as they provide broad coverage against highly diverse strains in serogroup B and other groups. Early safety, effectiveness and impact data of these vaccines are encouraging. These novel serogroup B vaccines should be actively considered for individuals at increased risk of disease and to control serogroup B outbreaks occurring in institutions or specific regions, as they are likely to save lives and prevent severe sequelae. Incorporation into national programs will require thorough country-specific analysis.
KEYWORDS: Neisseria meningitidis, epidemiology, meningococcal serogroup B, meningococcal vaccines, outbreaks
Brief overview of meningococcal infection/disease
Meningococcal disease (MD) is a major public health problem and remains a leading cause of meningitis and sepsis in many countries1,2 Case fatality rates (CFR) reach 10–20% despite aggressive treatment, 10–20% of survivors will develop major long-term sequelae, including deafness, neurological deficit, seizures, limb amputation, and up to 36% of survivors may have one or more deficits in physical, cognitive, and psychological functioning.1-3 MD occurs in all age groups, although incidence rates are highest in young children and teenagers. MD is mostly sporadic with seasonal variations, with occasional epidemics in large regions or smaller outbreaks in specific settings, which occur at rather unpredictable intervals. During these epidemics an increased number of cases usually occurs among adolescents and young adults.4,5
Neisseria meningitidis (N. meningitidis) is a Gram-negative, aerobic, encapsulated, non-mobile diplococcus, belonging to the Neisseriaceae family. The antigenic composition of the polysaccharide capsule defines 12 serogroups: A, B, C, H, I, K, L, W, X, Y, Z and E6. Currently, six serogroups, A, B, C, Y, W and X, are responsible for virtually all cases of disease reported worldwide.1,2,4,5 Meningococci are also classified into serotypes and serosubtypes according to the antigenic composition of the outer membrane proteins (OMP) PorB and PorA, respectively. Meningococci can exchange genetic material encoding for capsule synthesis, modifying the capsular antigenic composition of a specific strain. Antigenically distinct strains due to allelic replacement of the siaD gene can lead to outbreaks.7-10 Genetic multilocus sequence typing targeting polymorphisms within multiple genes, polymerase chain reaction, and whole-genome sequencing are currently the most widely used methods to detect and characterize meningococcal strains.5,6 Meningococci infect only humans and are transmitted from person to person by aerosolized or direct contact with respiratory secretions or saliva. Acquisition of meningococci can lead to transient carriage, persistent colonization, or result in invasive disease. Most carriers will remain asymptomatic with the microorganism in their nasopharynx throughout their lifetime; invasive MD is a rare outcome of meningococcal infection. For most individuals, carriage is an immunizing process eliciting protective antibodies.11,12
Asymptomatic nasopharyngeal carriage of N. meningitidis is common, with a population prevalence of approximately 5–10% in non-epidemic settings. Carriage prevalence varies with age, being low during the first years of life, increasing in teenagers and young adults when rates of up to 20–50% are reported, followed by a decline during adulthood.11-13 Major differences in meningococci phenotypic and genotypic distribution between invasive and carriage strains are usually observed, with only a small proportion of carriage strains representing hyper invasive lineages. Carriage rates of meningococci can be considerably higher in outbreak situations, household contacts of people with MD and in closed institutional settings, particularly in military personnel.11,12 Most carriers have relatively few organisms detectable with a minority having much larger numbers at any one time.14
Several host-, organism- and environmental-factors have been associated with an increased risk for MD. Deficiencies in the common complement pathway (e.g., C3, properdin, Factor D, Factor H, or C5–C9), eculizumab therapy, functional or anatomic asplenia (including sickle cell disease), chronic underlying illness, infection with the human immunodeficiency virus,12 preceding viral infections (particularly influenza), household crowding, men who have sex with men, microbiology profession, active and passive smoking, and bar attendance, are all risk factors for meningococcal disease.12,15
Criteria used for case definition of MD vary from one place to another, limiting the reliability of comparisons of incidence rates among different regions. The European Centre for Disease Prevention and Control (ECDC) surveillance network considers a case of invasive meningococcal disease confirmed when at least one of the following criteria is met: isolation of/or detection of N meningitidis nucleic acid in a normally sterile site; N meningitidis detection in cerebrospinal fluid (CSF) by antigen detection test; or visualization of a Gram negative diplococci in CSF.16 In the United States of America (USA), as well as in South Africa, a case is confirmed if the bacteria is isolated from a specimen obtained from a normally sterile site.17,18 In Australia and Canada the criteria includes nucleic acid amplification from a usually sterile site.19,20 In Latin America, despite the lack of uniform criteria across countries, the Pan American Health Organization includes confirmed cases (either detection of bacterial antigen(s) in CSF or positive culture laboratory proven), probable (suspected case plus turbid CSF or link to a confirmed case), and suspected cases (sudden onset of fever plus meningeal sign or petechial or purpuric rash).21
MD occurs worldwide, but there are marked geographical differences in incidence and serogroup distribution.22 In North America, serogroups B (MenB), C (MenC) and more recently Y (MenY) have been the main serogroups causing MD, whereas in Africa, serogroup A was the main cause of epidemics until 15 years ago when serogroups C, W and X emerged.22,23 In Europe, serogroups B and Y, and more recently W (MenW) in some areas have predominated, although serogroup C remains prevalent in some countries lacking meningococcal C conjugate (MCC) vaccination programs.22,24 In Latin America MenB, MenC and, during the past decade, MenW are currently responsible for the majority of reported MD cases.21 Serogroups A, B, C, Y, and W have all been present, without apparent particular predominance in Asia. In Australia and New Zealand MenB has predominated during the last decades. However, in 2016, MenW became the predominant meningococcal serogroup in Australia.25-27
Despite the availability of safe and effective meningococcal conjugate vaccines against serogroups A, C, W and Y for several years, only recently two serogroup B recombinant protein meningococcal vaccines were licensed and recommended for prevention of serogroup B meningococcal disease (B-MD) across different age groups in several countries. The aim of this article is to describe the global burden of B-MD, briefly review the data on vaccines development, and “real world” experience with these vaccines, including the first estimates of effectiveness, safety and impact data based on the as to yet rather limited use of these vaccines in routine immunization programs and for outbreak control.
Serogroup B meningococcal disease, an ever changing, unpredictable epidemiology
Incidence rates (IR) of B-MD have declined during the past years, in the absence of any vaccine intervention. A recently published systematic review reports an average rate ranging from 0.01 to 4.26 per 100,000 population, with a decreasing overall trend, particularly in countries where data collection is more consistently collected (Fig. 1).28 From 2000 to 2015, only two countries, New Zealand and Ireland, reported mean annual IR of B-MD disease above 2/100,000 habitants per year. Australia, Iceland, Netherlands and UK report IR from 1–2/100,000, while the remaining countries report rates < 1/100,000. Case -fatality ratios ranged from 3% to 10% in most countries. Three major hyper-invasive genotypes (clonal complex (cc) 32, cc41/44, and cc269) were responsible for most endemic B-MD cases globally.28 In 2014, 2,760 confirmed cases of invasive MD were reported in Europe, with highest IR in infants (10.1 cases per 100,000). Serogroup B was responsible for most reported cases (64%), while MenC was more prominent in countries that had not implemented MCC vaccination.22,24 In the USA, 375 cases of MD (IR 0.12/100,000) and 60 deaths (CFR 16%) were reported in 2015, with serogroup B responsible for approximately 60% of the cases among children younger than 5 years of age.29 In Canada, serogroup B remains as the predominant serogroup, particularly at younger ages. However, increased incidence of serogroup W has been reported recently.30 In Latin America, incidence rates of MD have varied widely during past years, from < 0.1 cases per 100,000 in Mexico, Peru, Paraguay and Bolivia, to 2/100,000 in Brazil, with the highest incidence rates observed in infants. Misnotification and poor surveillance in some countries of the region, especially in those with low IR, are issues that introduce some bias in the analysis of these data. Regarding serogroup distribution, serogroups B and C are responsible for the majority of cases reported in the region, with an increased number of serogroup W cases associated with the cc ST-11, reported in Argentina and Chile. The highest incidence rates of B-MD disease in the region are reported in Argentina, Brazil, Chile, Colombia and Uruguay.31 In New Zealand, during the 2000s a serogroup B epidemic occurred with incidence rates reaching 17.4 per 100,000 total inhabitants in 2003. A tailor-made, strain-specific serogroup B vaccine using outer membrane vesicles (OMV) from cc ST-41/44 (MeNZB®, Norwegian Institute of Public Health and Novartis Vaccines) was introduced in 2004. A significant decrease in incidence rates of the B-MD epidemic strain during the first years after program implementation was observed, leading to discontinuation of vaccination.32
Figure 1.
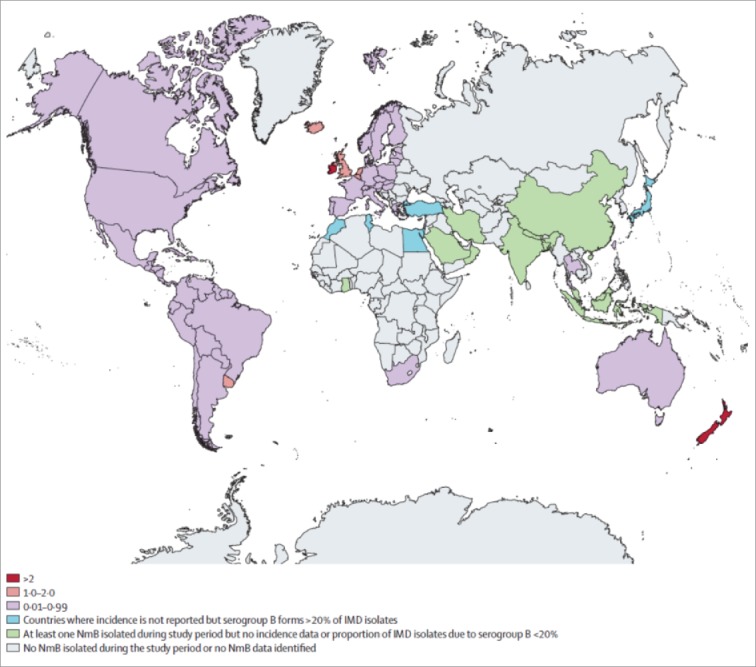
Annual incidence per 100,000 people of serogroup B invasive meningococcal disease worldwide from Jan 1, 2000, to March 1, 2015 (28). Footnote: authorization obtained by Lancet Infectious Diseases, license number 4253831337542.
In Asia, although the true burden of MD is unknown, reported IR are low in all countries although B-MD has been reported causing sporadic cases in Bangladesh, China, India, Indonesia, Japan, Malaysia, the Philippines, Singapore, Taiwan, and Thailand.28 However, the degree of underreporting has not been fully evaluated in this and to some extent also in other regions. Few studies and low numbers of publications, poorly implemented surveillance programs, lack of guidelines and standard case definitions, and inappropriate laboratory methods are significant issues in Asia, with some notable exceptions.22,33,34
In Africa, endemic B-MD disease has been reported only in Ghana and South Africa, with almost no B-MD cases reported from the remaining countries. In the 26 countries of the sub-Saharan meningitis belt the incidence of serogroup A decreased dramatically after MenAfriVac® introduction; serogroup W and more recently C have become predominant.35
Outbreaks of B-MD disease
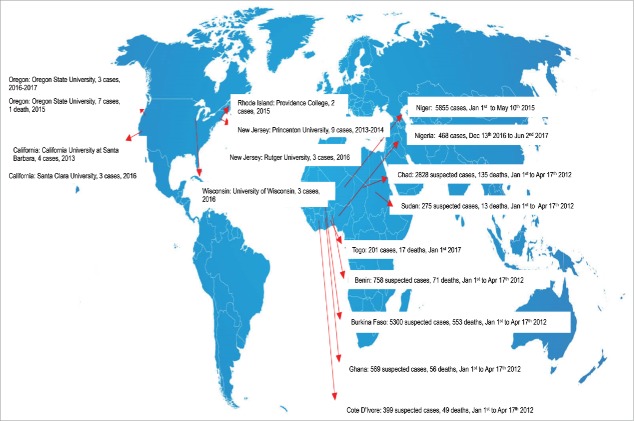
A small fraction of reported MD cases occur within the context of meningococcal outbreaks. They are unpredictable and associated with severe outcomes, which can be emotionally devastating within affected communities or institutions. According to the USA Centers for Disease Control and Prevention (CDC), an outbreak is defined by the occurrence of at least three confirmed or probable primary cases of MD caused by the same serogroup in ≤3 months, with a resulting primary attack rate of ≥10 cases per 100,000 population.36 This definition is used primarily to guide vaccination and antibiotic intervention recommendations. However, in organization-based outbreaks, such as those that occur in universities, schools, daycare centers, occupational training centers, or correctional facilities, with three or even just two cases of disease, rates may reach >10 cases/100,000 population. In such situations, public health officials may also consider vaccination after only two primary cases are identified. In Fig. 2, we depict outbreaks reported to the World Health Organization (WHO) from 2012 to 2017; most were caused by serogroup B, affecting high schools, colleges and universities.
Figure 2.
Meningococcal Outbreaks reported to the WHO from 2012 to 2017.
Group B meningococcal vaccines
The first attempts to prevent MD by vaccination occurred at the beginning of the twentieth century with whole cell formulations, which were used until sulfa chemoprophylaxis became available.37 Development of polysaccharide vaccines began in the 1960s with the hallmark finding that susceptibility to invasive disease was associated with low levels of serum bactericidal antibodies (SBA) to meningococci.38 Due to the relatively low incidence of endemic meningococcal disease, vaccine efficacy studies would require hundreds of thousands of subjects. Accordingly, the WHO has accepted an SBA titer using exogenous human complement (hSBA) ≥ 1:4 as correlate of protection.39,40 Meningococcal A, C, W and Y conjugated polysaccharide vaccines have been developed and licensed, in mono, bivalent and multivalent formulations since the year 2000.40 The capsular polysaccharide of serogroup B strains is poorly immunogenic due to its antigenic structure, which resembles glycosylated neural cell adhesion molecule which is expressed in the developing human brain; as well as immunological tolerance, this antigenic mimicry with human tissue raises the potential for the induction of autoimmunity.34 For this reason, serogroup B vaccine antigen selection strategy moved towards OMPs, mainly PorA and PorB, which can elicit strain-specific protective antibodies measurable in human sera. These OMPs were first obtained from OMVs generated in laboratory conditions and shed during the growth process of N. meningitidis. They mimic the structure of the outer membrane of the specific meningococcal B strain, are soluble and induce a robust immune response by presenting proteins in their native structural conformation.34,40-43 OMV vaccines were successfully used in specific clonal outbreaks in Cuba, Norway and New Zealand.34,39,40,43,44 However, monovalent OMV vaccines elicit highly specific immune responses to the PorA subtype, and these subtypes are highly variable among different isolates of N. meningitidis circulating worldwide.
Studies showed disparity in population immunogenicity, effectiveness and persistence between OMVs, mainly in infants and toddlers.39,40,44 Multivalent PorA candidates were evaluated, but SBA responses and direct bactericidal activity against strains varied depending on the PorA type.40 Thus a universal OMV vaccine was not deemed feasible.34,39,40 Using genomic based approaches, specific OMPs have been synthesized and used with the aim of broadening strain coverage as they should be functionally relevant, immunogenic, and more conserved among geographically diverse strains, compared to OMVs.34 For MenB, the antigenic variability and level of surface expression of OMPs presents a challenge for determining vaccine coverage against the myriad of circulating strains.34,45,46 These novel B-MD vaccines, denominated 4CMenB (Bexsero®, GlaxoSmithKline (GSK)) and bivalent rLP2086 (Trumenba®, Pfizer) are now licensed in the USA, Canada and Europe as well as in other countries (Figure 3).
Figure 3.
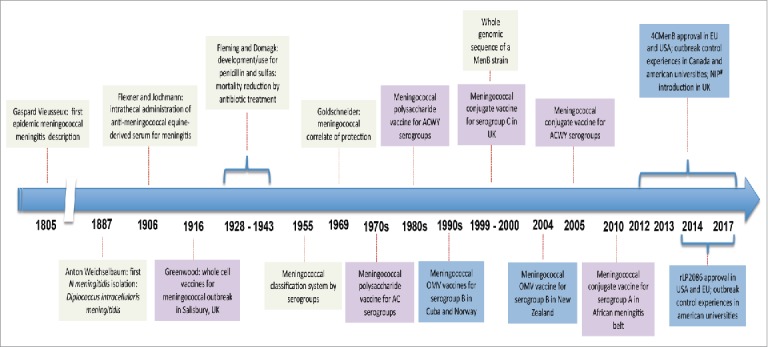
Timeline of some relevant moments in meningococcal disease control and prevention.
The multicomponent vaccine 4CMenB has three primary recombinant antigens obtained using an approach denominated “reverse vaccinology”, based on DNA sequence data from serogroup B strains which identified surface-exposed proteins, with capacity to induce bactericidal antibodies in animal models42,47: factor H binding protein (fHbp), subfamily B/v1, neisserial adhesin A (NadA), and Neisseria heparin binding antigen (NHBA). In addition it includes the OMV expressing PorA from the New Zealand strain, NZ PorA P1.4. Native OMVs have a potential advantage, as detergent extraction solubilizes phospholipids and membrane-associated lipoproteins that enhance the immune response.48 The selection of the OMV-NZ strain was based on the experience from the OMV-based vaccine MeNZB®, which proved to be safe and efficacious in the control of the clonal MenB epidemic in New Zealand,42 in combination with immunogenicity results from a study performed in healthy adults.49 The latter provided evidence that the addition of OMV-NZ to the other three antigens (fHbp, NadA and NHBA) enhanced the coverage against ST-41/44 complex/lineage III strains. Pivotal phase II/III studies are summarized in Table 1. The vaccine has been demonstrated to provide a robust priming and anamnestic immune response in all age groups against four laboratory serogroup B reference strains chosen to measure responses against each antigen included in the vaccine, even using a reduced vaccine schedule in young infants,50 and allows concomitant administration with other routine vaccines. Concomitant administration of MCC-CRM vaccine with 4CMenB was performed in Brazilian infants at 3 and 5 months with a booster at 12 months of age. Although the geometric mean titers against meningococcal serogroup C were lower among subjects that received 4CMenB, the proportion that achieved seroconversion was identical, and considered sufficient to MenB, after primary and booster vaccination. Reactogenicity was higher for concomitant vaccines administration, but no safety concerns were identified.51 The safety profile of 4CMenB has been considered acceptable, although it is reactogenic. Injection site pain/tenderness and fever in infants, and injection site pain, malaise, and headache in adolescents are relatively common.52-54 Among infants, side effects can become a cause for emergency room visits, hospitalizations, and increase antimicrobial misuse.55,56 The European Medicines Agency (EMA) approved 4CMenB in 2013 for infants from 2 months of age, as a three-dose primary schedule followed by a booster in the second year of life, and a two-dose schedule in children, adolescents and adults.57 The USA Food and Drug Administration (FDA) approved the vaccine in 2015 as a two-dose schedule for use in individuals aged 10 through 25 years of age.58 This vaccine was introduced into the United Kingdom (UK) universal immunization schedule at 2, 4 and 12 months of age in 201559,60. Other countries with approval include Australia, Canada, Chile, Brazil, and Argentina.
Table 1.
Immunogenicity and safety data from pivotal phase II/III studies for novel recombinant B-MD vaccines.
| Author, vaccine, study design | Immunogenicity | Safety | Concomitant vaccines |
|---|---|---|---|
| Gossger N, et al. 4CMenB53 | After priming ≥ 99% reached protective titers (hSBA ≥ 5) against Nad A, fHbp; and 79 – 86,1% for PorA tests strains. | Fever was more frequent after 4CMenBdoses (26%-41%) compared with routine vaccines (23%-36%); severe erythema, swelling, or induration were seen in ≤ 1%; severe pain was registered in 16% in the accelerated groups. | PCV7 and DTaP-HBV-IPV/Hib |
| Phase IIb, multicenter, open-label, parallel group, randomized controlled trial; infants 2 months of age including two different 3 +1 schedules (sole or co-administered with routine vaccines); N = 1,885 | Immune responses were non-inferior by concomitant vaccination; and its immunogenicity was non-inferior to routine vaccines alone for all antigens. | No evidence of immune interference, except for pertactin and pneumococcal serotype 6B, of unlikely clinical significance. | |
| Sole administration elicited higher hSBA GMTs for all strains compared to coadministration with routine vaccines; 2,4, and 6-month schedule, rather than an accelerated 2-, 3-, and 4-month schedule, resulted in higher hSBA GMTs for the Nad A test strain. | Increase in reactogenicity in the coadministration group, mainly fever (51%-61%) and severe pain at the injection site (12%). | ||
| Vesikari T, et al, 4CMenB52 | After priming 100% reached hSBA ≥ 5 against fHbp and Nad A, and 84% for New Zealand OMV and NHBA tests strains. | Fever, injection-site reactions and tenderness were the most frequent adverse events reported | PCV7 and DTaP-HBV-IPV/Hib; MMRV |
| Phase III, multicenter, randomized, open label for immunogenicity and observer blind for safety; infants 2 – 12 months of age; 3 +1 schedule | After booster dose: 95−100% of children had hSBA ≥ 5 for all antigens. | Fever: usually on day 1, returning to normal by day 3. | No evidence of immune interference, except for poliovirus type 2 responses, with unknown clinical significance. |
| N = 3,360 | Injection-site reactions: peaked on day 1, with a steep decrease on day 2 and lower incidence after booster. | Increase in reactogenicity in the coadministration group; mainly fever (77% vs. 45%). | |
| Martinón-Torres F, et al, 4CMenB50 | After priming for groups 1 to 3, and after the catch up series for group 4 100% and 99% reached hSBA ≥ 4 against fHbp and Nad A; | In infants local tenderness, erythema and pain were the most commonly reported adverse effects | None |
| Phase IIIb, multicenter, randomized, open-label; healthy subjects from 2 ½ months to 10 years old, divided in four groups: | After primary/catch-up series hSBA ≥ 4 against Por A was reached in groups 1,3 and 4 in 99% and in 98% for group 2. | Rates of systemic adverse reactions in infants were similar across the 3 groups and highest after the first vaccination. Across all groups, no increased reactogenicity was observed following subsequent vaccinations | |
| Group 1: doses at 2½, 3½, and 5 + 11 months of age; | NHBA 1 month after primary/catch-up series reached 59% for group 1, 49% for group 2, 77% for group 3, and 95% for group 4 | ||
| Group 2: doses at 3½ and 5 + 11 months of age; | After booster response (measured in groups 1,2 and 3) 100% reached hSBA titers ≥ 4 for fHbp and Nad A, ≥99% for Por A | ||
| Group 3: dose at 6 and 8 + 11 months of age. | NHBA reached hSBA titers > 4 in 84%, 88% and 87% in groups 1, 2 and 3 respectively. | ||
| Group 4: 2-dose-catch-up series, administered 2 months apart. | |||
| N = 754 infants and 404 children | |||
| Santolaya ME, et al., 4CMenB54 | After 1 dose: 92–97% had hSBA titers ≥ 4 against test strains | Local and systemic reaction rates were similar after each injection and did not increase with subsequent doses, but remained higher than placebo. | None |
| Phase IIb/III, multicenter, randomized, observer-blind, placebo-controlled study; adolescents from 11 to 17 years old; 1, 2 or 3 dosing schedule with 1,2 and/or 6 month intervals; N = 1,631 | After 2 or 3 doses: 99–100% had hSBA titers ≥ 4 against test strains; reaching seroresponse rates of 99–100% for each strain at 6 months. | Pain was reported in 86% of which 17% was severe, most resolving at day 3; malaise: 51%; headache: 42% and fever: 4%; all the above were statistically significant compared to placebo. | |
| A third dose had a small incremental effect on geometric mean titers, especially when given at 6 months, but did not increase the proportion of participants achieving protective titers | No vaccine-related SAEs were reported and no significant safety signals were identified. | ||
| Vesikari T, el al. rLP208663 | Participants whom elicited hSBA titers ≥1: 8 for serogroup B test strains expressing vaccine-heterologous fHbp variants A22, A56, B24, and B44: After 2 doses: 90–93%, 98–100%, 69–81%, and 70–77%. | Pain at the injection site, redness and swelling were the most common reactions and were mild to moderate. Severe pain was reported by 9.9% of subjects | None |
| Phase II, randomized, multicenter, single-blind study; adolescents from 11 – <19 years old; 2- or 3-dose schedules (0,1,6-month; 0,2,6-month; 0,2-month; 0,4-month; 0,6-month); N = 1,713 | After 3 doses: 92–95%, 98–99%, 88–89%, and 86–88%. | Headache and fatigue were the most common systemic symptoms and were mild or moderate in severity. Fever was infrequent (1.7%–4.3%). | |
| GMTs were similar between the 2- and 3-dose regimens. | There were no differences in the incidence of SAEs between bivalent rLP2086 and saline recipients. | ||
| Muse D, et al.; rLP208664 | Immune responses to MCV4 + Tdap + rLP2086 were non-inferior to MCV4 + Tdap or rLP2086 alone. Seroprotective hSBA (>1:4) were documented for 62.3%–68.0% and 87.5%–90% of MCV4 + Tdap + bivalent rLP2086 recipients after doses 2 and 3, respectively. A ≥4-fold rise in hSBA titers from baseline was achieved by 56.3% – 64.3% and 84.0%–85.7% of subjects after doses 2 and 3, respectively. Bivalent rLP2086 alone induced similar responses. | Concomitant administration did not substantially increase reactogenicity compared with rLP2086 alone. | MCV4 and Tdap |
| Phase II, multicenter, randomized, active-controlled, observer-blinded study; adolescents from 10 to <13 years old; concomitant or standalone schedule: 0,2 and 6 months; N = 2,648 | Bivalent rLP2086 concomitantly with MCV4 + Tdap met all noninferiority immunogenicity criteria without an increase in reactogenicity. | ||
| Ostergaard L, et al.; rLP208667 | Immunogenicity data were not collected | SAEs: 1.6% of rLP2086 recipients | None |
| Phase III, multicenter, randomized, active-controlled, observer-blind study; adolescents and young adults ≥ 10 – <26 years old; 3 doses schedule (0, 2, and 6 months); N = 5,712 | Medically attended AEs were similar between rLP2086 and hepatitis A vaccine/placebo group (5,5% – 7%) and decreased after each consecutive dosing | ||
| Subjects reporting ≥ 1 AE were greater in the rLP2086 vaccine group. | |||
| Ostergaard L, et al.; rLP208668 | After 2 doses hSBA titer ≥ 4 against each primary strain ranged from 56.0 to 85.3% in subjects 10 – 18 years old and from 78.8 to 90.2% after dose 3 | Pain was the most common reaction in the two trial groups, mainly after dose 1, and ≤1.1% reported increased severity of reaction with subsequent doses | None |
| Phase III, multicenter, randomized, controlled, observer-blinded, adolescents from 10 – 18 years old and from 18 to 25 years old; 3 doses (0,2 and 6 months) | Young adults with hSBA titers ≥ 4 ranged from 54.6 to 85.6% and 78.9 to 89.7%, after doses 2 and 3, respectively | Headache and fatigue were the most common systemic events among both adolescents and young adults | |
| N = 3596 adolescents (10 to 18 years of age); 3304 young adults (18 to 25 years of age) | Composite responses after doses 2 and 3 in adolescents were 53.7% and 82.7%, respectively, and those in young adults were 63.3% and 84.5%, respectively |
4CB-MD: Bexsero®; fHbp: factor H binding protein; Nad A: neisserial adhesin A; neisseria heparin binding antigen: NHBA; OMV: outer-membrane vesicle; hSBA: human complement serum bactericidal activity; GMTs: geometric mean titres; SAE: serious adverse events; AE: adverse event; SBA: serum bactericidal assays; hSBA: serum bactericidal assays using human complement; PCV7: heptavalent pneumococcal conjugate vaccine; DTaP-HBV-IPV/Hib: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b, inactivated poliomyelitis, hepatitis B vaccine; MMRV: measles, mumps, rubella, varicella; MCV4: meningococcal ACWY conjugate vaccine; Tdap: tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine adsorbed.
The bivalent rLP2086 includes two variants of the surface-exposed fHbp protein. This protein segregates into two genetically and immunologically distinct subfamilies, A (A05) and B (B01), which have been found to be expressed in nearly all strains isolated from invasive disease caused by serogroup B in reference laboratories in Europe and USA.41 Clinical studies included more than 15,000 subjects aged 10 through 25 years of age in 11 clinical studies conducted in the US, Europe, Canada, Chile, and Australia. These studies demonstrated that the bivalent rLP2086 elicits SBA capable of killing serogroup B strains expressing fHbps that are homologous and heterologous to vaccine components. In phase II trials, two doses of rLP2086 provided robust immunogenicity in healthy adolescents which increased after a third dose61-64 (Table 1). The vaccine can be administered to adolescents concomitantly with other licensed vaccines, including ACWY meningococcal (MenACWY) conjugate vaccine, quadrivalent human papillomavirus vaccine (HPV), reduced diphtheria toxoid, tetanus toxoid, acellular pertussis and inactivated polio virus vaccine (TdaP-IPV) and tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine adsorbed (Tdap).64-66 The vaccine has been reported to be well tolerated in clinical trials, although mild to moderate adverse reactions such as pain, redness and swelling at the site of injection are common, followed by headache, fatigue and fever as systemic reactions.62,64,67,68 The vaccine was approved by the FDA in 2014 for use in adolescents and young adults aged 10–25 years in a three-dose schedule, 0, 1–2 and 6 months or a two-dose schedule, 0 and 6 months, depending on the risk of exposure and the patient's susceptibility to meningococcal serogroup B disease.65 EMA approved the vaccine in 2017 for subjects aged 10 years and older, in a two-dose schedule administered at a 0 and 6 months interval, or in a three-dose schedule, 0, 1-2 and 6 months. At the time of writing, the vaccine had not been submitted for use in children below 10 years of age. A booster dose should be considered following either dosing regimen for individuals at continuing risk of invasive MD.66
4CMenB in infants is associated with higher rates of local and systemic reactions when given with other routine infant vaccinations, with a significantly increased the risk of serious adverse events (SAEs) compared with control vaccines (odds ratio 4.36 [95% CI 1.05–18.1]; p = 0.043), but the incidence of potentially vaccine-related acute SAEs in individuals receiving 4CMenB was low (5.4 per 1,000 individuals).69 Fever, local tenderness, erythema and pain have been the most commonly reported adverse effects across the studies. Fever was seen up to 41%, usually on day 1, returning to normal by day 3. Prophylactic administration of paracetamol before and 4–6 hours after vaccination significantly reduces post vaccination fever without affecting immunological responses.70 Severe erythema, swelling, or induration were seen in < 1%, with a peak on day 1, with a steep decrease on day 2 and lower incidence after booster (Table 1). The most commonly reported SAE were febrile convulsions, Kawasaki disease and arthritis. Seven cases of febrile seizures occurred after vaccination with 4CMenB, with a rate of 0.1 events/1,000 vaccination visits in the 4CMenB study arms, mainly during primary infant series, on the day of or day following vaccination.69,71 Arthritis was reported in 4 subjects, three of them after 4CMenB vaccination.69 Finally, Kawasaki disease was reported in 7 subjects (1 in the control group and 6 in the 4CMenB groups) in the pre-licensure studies. The onset varied from 1 day to 5.5 months after vaccination and all cases were adjudicated by a panel of outside experts and estimated annual incidence post-vaccination was 72 (95% CI 23–169) per 100,000 subject-years after 4CMenB versus 56 (95% CI 1–311) after control vaccines, which were similar to rates of other rare adverse events observed in other pre-licensure clinical programs,46,57,58,69,71 however, post-licensure safety surveillance will be of paramount importance. In phase 1/2 study in infants, rLP2086 was considered not acceptable due to the high fever rate experienced (64-90%) in the 20 and 60 μg groups so formulation were discontinued.72
In adolescents headache and fatigue were the most common systemic events for both vaccines. Fever was as infrequent as 4%. Local and systemic reaction rates were similar after each injection and did not increase with subsequent doses, but remained higher than placebo. Pain was most frequently reported in pre-licensure studies with 4CMenB than rLP2086 recipients (86% versus 9,9%), however, an observational study of adverse events during a college outbreak after a campaign vaccination with rLP2086 published recently, reported that the most commonly event following vaccination was injection site pain, reaching 77,6% after the first dose, but only 4% was considered severe.54,57,58,62,65-68,73,74
Persistence of bactericidal antibodies among infants vaccinated with 4CMenB wanes at 12 months of age although remaining higher than baseline; the magnitude of waning varied for each antigen being less for NadA and fHbp.75,76 Actually, at 12 months of age, after three priming doses and before the booster dose, the proportions with hSBA titers ≥ 1:5 for fHbp was 57%-85%, ≥ 96% for NadA and between 18–35% for PorA. Following a 12-month booster-dose ≥ 95% of previously immunized participants had titers ≥1:5 for all strains, independent of the priming schedule interval.75 Similar results were observed in a study that assessed the persistence of immune responses after one year in participants vaccinated as infants, and responses in vaccine-naïve children. Antibodies waned over 12 months, particularly against strain NZ98/254 (PorA), although higher GMTs were observed compared to the unvaccinated control group.76 Even in children vaccinated with 5 doses, at 2, 4, 6, 12 and 40 months of age, waning of immunity was observed by 5 years of age, with protective antibodies ranging from 44% to 88% against matched strains and from 13% to 81% against mismatched strains.77 In children vaccinated between 12 and 24 months of age waning of hSBA titers was also observed by 4 years of age, with a robust response after a booster dose at 40 months of age.78 For adolescents, protective hSBA titers 18–24 months after completing a two-dose vaccine schedule with the 4CMenB vaccine were detected in 64% for all vaccine-related antigens and in 85% for two of the three target antigens; a third dose did not provide additional benefit.79
Antibody persistence after the bivalent rLP2086 vaccine was assessed in an open-label, follow-up study of subjects previously enrolled in a primary study. The decline in antibody levels among individuals 11 to 18 years of age, 4 years after a primary series on a 2 or 3-dose schedules, follows a similar pattern. A decrease in response was evident for all test strains from month 6 to month 12, followed by a plateau thereafter up to month 48. Subjects achieving protective hSBA titers for four fHbp variants strains combined ranged from 15.7% to 18.2%. Taking into account the importance of circulating serum antibodies to maintain protection against invasive meningococcal disease, the persistence data suggest that booster doses would be required to maintain long-term protection. All subjects showed a robust immune response one month after a booster dose for different fHbp variants66,74,80
The capacity of these protein based vaccines to prevent acquisition of serogroup B carriage, or otherwise to interrupt transmission and thus provide herd protection, once targeting the age groups responsible for carriage, is currently unclear. In UK university students no significant difference in serogroup B carriage prevalence was detected between the 4CMenB vaccinated and non-vaccinated control groups one month after the second dose, however three to twelve months after vaccination, meningococcal carriage prevalence was reduced for all N meningitidis and capsular groups BCWY in vaccinated individuals, although to a lower extent compared to carriage reduction conferred by ACWY conjugated vaccines.81 Another meningococcal carriage study was performed in 3,082 students at the Oregon University following a meningococcal B vaccination campaign with both novel B-MD vaccines, in response to an outbreak in 2015. After 4 carriage surveys over a period of 11 months, no impact on meningococcal carriage was shown, suggesting that novel B-MD vaccines may not provide herd protection in the context of an outbreak response.82 Similar experience was seen in Rhode Island after a mass vaccination campaign in a college with rLP2086, reinforcing the need for high vaccination coverage to protect vaccinated individuals and chemoprophylaxis for close contacts during outbreaks.83 A recent study performed in Spain found that the potential impact of the 4CMenB vaccine on Spanish asymptomatic carrier strains appears to be due to the NHBA antigen.84
Effectiveness of the protein-based vaccines will depend on strain coverage, which can be estimated from hSBA responses performed with a panel of serogroup B strains representative of antigenically and epidemiologically diverse invasive disease isolates. However, the logistical limitations associated with the use of hSBA led to the development of alternative nonfunctional assays to infer vaccine breadth of coverage, such as the meningococcal antigen typing system (MATS) and/or the meningococcal antigen surface expression (MEASURE) flow cytometry-monoclonal antibody based method. 4CMenB is conservatively estimated to provide 66–91% coverage against serogroup B strains worldwide46,85 based on analysis of pooled sera from vaccinated infants that meet a minimum threshold of reactivity in the MATS ELISA and/or contain the PorA 1.4 antigen. For the bivalent rLP2086, MEASURE uses immune sera specific to a surface-expressed epitope common to variants within both fHbp subfamilies included in the bivalent vaccine50; at the time of writing, testing of strains had not yet been published. For rLP2086, pairwise identity analysis between a test strain and fHbp sequences from the same subgroup indicate high sequence conservation, estimating a coverage from 84.8% to 88.5%.86 In the future, these types of analysis may provide temporal and regional data for serogroup B vaccine policy related decisions. The MATS could potentially underestimate coverage, as it was shown in a study when MenB isolates from England and Wales were assessed, reaching a 73% to 88% coverage.45-48 Another study performed in the UK following 4CMenB introduction in infants showed >80% vaccine-mediated protection against all current MenB strains circulating in the country.87,88 This could be explained by the existence of cross-protective epitope on fHbp variant 1.1 that elicit bactericidal neutralizing antibodies to antigen-binding fragment 1A12 which is cross-reactive and targets an epitope highly conserved across the repertoire of three naturally occurring fHbp variants89
These vaccines may prove to be effective against non-serogroup B strains due to cross-protection provided by the highly conserved antigenic proteins included in the vaccines,85,90 as has been reported in the UK in relation with the hyper virulent Neisseria meningitidis serogroup W strain circulating, especially in infants. The technique to predict non-MenB vaccine strain coverage using MATS positive bactericidal threshold has not yet been validated. Therefore to evaluate the impact that 4CMenB vaccination may have on non-serogroup B disease SBA activity of sera from vaccinated subjects to kill meningococcal strains belonging to serogroups C, Y, and W isolates have been used, provided by the reference laboratories in UK, Germany, France and Brazil. An overall proportion of serogroups C, Y and W strains killed at hSBA titers ≥8 ranged from near 45% to 90%.91 Another study, performed by Gorla et al, with strains representing the total MD cases occurring during 2012 in Brazil showed differences between adolescents and infants coverage with a 100% coverage for MenW and MenY, but no coverage for MenC strains tested with pooled infant immune sera and 100% for MenC, 86% for MenW, and 67% for MenY using adolescent immune sera.92 Regarding serogroup X, the same analysis was performed for strains from Niger, Chad, Burkina Faso and France, suggesting coverage for african isolates but not for X isolates from France which expressed unrelated fHbp sub variants belonging to variants 2 and 3.93 The universal presence of full-length NadA genes within currently circulating MenW cc 11 clones, English/Welsh strain, indicates that 4CMenB may afford protection. Tested invasive MenW:cc11 isolates from patients 4 months to 91 years old in England and Wales during 2011–2012 with pooled sera from vaccinated children showed that hSBA titers were high (>1:32) against all MenW isolates.8,90 In addition, 4CMenB variants for NadA and NHBA, unless different from these alleles peptides in MenW English/Welsh strain, can induce cross-protection, and collaborate in the complement-mediated bactericidal killing.90
Considering the commonly accepted threshold of €50,000 per QALY, novel MenB vaccines are not expected to be cost-effective in a National Immunization Program (NIP) unless a considerable increase in MenB incidence occurs or new information that clarify its role in herd protection or persistence of immune protection becomes available. Infant vaccination could further reduce the burden of disease and prevent more deaths as compared to adolescent schedules, but at a substantially higher cost; so cost- effectiveness and feasibility of introducing a novel MenB vaccine into a NIP needs to be based on country-specific assessments.94-100 This issue requires further analysis and studies, which should be obtained and complemented from the UK experience.
Regarding vaccines comparison, 4CMenB and rLP2086 are both proteins based vaccines, developed using different strategies, aiming to provide broad strain coverage. 4CMenB includes three antigens (including only one subtype of fHbp) in addition to OMV while rLP2086 includes the two main subtypes of fHbp. Comparisons of potential strengths and weaknesses have to be made with caution as different techniques have been used to evaluate these vaccines. Face to face efficacy studies are not available and thus vaccines cannot be fairly compared. Immunogenicity studies use different antigens and criteria and thus for are not comparable. For rLP2086 vaccines protective antibody titers surpass 73% and 80% after two or three doses respectively in adolescents74 and for 4CMenB, protective levels ranged from 99% to 100% for PorA, fHbp, and NadA antigens.54 Only the 4CMenB vaccine was licensed in infants with composite antibody levels reaching 85–95% after the primary series with three doses.57 Studies of antibody persistence revealed a bactericidal activity lasting 18–24 months in over 64% of adolescents for all three tested 4CMenB vaccine-related antigens79 and a sharp decline for antigens expressing fHbp subfamilies A and B ranging from near 25% to 60% in the percentage of subjects with protective antibodies since 12 to 48 months after priming with rLP208680; indicating that immunity wanes for both vaccines. In children, a recently published meta-analysis assessed 4CMenB persistence of immunogenicity against the four reference strains finding that it remained high 6 months after the booster dose just for NadA and NHBA reference strains and then decreased till values obtained before booster dose69,75,78
The potential for cross-protection against non-B meningococcal strains has been described for 4CMenB, specifically for C, W, Y and X strains, but not yet for rLP2086. For both vaccines, the impact of vaccination on nasopharyngeal carriage is uncertain. 4CMenB has demonstrated a reduction only in one study performed in UK from 3 months after dose two, of 18.2% on any meningococcal strain and 26.6% for capsular groups BCWY, with a reduction in prevalence of carriage observed over 1 year of follow-up.81 For rLP2086 no impact on carriage has been demonstrated, regardless the number of doses administrated during outbreaks in American universities and colleges.82,83 Both vaccines can be coadministered with other childhood or adolescent vaccinations. 4CMenB can be coadministered with monovalent or combination vaccines including: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b, inactivated poliomyelitis, hepatitis B, heptavalent pneumococcal conjugate, measles, mumps, rubella, varicella, and MCC-CRM vaccine in infants and children; coadministration studies are not available for adolescents.57,58 For rLP2086 the following vaccines can be given concomitantly in adolescents, TdaP-IPV, quadrivalent HPV, MenACWY conjugate vaccine and tetanus toxoid, Tdap. Bivalent rLP2086 vaccine is not approved for infants.65,66 Both vaccines are relatively reactogenic as has been described above, causing pain at the site of injection in adolescents and infants, and fever in infants; it is unclear if one vaccine is more reactogenic than the other as no head to head studies are available. 4CMenB has been included in five NIPs59,60,101-104 and has been used in regional programs and to control specific outbreaks,105-110 especially in colleges, while rLP2086 has not yet been incorporated into a NIP, but has been used in college outbreaks.110-112 Finally, both vaccines are currently of relatively high cost.97,98,100
For the investigational vaccine against serogroups A, B, C, W and Y (MenABCWY), phase 2 studies performed in adolescents have showed that two or three doses are able to elicit a robust immune response against ACWY serogroups, at least comparable with those after one dose of MenACWY-CRM vaccine, and for serogroup B test strains with an acceptable reactogenicity and safety profile, similar to 4CMenB. In addition, MenABCWY vaccine may be suitable for booster doses after priming with MenABCWY vaccine or 4CMenB vaccine.113-116 Further clinical development is necessary but promising to give us the opportunity to control MD.
Consideration for use of the serogroup B protein based vaccines for N. meningitidis outbreaks
Massive vaccination campaigns can be implemented for outbreak control. Importantly, vaccination does not replace recommended chemoprophylaxis.36,105,117 Before vaccine implementation, appropriate surveillance systems including follow-up of close contacts should be available. Ideally, the infecting strain should be characterized, but, this should not delay the decision regarding vaccine introduction during outbreaks.36 There is no current preference for one of the two available vaccines according to CDC recommendations and vaccine interchangeability cannot currently be endorsed.118
Effectiveness and safety of serogroup B protein-based vaccines from “early adopter” countries/regions
The UK experience
For the last several decades capsular group B endemic meningococcal disease has predominated in the British Isles at incidence rates that are higher than those seen elsewhere in Europe.33 Numerous clonal types of group B causing invasive disease have circulated.119 This is in contrast to some other capsular groups, notably group C in the 1990s and group W in the second decade of the twenty first century, each of which showed rapid rises in incidence associated with spread of single hyper invasive clones.8 While these latter problems were, respectively, solved120 and, at the time of writing, are being tackled by widespread deployment of conjugate vaccines in the adolescent age group,121 who are most commonly carriers and thus onwards transmitters of meningococci in the population, this option does not currently exist for group B.
Accordingly, in the context of recognition that efforts to improve early diagnosis and to improve outcomes through more aggressive or novel adjunctive therapy were unlikely further to reduce case fatality rates or the frequency and severity of long term morbidity and buoyed by strong public and political support for action, the pediatric academic community in Europe engaged actively with the company developing 4CMenB and contributed to advancing the pre-licensure development program.122
Following European licensure, initial evaluation of the cost effectiveness of using the vaccine, based on estimates of likely coverage of UK invasive strains,122 cast doubt as to whether it would meet the stringent criteria set by the UK government for introduction of healthcare interventions within the National Health Service in the country.60 However, after further evidence was gathered, it became clear that the threshold could be met if a slightly modified schedule from the one specified in the product license was used and the vaccine was introduced for infants in September 2015.59 Infants receive 2 doses at 2 and 4 months of age alongside other primary schedule vaccines and receive a third dose at 12–13 months. The uptake reported from August to December of 2017 in UK, reached 95.9% for one dose and 88.4% for two doses by six months of age and 87.4% for the booster dose.123 Advice concerning routine antipyretic-analgesic use was modified to advise that paracetamol should be given at the time of the first two doses.
Early ecological data comparing changes in rates of meningococcal group B disease over time in the immunized cohort with those in older age groups indicate that the vaccine induces protection more effectively than was predicted pre-implementation.87 The rates of uptake of the vaccine have been consistently high but concerns that its tendency to cause fever in many infants when given with other vaccines would lead to increases in rates of hospital attendance have been confirmed, reinforcing the importance of paracetamol prophylaxis.56
Whether deployment of the vaccine in adolescents would reduce carriage and result in herd protection as seen following conjugate vaccine programs remains an unanswered question at the time of writing. If 4CMenB and/or the more recently licensed bivalent fHbp vaccine can do this will determine whether cost-benefit thresholds can be met for their use in this age group. An on-going study in South Australia (Marshall et al., manuscript submitted for publication) and a planned study in the UK may provide the answer.
The Saguenay-Lac-Saint-Jean experience in Canada
The Saguenay-Lac-Saint-Jean (SLSJ) region was affected by a 269 (ST-269) serogroup B clone with an average incidence rate of 3.4 per 100,000 person-years from 2006 to 2013, which surpassed Canadian average rates by more than 10-fold (0.3/100,000). Cases were concentrated among individuals ≤ 20 years of age. To control the spread of this clone, a massive vaccination campaign using 4CMenB according to its locally approved schedule, was undertaken, targeting more than 50,000 people between the ages of 2 months and 20 years residing in, or attending an educational institution in the region.109 The vaccine uptake rate for one dose was 82%, but only 70% for at least two doses, mainly due to low uptake in older adolescents and young adults. The vaccination campaign was estimated to have reduced disease incidence by 77% and new serogroup B cases have not been reported among vaccinees, with two cases observed among non-vaccinated adults. An enhanced surveillance system was also implemented to monitor the onset of adverse events following immunization in real time.109,124 Fever was reported in 12% of vaccinees and was more frequent in young children. Antipyretic prophylaxis (paracetamol mainly) was 50% effective in preventing the occurrence of fever in children less than 5 years of age but not in older age groups. There was no death and no major adverse event with or without sequelae associated with vaccination. During the two-year period following the immunization campaign in SLSJ, no IMD case was recorded among unvaccinated individuals, including infants, thus it seems that at least some herd effect may be occurring.109
Vaccine use for control of university outbreaks in the USA
From 2009 to 2014 five serogroup B outbreaks on college campuses were reported to CDC. In 2013, 4CMenB vaccination campaigns were implemented in response to ongoing serogroup B outbreaks at the Universities of Princeton and Santa Barbara, due to sustained transmission during 2 academic years. The FDA approved the use of 4CMenB before national licensure under an expanded access investigational new drug protocol in December 2013.106,108 The attack rate in Princeton was 134/100,000 among undergraduates living in dorms, with individual cases occurring 2 to 4 weeks apart. The strain isolated expressed two of the 4 antigens (fHbp and NHBA) in sufficient quantities to suggest that 4CMenB might be protective.108 Vaccine was offered to nearly 5,800 individuals including undergraduate students, faculty, staff and graduate students at increased risk of meningococcal disease, and spouses and caregivers of graduate and undergraduate students living in a dormitory with students. Uptake was very high, reaching 95% for the first dose and 89% for the second dose, with approximately 5,200 individuals who received al least one dose.106 The rate of reported SAEs was near 2.0/1,000 vaccinees following the first dose and 0.2/1,000 following the second dose, with no causality related to 4CMenB observed.108 In the University of California at Santa Barbara outbreak, the attack rate was 21.1/100,000; despite the fact that the strain was different from the Princeton strain, protection was expected from 4CMenB based on killing properties of pooled post-immunization sera. The target groups were similar to Princeton with nearly 20,000 subjects eligible and nearly 17,000 vaccinated with no cases occurring in vaccinated students.34,108,117 Parallel to this immunization campaign, an immunogenicity study was performed in a subset of 607 subjects showing that 66.1% had hSBA protective titers against the outbreak strain.125 This result was lower than that predicted by the MATS test, suggesting that hSBA underestimates protective immunity. The bivalent rLP2086 vaccine was administered to 3,525 subjects in Providence College and to nearly 22,0000 students in the University of Oregon during outbreaks occurring in 2015, achieving a 94% first dose uptake among eligible students. College-associated cases were not identified during the 4 months follow up period.82,83,107,126
Despite the success achieved after the widespread vaccination campaigns to control outbreaks of serogroup B-MD, with no further cases reported among vaccinated subjects, the long-term duration and breadth of protection, as well as the impact of these vaccines on prevention of carriage remains to be determined.
Conclusions
Rates of invasive B-MD have been declining worldwide, although in certain geographical areas and populations incidence rates can be particularly high. In addition, outbreaks of B-MD occur in an unpredictable manner in a number of settings where people gather, especially universities. Although several possibilities were raised to explain the declining trend in B-MD incidence observed during the last decades, including declining smoking rates, changes in population immunity, bacterial virulence and a natural cyclical pattern of meningococcal serogroup distribution,25 reasons remain unclear. The high CFR and the significant sequelae in surviving individuals, as well as its potential epidemic nature are the main reasons for primary prevention, for which vaccination is the only effective tool. Age groups at highest risk are young children, especially infants under one year of age, followed by adolescents in areas where close contact is frequent such as university dorms in the USA. Meningococcal vaccines against non-serogroup B strains have been based on protein conjugated capsular polysaccharides targeting specific serogroups (A, C, W and Y) with great success. The rapid and sustained reduction in serogroup C disease rates in the UK and other countries after vaccine implementation into national immunization programs has been a significant achievement. Flexible vaccine schedules focusing not only on direct protection, targeting the age groups with the highest incidence rates of disease (usually infants), but also vaccinating the age groups that act as the reservoir for infection (usually adolescents and young adults) and thus reducing carriage rates and interrupting transmission in the community, have proven effective for serogroups susceptible to be prevented by this vaccine strategy.
The antigenic mimicry between serogroup B polysaccharide and human neural tissue antigens curtailed development of polysaccharide-based vaccines against this pathogen, and left it unprevented for decades. The solution was found in targeting outer membrane proteins, first in the form of OMVs, which are a laboratory-obtained simile of the outer membrane (of which porins are the main antigenic target). This vaccine proved to be immunogenic, protective, and effective in controlling regional outbreaks, with the caveat that the immune response, based on the induction of SBA, was highly strain-specific for the porin within the OMV. The significant variability of porins among strains circulating worldwide precluded this strategy for universal vaccination. But the proof of concept that protein antigen vaccines can be highly protective had been established. Second generation protein-based vaccine candidates aimed to identify antigens expressed in as many serogroup B strains as possible in order to provide “broad strain” protection. These candidates are now licensed vaccines although in a stage of post-licensing “field evaluation” as they were approved based on safety and immunogenicity studies and their use is currently limited to only one country in a routine national program, to some regions, and a few outbreak situations. The fact that in all the experiences to date results have been encouraging is promising. The 4 component vaccine targeting four different proteins from GSK (developed by Novartis vaccines) and the two component vaccine targeting the two subfamilies of one relevant protein, from Pfizer, elicit serum bactericidal antibodies although in variable quantities depending on the antigen. It seems that antibodies to any of the protein antigens in the vaccine may be sufficient to protect, but this is uncertain. The exact protective effectiveness for each vaccine is thus currently unknown although for 4CMenB it seems to surpass 85% for the first year after the primary series. Antibodies wane and because MD is fulminant, depending on memory cells is not sufficient to avoid severe disease if a vaccinated individual is exposed several years after vaccination. Thus, booster doses are likely to be needed to provide sustained individual protection. In addition, the breadth of strain coverage, which seems quite inclusive based on MATS testing for 4CMenB (over 60% of strains) and probably also for rLP2086, will require persistent strain surveillance over time. One limitation associated with both vaccines is their high reactogenicity profile both local and systemic, especially among infants. Only one of the two vaccines, 4CMenB, has been licensed for use in infants, and increased medical visits and even hospitalization due to febrile episodes have been reported. Importantly though, this reactogenicity profile has not been associated with severe outcomes to date, and hopefully will be adequately dealt with through parental education, as we gain more experience with both vaccines.
The two-serogroup B vaccines are a significant addition to our armamentarium against highly significant pathogens for humans. The vaccines should certainly be considered for persons at increased risk for MD and when dealing with serogroup B outbreaks occurring in institutions or specific regions, as they will most likely save lives and prevent severe sequelae. Incorporation into national programs will require thorough analysis such as has been done in the UK; the world is once again watching closely the vaccine experience of this country (a post licensure field experiment) which will be of significant help to other countries with relatively high prevalence rates, who may be considering the incorporation of a serogroup B vaccine for the benefit of their populations.
Disclosure of potential conflicts of interest
RV: has received grants to support research projects from GSK.
M.A.P.S. has received grants to support research projects and consultancy fee from GSK, Pfizer and Sanofi Pasteur.
A.F. is an investigator in vaccine research projects funded by GSK, Pfizer and Sanofi Pasteur (funding paid to his employers).
MO: has received grants to support research projects from GSK.
JPT and MTV do not report any conflicts of interest.
References
- 1.American Academy of Pediatrics Meningococcal infections In: Kimberlin DW, Brady M, Jackson MA, Long SS, Red book: Report of the 2015 committee on infectious diseases. 30th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2015. p. 547–57. [Google Scholar]
- 2.Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344(18):1378–88. doi: 10.1056/NEJM200105033441807. PMID:11333996. [DOI] [PubMed] [Google Scholar]
- 3.Viner RM, Booy R, Johnson H, Edmunds WJ, Hudson L, Bedford H, Kaczmarski E, Rajput K, Ramsay M, Christie D. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): A case-control study. Lancet Neurol. 2012;11(9):774–83. doi: 10.1016/S1474-4422(12)70180-1. PMID:22863608. [DOI] [PubMed] [Google Scholar]
- 4.Rouphael NG, Stephens DS. Neisseria meningitidis. Methods Mol Biol. 2012;799(3):1–20. PMID:21993636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369(9580):2196–210. doi: 10.1016/S0140-6736(07)61016-2. PMID:17604802. [DOI] [PubMed] [Google Scholar]
- 6.Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, Corton C, Care R, Poolman JT, Zollinger W, et al.. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis. 2013;19(4):566–73. doi: 10.3201/eid1904.111799. PMID:23628376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucidarme J, Lekshmi A, Parikh SR, Bray JE, Hill DM, Bratcher HB, Gray S, Carr A, Jolley K, Findlow J, et al.. Frequent capsule switching in “ ultra-virulent ” meningococci – Are we ready for a serogroup B ST-11 complex outbreak? J Infect. 2017;75(2):95–103. doi: 10.1016/j.jinf.2017.05.015. PMID:28579305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucidarme J, Hill DMC, Bratcher HB, Gray SJ, du Plessis M, Tsang RSW, Vazquez J, Taha M, Ceyhan M, Efron A, et al.. Genomic resolution of an aggressive, widespread, diverse and expanding meningococcal serogroup B, C and W lineage. J Infect. 2015;71(5):544–52. doi: 10.1016/j.jinf.2015.07.007. PMID:26226598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucidarme J, Scott KJ, Ure R, Smith A, Lindsay D, Stenmark B, Jacobsson S, Fredlund H, Cameron JC, Smith-Palmer A, et al.. An international invasive meningococcal disease outbreak due to a novel and rapidly expanding serogroup w strain, Scotland and Sweden, July to August 2015. Eurosurveillance. 2016;21(45):1–9. doi: 10.2807/1560-7917.ES.2016.21.45.30395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lancellotti M, Guiyoule A, Ruckly C, Hong E, Alonso J, Taha M. Conserved virulence of C to B capsule switched Neisseria meningitidis clinical isolates belonging to ET-37/ST-11 clonal complex. Microbes Infect. 2006;8(1):191–6. doi: 10.1016/j.micinf.2005.06.012. PMID:16182586. [DOI] [PubMed] [Google Scholar]
- 11.Jeppesen CA, Snape MD, Robinson H, Gossger N, John TM, Voysey M, Ladhani S, Okike I, Oeser C, Kent A, et al.. Meningococcal carriage in adolescents in the United Kingdom to inform timing of an adolescent vaccination strategy. J Infect. 2015;71(1):43–52. doi: 10.1016/j.jinf.2015.02.006. PMID:25709085. [DOI] [PubMed] [Google Scholar]
- 12.Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker C, Messonnier N. Prevention and control of meningococcal disease recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2013;62(2):1–32. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6202a1.htm. PMID:23515099. [PubMed] [Google Scholar]
- 13.Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: A systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853–61. doi: 10.1016/S1473-3099(10)70251-6. PMID:21075057. [DOI] [PubMed] [Google Scholar]
- 14.Finn A, Morales-Aza B, Sikora P, Giles J, Lethem R, Marlais M, Thors V, Pollard A, Faust S, Heath P, et al.. Density distribution of pharyngeal carriage of meningococcus in healthy young adults: New approaches to studying the epidemiology of colonization and vaccine indirect effects. Pediatr Infect Dis J. 2016;35(10):1080–5. doi: 10.1097/INF.0000000000001237. PMID:27228196. [DOI] [PubMed] [Google Scholar]
- 15.Kratz MM, Weiss D, Ridpath A, Zucker JR, Geevarughese A, Rakeman J, Varma JK. Community-based outbreak of Neisseria meningitidis serogroup C infection in men who have sex with men, New York City, New York, USA, 2010–2013. Emerg Infect Dis. 2015;21(8):1379–86. doi: 10.3201/eid2108.141837. PMID:26197087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Center for Disease Prevention and Control Case definitions; 2012. (accessed 2017September29) https://ecdc.europa.eu/en/infectious-diseases-public-health/surveillance-and-disease-data/eu-case-definitions.
- 17.US Centers for Disease Control and Prevention Active Bacterial Core surveillance (ABCs). (accessed 2017September29) http://www.cdc.gov/abcs/overview/background.html.
- 18.National Institute for Communicable Diseases GERMS, South Africa; 2014. (accessed 2017September29) http://www.nicd.ac.za/assets/files/GERMSSA%Case%20definition%20Non%20ESS%20poster_V7%20May%202014.pdf.
- 19.Public Health Agency of Canada Case definitions for communicable diseases under national surveillance; 2009. (accessed 2017September29) http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/09vol35/35s2/index-eng.php.
- 20.Australian Government, Department of Health Meningococcal—Australian Meningococcal Surveillance Programme annual reports. (accessed 2017September29) http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-pubs-annlrpt-menganrep.htm.
- 21.Sáfadi MAP, de los Monteros LEE, López EL, Sàez-Llorens X, Lemos AP, Moreno-Espinosa S, Ayala SG, Torres JP, de Moraes JC, Vázquez JA. The current situation of meningococcal disease in Latin America and recommendations for a new case definition from the Global Meningococcal Initiative. Expert Rev Vaccines. 2013;12:903–15. doi: 10.1586/14760584.2013.814879. PMID:23909747. [DOI] [PubMed] [Google Scholar]
- 22.Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, Ladhani SN, McIntyre P, Ramsay M, Safadi M. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30(suppl. 2):B26–36. doi: 10.1016/j.vaccine.2011.12.032. PMID:22178525. [DOI] [PubMed] [Google Scholar]
- 23.WHO Epidemic meningitis control in countries of the African meningitis belt, 2016. Wkly Epidemiol Rec. 2017;3(92):145–64. http://apps.who.int/iris/bitstream/10665/254901/1/WER9213.pdf?ua = 1. [PubMed] [Google Scholar]
- 24.European Center for Disease Prevention and Control Annual Epidemiological Report 2016 – Invasive meningococcal disease 2016. [accessed 2017October1]. http://ecdc.europa.eu/en/healthtopics/meningococcaldisease/Pages/Annualepidemiologicalreport2016.
- 25.Martin NV, Ong KS, Howden BP, Lahra MM, Lambert SB, Beard FH, Dowse GK, Saul N, Communicable Diseases Network Australia MenW Working Group . Rise in invasive serogroup W meningococcal disease in Australia, 2013–2015. Commun Dis Intell Q Rep. 2016;40(4):2013–5. [DOI] [PubMed] [Google Scholar]
- 26.Archer BN, Chiu CK, Jayasinghe SH, Richmond PC, McVernon J, Lahra MM, Andrews R, McIntyre P, Nissen M, Campbell-Lloyd S, et al.. Epidemiology of invasive meningococcal b disease in Australia, 1999–2015: Priority populations for vaccination. Med J Aust. 2017;207(9):382–7. doi: 10.5694/mja16.01340. PMID:29092704. [DOI] [PubMed] [Google Scholar]
- 27.Australian Government, Department of Health Invasive menongococcal disease national surveillance report 2017. (accessed 2017December28) http://www.health.gov.au/internet/main/publishing.nsf/Content/5FEABC4B495BDEC1CA25807D001327FA/$File/Sept-2017-IMD-Surveillance-report.pdf.
- 28.Sridhar S, Greenwood B, Head C, Plotkin SA, Sáfadi MA, Saha S, Taha MK, Tomori O GB, Gessner B. Global incidence of serogroup B invasive meningococcal disease: a systematic review. Lancet Infect Dis. 2015;15(11):1334–46. doi: 10.1016/S1473-3099(15)00217-0. PMID:26453240. [DOI] [PubMed] [Google Scholar]
- 29.US Centers for Disease Control and Prevention Meningococcal Disease. 2017. [accessed 2017October1]. http://www.cdc.gov/meningococcal/surveillance.
- 30.Tsang RSW, Hoang L, Tyrrell GJ, Horsman G, Van Caeseele P, Jamieson F, Lefebvre B, Haldane D, Gad RR, German GJ, et al.. Increase in Neisseria meningitidis serogroup W invasive disease in Canada: 2009–2016. CCDR. 2017;43(3):144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sáfadi MAP, O'Ryan M, Valenzuela Bravo MT, Brandileone MCC, Gorla MCO, de Lemos APS, Moreno G, Vazquez JA, López EL, Taha MK, et al.. The current situation of meningococcal disease in Latin America and updated Global Meningococcal Initiative (GMI) recommendations. Vaccine. 2015;33(48):6529–36. doi: 10.1016/j.vaccine.2015.10.055. PMID:26597036. [DOI] [PubMed] [Google Scholar]
- 32.Galloway Y, Stehr-Green P, Mcnicholas A, O'Hallahan J. Use of an observational cohort study to estimate the effectiveness of the New Zealand group B meningococcal vaccine in children aged under 5 years. Int J Epidemiol. 2009;38(2):413–8. doi: 10.1093/ije/dyn228. PMID:18988650. [DOI] [PubMed] [Google Scholar]
- 33.Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, Eskola J, Fermon F, Klugman K, Ramsay M, Sow S, et al.. Global epidemiology of invasive meningococcal disease. Popul Health Metr. 2013;11(1):17. doi: 10.1186/1478-7954-11-17. PMID:24016339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borrow R, Alarcón P, Carlos J, Caugant DA, Debbag R, Wals P De, Echániz-Aviles G, Findlow J, Head C, Holt D, et al.. The Global Meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;0(0):1–16. [DOI] [PubMed] [Google Scholar]
- 35.Mohammed I, Iliyasu G. Emergence and control of epidemic meningococcal meningitis in sub-Saharan Africa. Pathog Glob Heal. 2017;1(111):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US Centers for Disease Control and Prevention Interim guidance for control of serogroup B meningococcal disease outbreaks in organizational settings. [accessed 2017October1]. https://www.cdc.gov/meningococcal/downloads/interim-guidance.pdf.
- 37.Labrie JE, Keiser PB. Whole cell vaccination for meningococcus: Lessons from an idea whose time has gone. Hum Vaccin. 2010;6(4):360–5. doi: 10.4161/hv.6.4.11055. PMID:20372072. [DOI] [PubMed] [Google Scholar]
- 38.Goldschneider I, Gotschlich E, Artenstein M. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129(6):1307–26. doi: 10.1084/jem.129.6.1307. PMID:4977280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization Meningococcal vaccines: WHO position paper. Wkly Epidemiol Rec. 2011;86(47):521–40. Available from: http://www.who.int/entity/wer/2011/wer8647.pdf?ua=1 PMID:22128384. [PubMed] [Google Scholar]
- 40.Vipond C, Care R, Feavers IM. History of meningococcal vaccines and their serological correlates of protection. Vaccine. 2012;30(suppl. 2):B10–7. doi: 10.1016/j.vaccine.2011.12.060. PMID:22607894. [DOI] [PubMed] [Google Scholar]
- 41.Shirley M, Dhillon S. Bivalent rLP2086 Vaccine (Trumenba): A review in active immunization against invasive meningococcal group B disease in individuals aged 10–25 Years. BioDrugs. 2015;29(5):353–61. doi: 10.1007/s40259-015-0139-0. PMID:26394633. [DOI] [PubMed] [Google Scholar]
- 42.Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: Immunological, functional and structural characterization of the antigens. Vaccine. 2012;30(suppl. 2):B87–97. doi: 10.1016/j.vaccine.2012.01.033. PMID:22607904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masforrol Y, Gil J, García D, Noda J, Ramos Y, Betancourt L, Guirola O, González S, Acevedo B, Besada V, et al.. A deeper mining on the protein composition of VA-MENGOC-BC®: An OMV-based vaccine against N. meningitidis serogroup B and C. Hum Vaccines Immunother. 2017;13(11):2548–60. doi: 10.1080/21645515.2017.1356961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tappero J, Lagos R, Maldonado A, Plikaytis B, Williams D, Dykes J, Gheesling L, Carlone G, Høiby EA, Poolman JT, et al.. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA. 1999;281(16):1520–7. doi: 10.1001/jama.281.16.1520. PMID:10227322. [DOI] [PubMed] [Google Scholar]
- 45.Harder T, Koch J, Wichmann O. Predicted vs observed effectiveness of outer membrane vesicle (OMV) vaccines against meningococcal serogroup B disease: Systematic review. J Infect. 2017;75(2):81–94. doi: 10.1016/j.jinf.2017.05.001. PMID:28487177. [DOI] [PubMed] [Google Scholar]
- 46.O'Ryan M, Stoddard J, Toneatto D, Wassil J, Dull PM. A multi-component meningococcal serogroup B vaccine (4CMenB): The clinical development program. Drugs. 2014;74(1):15–30. doi: 10.1007/s40265-013-0155-7. PMID:24338083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rappuoli R, Bottomley MJ, D'Oro U, Finco O, De Gregorio E. Reverse vaccinology 2.0: Human immunology instructs vaccine antigen design. J Exp Med. 2016;213(4):469–81. doi: 10.1084/jem.20151960. PMID:27022144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feavers IM, Maiden MCJ. Recent Progress in the Prevention of Serogroup B Meningococcal Disease. Clin Vaccine Immunol. 2017;24(5):1–13. doi: 10.1128/CVI.00566-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toneatto D, Ismaili S, Ypma E, Vienken K, Oster P, Dull P. The first use of an investigational multicomponent meningococcal serogroup B vaccine (4CMenB) in humans. Hum Vaccin. 2011;7(6):646–53. doi: 10.4161/hv.7.6.15482. PMID:21904120. [DOI] [PubMed] [Google Scholar]
- 50.Martinón-Torres F, Safadi MAP, Martinez AC, Marquez PI, Torres JCT, Weckx LY, Moreira ED, Mensi I, Calabresi M, Toneatto D, et al.. Reduced schedules of 4CMenB vaccine in infants and catch-up series in children: Immunogenicity and safety results from a randomised open-label phase 3b trial. Vaccine. 2017;35(28):3548–57. doi: 10.1016/j.vaccine.2017.05.023. PMID:28533054. [DOI] [PubMed] [Google Scholar]
- 51.Safadi MA, Martinon-Torres F, Weckx LY, Moreira ED, da Fonseca Lima EJ, Mensi I, Calabresi M, Toneatto D. Immunogenicity and safety of concomitant administration of meningococcal serogroup B (4CMenB) and serogroup C (MenC-CRM) vaccines in infants: A phase 3b, randomized controlled trial. Vaccine. 2017;35(16):2052–9. doi: 10.1016/j.vaccine.2017.03.002. PMID:28318767. [DOI] [PubMed] [Google Scholar]
- 52.Vesikari T, Esposito S, Prymula R, Ypma E, Kohl I, Toneatto D, Dull P, Kimura A. for the EU Meningococcal B Infant Vaccine Study group Summary Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: Results of two randomised trials. Lancet. 2013;381(9869):825–35. doi: 10.1016/S0140-6736(12)61961-8. PMID:23324563. [DOI] [PubMed] [Google Scholar]
- 53.Gossger N, Snape MD, Yu LM, Finn A, Bona G, Esposito S, Principi N, Diez-Domingo J, Sokal E, Becker B, Kieninger D, et al.. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA. 2012;6(307):573–82. [DOI] [PubMed] [Google Scholar]
- 54.Santolaya ME, O'Ryan M, Valenzuela MT, Prado V, Vergara R, Muñoz A, Toneatto D, Graña G, Wang H, Clemens R, et al.. Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet. 2012;379(9816):617–24 doi: 10.1016/S0140-6736(11)61713-3. PMID:22260988. [DOI] [PubMed] [Google Scholar]
- 55.Nainani V, Galal U, Buttery J, Snape MD. An increase in accident and emergency presentations for adverse events following immunisation after introduction of the group B meningococcal vaccine: an observational study. Arch Dis Child. 2017;102:958–62. doi: 10.1136/archdischild-2017-312941. [DOI] [PubMed] [Google Scholar]
- 56.Murdoch H, Wallace L, Bishop J, Robertson C, Claire Cameron J. Risk of hospitalisation with fever following MenB vaccination: self-controlled case series analysis. Arch Dis Child. 2017;102(10):894–8. doi: 10.1136/archdischild-2017-313079. PMID:28931535. [DOI] [PubMed] [Google Scholar]
- 57.EMA Bexsero: EPAR – Product Information 2013. [accessed 2017November17]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002333/WC500137881.pdf.
- 58.Food and Drug Administration BEXSERO ® [Internet]. 2015. [accessed 2017November17]. p. 1–11. Available from: https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm431374.htm.
- 59.Ladhani SN, Campbell H, Parikh SR, Saliba V, Borrow R, Ramsay M. The introduction of the meningococcal B (MenB) vaccine (Bexsero??) into the national infant immunisation programme – New challenges for public health. J Infect. 2015;71(6):611–4. doi: 10.1016/j.jinf.2015.09.035. PMID:26433141. [DOI] [PubMed] [Google Scholar]
- 60.JCVI interim position statement on use of Bexsero® meningococcal B vaccine in the UK. July 2013. page 5 (accessed 3rdMarch2018) https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/224896/JCVI_interim_statement_on_meningococcal_B_vaccination_for_web.pdf.
- 61.Richmond PC, Marshall HS, Nissen MD, Jiang Q, Jansen KU, Garcés-sánchez M, Martinón-Torres F, Beeslaar J, Szenborn L, Wysocki J, et al.. Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet. 2012;3099(12):1–11. [DOI] [PubMed] [Google Scholar]
- 62.Vesikari T, Wysocki J, Beeslaar J, Eiden J, Jiang Q, Jansen KU, Jones TR, Harris SL, O'Neill RE, York LJ, et al.. Immunogenicity, safety, and tolerability of bivalent rLP2086 meningococcal group B vaccine administered concomitantly with diphtheria, tetanus, and acellular pertussis and inactivated poliomyelitis vaccines to healthy adolescents. J Pediatric Infect Dis Soc. 2016;5(2):180–7. doi: 10.1093/jpids/piv064. PMID:26803328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vesikari T, Østergaard L, Diez-Domingo J, Wysocki J, Flodmark C-E, Beeslaar J, Eiden J, Jiang Q, Jansen KU, Jones TR, et al.. Meningococcal Serogroup B Bivalent rLP2086 Vaccine Elicits Broad and Robust Serum Bactericidal Responses in Healthy Adolescents. J Pediatric Infect Dis Soc. 2016;5(2):152–60. doi: 10.1093/jpids/piv039. PMID:26407272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muse D, Christensen S, Bhuyan P, Absalon J, Eiden J, Jones TR, York LJ, Jansen KU, O'Neill RE, Harris SL, et al.. A Phase 2, Randomized, Active-controlled, Observer-blinded Study to Assess the Immunogenicity, Tolerability and Safety of Bivalent rLP2086, a Meningococcal Serogroup B Vaccine, Coadministered With Tetanus, Diphtheria and Acellular Pertussis Vaccine and Serogroup A, C, Y and W-135 Meningococcal Conjugate Vaccine in Healthy US Adolescents. Pediatr Infect Dis J. 2016;35(6):673–82. doi: 10.1097/INF.0000000000001124. PMID:26974889. [DOI] [PubMed] [Google Scholar]
- 65.Food and Drug Administration Trumenba. [accessed 2017November17]. p. 1–15. https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm421020.htm.
- 66.EMA Trumenba: EPAR – Product Information 2017. (accessed 2017October1) http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004051/WC500228995.pdf.
- 67.Ostergaard L, Lucksinger GH, Absalon J, Beeslaar J, Eiden J, Jansen KU, York LJ, Quinn A, Graversen ME, Perez JL. A phase 3, randomized, active-controlled study to assess the safety and tolerability of meningococcal serogroup B vaccine bivalent rLP2086 in healthy adolescents and young adults. Vaccine. 2017;34(12):1465–71. doi: 10.1016/j.vaccine.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 68.Ostergaard L, Vesikari T, Absalon J, Beeslaar J, Ward BJ, Senders S, Eiden JJ, Jansen KU, Anderson AS, York LJ, et al.. A Bivalent Meningococcal B Vaccine in Adolescents and Young Adults. N Engl J Med. 2017;377(24):2349–62. doi: 10.1056/NEJMoa1614474. PMID:29236639. [DOI] [PubMed] [Google Scholar]
- 69.Flacco ME, Manzoli L, Rosso A, Marzuillo C, Bergamini M, Stefanati A, Cultrera R, Villari P, Ricciardi W, Ioannidis J, et al.. Immunogenicity and safety of the multicomponent meningococcal B vaccine (4CMenB) in children and adolescents: A systematic review and meta-analysis. Lancet Infect Dis. 2018;3099(18):1–12. [DOI] [PubMed] [Google Scholar]
- 70.Prymula R, Esposito S, Zuccotti GV, Xie F, Toneatto D, Kohl I, Dull P. A phase 2 randomized controlled trial of a multicomponent meningococcal serogroup B vaccine (I): Effects of prophylactic paracetamol on immunogenicity and reactogenicity of routine infant vaccines and 4CMenB. Hum Vaccines Immunother. 2014;10(7):1993–2004. doi: 10.4161/hv.28666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esposito S, Tagliabue C, Bosis S. Meningococcal B Vaccination (4CMenB) in Infants and Toddlers. J Immunol Res. 2015;2015(Article ID 402381):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martinon-Torres F, Gimenez-Sanchez F, Bernaola-Iturbe E, Diez-Domingo J, Jiang Q, Perez JL. A randomized, phase 1/2 trial of the safety, tolerability, and immunogenicity of bivalent rLP2086 meningococcal B vaccine in healthy infants. Vaccine. 2014;32(40):5206–11. doi: 10.1016/j.vaccine.2014.07.049. PMID:25077420. [DOI] [PubMed] [Google Scholar]
- 73.Fiorito TM, Baird GL, Alexander-Scott N, Bornschein S, Kelleher C, Du N, Dennehy P. Adverse Events Following Vaccination with Bivalent rLP2086 (Trumenba®). Pediatr Infect Dis J. 2017;37(1):1. [DOI] [PubMed] [Google Scholar]
- 74.Shirley M, Taha M-K. MenB-FHbp Meningococcal Group B Vaccine (Trumenba®): A Review in Active Immunization in Individuals Aged ≥ 10 Years. Drugs. 2018;78(2):257–68. doi: 10.1007/s40265-018-0869-7. PMID:29380290. [DOI] [PubMed] [Google Scholar]
- 75.Snape MD, Voysey M, Finn A, Bona G, Esposito S, Principi N, Diez-Domingo J, Sokal E, Kieninger D, Prymula R, et al.. Persistence of Bactericidal Antibodies After Infant Serogroup B Meningococcal Immunization and Booster Dose Response at 12, 18 or 24 Months of Age. Pediatr Infect Dis J. 2016;35(4):e113–23. doi: 10.1097/INF.0000000000001056. PMID:26756390. [DOI] [PubMed] [Google Scholar]
- 76.Vesikari T, Prymula R, Merrall E, Kohl I, Toneatto D, Dull PM. Meningococcal serogroup B vaccine (4CMenB): Booster dose in previously vaccinated infants and primary vaccination in toddlers and two-year-old children. Vaccine. 2015;33(32):3850–8. doi: 10.1016/j.vaccine.2015.06.079. PMID:26141011. [DOI] [PubMed] [Google Scholar]
- 77.McQuaid F, Snape MD, John TM, Kelly S, Robinson H, Yu LM, Toneatto D, D'Agostino D, Dull P, Pollard A. Persistence of specific bactericidal antibodies at 5 years of age after vaccination against serogroup B meningococcus in infancy and at 40 months. Cmaj. 2015;187(7):E215–25. doi: 10.1503/cmaj.141200. PMID:25802309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sadarangani M, Sell T, Iro MA, Snape MD, Voysey M, Finn A, Heath PT, Bona G, Esposito S, Diez-Domingo J, et al.. Persistence of immunity after vaccination with a capsular group B meningococcal vaccine in 3 different toddler schedules. Cmaj. 2017;189(41):E1276–85. doi: 10.1503/cmaj.161288. PMID:29038320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Santolaya ME, O'Ryan ML, Valenzuela MT, Prado V, Vergara RF, Muñoz A, Toneatto D, Grana G, Wang H, Dull P. Persistence of antibodies in adolescents 18–24 months after immunization with one, two, or three doses of 4CMenB meningococcal serogroup B vaccine. Hum Vaccines Immunother. 2013;9(11):2304–10. doi: 10.4161/hv.25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patton ME, Stephens D, Moore K, MacNeil JR. Updated recommendations for use of MenB-FHbp serogroup B meningococcal vaccine – Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(19):509–13. doi: 10.15585/mmwr.mm6619a6. PMID:28520709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Read RC, Baxter D, Chadwick DR, Faust SN, Finn A, Gordon SB, Heath PT, Lewis D, Pollard A, Turner D, et al.. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2017 Nov 24;384(9960):2123–31. doi: 10.1016/S0140-6736(14)60842-4. [DOI] [PubMed] [Google Scholar]
- 82.McNamara L, Dolan Thomas J, MacNeil J, Chang HY, Day M, Fisher E, Martin S, Poissant T, Schmink S, Steward-Clark E, et al.. Meningococcal Carriage Following a Vaccination Campaign With MenB-4C and MenB-FHbp in Response to a University Serogroup B Meningococcal Disease Outbreak—Oregon, 2015–2016. J Infect Dis. 2017;216(November):1130–40. PMID:28968661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Soeters HM, Whaley M, Alexander-Scott N, Kanadanian KV, MacNeil JR, Martin SW, McNamara LA, Sicard K, Vanner C, Vuong J, et al.. Meningococcal carriage evaluation in response to a Serogroup B meningococcal disease outbreak and mass vaccination campaign at a College-Rhode Island, 2015–2016. Clin Infect Dis. 2017;64(8):1115–22. doi: 10.1093/cid/cix091. PMID:28158417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abad R, Medina V, Fariñas M, del C, Martínez-Martínez L, Bambini S, Dari A, Medini D, Pizza M, Vázquez J. Potential impact of the 4CMenB vaccine on oropharyngeal carriage of Neisseria meningitidis. J Infect. 2017;75(6):511–20. doi: 10.1016/j.jinf.2017.09.021. PMID:28987549. [DOI] [PubMed] [Google Scholar]
- 85.Medini D, Stella M, Wassil J. MATS: Global coverage estimates for 4CMenB, a novel multicomponent meningococcal B vaccine. Vaccine. 2017;33(23):2629–36. doi: 10.1016/j.vaccine.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 86.Donald R, Hawkins JC, Hao L, Liberator P, Jones TR, Harris SL, Perez J, Eiden JJ, Jansen K, Anderson A. Meningococcal serogroup B vaccines: Estimating breadth of coverage. Hum Vaccines Immunother. 2017;13(2):255–65. doi: 10.1080/21645515.2017.1264750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parikh SR, Andrews NJ, Beebeejaun K, Campbell H, Ribeiro S, Ward C, White J, Borrow R, Ramsay ME, Ladhani SN. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet. 2016;388(10061):2775–82. doi: 10.1016/S0140-6736(16)31921-3. PMID:28100432. [DOI] [PubMed] [Google Scholar]
- 88.Basta NE, Christensen H. 4CMenB vaccine effectiveness: reasons for optimism. Lancet. 2016;388(10061):2719–21. doi: 10.1016/S0140-6736(16)32061-X. PMID:28100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.López-Sagaseta J, Beernink PT, Bianchi F, Santini L, Frigimelica E, Lucas AH, Pizza M, Bottomley MJ. Crystal structure reveals vaccine elicited bactericidal human antibody targeting a conserved epitope on meningococcal fHbp. Nat Commun. 2018;9(1):528. doi: 10.1038/s41467-018-02827-7. PMID:29410413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ladhani SN, Giuliani MM, Biolchi A, Pizza M, Beebeejaun K, Lucidarme J, Findlow J, Ramsay ME, Borrow R. Effectiveness of meningococcal B vaccine against endemic hypervirulent neisseria meningitidis W strain, England. Emerg Infect Dis. 2016;22(2):309–11. doi: 10.3201/eid2202.150369. PMID:26811872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tomei S, Biolchi A, Brunelli B, De Angelis G, Masignani V, Budroni S, Claus H, Vogel U, Taha K, Hong E, et al.. Potential coverage of the BEXSERO® MenB vaccine on non-B meningococci. 2014. XIXth International Pathogenic Neisseria Conference; Asheville, NC, USA, Oct 12 2014; Available from: http://neisseria.org/ipnc/2014/IPNC_2014_abstracts.pdf (accessed 2017March4). [Google Scholar]
- 92.Gorla MCO, Lemos A, Biolchi A, Tomei S, De Angelis G, Brandileone MCC. Impact vaccination with the Novartis meningococcal serogroup B vaccine 4CMenB (Bexsero®) on non-serogroup b disease burden in Brazil. 2014. ESPID 32nd Annual Meeting, Dublin May 6-10, 2014Available from: http://espid2015.kenes.com/PublishingImages/scientific-information/espid-abstracts/ESPID 2014 abstracts.pdf%0D. (accessed 2017March4). [Google Scholar]
- 93.Hong E, Giuliani MM, Deghmane AE, Comanducci M, Brunelli B, Dull P, Pizza M, Taha M. Could the multicomponent meningococcal serogroup B vaccine (4CMenB) control Neisseria meningitidis capsular group X outbreaks in Africa? Vaccine. 2013;31(7):1113–6. doi: 10.1016/j.vaccine.2012.12.022. PMID:23261039. [DOI] [PubMed] [Google Scholar]
- 94.Tirani M, Meregaglia M, Melegaro A. Health and economic outcomes of introducing the new MenB vaccine (Bexsero) into the Italian routine infant immunisation programme. PLoS One. 2015;10(4):1–21. doi: 10.1371/journal.pone.0123383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sadarangani M, Pollard AJ. Can we control all-cause meningococcal disease in Europe? Clin Microbiol Infect. 2016;22:S103–12. doi: 10.1016/j.cmi.2016.03.006. PMID:27129415. [DOI] [PubMed] [Google Scholar]
- 96.Izquierdo G, Torres JP, Santolaya ME, Valenzuela MT, Vega J, Chomali M. Cost-effectiveness analysis of a multicomponent meningococcal serogroup B vaccine in hypothetic epidemic situation in a middle-income country. Hum Vaccines Immunother. 2015;11(4):875–83. doi: 10.1080/21645515.2015.1010885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Christensen H, Irving T, Koch J, Trotter CL, Ultsch B, Weidemann F, Wichmann O, Hellenbrand W. Epidemiological impact and cost-effectiveness of universal vaccination with Bexsero®to reduce meningococcal group B disease in Germany. Vaccine. 2016;34(29):3412–9. doi: 10.1016/j.vaccine.2016.04.004. PMID:27109566. [DOI] [PubMed] [Google Scholar]
- 98.Christensen H, Trotter CL. Modelling the cost-effectiveness of catch-up “MenB” (Bexsero) vaccination in England. Vaccine. 2017;35(2):208–11. doi: 10.1016/j.vaccine.2016.11.076. PMID:27923519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaaijk P, Van der Ende A, Luytjes W. Routine vaccination against MenB Considerations for implementation. Hum Vaccines Immunother. 2014;10(2):310–6. doi: 10.4161/hv.26816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Whittaker R, Dias JG, Ramliden M, Ködmön C, Economopoulou A, Beer N, Pastore Celentano L. The epidemiology of invasive meningococcal disease in EU/EEA countries, 2004–2014. Vaccine. 2017;35(16):2034–41. doi: 10.1016/j.vaccine.2017.03.007. PMID:28314560. [DOI] [PubMed] [Google Scholar]
- 101.Govern dAndorra Pla de vacunacions. (accessed 6thMarch2018) Available from https://www.salut.ad/temes-de-salut/vacunacio.
- 102.Ministero della Salute, Italia; Piano Nazionale Prevenzione Vaccinale PNPV 2017–2019. (accessed 6thMarch2018) Available from http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf.
- 103.Ministry of Health of The Republic of Lithuania, (accessed 6thMarch2018) Available from https://sam.lrv.lt/en/news/minister-of-health-aurelijus-veryga-signed-an-order-by-which-vaccines-against-type-b-meningococcus-are-to-be-included-in-the-schedule-for-the-preventive-vaccination-of-children-the-start-of-the-vaccination-is-scheduled-as-of-july-this-year.
- 104.National immunisation advisory committee royal college of physicians of Ireland, immunisation guidelines. (accessed 6thMarch2018) Available from https://www.hse.ie/eng/health/immunisation/hcpinfo/guidelines/chapter13.pdf.
- 105.Basta N, Mahmoud A, Wolfson J, Ploss A, Heller BL, Hanna S, Johnsen P, Izzo R, Grenfell B, Findlow J, et al.. Vaccine during a university outbreak. N Engl J Med. 2016;375;220–8. doi: 10.1056/NEJMoa1514866. PMID:27468058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McNamara LA, Shumate AM, Johnsen P, MacNeil JR, Patel M, Bhavsar T, Cohn AC, Dinitz-Sklar J, Duffy J, Finnie J, et al.. First use of a serogroup B meningococcal vaccine in the US in response to a university outbreak. Pediatrics. 2015;135(5):798–804. doi: 10.1542/peds.2014-4015. PMID:25917990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harrison LH. Vaccines for prevention of Group B meningococcal disease. Am J Prev Med. 2015;49(6):S345–54. doi: 10.1016/j.amepre.2015.09.007. PMID:26590434. [DOI] [PubMed] [Google Scholar]
- 108.Advisory Committee on Immunization Practices (ACIP) Department of health and human services centers for disease control and prevention Summary Report 2014. [accessed 2017 Nov 17]. p. 51–7. https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2014-02.pdf.
- 109.De Wals P, Deceuninck G, Lefebvre B, Tsang R, Law D, De Serres G, Gilca V, Gilca RBN, Serres D, Gilca V, Gilca R, Boulianne N. Impact of an Immunization Campaign to Control an Increased Incidence of Serogroup B Meningococcal Disease in One Region of Quebec, Canada. Clin Infect Dis. 2017;9(64):1263–7. [DOI] [PubMed] [Google Scholar]
- 110.Grogan J, Roos K. Serogroup B Meningococcus Outbreaks, Prevalence, and the Case for Standard Vaccination. Curr Infect Dis Rep. 2017;19:3. doi: 10.1007/s11908-017-0587-4. PMID:28210940. [DOI] [PubMed] [Google Scholar]
- 111.Wilkins AL, Snape MD. Emerging clinical experience with vaccines against group B meningococcal disease. Vaccine. 2017; pii. S0264-410X(17)30964-7. doi: 10.1016/j.vaccine.2017.07.056. PMID:28778616. [DOI] [PubMed] [Google Scholar]
- 112.Baker CJ. Prevention of Meningococcal Infection in the United States: Current Recommendations and Future Considerations. J Adolesc Heal [Internet]. Elsevier Inc.; 2016;59(2):S29–37. Available from: https://doi.org/ 10.1016/j.jadohealth.2016.03.040. doi: 10.1016/j.jadohealth.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 113.Saez-llorens X, Catalina D, Vaca A, Abarca K, Maho E, Graña MG, Heijnen E, Smolenov I, Dull PM. Immunogenicity and safety of investigational vaccine formulations against meningococcal serogroups A, B, C, W, and Y in healthy adolescents Immunogenicity and safety of investigational vaccine formulations against meningococcal serogroups A, B, C, W, and Y in healthy adolescents. 2015;5515 (August 2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saez-Llorens X, Aguilera Vaca DC, Abarca K, Maho E, Han L, Smolenov I, Dull P. Persistence of meningococcal antibodies and response to a third dose after a two-dose vaccination series with investigational MenABCWY vaccine formulations in adolescents. Pediatr Infect Dis J. 2015;34(10):e264–78. doi: 10.1097/INF.0000000000000822. PMID:26135245. [DOI] [PubMed] [Google Scholar]
- 115.Szenborn L, Block SL, Jackowska T, Konior R, D'Agostino D, Smolenov I, Toneatto D, Welsch JA. Immune Responses to Booster Vaccination with Meningococcal ABCWY Vaccine Following Primary Vaccination with Either Investigational or Licensed Vaccines. Pediatr Infect Dis J. 2018;1. doi: 10.1097/INF.0000000000001896. [DOI] [PubMed] [Google Scholar]
- 116.Block SL, Szenborn L, Daly W, Jackowska T, D'Agostino D, Han L, Dull P, Smolenov I. A comparative evaluation of two investigational meningococcal ABCWY vaccine formulations: Results of a phase 2 randomized, controlled trial. Vaccine. 2015;33(21):2500–10. doi: 10.1016/j.vaccine.2015.03.001. PMID:25795256. [DOI] [PubMed] [Google Scholar]
- 117.Nolan T, O'Ryan M, Wassil J, Abitbol V, Dull P. Vaccination with a multicomponent meningococcal B vaccine in prevention of disease in adolescents and young adults. Vaccine. 2015;33(36):4437–45. doi: 10.1016/j.vaccine.2015.06.011. PMID:26187261. [DOI] [PubMed] [Google Scholar]
- 1118.US Centers for Disease Control and Prevention Guidance for the Evaluation and Public Health Management of Suspected Outbreaks of Meningococcal Disease. 2017. (accessed 2017December28) https://www.cdc.gov/meningococcal/downloads/meningococcal-outbreak-guidance.pdf.
- 119.Hill DMC, Lucidarme J, Gray SJ, Newbold LS, Ure R, Brehony C, Harrison OB, Bray JE, Jolley KA, Bratcher HB, et al.. Genomic epidemiology of age-associated meningococcal lineages in national surveillance: An observational cohort study. Lancet Infect Dis. 2015;15(12):1420–8. doi: 10.1016/S1473-3099(15)00267-4. PMID:26515523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Campbell H, Borrow R, Salisbury D, Miller E. Meningococcal C conjugate vaccine: The experience in England and Wales. Vaccine. 2009;27(suppl. 2):20–9. doi: 10.1016/j.vaccine.2009.04.067. [DOI] [PubMed] [Google Scholar]
- 121.Campbell H, Saliba V, Borrow R, Ramsay M, Ladhani SN. Targeted vaccination of teenagers following continued rapid endemic expansion of a single meningococcal group W clone (sequence type 11 clonal complex), United Kingdom 2015. Eurosurveillance. 2015;20(28):1–5. doi: 10.2807/1560-7917.ES2015.20.28.21188. PMID:26132766. [DOI] [PubMed] [Google Scholar]
- 122.Parikh SR, Newbold L, Slater S, Stella M, Moschioni M, Lucidarme J, Paola R, Giuliani M, Serino L, Gray S, et al.. Articles Meningococcal serogroup B strain coverage of the multicomponent 4CMenB vaccine with corresponding regional distribution and clinical characteristics in England, a qualitative and quantitative assessment. Lancet Infect Dis. 2017;3099(17):1–9. [DOI] [PubMed] [Google Scholar]
- 123.England Public Health Preliminary vaccine coverage estimates for the meningococcal B (MenB) immunisation programme for England Preliminary vaccine coverage estimates for the meningococcal B (MenB) immunisation programme for England, update from August to December 2017. Health Protection Report. 2017. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/677275/hpr0318_menb.pdf.
- 124.De Serres G, Gariepy M, Billard M, Rouleau I. Initial Dose of a Multicomponent Serogroup B Meningococcal Vaccine in the Saguenay– Lac-Saint-Jean Region, Québec, Canada: An Interim Safety Surveillance Report. 2014. (accessed 2017September12) https://www.inspq.qc.ca/pdf/publications/1902_SerogroupB_Meningococcal_Vaccine.pdf.
- 125.Basta N, Mahmoud A, Wolfson J, Ploss A, Heller H, Hanna S, Johnsen P, Izzo R, Grenfell BT, Findlow J, et al.. Immunogenicity of a meningococcal B vaccine during a university outbreak. N Engl J Med. 2016;375(3):220–8. doi: 10.1056/NEJMoa1514866. PMID:27468058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Folaranmi T, Rubin L, Martin SW, Patel M, MacNeil JR. US Centers for Disease and Control. Use of Serogroup B Meningococcal Vaccines in Persons Aged >/ = 10 Years at Increased Risk for Serogroup B Meningococcal Disease: Recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(22):608–12. http://www.ncbi.nlm.nih.gov/pubmed/26068564 PMID:26068564. [PMC free article] [PubMed] [Google Scholar]