ABSTRACT
The endocrine pancreas expands markedly in the first postnatal days and the insulin producing β-cells initiate a functional maturation preceded by a morphological change of the islets of Langerhans. Trefoil factor 3 (TFF3) is a secreted peptide expressed in intestinal epithelia, where it promotes migration, but its role in the pancreas is not characterized. The aim of this study was to examine the expression and function of TFF3 in perinatal rat pancreas, ex vivo cultured fetal rat pancreas and in the rat β-cell line INS-1E.
Control or gestational low-protein diet perinatal rat pancreas was harvested at embryonic day 20 (E20), day of birth (P0) and postnatal day 2 (P2). TFF3 mRNA was upregulated 4.5-fold at P0 vs. E20 and downregulated again at P2. In protein-undernourished pups induction of TFF3 at P0 was further increased to 9.7-fold and was increased at P2. TFF3 caused tyrosine phosphorylation of EGFR in INS-1E β-cells, and purified recombinant TFF3 increased both attachment and spreading of INS-1E β-cells. In ex vivo cultures of collagenase digested fetal rat pancreas, a model of perinatal β-cell maturation, TFF3 increased cellular spreading as well as insulin mRNA levels. TFF3 also increased the expression of Pref1/Dlk1 that shares similarities in expression and regulation with TFF3.
These results suggest that TFF3 may promote adhesion and spreading of cells to accelerate β-cell maturation. This study indicates a functional role for TFF3 in pancreatic β-cell maturation in the perinatal period, which is altered by low protein diet during gestation.
KEYWORDS: adhesion, EGFR, insulin, islets of Langerhans, maturation, perinatal pancreas, Pref1/Dlk1, TFF3 (ITF), β-catenin
Introduction
In pancreas, a transient burst of β-cell replication occurs around birth. Moreover, a temporary rise in β-cell neogenesis is followed by functional maturation of the endocrine pancreas.1 In the period shortly before and after birth the pancreas goes through major changes resulting in the aggregation of endocrine cells into islets of Langerhans followed by the functional maturation of β-cells.2-5 The exocrine cells of the pancreas are activated as the nutrition changes to lipid and carbohydrate rich milk.
Trefoil factor 3 (TFF3, also known as intestinal trefoil factor, ITF) is a small mucin-associated protein.6 expressed in the intestines,7 the airway epithelia,8 kidney and pancreatic β-cells.9,10 TFF3 has multiple actions on mucosal epithelial cells, such as anti-apoptotic effects, intestinal cell migration and adhesion, bronchial cell migration and plays a major and necessary role in the repair processes in the gastrointestinal tract.7 TFF3 increases cellular adhesion and migration in a number of different mucosal epithelial cell line models: Human colon cancer cells,11,12 bronchial epithelial cells,8 as well as primary intestinal and corneal epithelium of mice.13,14 Although no specific receptor has been conclusively identified, TFF3 administration has been shown to cause changes in intracellular signaling pathways and phosphorylation of the epidermal growth factor receptor (EGFR), β-catenin,11,15 and decreased activation of the MAP kinase pathway.16
β-cells of human and rat pancreas express TFF3, which is upregulated by growth hormone (GH) in the rat, and increases spreading of neonatal rat islets of Langerhans.9 In human development, TFF3 co-localizes with insulin and glucagon in gestational week 14.9 However, there has been no investigation of the expression or role of TFF3 in pancreas during late fetal development.
The aim of this study was to examine the localization and possible functions of TFF3 in perinatal rat pancreas and neonatal islets of Langerhans. Major changes in islet function and structure take place around birth, where the islet cells migrate into firm islets, mature and progressively become more glucose-responsive.4,17 Based on the role of TFF3 in intestinal cell migration and restitution, we hypothesized that TFF3 is involved in migration or adhesion of β-cells promoting the generation and maturation of islets occurring at birth. The rate of β-cell replication in the perinatal pancreas is an order of magnitude larger than in the adult pancreas. It is thus relevant to identify the physiological mechanisms responsible for this large expansion of the functional β-cell mass in order to identify points of intervention for the treatment of β-cell failure during type 2 diabetes or for generation of β-cells as a supply for β-cell replenishment in type 1 diabetes.
Results
TFF3 is upregulated in rat pancreas at the day of birth and is increased by fetal malnutrition
Using an array data-set of mRNAs expressed and regulated in rat perinatal pancreas over the time course of embryonic day 20 (E20), at the day of birth (P0) and postnatal day 2 (P2) we identified Trefoil Factor 3 (TFF3) as a transcript upregulated specifically at P0.18 The expression levels of TFF3 mRNA were verified by qPCR (Fig. 1A). TFF3 was upregulated 4.5 ± 0.7-fold at P0 compared with E20 (p < 0.001) and decreased again at P2 to the level found at E20. TFF3 expression was further increased to 9.7 ± 0.4-fold at P0 in a low protein (LP) fetal malnutrition model compared with the 4.5 ± 0.7-fold up-regulation in the control animals (LP P0 vs. Ctrl P0, p < 0.001) (Fig. 1A). In the low protein model TFF3 expression was also temporally prolonged and was increased also at P2 (Ctrl P2 vs. LP P2, p < 0.001).
Figure 1.
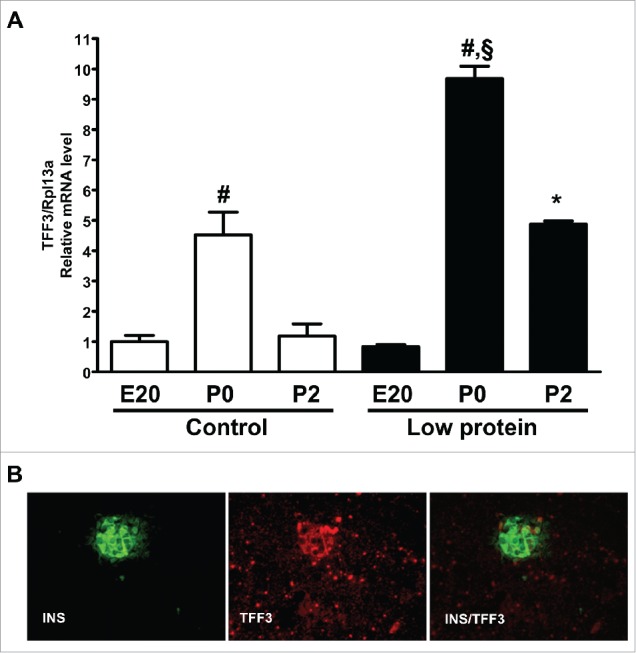
TFF3 mRNA regulation and protein expression in perinatal rat pancreas. A) Levels of TFF3 mRNA in pancreas at E20, P0 and P2 in rat pups from dams receiving control chow (white bars) or low protein diet (black bars). Messenger RNA levels of TFF3 relative to Rpl13α was determined by qPCR. Data are from three experiments performed in replicates of 3 (n = 3). #: p < 0.001 P0 vs. E20 or P2 (control or low protein, respectively). §: p < 0.001 vs. control P0. *: p < 0.05 vs. control P2, B) Immunohistochemical staining for insulin (green) and TFF3 (red) in rat pancreas at P0. TFF3 is expressed throughout the pancreas in a punctate pattern, but the level is highest in islets, where it co-localizes with insulin. Magnification: 400x.
In perinatal pancreas TFF3 protein is expressed throughout the exocrine and endocrine tissue and a substantial part of the signal originates from non-endocrine tissue (exocrine, fibroblasts, ductal tissue). Images from P0 are shown in Fig. 1B and the punctate staining pattern is similar at E19.5 and E21.5. In endocrine cells TFF3 is co-expressed with insulin but not with glucagon (Fig. 1B).
Effects of TFF3 treatment on ex vivo cultured fetal pancreas
In order to test the effect of TFF3 in fetal pancreatic cells, ex vivo pancreas cultures were established using the method of Hellerstrom et al.,19,20 in which fetal rat pancreas is dissociated by collagenase and cultured for up to 7 days. During this period the exocrine cells degenerate/dedifferentiate and β-cell mass expands. Islet-like-structures consisting of >90% β-cells form and differentiate into glucose-responsive mature islets, which grow on top of a proliferating layer of fibroblast-like cells (FBLCs) (Fig. 2, arrows). The special feature of this perinatal or fetal pancreas culture model is that cells retain their identities and cellular program. Thus, among the exocrine and fibroblast cells forming a monolayer, islets sprout up and grow for a period of up to one week. The islets are easily separated from the fibroblast cell layer by pipetting. This culture system can be used to model the growth and functional maturation of the endocrine pancreas observed in vivo in the perinatal period.
Figure 2.
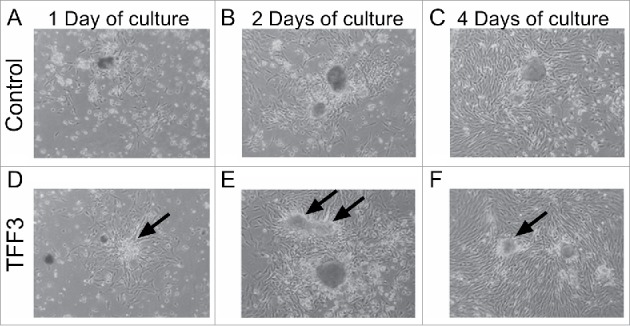
Morphology of fetal pancreas cultured ex vivo in the absence or presence of TFF3. In the fetal ex vivo pancreas culture model, islet-cells sprout from the underlying fibroblast-like cell layer (FBLCs) over the time course of the experiment. With TFF3 treatment, islets form more extended and spread-out structures (arrows). A-C) Control, D-F) TFF3 (200ng/mL). Light microscope images of ex vivo fetal pancreas culture. A, D) Day 1, B, E) Day 2, C, F) Day 4. Magnification: 100x.
Ex vivo fetal (E19.5) pancreas culture was treated with TFF3 protein in order to investigate the effects of TFF3 on cell morphology and various islet markers. After 24 hrs of culture (day 1), FBLCs were visible in the periphery of the islets (Ctrl: Fig. 2B and TFF3: Fig. 2E). TFF3 had morphological effects on islets, which form more extended and spread-out structures at day 2 and 4 (Fig. 2E-F). We have previously observed that TFF3 mediates spreading of islets using neonatal rat islets and adult human islets .9 Thus, this effect of TFF3 is conserved in the ex vivo fetal pancreas culture system.
Effects of TFF3 on insulin, Pref1 and Afp mRNA levels in ex vivo cultured fetal pancreas cells
We investigated the effect of TFF3 on markers of differentiation and maturation of β-cells in the fetal ex vivo cultured islet cells. TFF3 treatment significantly increased insulin mRNA levels in islet cells at day 2 (5.9 ± 1.1-fold) and at day 5 (24.7 ± 2.7-fold) compared with control at day 1 (2.2-fold compared with Ctrl at day 5, p < 0.001) (Fig. 3A). In FBLCs the level of insulin mRNA was low and decreased over time (p < 0.01), and the effect of TFF3 vs. Control was limited to day 2 where TFF3 treated cells expressed less insulin mRNA (0.4-fold vs. Ctrl.) (Fig. 3D). TFF3 did not affect insulin mRNA expression in neonatal rat islets (data not shown).
Figure 3.
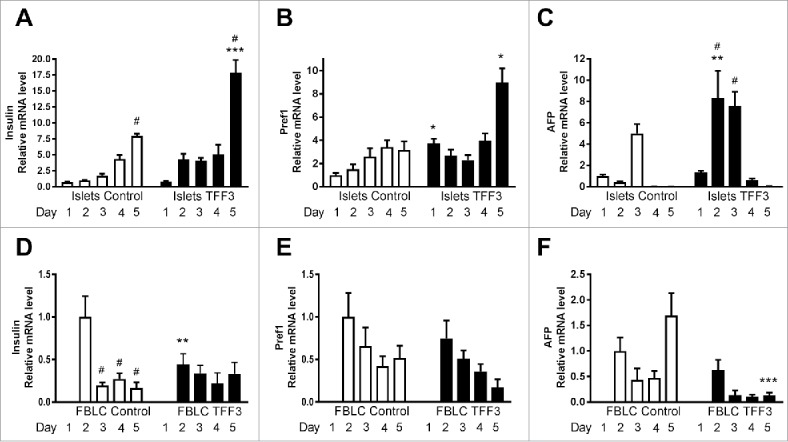
TFF3 treatment of ex vivo fetal pancreas cultures: Effects on mRNA levels of markers of differentiation in islet cells and FBLCs. In the fetal ex vivo pancreas culture model, islet-cells sprouting from the underlying fibroblast-like cell layer (FBLCs) were separated from the FBLC layer by pipette suction, and RNA from the two cell populations were extracted. Messenger RNA levels were determined by qPCR in islet cells (A-C) and FBLCs (D-F). A, D) Insulin mRNA, B, E) Pref1 mRNA, C, F) Afp mRNA. Levels were normalized to Rpl13α mRNA. Statistical comparisons indicated by asterisks (*: p < 0.05, **: p < 0.01, ***: p < 0.001) are between the mRNA levels of the respective transcript with or without TFF3 in the culture medium for the indicated day. Statistical comparisons indicated by hatches (#: p < 0.05) are between the mRNA levels of the respective transcript at the first day of culture compared with the indicated day. Data are from three experiments performed in replicates of 3.
TFF3 upregulated Pref1/Dlk1 mRNA levels at day 1 (by 3.7-fold, p < 0.05) and at day 5 (by 2.8-fold, p < 0.001) in the islet cell fraction (Fig. 3B), whereas in FBLCs there was no significant effect of TFF3 (Fig. 3E) and transcript levels of Pref1/Dlk1 decreased over time. Alpha fetoprotein (Afp) is regulated perinatally in liver21 and was tested in the fetal pancreas culture model. TFF3 treatment increased Afp mRNA levels markedly on day 2 (19-fold, p < 0.01) (Fig. 3C). In FBLCs the relative mRNA level of Afp was lower than in islet cells, decreased during the culture time and were decreased by TFF3 treatment (p = 0.0005). There was no impact of TFF3 on mRNA levels of Musculoaponeurotic Fibrosarcoma Oncogene Homolog (Maf) A, MafB or neurogenin 3 (Ngn3) (data not shown).
Basal insulin secretion from these cultures was unaffected by TFF3 (Fig. 4A) and there was no effect of TFF3 treatment on insulin secretion by neonatal rat islets (Fig. 4B (low glucose, 2mM) and Fig. 4C (high glucose, 20mM)) confirming our previous results.9
Figure 4.
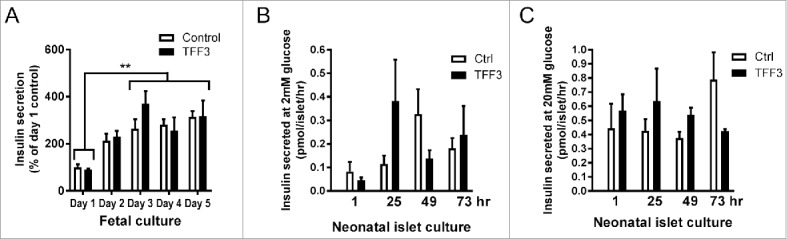
Insulin secretion from ex vivo fetal pancreas cultures and isolated rat neonatal islets. A) Collagenase-treated fetal pancreas were cultured ex vivo in the absence (white bars) or presence (black bars) of TFF3 (200ng/mL) for 5 days. Each day the culture medium was aspirated and the insulin secreted into the media the preceding 24 hrs was determined. **: p < 0.05 between insulin secretion at Day 1 of culture compared with Days 3 to 5. B) Insulin secretion of neonatal rat islets at 2mM glucose after 1, 25, 49 and 73 hrs of culture with TFF3 (200ng/mL). C) Insulin secretion of neonatal rat islets at 20mM glucose after 1, 25, 49 and 73 hrs of culture with TFF3 (200ng/mL). Data are from 4 experiments performed in replicates of 3.
TFF3 mRNA regulation in ex vivo cultured fetal pancreas
TFF3 mRNA levels were measured at different time-points during the culture period in sprouting islets and FBLCs. TFF3 mRNA levels increased1.6-fold by day 4 and 3-fold up-regulation by day 5 vs. day 0 (p < 0.01 by 2-way ANOVA) (Fig. 5). Human growth hormone (GH) treatment of fetal cultures markedly increased TFF3 mRNA levels in islet cells: 11.7 ± 0.7-fold by day 4 measured by qPCR (p < 0.001)(Fig. 5). Thus, in this ex vivo fetal culture system TFF3 mRNA expression levels are associated with the formation of islets. Moreover, TFF3 expression in fetal or immature islet cells appears to be more up-regulated by GH than adult islets, which increase TFF3 levels by 3-fold following GH treatment.9
Figure 5.
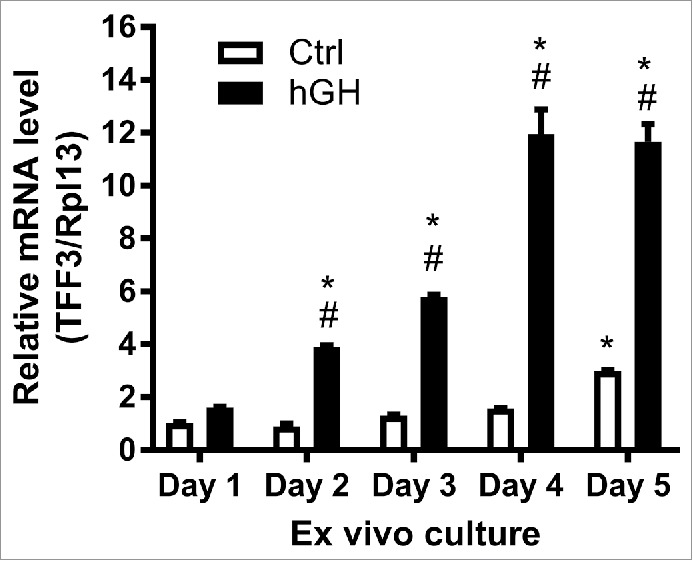
Expression of TFF3 mRNA in ex vivo fetal pancreas cultures. Fetal pancreas was cultured ex vivo in the presence or absence of human growth hormone (hGH) (500ng/mL). Islet-cells sprouting from the underlying fibroblast-like cell layer were harvested by pipette suction. Messenger RNA expression of TFF3 in islet-cells was measured by qPCR. #: p<0.001 for the comparison between Ctrl and hGH treated at the same time point, *:p<0.05 for the comparison between the mRNA level at Day 1 and the indicated day. Data are from 3 experiments.
Effects of TFF3 on adhesion, migration and proliferation of clonal β-cells
In order to determine the effects of TFF3 on β-cell adhesion, a real-time cell monitoring system (xCELLigence System) based on impedance was used to measure effects of exogenous TFF3 on cell spreading and growth. A typical experiment with INS-1E cells is shown in Fig. 6A: After seeding there is an initial rise in Cell Index, which corresponds to the initial adhesion of cells to the substrate. This period lasts for up to 2 hrs and is followed by a period of almost linear increase in Cell Index lasting for up to 10 hrs, which can be interpreted as a growth or spreading phase of cells occurring before cell division. TFF3 treatment significantly increased the rate of both initial cell attachment (Fig. 6B) (p < 0.001 by 2-way ANOVA) and later cellular spreading (Fig. 6C). The effect on cell spreading is significant at lower cell densities (17% increase at 30.000 cells/well and 30% increase at 50.000 cells/well) but not at higher densities of seeding (60.000 cells/well) (Fig. 6C); this most likely reflects the fact that confluent cell layers are obtained at higher densities of seeding. Thus, similarly to ex vivo cultured pancreas, INS-1E β-cells respond to TFF3 treatment by increasing attachment and spreading.
Figure 6.
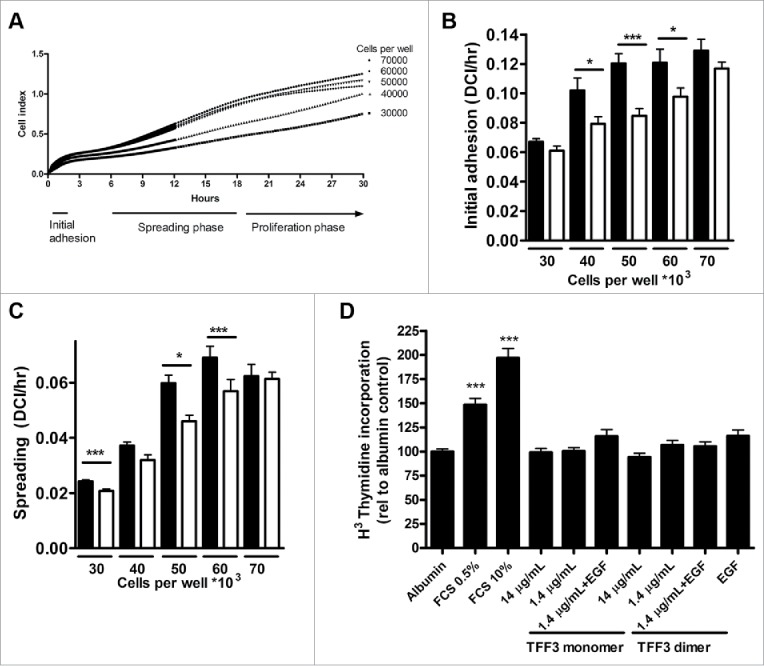
Effects of TFF3 on adhesion, spreading and proliferation Adhesion and spreading of clonal β-cells (INS-1E) were determined using a real-time cell analyzer (xCELLigence). A) Typical xCELLigence experiment curve showing Cell Index over time with increasing number (30.000 to 70.000 cells) of seeded INS-1E cells/96 well. Underneath is indicated 3 phases of cell growth: ‘initial adhesion’, ‘spreading phase’ and ‘proliferation phase’. B) Quantification of initial adhesion: Slopes of curves during initial adhesion phase (unit is delta Cell Index (DC)/hr). Black bars: TFF3 (200ng/mL), white bars: Control. C) Quantification of cell spreading: Slopes of curves during spreading phase (unit is delta Cell Index (DC)/hr). Black bars: TFF3 (200ng/mL), white bars: Control. Data are from three experiments performed in replicates of 3–6. *: p < 0.05, ***: p < 0.001. D) Effects of TFF3 on proliferation using H3 thymidine incorporation in INS-1E cells treated with TFF3 monomer or dimer. Concentrations of TFF3 were as indicated, and EGF was used at 200pM. Data are from 5 experiments performed in replicates of 3–6. *: p < 0.05, ***: p < 0.001.
Impedance measurements do not distinguish between cellular spreading and cellular proliferation, as both features increase the area covered by cells in the growth vessel. In order to determine the effect of TFF3 on cellular proliferation we performed 3H-thymidine incorporation assays. Addition of TFF3 in various amounts did not increase proliferation of INS-1E cells, whereas addition of fetal calf serum (10%) increased labeling 2-fold (p < 0.001 vs. albumin control) (Fig. 6D).
Intracellular signaling elicited by TFF3
TFF3 has been reported to induce tyrosine phosphorylation of the epidermal growth factor receptor (EGFR) and β-catenin in epithelial cell types in connection with its stimulation of migration,15 but this has not been determined in pancreatic β-cells. Immunoprecipitation experiments of EGFR in INS-1E cells stimulated with TFF3 and immunoblotted for phosphotyrosine show a consistent tyrosine phosphorylation of EGFR (Fig. 7A, B). The EGFR tyrosine phosphorylation is time and concentration dependent with TFF3 stimulating phosphorylation at 100nM (Fig. 7A) and showing maximal stimulation after 5 min (Fig. 7B). Immunoprecipitation of β-catenin followed by phosphotyrosine immunoblotting indicates that TFF3 might also dose-dependently stimulate phosphorylation of β-catenin in INS-1E cells, but these findings were not statistically significant (Fig. 7C). EGF induced phosphorylation of the EGFR was robust in the INS-1E cell line (Fig. 7D). Thus, intracellular signaling using the EGFR pathway was initiated following TFF3 treatment of cells. The phosphorylation of EGFR suggests that TFF3 may enhance cytoskeletal re-arrangements, which is in accordance with its effect on spreading.
Figure 7.
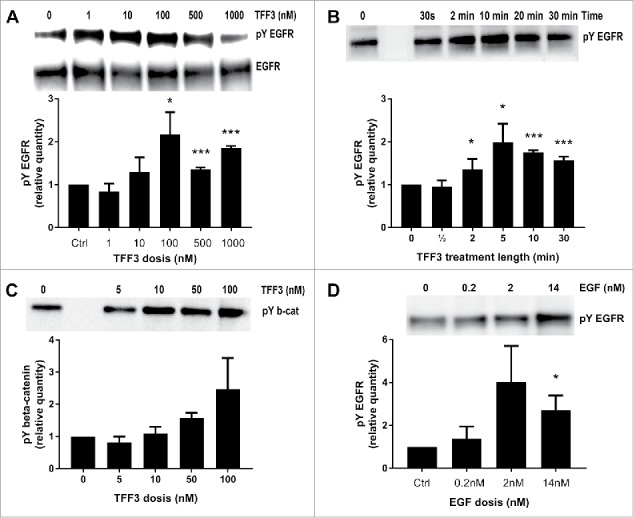
TFF3 induced tyrosine phosphorylation of EGFR and β-catenin. Images from western blots of lysates from INS-E cells stimulated with TFF3 or epidermal growth factor (EGF) and immunoprecipitated with anti-EGFR or anti-β-catenin antibodies. Shown are representative images (top) and quantification of 2–5 blots (bar diagrams below). A) INS-1E cells were stimulated with increasing concentrations of TFF3 (0-1000nM) for 10 min before protein extraction, EGFR immunoprecipitation and blotting for phosphotyrosine (pY EGFR) (top pane). Blots were stripped and reprobed with EGFR antibody (EGFR, middle pane). B) INS-1E cells were stimulated with TFF3 (100nM) for up to 30 min before protein extraction, EGFR immunoprecipitation and blotting for phosphotyrosine (pY EGFR). C) INS-1E cells were stimulated with increasing concentrations of TFF3 for 10 min before protein extraction, β-catenin immunoprecipitation and blotting for phosphotyrosine (pY b-cat). D) INS-1E cells were stimulated with EGF (0-14nM) for 5 min before protein extraction, EGFR immunoprecipitation and blotting for phosphotyrosine (pY EGFR). Representative blots are shown, n = 2-5 for each experiment. *: p < 0.05, ***: p < 0.001 for the comparisons between indicated treatments and the corresponding control condition.
Discussion
The regulation of TFF3 mRNA in perinatal pancreas occurs in context with the large change in pancreas and islet function during and after birth, where the nutritional status of the newborn changes. The prolonged and higher expression of TFF3 transcript in the pancreas from the offspring of dams fed a low protein diet during gestation may be a compensatory, though inadequate, mechanism aiming at enhancing β-cell maturation, which is consistent with premature maturation of β-cells in individuals malnourished during fetal life.20,22,23
Similarly, TFF3 does not induce proliferation of clonal β-cells (Fig. 6D), which is concordant with earlier findings in isolated islets.9 Thus, the increase in Cell Index by TFF3 treatment is due to an increase in cell attachment and spreading rather than an increase in cell number (Fig. 6B and Fig. 6C). These findings are in contrast to Fueger et al.,10 who found that adenovirus mediated TFF3 over-expression in β-cells lead to proliferation. This may indicate that endogenous TFF3 could have different functions than exogenous added TFF3. When TFF3 is administered as a recombinant, purified protein, it does not increase proliferation in INS-1E cells, underscoring the observation that TFF3 increases adhesion and spreading of β-cells. Cellular spreading was also increased by TFF3 in the ex vivo fetal pancreas culture system (Fig. 2), coinciding with a marked increase in the expression of insulin mRNA (Fig. 3A) in the sprouting islet cells. However, since insulin content was not measured in these experiments, it remains to be determined if the actual insulin content is increased by TFF3. Also, TFF3 treatment did not increase insulin secretion from ex vivo cultured fetal pancreas (Fig. 4A) or rat neonatal islets (Fig. 4B and Fig. 4C), in accordance with previous data.9
There could be a limited perinatal time window for the action of TFF3, which mirrors the remarkable up-regulation of TFF3 transcript on the day of birth. TFF3 treatment seems to accelerate islet cell maturation during the culture period: Insulin mRNA levels in TFF3 treated islet-fractions at day 2 are at level with control levels of insulin mRNA at day 4 (Fig. 3A). Pref1/Dlk1 mRNA levels in TFF3 treated islet-fractions at day 1 are at level with control levels of Pref1/Dlk1 mRNA at day 3 (Fig. 3B).
Pref1/Dlk1 is among the 10 most β-cell enriched transcripts24 and is highly expressed in β-cells in the perinatal period and in pregnancy and is upregulated by GH and prolactin.25 very much like TFF3. Pref1/Dlk1 was recently shown to induce differentiation of pancreatic ductal cells to insulin producing cells.26 Pref1/Dlk1 has been shown to act as a non-canonical inhibitor of Notch1.27 and is therefore conceivable that both TFF3 and Pref1/Dlk1 contribute to β-cell development. Membrane bound Pref1/Dlk1 inhibits the growth of adipose tissue by inhibition of preadipocyte proliferation.28 and over-expression of Pref1/Dlk1 inhibits proliferation of the clonal β-cell line RINm5F.29 However, the proliferation and protein synthetic activity of neonatal b-cells did not differ between high and low Pref1/Dlk1 expressing b-cells indicating that Pref1/Dlk1 is not a direct regulator of b-cell maturation.30 In neonatal islets, the expression of Pref1/Dlk1 was higher in the low insulin containing cells and vice versa.29 Furthermore, TFF3 was highly expressed in adult human pancreatic duct cells.9 and over-expression of Pref1/Dlk1 promoted the differentiation of pancreatic ductal cells to insulin-secreting cells.26 Thus, the increased Pref1/Dlk1 expression promoted by TFF3 in the ex vivo fetal pancreas culture model (Fig. 3B) may suggest that both factors have permissive functions in β-cell differentiation. The stimulation of EGFR activation by TFF3 is shared with several β-cell growth and differentiation factors like GLP-1.31 and placental lactogen.32 Thus EGFR activation seems to be required but not sufficient for proliferation and differentiation of β-cells and may therefore play a permissive role in these processes.
Interestingly, liver TFF3 expression was shown to be highly suppressed by galactose metabolism and after high fat-high sucrose feeding,33 which is in accordance with the down-regulation of TFF3 levels observed in pancreas at P2 (Fig. 1A). However, the specific metabolites and/or transcriptional pathways controlling the TFF3 response to nutrients have not been identified.33 Since the low protein malnutrition model shows delayed β-cell development and fewer adult β-cells,22,23 the increased levels of TFF3 mRNA may be a compensation, which promotes β-cell maturation in order to cope with physiological demands for insulin after birth. In the case of fetal low protein malnutrition, however, this maturation then occurs before enough β-cells have formed resulting in a lower number of β-cells in the mature pancreas. Thus, we suggest that TFF3 could be a useful factor in promoting maturation of in vitro generated β-cells from embryonic or pluripotent stem cells possibly in synergy with Pref1/Dlk1. However, the specific molecular mechanisms remain to be determined.
Materials and methods
Materials
Chemicals were from Sigma-Aldrich and media from Invitrogen/Life Technologies unless otherwise stated. Human GH was a gift from Novo Nordisk A/S. Recombinant rat TFF3 and antibodies for TFF3 have been described.9,34 Antibodies or staining reagents: anti-EGFR (sc-03, Santa Cruz Biotechnology), anti-phospho tyrosine 4G10 (05-321, Millipore), anti-β-catenin (610153, BD Biosciences), anti-insulin, anti-rabbit and anti-mouse HRP or AP conjugated antibodies (DAKO).
Perinatal tissue sampling, cell culture and tissue treatments
Time-mated Wistar rats (age 10–11 weeks) (Taconic Europe) were transferred to local facilities at least one week prior to experiments. Animals had free access to food and water and were kept at a 12 hr light-12 hr dark cycle. Two isocaloric diets were used: Low protein diet (8% casein; LP; Hope Farms 4400.00, Woerden, NL) or Normal protein (20% casein; NP; Hope Farms 4400.01).23 Rats were killed at embryonic day 20 (E20), postnatal day 0 (P0) and P2, offspring decapitated and their pancreas removed and placed directly in cold TriReagent (SigmaAldrich) for subsequent RNA isolation. The animal handling and experiments were approved by the local animal care and use committee and approved by the Animal Experiments Inspectorate in Denmark. Thus, all institutional and national guidelines for the care and use of laboratory animals were followed.
The insulin-producing β-cell line INS-1E was kindly provided by Dr. Claes Wollheim, University of Geneva, Switzerland and cultured as described previously.35
Ex vivo cultures of fetal rat pancreas were obtained from embryos at E21.5 according to the procedure described by Hellerstrom et al.19 Briefly, embryos were rapidly dissected, pancreata removed and placed in chilled HBSS. Pancreata were subsequently hand shaken for 4 min in collagenase (1.5µg/mL), washed 3 times in HBSS and placed in cell culture dishes (6cm), 4 pancreata per dish in RPMI 1640 supplemented with NaHCO3, penicillin-streptomycin, HEPES and 10% newborn calf serum. Cultures were incubated at 37°C in 5% CO2, and medium was changed daily. Harvested medium was saved for determination of insulin. Islets and fibroblast-like cells (FBLCs) were harvested separately at day 1, 2, 3, 4 and 5. This was done following media change by carefully suctioning off the islets before harvesting the fibroblasts. Cultures were inspected by microscopy to ensure that all islets were removed and that the fibroblasts remained.
Immunohistochemistry
Tissue samples for immunohistochemistry were fixed in 4% paraformaldehyde overnight and embedded in paraffin. Sections of 3µm were blocked with Protein Block (Dako, Agilent, Glostrup, Denmark), and incubated with primary antibodies overnight (guinea pig anti swine insulin, 1:1500 dilution (Dako, Agilent, Glostrup, Denmark)) and rabbit anti-TFF3 dimer as described previously9 and secondary antibodies for 2hrs.9 Unfortunately, due to the scarcity of the material immunohistochemistry was performed only in pancreatic tissue from pups of dams fed a normal (chow) diet during gestation.
Immunoprecipitation and western blotting
INS-1E cells were lysed in RIPA buffer as described previously.36 One milligram of protein extracts were incubated with antibody as indicated overnight at 4°C. Complexes were precipitated using Dynabeads M-280 sheep anti-rabbit or anti-mouse IgG according to instructions from the manufacturer. The antigens were eluted by incubation for 5 min at 95°C in RIPA buffer containing 0.11 μM β-mercaptoethanol and 1x NuPage LDS sample buffer (Invitrogen). Proteins were separated by electrophoresis and transferred to nitrocellulose membranes by electroblotting. After blocking the membrane was exposed to primary antibody overnight at 4°C (anti-EGFR, anti-β-catenin, anti-phosphotyrosine and subsequently washed in Tris-buffered saline containing 0.1% Tween20, incubated with peroxidase-conjugated secondary antibody and proteins detected by chemiluminescence using ECL plus Western blotting detection reagent (GE Healthcare). Following phosphotyrosine blotting, blots were stripped (Pierce stripping buffer) and exposed to EGFR or β-catenin antibody to ascertain equal loading. Blots were quantified using Fiji (https://fiji.sc/),37 controlled for loading differences and normalized to the control condition.
RNA isolation and qPCR
Total RNA was isolated using TriReagent according to manufacturer's instructions. The integrity of each RNA sample was tested using an Agilent 2100 Bioanalyzer (Agilent). Samples were only used if the 28S/18S RNA ratio was >2 and the RNA integrity number was >7. RNA for RT-qPCR was reverse transcribed using iScript (BioRad). Quantitative PCR was carried out using the LightCycler 2.0 (Roche) with the FastStart DNA MasterPLUS SYBR Green I kit according to the recommended protocol. Real-time PCR runs were all performed using the same reaction conditions for denaturation, amplification, and extension [initial denaturation for 15 min at 95°C; three-cycling step for 35–45 cycles: 15 s denaturation at 94°C, 20 s annealing at 55°C, 20 s extension at 72°C]. Gel electrophoresis and/or melting curve analysis were used to confirm the fidelity of the PCR. Rpl13α was used for normalization as its expression is unchanged perinatally (Primer sequences are available on request).
Insulin secretion
Rat neonatal islets were isolated and cultured as described previously.36 Aliquots of islets (500/dish) were cultured for 1, 25, 49 and 73 hrs with change of media daily and treated with TFF3 dimer (200ng/mL). The medium was sampled for determination of accumulated insulin. For assessment of insulin secretion 10 islets were pre-incubated 90 min in KRBH supplemented with 2mM glucose, and incubated for another 30 min in KRBH supplemented with 2mM glucose after which islets were transferred to KRBH supplemented with 20mM glucose. The level of insulin in media (from fetal cultures) or KRBH (from islet studies) was determined by competitive ELISA with a rat insulin standard curve38; the first antibody was rabbit polyclonal secondary antibody to guinea pig IgG, IgM and IgA (1µg/mL, ab8522, Abcam) and the second antibody was guinea pig anti-insulin (diluted 1:300,000 not commercially available).
Assays of cell adhesion, proliferation and migration
The xCELLigence System (Roche) was used to evaluate the effects of TFF3 in real-time by measuring electrical impedance across micro-electrodes incorporated in the cell culture plate. The resulting Cell Index is proportional to the area covered by cells and is thus an estimate of average cell area or cell numbers. INS-1E cells (30.000-70.000/well) were seeded in poly-D-lysine coated xCELLigence 96 well plates with or without TFF3 (200ng/mL) in media and placed in an xCELLigence System. Cell Index was monitored for up to 35 hrs; the first 12 hrs every minute and the remaining time every 15 min. The effect of TFF3 on Cell Index was evaluated by calculating slopes of growth curves of individual wells in the intervals of linear increase of Cell Index after seeding (1-2 hrs (attachment phase) and 6–18 hrs (spreading phase)).
For thymidine incorporation assays INS-1E cells were cultured in normal medium in poly-D-lysine coated 96-well plates for 24 hrs. The medium was then changed into 0.5% serum containing medium to synchronize cell cycle. TFF3 or other stimulators was added and 20 hrs later, each well was supplemented with 1μCi 3H-thymidine (GE Healthcare). After 4 hrs of incorporation, cells were harvested in an Automash 2000 (Dynex) and counted using liquid scintillation. In all setups internal control conditions consisted of medium with 0.25% BSA or 10% FCS, and all conditions were made in replicates of 3–5.
Statistical analysis
Results are expressed as mean value ± SEM. Statistical analysis was performed in GraphPad Prism software. Effects of time or concentration were tested using 1-way ANOVA with Tukey's post-tests (Fig. 1, 4, 5, 7). Effects of treatments and time/cell numbers were evaluated using 2-way ANOVA with Bonferroni post-tests (Fig. 3, 6). Differences between treatments were considered significant at a p-value<0.05 (two-tailed).
Funding Statement
This work was supported by the Danish Medical Research Council, the Novo Nordisk Foundation, Juvenile Diabetes Foundation International and the EU Concerted Action: BETACELLTHERAPY.
Abbreviations
- Afp
alpha fetoprotein
- Dlk1
delta-like 1
- EGFR
epidermal growth factor receptor
- FBLC
fibroblast-like cell
- GH
growth hormone
- LP
low protein
- Pref1
preadipocyte factor 1
- TFF3
trefoil factor 3
Disclosure of potential conflicts of interest
LT, LL and LW are employed by Novo Nordisk A/S, a health care company performing research in and producing products for treatment of diabetes mellitus. There are no other conflicts of interests.
Acknowledgments
We are very grateful for the skilled technical assistance of Susanne Nørskov Sørensen, Jacqueline Tybjerg and Kirsten Olesen.
References
- 1.Nielsen JH, Haase TN, Jaksch C, Nalla A, Sostrup B, Nalla AA, Larsen L, Rasmussen M, Dalgaard LT, Gaarn LW, et al. Impact of fetal and neonatal environment on beta cell function and development of diabetes. Acta Obstet Gynecol Scand. 2014;93(11):1109–22. [DOI] [PubMed] [Google Scholar]
- 2.Hellerstrom C. The life story of the pancreatic B cell. Diabetologia. 1984;26(6):393–400. doi: 10.1007/BF00262208. PMID:6381187. [DOI] [PubMed] [Google Scholar]
- 3.Sodoyez-Goffaux F, Sodoyez JC, De Vos CJ, Foa PP. Insulin and glucagon secretion by islets isolated from fetal and neonatal rats. Diabetologia. 1979;16(2):121–3. doi: 10.1007/BF01225461. PMID:365657. [DOI] [PubMed] [Google Scholar]
- 4.Miller K, Kim A, Kilimnik G, Jo J, Moka U, Periwal V, Hara M. Islet formation during the neonatal development in mice. PLoS One. 2009;4(11):e7739. doi: 10.1371/journal.pone.0007739. PMID:19893748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson M, Andersson A, Carlsson PO, Jansson L. Perinatal development of the pancreatic islet microvasculature in rats. J Anat. 2006;208(2):191–6. doi: 10.1111/j.1469-7580.2006.00520.x. PMID:16441563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thim L, May FE. Structure of mammalian trefoil factors and functional insights. Cell Mol Life Sci. 2005;62(24):2956–73. doi: 10.1007/s00018-005-5484-6. PMID:16374584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4(9):721–32. doi: 10.1038/nrm1203. PMID:14506475. [DOI] [PubMed] [Google Scholar]
- 8.Oertel M, Graness A, Thim L, Buhling F, Kalbacher H, Hoffmann W. Trefoil factor family-peptides promote migration of human bronchial epithelial cells: synergistic effect with epidermal growth factor. Am J Respir Cell Mol Biol. 2001;25(4):418–24. doi: 10.1165/ajrcmb.25.4.4429. PMID:11694446. [DOI] [PubMed] [Google Scholar]
- 9.Jackerott M, Lee YC, Mollgard K, Kofod H, Jensen J, Rohleder S, Neubauer N, Gaarn LW, Lykke J, Dodge R, et al. Trefoil factors are expressed in human and rat endocrine pancreas: differential regulation by growth hormone. Endocrinology. 2006;147(12):5752–9. doi: 10.1210/en.2006-0601. PMID:16973727. [DOI] [PubMed] [Google Scholar]
- 10.Fueger PT, Schisler JC, Lu D, Babu DA, Mirmira RG, Newgard CB, Hohmeier HE. Trefoil factor 3 stimulates human and rodent pancreatic islet beta-cell replication with retention of function. Mol Endocrinol. 2008;22(5):1251–9. doi: 10.1210/me.2007-0500. PMID:18258687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinoshita K, Taupin DR, Itoh H, Podolsky DK. Distinct pathways of cell migration and antiapoptotic response to epithelial injury: structure-function analysis of human intestinal trefoil factor. Mol Cell Biol. 2000;20(13):4680–90. doi: 10.1128/MCB.20.13.4680-4690.2000. PMID:10848594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taupin DR, Kinoshita K, Podolsky DK. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci U S A. 2000;97(2):799–804. doi: 10.1073/pnas.97.2.799. PMID:10639160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274(5285):262–5. doi: 10.1126/science.274.5285.262. PMID:8824194. [DOI] [PubMed] [Google Scholar]
- 14.Paulsen FP, Woon CW, Varoga D, Jansen A, Garreis F, Jager K, Amm M, Podolsky DK, Steven P, Barker NP, et al. Intestinal trefoil factor/TFF3 promotes re-epithelialization of corneal wounds. J Biol Chem. 2008;283(19):13418–27. doi: 10.1074/jbc.M800177200. PMID:18326859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D, el-Hariry I, Karayiannakis AJ, Wilding J, Chinery R, Kmiot W, McCrea PD, Gullick WJ, Pignatelli M, et al. Phosphorylation of beta-catenin and epidermal growth factor receptor by intestinal trefoil factor. Lab Invest. 1997;77(6):557–63. PMID:9426392. [PubMed] [Google Scholar]
- 16.Kanai M, Mullen C, Podolsky DK. Intestinal trefoil factor induces inactivation of extracellular signal-regulated protein kinase in intestinal epithelial cells. Proc Natl Acad Sci U S A. 1998;95(1):178–82. doi: 10.1073/pnas.95.1.178. PMID:9419349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jermendy A, Toschi E, Aye T, Koh A, guayo-Mazzucato C, Sharma A, Weir GC, Sgroi D, Bonner-Weir S. Rat neonatal beta cells lack the specialised metabolic phenotype of mature beta cells. Diabetologia. 2011;54(3):594–604. doi: 10.1007/s00125-010-2036-x. PMID:21240476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen L, Rosenstierne MW, Gaarn LW, Bagge A, Pedersen L, Dahmcke CM, Nielsen JH, Dalgaard LT. Expression and Localization of microRNAs in Perinatal Rat Pancreas: Role of miR-21 in Regulation of Cholesterol Metabolism. PLoS One. 2011;6(10):e25997. doi: 10.1371/journal.pone.0025997. PMID:22022489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellerstrom CH, Lewis NJ, Borg H, Johnson R, Freinkel N. Method for large-scale isolation of pancreatic islets by tissue culture of fetal rat pancreas. Diabetes. 1979;28(8):769–76. doi: 10.2337/diab.28.8.769. PMID:376384. [DOI] [PubMed] [Google Scholar]
- 20.Hellerstrom C, Swenne I. Functional maturation and proliferation of fetal pancreatic beta-cells. Diabetes. 1991;40(Suppl)2:89–93. doi: 10.2337/diab.40.2.S89. [DOI] [PubMed] [Google Scholar]
- 21.Spear BT, Jin L, Ramasamy S, Dobierzewska A. Transcriptional control in the mammalian liver: liver development, perinatal repression, and zonal gene regulation. Cell Mol Life Sci. 2006;63(24):2922–38. doi: 10.1007/s00018-006-6258-5. PMID:17041810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reusens B, Theys N, Dumortier O, Goosse K, Remacle C. Maternal malnutrition programs the endocrine pancreas in progeny. Am J Clin Nutr. 2011;94(6 Suppl):1824S–9S. doi: 10.3945/ajcn.110.000729. PMID:21562089. [DOI] [PubMed] [Google Scholar]
- 23.Haase TN, Rasmussen M, Jaksch CA, Gaarn LW, Petersen CK, Billestrup N, Nielsen JH. Growth arrest specific protein (GAS) 6: a role in the regulation of proliferation and functional capacity of the perinatal rat beta cell. Diabetologia. 2013;56(4):763–73. doi: 10.1007/s00125-012-2821-9. PMID:23334461. [DOI] [PubMed] [Google Scholar]
- 24.Nica AC, Ongen H, Irminger JC, Bosco D, Berney T, Antonarakis SE, Halban PA, Dermitzakis ET. Cell-type, allelic, and genetic signatures in the human pancreatic beta cell transcriptome. Genome Res. 2013;23(9):1554–62. doi: 10.1101/gr.150706.112. PMID:23716500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlsson C, Tornehave D, Lindberg K, Galante P, Billestrup N, Michelsen B, Larsson LI, Nielsen JH. Growth hormone and prolactin stimulate the expression of rat preadipocyte factor-1/delta-like protein in pancreatic islets: molecular cloning and expression pattern during development and growth of the endocrine pancreas. Endocrinology. 1997;138(9):3940–8. doi: 10.1210/endo.138.9.5408. PMID:9275085. [DOI] [PubMed] [Google Scholar]
- 26.Rhee M, Lee SH, Kim JW, Ham DS, Park HS, Yang HK, Shin JY, Cho JH, Kim YB, Youn BS, et al. Preadipocyte factor 1 induces pancreatic ductal cell differentiation into insulin-producing cells. Sci Rep. 2016;6:23960. doi: 10.1038/srep23960. PMID:27044861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traustadottir GA, Jensen CH, Thomassen M, Beck HC, Mortensen SB, Laborda J, Baladrón V, Sheikh SP, Andersen DC. Evidence of non-canonical NOTCH signaling: Delta-like 1 homolog (DLK1) directly interacts with the NOTCH1 receptor in mammals. Cell Signal. 2016;28(4):246–54. doi: 10.1016/j.cellsig.2016.01.003. PMID:26791579. [DOI] [PubMed] [Google Scholar]
- 28.Mortensen SB, Jensen CH, Schneider M, Thomassen M, Kruse TA, Laborda J, Baladrón V, Sheikh SP, Andersen DC. Membrane-Tethered Delta-Like 1 Homolog (DLK1) Restricts Adipose Tissue Size by Inhibiting Preadipocyte Proliferation. Diabetes. 2012;61(11):2814–22. doi: 10.2337/db12-0176. PMID:22891218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedrichsen BN, Carlsson C, Moldrup A, Michelsen B, Jensen CH, Teisner B, Nielsen JH. Expression, biosynthesis and release of preadipocyte factor-1/ delta-like protein/fetal antigen-1 in pancreatic beta-cells: possible physiological implications. J Endocrinol. 2003;176(2):257–66. doi: 10.1677/joe.0.1760257. PMID:12553874. [DOI] [PubMed] [Google Scholar]
- 30.Martens GA, Motte E, Kramer G, Gaarn LW, Stange G, Hellemans K, Nielsen JH, Aerts JM, Ling Z, Pipeleers D. Functional characteristics of neonatal rat beta cells with distinct markers. J Mol Endocrinol. 2013;52(1):11–28. doi: 10.1530/JME-13-0106. PMID:24049066 [DOI] [PubMed] [Google Scholar]
- 31.Miettinen P, Ormio P, Hakonen E, Banerjee M, Otonkoski T. EGF receptor in pancreatic beta-cell mass regulation. Biochem Soc Trans. 2008;36(Pt 3):280–5. doi: 10.1042/BST0360280. PMID:18481942. [DOI] [PubMed] [Google Scholar]
- 32.Hakonen E, Ustinov J, Palgi J, Miettinen PJ, Otonkoski T. EGFR signaling promotes beta-cell proliferation and survivin expression during pregnancy. PLoS One. 2014;9(4):e93651. doi: 10.1371/journal.pone.0093651. PMID:24695557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Y, Zhao S, Deng Y, Gordillo R, Ghaben AL, Shao M, Zhang F, Xu P, Li Y, Cao H. Hepatic GALE Regulates Whole-Body Glucose Homeostasis by Modulating Tff3 Expression. Diabetes. 2017;66(11):2789–99. doi: 10.2337/db17-0323. PMID:28877911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thim L, Woldike HF, Nielsen PF, Christensen M, Lynch-Devaney K, Podolsky DK. Characterization of human and rat intestinal trefoil factor produced in yeast. Biochemistry. 1995;34(14):4757–64. doi: 10.1021/bi00014a033. PMID:7718582. [DOI] [PubMed] [Google Scholar]
- 35.Merglen A, Theander S, Rubi B, Chaffard G, Wollheim CB, Maechler P. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology. 2004;145(2):667–78. doi: 10.1210/en.2003-1099. PMID:14592952. [DOI] [PubMed] [Google Scholar]
- 36.Jensen J, Galsgaard ED, Karlsen AE, Lee YC, Nielsen JH. STAT5 activation by human GH protects insulin-producing cells against interleukin-1beta, interferon-gamma and tumour necrosis factor-alpha-induced apoptosis independent of nitric oxide production. J Endocrinol. 2005;187(1):25–36. doi: 10.1677/joe.1.06086. PMID:16214938. [DOI] [PubMed] [Google Scholar]
- 37.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–82. doi: 10.1038/nmeth.2019. PMID:22743772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen K, Karlsen AE, Deckert M, Madsen OD, Serup P, Mandrup-Poulsen T, Nerup J. Beta-cell maturation leads to in vitro sensitivity to cytotoxins. Diabetes. 1999;48(12):2324–32. doi: 10.2337/diabetes.48.12.2324. PMID:10580420. [DOI] [PubMed] [Google Scholar]


