Abstract
Purpose
To examine the associations of energy, macronutrient and food intakes with GWG on 960 pregnant women from the Growing Up in Singapore Towards healthy Outcomes (GUSTO) mother-offspring cohort.
Methods
Dietary intake was assessed at 26–28 weeks’ gestation with a 24-hour recall and 3-day food diary. GWG z-scores were calculated from first (4-13 weeks’ gestation) and last (30-40 weeks gestation) measured weights; inadequate and excessive GWG were defined using the Institute of Medicine recommendations based on weights between 15 and 35 weeks’ gestation. Associations were examined using substitution models for macronutrient composition, with linear or multinomial logistic regressions.
Results
Mean±SD daily energy intake was 1868±598 kcal, and percentage energy intakes were 51.8±8.9% from carbohydrate, 15.7±3.9% from protein and 32.6±7.7% from fat. Higher energy intake (per 500kcal increment) was associated with 0.18 SD higher GWG. In isocaloric diets, higher-carbohydrate and lower-fat intakes (at 5% energy substitution) were associated with 0.07 SD higher GWG, and 14% higher likelihood of excessive GWG. Concordantly, the highest tertile of carbohydrate-rich foods intake was associated with 0.20 SD higher GWG, but the highest tertile of fruit and vegetable intake was independently associated with 40% lower likelihood of inadequate GWG. Additionally, the highest tertile of dairy intake was associated with 0.18 SD lower GWG; and the highest tertile of plant-based protein foods intake was associated with 60% and 34% lower likelihood of inadequate and excessive GWG.
Conclusions
Balancing the proportions of carbohydrates and fat, and a higher intake of plant-based protein foods may be beneficial for achieving optimal GWG.
Keywords: energy, macronutrients, food group, pregnancy, gestational weight gain
Introduction
The associations of inadequate or excessive gestational weight gain (GWG) with adverse pregnancy and child health outcomes are well-documented [1]. Inadequate GWG increases the risks of preterm birth and delivering small-for-gestational-age babies, whereas excessive GWG increases risks of gestational diabetes and caesarean delivery in mothers [1], and increases the offspring's risks of obesity, insulin resistance and cardiovascular diseases in later life [2]. Despite this, approximately 20-30% of women do not gain enough weight, while another 30-50% of women gain excessive weight during pregnancy with a disproportionately higher prevalence of excessive weight gain in women with overweight or obesity [3–5] suggesting a need for effective dietary strategies to manage gestational weight gain, and specifically targeting overweight and obese women.
Human metabolic studies have demonstrated that macronutrient composition plays an important role in weight gain by influencing satiation and thermogenesis [6,7]. For example, carbohydrates are known to be less satiating [7] and produce a smaller thermogenic response than protein [6], thus a high-carbohydrate, low-protein diet may induce excessive energy intake. A recent systematic review of intervention and observational studies examining macronutrient composition and GWG revealed that while energy intake was associated with GWG, the evidence relating macronutrient composition to GWG was less consistent [8]. The overall quality of the included studies was low, with many not adjusting for energy intake and important confounding variables such as pre-pregnancy BMI and physical activity during pregnancy, and failing to account for the inter-relationships among macronutrients.
It is important to investigate the effects of different food components on GWG because individuals consume a combination of foods rather than individual nutrients. Yet, studies examining the types of foods consumed during pregnancy and their influence on GWG are scarce and existing results are conflicting. Furthermore, the food groups examined vary for each study with minimal overlap, making it difficult to conclude which food components are beneficial for adequate GWG. Evidence from observational studies on fried food [9,10] and sugar-sweetened beverages [11,12] are generally in line, showing higher intakes to be associated with greater weight gain or higher risks of excessive GWG. Observational findings on milk/dairy, fish, fruit and vegetables, and red meat in relation to GWG are less consistent [11,13,10]. One intervention study found dietary counseling (to reduce intakes of fast food and sweets and increase intakes of fruit, low-fat dairy and whole-wheat grains) to have no effect on GWG [14], likely due to poor compliance – a limitation inherent to intervention study [15]. Findings from cohort studies may help address this limitation by comparing usual healthy or unhealthy eating patterns. No studies to date have examined food group intakes and GWG in Asian populations, who have different food and beverage intake patterns to their Western counterparts, and thus may have different associations with GWG.
In view of the inconsistent study findings, the limitations of existing studies on macronutrient composition and the lack of Asian studies examining food groups, our study aims to address these research gaps in a multi-ethnic Asian population by 1) examining the associations of energy and macronutrient intakes during pregnancy with GWG with the use of substitution models, and 2) examining the associations between different food groups and GWG.
Subjects and Methods
Subjects
Data for the present analysis were drawn from the Growing Up in Singapore Towards healthy Outcomes (GUSTO) study, a prospective mother-offspring cohort study in Singapore [16]. This study monitored the health and mental wellbeing of mothers and growth of their offspring during the antenatal period until the children reach nine years of age. Detailed descriptions of the GUSTO study have been published previously [17,16]. In brief, a total of 1247 pregnant women in their first trimester (<14 weeks) were recruited from KK Women’s and Children’s Hospital (KKH) or National University Hospital (NUH) between June 2009 and September 2010. To be eligible for the study, potential participants had to 1) be Singapore citizens or permanent residents of Chinese, Malay or Indian ethnicity with homogenous parental ethnic background; 2) have the intention to reside in Singapore for the next 5 years; 3) agree to donate birth tissues at delivery; and 4) not be receiving chemotherapy, psychotropic drugs or having type 1 diabetes mellitus. The GUSTO study has received ethical approval from the Institutional Review Board of KKH and NUH. Written informed consent was obtained before participants were enrolled in the study.
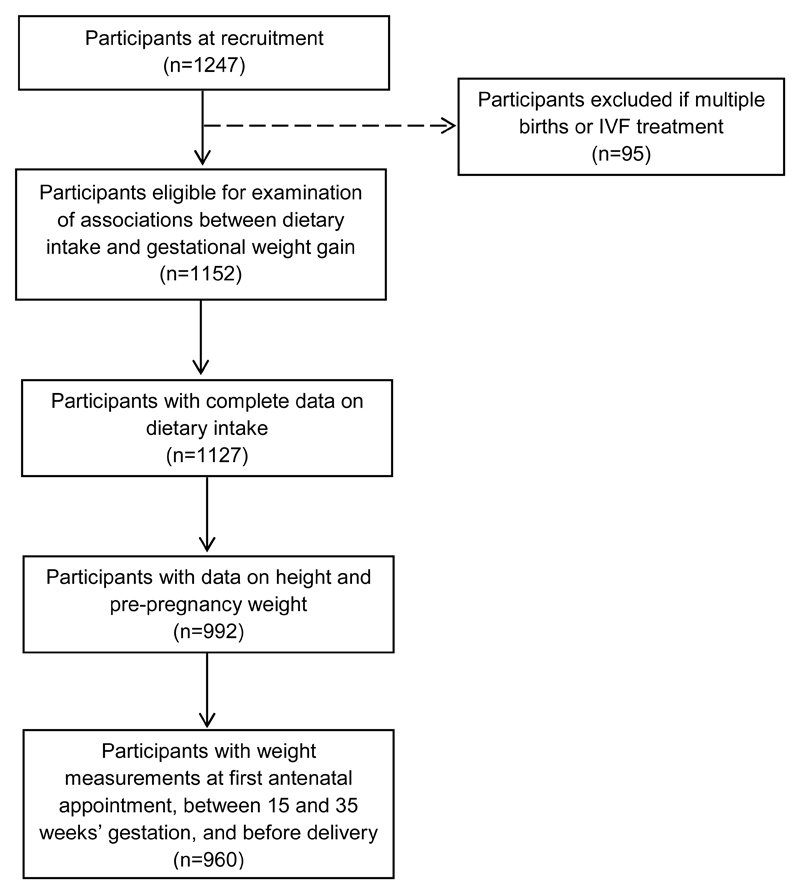
Of the 1247 pregnant women initially recruited, our sample consists of the 960 participants who conceived naturally with singleton pregnancies and provided data on dietary intake during pregnancy, height, reported pre-pregnancy weight, and had weight measurements recorded at their first antenatal appointment, at week 15 and week 35 of gestation, and before delivery. We excluded 287 participants who did not meet these criteria (Figure 1). Those excluded from analyses tended to be younger (mean ± SD: 29.4 ± 5.4 vs 30.5 ± 5.1), of lower educational level (46.2% vs 29.5% attained secondary education or less) and had lower household incomes (28.9% vs 14.2% received less than $2000 household monthly income), compared to those included.
Fig. 1.
Participant flow diagram for analysis of associations between maternal dietary intake and gestational weight gain in the Growing Up in Singapore Towards healthy Outcomes study. IVF, in-vitro fertilization
Dietary intake
Maternal dietary intake was assessed at 26-28 weeks’ gestation using a single 24-hour recall and a 3-day food diary. The 24-hour recall was administered by trained clinical staff on a weekday or weekend day using the 5-stage, multiple-pass interviewing technique [18]. Standard household measuring utensils and food pictures of various portion sizes were presented to assist participants in quantifying their food and beverage intakes. Participants were then given the 3-day food diary (2 weekdays and 1 weekend day) to complete at home with clear instructions from the clinical staff. Completed diaries were collected at the next clinic visit. All participants completed the 24-hour recall, while only a subset (n=193) completed the 3-day food diary. Both methods of dietary assessments were completed on separate occasions. Due to the small number of participants having completed 3-day food diary, the main analyses were based on dietary intakes estimated from the 24-hour recall and subgroup analyses were based on dietary intakes estimated from 24-hour recall and 3-day food diary (refer to Statistical Analysis)
Nutrient intakes were estimated using nutrient analysis software (Dietplan, Forestfield Software, UK) based on a food composition database containing local foods with slight modifications to correct for inaccuracies. For local dishes not found in the food composition database, their nutrient compositions were calculated based on nutrition values of the ingredients from generic recipes. For individual food items not found in the database, nutrient information was obtained from either food labels or the US Department of Agriculture nutrient database (for food products commonly imported from the Western countries) [19].
Additionally, food and beverage items with similar nutrient composition and common culinary uses were combined to produce 68 sub-groups of food, which were further grouped into 8 major food groups a priori: animal-based protein foods (e.g. poultry, meat and fish), plant-based protein foods (e.g. legumes and nuts), fruit and vegetables, grains (e.g. bread, rice and noodles), dairy products (e.g. milk, yoghurt and cheese), fast foods and savoury snacks (e.g. burger, pizza and hot chips), desserts and sweet snacks (e.g. pastries, cakes and biscuits), and sugar-sweetened beverages (e.g. carbonated drinks, fruit juices and cordials) (Supplemental Table 1, Online Resource).
Gestational Weight Gain
Serial pregnancy weight measurements with the corresponding gestational age and date were extracted from clinical obstetric records by clinically trained midwives at various time points throughout pregnancy.
GWG z-score was calculated for each participant using the formula z = (observed weight gain - mean)/SD provided by Hutcheon et al [20]. Each participant’s observed weight gain was defined as the difference between the weight measured at time of delivery and the weight measured at first antenatal visit, which was first natural-log transformed before substituting into the formula. Gestational-age-specific means and SDs (natural-log transformed) of the reference population were also obtained from the chart provided by Hutcheon et al [20], and then entered into the formula. Compared to conventional measures of GWG, the use of GWG z-score is advantageous as it is standardized for gestational age [20]. This is particularly useful in our cohort as there were large variations in timings of the weight measured at first antenatal appointment (between 4-13 weeks’ gestation) and the weight measured at the time of delivery (between 30-40 weeks’ gestation).
Additionally, participants were classified into groups of inadequate, adequate and excessive weight gain based on the Institute of Medicine (IOM) recommended rate of weight gain (kg/week) in the second and third trimesters according to pre-pregnancy BMI category [1]. Pre-pregnancy BMI (kg/m2) were based on self-reported pre-pregnancy weights collected during recruitment, and height measured with a stadiometer (SECA model 213) at the 26-28 weeks’ gestation follow-up visit. To compute rate of weight gain, linear mixed-effects model with the Best Linear Unbiased Predictor was used to estimate linear trajectory of GWG per week [21], using weight measurements recorded at multiple time points (≥2 time points) between 15 to 35 weeks’ gestation for each individual. Inadequate weight gain was defined as a weight gain rate less than the recommended lower limit; whereas excessive weight gain was defined as a weight gain rate greater than the recommended upper limit. To minimize self-reporting bias, pre-pregnancy weights were not used in the estimation of weight gain rate.
Co-variates
Potential confounding variables were identified a priori from previous studies [22,13,23,9,10]. Information on maternal age, ethnicity (Chinese, Malay, Indian), highest education level (secondary or lower, technical college/GCE “A” levels, University), monthly household income ($<1999, $2000-5999, $>6000) and parity (0, ≥1) were obtained at study recruitment. Participants returned for a follow-up visit at 26–28 weeks’ gestation, during which self-reported physical activity (defined as minutes of metabolic equivalents of task (MET.mins), alcohol consumption, and cigarette smoking habits during pregnancy were ascertained. Oral glucose tolerance tests were performed at the same clinic visit to determine if participants developed gestational diabetes mellitus (GDM) based on the 1999 WHO criteria [24].
Statistical analysis
Energy (per 500kcal increment) and macronutrient intakes were treated as continuous variables. Macronutrient intakes were adjusted for energy intake using the nutrient density method, and expressed per 5% increment in energy intake. Food group intakes were divided into tertiles. A large proportion of participants reported not consuming food items in the following food groups: plant-based protein foods, dairy, fast foods and savoury snacks, desserts and sweet snacks, and sugar-sweetened beverages. Hence, for these food groups, participants with no intake were allocated into Tertile 1, and the remaining participants were allocated into Tertiles 2 and 3 with approximately the same number per tertile. For all analyses, they were entered into the statistical models as tertiles (using the lowest tertile as reference) and as ordinal variables (tests of linear trend across tertiles).
Participant characteristics, nutrients and food groups intakes according to GWG status were compared using χ2 test for categorical variables, oneway ANOVA (for continuous variable with normal distribution) or Kruskal-Wallis (for continuous variable with skewed distribution) tests. For significant ANOVA or Kruskal-Wallis test results, post hoc analysis with Bonferroni correction was carried out to identify the group(s) which differed.
Multinomial logistic regression was used to examine the associations between dietary intake and the polytomous GWG status (inadequate, adequate and excessive) with adequate GWG as reference, while linear regression was used to examine associations with GWG z-scores. For analysis of macronutrient composition, substitution models [25] were used to evaluate the effects of one macronutrient relative to another in isocaloric diets (total energy held constant). Carbohydrate was entered into the model together with protein and total energy intake (Model 1) such that an increase in percentage of energy intake from carbohydrate is accompanied by a decrease in percentage energy intake from fat. The effect estimate can be interpreted as the effect of increasing carbohydrate intake at the expense of fat, while keeping total energy constant. Likewise, the effect of increasing carbohydrate intake at the expense of protein (including carbohydrate, fat and energy intakes in the model), and the effect of increasing protein intake at the expense of fat (including protein, carbohydrate and energy intakes in the model) in isocaloric diets were examined. The substitution models then included adjustment for maternal age, ethnicity, education, income, physical activity, alcohol consumption, smoking, parity and GDM status (Model 2). The models examining associations with GWG z-scores included an additional adjustment for pre-pregnancy BMI. For analysis of food groups, an unadjusted model was first created for each food group (Unadjusted); then included adjustment for maternal energy intake and confounders as per Model 2 above.
In order to test the robustness of main analyses findings, subgroup analyses were performed combining data from the 24-hour recall and 3-day food diary using the Multiple Source Method (MSM) [26], in the subset of participants who has completed both dietary assessments (n=193). The MSM provides an estimation of usual dietary intake combining data from multiple short-term dietary assessments and consumption frequency information. As there was no consumption frequency information available, a few a priori assumptions were made: (1) all individuals have frequent consumption of energy, macronutrients, and core food groups (i.e. animal-based protein foods, fruit and vegetables, grains and dairy products) based on a reported intake in 24-hour recall and 3-day food diary; (2) assume 50% of individuals not reporting an intake in the 24-hour recall and 3-day food diary (e.g. fast foods and savoury snacks, desserts and sweet snacks, and sugar-sweetened beverages) will consume during other periods not captured by both dietary assessments.
Upon observing significant associations of higher-carbohydrate, lower-fat intakes with higher GWG z-scores and higher likelihood of excessive GWG, an ad hoc analysis was conducted to examine associations of ‘carbohydrate-rich foods’ intake (grains, desserts and sweet snacks, and sugar-sweetened beverages) with GWG status and z-scores, to determine whether the quality of carbohydrate play a role in GWG.
The IOM recommendations for GWG were based on World Health Organization’s international BMI classification rather than classifications applicable to Asian populations [40] and may result in misclassification bias. As such, we further performed a sensitivity analysis using GWG status classified according to Asian BMI cut-offs.
Missing data were imputed 20 times using multiple imputation technique with chained equations (20 times) for the following confounding variables: n=32 education, n=50 income, n=72 physical activity, n=23 alcohol consumption and n=59 GDM. The results of the 20 analyses were pooled using the Rubin's rule [27]. All analyses were conducted using Stata version 14 (StataCorp LP, College Station, TX, USA). Two sided P-values<0.05 were accepted as statistically significant.
Results
Participant characteristics
Approximately 12% of participants had inadequate GWG and 64% had excessive GWG. Women with inadequate GWG were more likely to be Indian and have GDM, less likely to be overweight or obese, and had the highest physical activity level (Table 1). These women also had the lowest energy and fruit and vegetables intakes, consumed less grains compared to those with excessive GWG, and least likely to consume plant-based protein foods. Women with excessive GWG were more likely to be Malay and to be overweight or obese, less likely to have GDM, and had the lowest physical activity level. Additionally, these women had the highest energy and grains intakes, consumed more fruit and vegetables compared to those with inadequate GWG, and tended to consume less plant-based protein foods than those with adequate GWG but more than those with inadequate GWG.
Table 1.
Participant characteristics, nutrients and food groups intakes according to gestational weight gain (GWG) status in the Growing Up in Singapore Towards healthy Outcomes (GUSTO) study
| Characteristics | Alla n=960 |
Inadequate GWG n=114 |
Adequate GWG n=231 |
Excessive GWG n=615 |
Pb |
|---|---|---|---|---|---|
| Age, year | 30.5 ± 5.1 | 30.4 ± 5.2 | 30.7 ± 5.1 | 30.5 ± 5.1 | 0.791 |
| Ethnicity | |||||
| Chinese | 531 (55.3) | 68 (59.6) | 143 (61.8) | 319 (52.1) | 0.006* |
| Malay | 252 (26.3) | 22 (19.3) | 44 (19.1) | 185 (30.2) | |
| Indian | 177 (18.4) | 24 (21.1) | 44 (19.1) | 108 (17.7) | |
| Education | |||||
| Secondary or lower | 274 (29.5) | 30 (27.0) | 73 (33.0) | 171 (28.7) | 0.124 |
| Post-secondary | 341 (36.8) | 34 (30.6) | 73 (33.0) | 234 (39.3) | |
| University | 313 (33.7) | 47 (42.3) | 75 (34.0) | 191 (32.0) | |
| Monthly household income | |||||
| <1999 | 129 (14.2) | 18 (16.5) | 31 (14.4) | 80 (13.7) | 0.163 |
| 2000-5999 | 518 (56.9) | 50 (45.9) | 127 (58.8) | 341 (58.3) | |
| >6000 | 263 (28.9) | 41 (37.6) | 58 (26.8) | 164 (28.0) | |
| Pre-pregnancy BMI (kg/m2) | |||||
| Underweight (<18.5) | 116 (12.1) | 41 (35.3) | 55 (47.4) | 20 (17.2) | <0.001* |
| Normal weight (18.5-24.9) | 606 (63.1) | 63 (10.4) | 159 (26.2) | 384 (63.4) | |
| Overweight (25-29.9) | 169 (17.6) | 9 (5.5) | 14 (8.5) | 142 (86.1) | |
| Obese (>30) | 69 (7.2) | 1 (1.4) | 3 (4.1) | 69 (94.5) | |
| Smoked during pregnancy | |||||
| Yes | 21 (2.2) | 5 (4.4) | 4 (1.7) | 12 (2.0) | 0.229 |
| No | 939 (97.8) | 109 (95.6) | 227 (98.3) | 602 (98.0) | |
| Alcohol intake during pregnancy | |||||
| Yes | 17 (1.8) | 4 (3.6) | 5 (2.2) | 8 (1.3) | 0.227 |
| No | 920 (98.2) | 108 (96.4) | 218 (97.8) | 594 (98.7) | |
| Physical activity, MET.mins/week | 1040 (329, 2673) | 1386 (681, 3119) | 1040 (297, 2142)# | 891 (297, 2349)# | 0.004* |
| Nulliparous | |||||
| Yes | 300 (31.3) | 33 (29.0) | 63 (27.3) | 204 (33.2) | 0.219 |
| No | 660 (68.7) | 81 (71.0) | 168 (72.7) | 411 (66.8) | |
| Gestational Diabetes | |||||
| Yes | 172 (19.1) | 38 (35.2) | 50 (23.3) | 84 (14.5) | <0.001* |
| No | 729 (80.9) | 70 (64.8) | 165 (76.7) | 494 (85.5) | |
| Energy, kcal/day | 1868 ± 598 | 1770 ± 469# | 1822 ± 573#, + | 1904 ± 625+ | 0.036* |
| Carbohydrate, %E | 51.8 ± 8.9 | 50.6 ± 8.6 | 51.1 ± 9.0 | 52.2 ± 8.8 | 0.093 |
| Protein, %E | 15.7 ± 3.9 | 15.8 ± 4.2 | 15.7 ± 3.8 | 15.6 ± 3.8 | 0.810 |
| Total Fat, %E | 32.6 ± 7.7 | 33.6 ± 6.9 | 33.2 ± 7.8 | 32.2 ± 7.7 | 0.091 |
| Animal-based protein foods, g/day | 146.0 (76.9, 232.5) | 135.6 (67.9, 225.6) | 145.5 (63.0, 218.7) | 147.1 (80.0, 236.1) | 0.569 |
| Fruit and vegetables, g/day | 150 (52.9, 291.5) | 111.8 (36.0, 226.2) | 165.9 (56.1, 287.8)# | 153.1 (61.1, 301.0)# | 0.012* |
| Grains, g/day | 360.0 (212.0, 478.7) | 357.2 (212.0, 484.0)# | 305.6 (157.8, 453.1)#, + | 375.0 (218.4, 484.5)+ | 0.044* |
| Plant-based protein foods | |||||
| No intake | 600 (62.5) | 83 (72.8) | 132 (57.1) | 385 (62.5) | 0.018* |
| Some intake | 360 (37.5) | 31 (27.2) | 99 (42.9) | 230 (37.4) | |
| Dairy products | |||||
| No intake | 282 (29.4) | 32 (28.1) | 70 (30.3) | 180 (29.3) | 0.908 |
| Some intake | 678 (70.6) | 82 (71.9) | 161 (69.7) | 435 (70.7) | |
| Fast food and savoury snacks | |||||
| No intake | 533 (55.5) | 70 (61.4) | 137 (59.3) | 326 (53.0) | 0.105 |
| Some intake | 427 (44.5) | 44 (38.6) | 94 (40.7) | 289 (47.0) | |
| Desserts and sweet snacks | |||||
| No intake | 495 (51.6) | 67 (58.8) | 123 (53.3) | 305 (49.6) | 0.166 |
| Some intake | 465 (48.4) | 47 (41.2) | 108 (46.7) | 310 (50.4) | |
| Sugar-sweetened beverages | |||||
| No intake | 432 (45.0) | 53 (46.5) | 104 (45.0) | 275 (44.7) | 0.941 |
| Some intake | 528 (55.0) | 61 (53.5) | 127 (55.0) | 340 (55.3) |
Values are n (%), means ± SDs, or medians (inter-quartile ranges).
BMI, body mass index; %E, percentage of energy intake; GWG, gestational weight gain; MET.mins/week, minutes of metabolic equivalents of task per week
Sample sizes vary due to missing data.
P-values (<0.05 with asterisk) were obtained from chi-square test, one-factor ANOVA or Kruskal-Wallis test with Bonferroni post hoc analysis (#, + groups with the same superscript symbol in a row indicate no significant difference).
Associations of energy and macronutrients intakes with GWG z-scores and status
A higher energy intake (per 500kcal increment) was associated with 0.18 SD higher GWG after adjusting for confounders (95% CI: 0.13, 0.23) (Table 2). Higher-carbohydrate, lower-fat intakes (per 5% energy substitution) in isocaloric diets were associated with 0.07 SD higher GWG (Table 2), and a 14% higher likelihood of excessive GWG in the adjusted models (Table 3). There was a weak trend towards higher-protein, lower-fat intakes and higher GWG z-scores in the adjusted model (95% CI: -0.01, 0.19) (Table 2). No significant associations were observed between a higher-carbohydrate and lower-protein intake and GWG status or z-score.
Table 2.
Associations of maternal energy, macronutrients, and food groups intakes, with gestational weight gain z-scores in 960 women of the Growing Up in Singapore Towards healthy Outcomes (GUSTO) study
| Model 1a | Model 2b | |||
| Energy and Macronutrients | β (95% CI) | P | β (95% CI) | P |
| Energy | 0.23 (0.18, 0.28) | <0.001* | 0.18 (0.13, 0.23) | <0.001* |
| Substitution of Carbohydrate for Fat | 0.05 (0.004, 0.10) | 0.033* | 0.07 (0.03, 0.12) | 0.003* |
| Substitution of Carbohydrate for Protein | -0.02 (-0.11, 0.06) | 0.560 | -0.01 (-0.09, 0.07) | 0.745 |
| Substitution of Protein for Fat | 0.08 (-0.02, 0.19) | 0.121 | 0.09 (-0.01, 0.19) | 0.072 |
| Unadjusted | Model 2b | |||
| Food Groups, g (Median; IQR) | β (95% CI) | P | β (95% CI) | P |
| Animal-based protein foods | ||||
| T1 (48; 0, 77) | (reference) | (reference) | ||
| T2 (146; 124, 171) | 0.13 (-0.03, 0.29) | 0.115 | 0.04 (-0.11, 0.19) | 0.636 |
| T3 (266; 233, 321) | 0.20 (0.04, 0.36) | 0.017* | -0.07 (-0.23, 0.09) | 0.396 |
| P-trend | 0.015* | P-trend | 0.394 | |
| Plant-based protein foods | ||||
| T1 (No intake) | (reference) | (reference) | ||
| T2 (15; 10, 22) | 0.19 (-0.01, 0.39) | 0.064 | 0.12 (-0.07, 0.31) | 0.220 |
| T3 (76; 50, 111) | 0.04 (-0.12, 0.20) | 0.637 | -0.06 (-0.22, 0.09) | 0.437 |
| P-trend | 0.429 | P-trend | 0.575 | |
| Dairy products | ||||
| T1 (No intake) | (reference) | (reference) | ||
| T2 (200; 150, 250) | -0.06 (-0.22, 0.10) | 0.479 | -0.14 (-0.29, 0.01) | 0.064 |
| T3 (450; 350, 500) | -0.02 (-0.19, 0.15) | 0.847 | -0.18 (-0.34, -0.03) | 0.022* |
| P-trend | 0.868 | P-trend | 0.024* | |
| Fruit and vegetables | ||||
| T1 (33; 0, 53) | (reference) | (reference) | ||
| T2 (150; 110, 184) | 0.07 (-0.09, 0.24) | 0.382 | 0.04 (-0.11, 0.19) | 0.626 |
| T3 (340; 292, 482) | 0.26 (0.10, 0.42) | 0.002* | 0.10 (-0.05, 0.26) | 0.191 |
| P-trend | 0.004* | P-trend | 0.210 | |
| Grains | ||||
| T1 (182; 99, 212) | (reference) | (reference) | ||
| T2 (360; 312, 400) | -0.01 (-0.18, 0.15) | 0.872 | -0.08 (-0.23, 0.06) | 0.257 |
| T3 (547; 479, 642) | 0.13 (-0.03, 0.29) | 0.117 | 0.04 (-0.11, 0.20) | 0.589 |
| P-trend | 0.108 | P-trend | 0.598 | |
| Fast food and savoury snacks | ||||
| T1 (No intake) | (reference) | (reference) | ||
| T2 (72; 50, 100) | 0.16 (-0.01, 0.34) | 0.058 | 0.002 (-0.15, 0.16) | 0.980 |
| T3 (231; 174, 316) | 0.16 (-0.01, 0.32) | 0.055 | -0.05 (-0.21, 0.10) | 0.482 |
| P-trend | 0.035* | P-trend | 0.521 | |
| Desserts and sweet snacks | ||||
| T1 (No intake) | (reference) | (reference) | ||
| T2 (45; 27, 68) | 0.04 (-0.11, 0.20) | 0.591 | -0.07 (-0.22, 0.07) | 0.310 |
| T3 (189; 143, 296) | 0.25 (0.08, 0.43) | 0.004* | -0.03 (-0.19, 0.13) | 0.731 |
| P-trend | 0.006* | P-trend | 0.570 | |
| Sugar-sweetened beverages | ||||
| T1 (No intake) | (reference) | (reference) | ||
| T2 (210; 150, 250) | 0.21 (0.05, 0.38) | 0.011* | 0.10 (-0.05, 0.25) | 0.201 |
| T3 (500; 397, 700) | 0.29 (0.13, 0.44) | <0.001* | 0.10 (-0.04, 0.25) | 0.166 |
| P-trend | <0.001* | P-trend | 0.144 | |
| Carbohydrate-rich foodsc | ||||
| T1 (363; 248, 450) | (reference) | |||
| T2 (621; 564, 707) | 0.23 (0.07, 0.40) | 0.006* | 0.09 (-0.06, 0.24) | 0.257 |
| T3 (979; 852, 1207) | 0.50 (0.33, 0.66) | <0.001* | 0.20 (0.03, 0.37) | 0.019* |
| P-trend | <0.001* | P-trend | 0.029* | |
CI, confidence interval; IQR, inter-quartile range; T1, tertile 1; T2, tertile 2; T3, tertile 3
Effect estimates for energy intake are per 500 kcal increment, for macronutrients are per 5% substitution for another macronutrient, and for food groups are per one tertile increase in median intake (P<0.05 with asterisk)
Model 1 adjusted for maternal energy intake only
Model 2 adjusted for maternal energy intake, age, ethnicity, education, income, smoking status, alcohol intake, physical activity, parity, gestational diabetes mellitus and pre-pregnancy BMI.
The combined sum of intakes from grains, desserts and sweet snacks, and sugar-sweetened beverages.
Table 3.
Associations of maternal energy, macronutrients and food groups intakes with gestational weight gain status in 960 women of the Growing Up in Singapore Towards healthy Outcomes (GUSTO) study
| Reference group: Adequate (n=231) | Inadequate (n=114) | Excessive (n=615) | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 1a | Model 2b | |||||
| Energy and Macronutrients | RRR (95% CI) | P | RRR (95% CI) | P | RRR (95% CI) | P | RRR (95% CI) | P |
| Energy | 0.92 (0.76, 1.11) | 0.380 | 0.88 (0.72, 1.07) | 0.206 | 1.10 (0.97, 1.24) | 0.078 | 1.10 (0.97, 1.25) | 0.151 |
| Substitution of Carbohydrate for Fat | 0.90 (0.76, 1.07) | 0.253 | 0.87 (0.72, 1.04) | 0.119 | 1.13 (1.01, 1.28) | 0.038* | 1.14 (1.00, 1.29) | 0.042* |
| Substitution of Carbohydrate for Protein | 0.89 (0.66, 1.19) | 0.423 | 0.88 (0.65, 1.19) | 0.930 | 1.00 (0.82, 1.22) | 0.978 | 0.95 (0.77, 1.17) | 0.619 |
| Substitution of Protein for Fat | 0.85 (0.60, 1.23) | 0.398 | 0.81 (0.56, 1.18) | 0.278 | 1.08 (0.84, 1.38) | 0.547 | 1.15 (0.89, 1.49) | 0.181 |
| Unadjusted | Model 2b | Unadjusted | Model 2b | |||||
| Food groups, g (Median; IQR) | RRR (95% CI) | P | RRR (95% CI) | P | RRR (95% CI) | P | RRR (95% CI) | P |
| Animal-based protein foods | ||||||||
| T1 (48; 0, 77) | (reference) | (reference) | (reference) | (reference) | ||||
| T2 (146; 124, 171) | 0.72 (0.42, 1.25) | 0.250 | 0.83 (0.46, 1.47) | 0.517 | 0.85 (0.59, 1.23) | 0.398 | 0.87 (0.59, 1.27) | 0.471 |
| T3 (266; 233, 321) | 0.90 (0.52, 1.57) | 0.722 | 1.11 (0.60, 2.05) | 0.740 | 1.06 (0.73, 1.55) | 0.751 | 1.03 (0.68, 1.55) | 0.891 |
| P-trend | 0.706 | P-trend | 0.746 | P-trend | 0.750 | P-trend | 0.891 | |
| Plant-based protein foods | ||||||||
| T1 (No intake) | (reference) | (reference) | (reference) | (reference) | ||||
| T2 (15; 10, 22) | 0.69 (0.34, 1.40) | 0.302 | 0.65 (0.31, 1.35) | 0.251 | 1.02 (0.64, 1.61) | 0.942 | 0.97 (0.61, 1.55) | 0.901 |
| T3 (76; 50, 111) | 0.41 (0.23, 0.75) | 0.003* | 0.40 (0.22, 0.75) | 0.004* | 0.70 (0.49, 0.99) | 0.046* | 0.66 (0.46, 0.94) | 0.024* |
| P-trend | 0.003* | P-trend | 0.003* | P-trend | 0.059 | P-trend | 0.031* | |
| Dairy products | ||||||||
| T1 (No intake) | (reference) | (reference) | (reference) | (reference) | ||||
| T2 (200; 150, 250) | 1.00 (0.57, 1.74) | 0.996 | 0.96 (0.54, 1.70) | 0.894 | 0.95 (0.66, 1.38) | 0.807 | 0.93 (0.64, 1.35) | 0.709 |
| T3 (450; 350, 500) | 1.27 (0.72, 2.24) | 0.415 | 1.24 (0.68, 2.25) | 0.490 | 1.18 (0.80, 1.73) | 0.408 | 1.09 (0.73, 1.63) | 0.668 |
| P-trend | 0.410 | P-trend | 0.481 | P-trend | 0.398 | P-trend | 0.663 | |
| Fruit and vegetables | ||||||||
| T1 (33; 0, 53) | (reference) | (reference) | (reference) | (reference) | ||||
| T2 (150; 110, 184) | 0.77 (0.45, 1.32) | 0.346 | 0.76 (0.43, 1.32) | 0.327 | 1.01 (0.69, 1.48) | 0.953 | 0.99 (0.67, 1.46) | 0.962 |
| T3 (340; 292, 482) | 0.43 (0.24, 0.76) | 0.004* | 0.40 (0.22, 0.73) | 0.003* | 0.84 (0.58, 1.22) | 0.370 | 0.83 (0.56, 1.23) | 0.362 |
| P-trend | 0.004* | P-trend | 0.003* | P-trend | 0.358 | P-trend | 0.351 | |
| Grains | ||||||||
| T1 (182; 99, 212) | (reference) | (reference) | (reference) | (reference) | ||||
| T2 (360; 312, 400) | 0.92 (0.54, 1.57) | 0.757 | 0.98 (0.56, 1.71) | 0.958 | 1.04 (0.72, 1.50) | 0.842 | 0.98 (0.67, 1.44) | 0.941 |
| T3 (547; 479, 642) | 0.76 (0.44, 1.33) | 0.342 | 0.84 (0.46, 1.53) | 0.572 | 1.09 (0.75, 1.58) | 0.637 | 1.01 (0.68, 1.49) | 0.974 |
| P-trend | 0.347 | P-trend | 0.586 | P-trend | 0.636 | P-trend | 0.974 | |
| Unadjusted | Model 2b | Unadjusted | Model 2b | |||||
| RRR (95% CI) | P | RRR (95% CI) | P | RRR (95% CI) | P | RRR (95% CI) | P | |
| Fast food and savoury snacks | ||||||||
| T1 (No intake) | (reference) | (reference) | (reference) | (reference) | ||||
| T2 (72; 50, 100) | 0.80 (0.45, 1.44) | 0.469 | 0.76 (0.41, 1.41) | 0.389 | 1.02 (0.70, 1.50) | 0.912 | 0.95 (0.64, 1.40) | 0.782 |
| T3 (231; 174, 316) | 1.05 (0.58, 1.87) | 0.878 | 1.15 (0.61, 2.16) | 0.656 | 1.61 (1.09, 2.38) | 0.016* | 1.46 (0.96, 2.20) | 0.076 |
| P-trend | 0.953 | P-trend | 0.866 | P-trend | 0.025* | P-trend | 0.120 | |
| Desserts and sweet snacks | ||||||||
| T1 (No intake) | (reference) | (reference) | (reference) | (reference) | ||||
| T2 (45; 27, 68) | 0.79 (0.47, 1.35) | 0.393 | 0.79 (0.46, 1.37) | 0.407 | 0.99 (0.70, 1.41) | 0.970 | 0.94 (0.65, 1.34) | 0.726 |
| T3 (189; 143, 296) | 0.80 (0.43, 1.51) | 0.502 | 0.89 (0.45, 1.75) | 0.738 | 1.43 (0.95, 2.14) | 0.086 | 1.29 (0.84, 1.98) | 0.248 |
| P-trend | 0.397 | P-trend | 0.582 | P-trend | 0.126 | P-trend | 0.357 | |
| Sugar-sweetened beverages | ||||||||
| T1 (No intake) | (reference) | (reference) | (reference) | (reference) | ||||
| T2 (210; 150, 250) | 0.93 (0.53, 1.63) | 0.800 | 0.91 (0.51, 1.63) | 0.753 | 1.09 (0.75, 1.58) | 0.660 | 1.04 (0.71, 1.54) | 0.820 |
| T3 (500; 397, 700) | 0.95 (0.56, 1.61) | 0.858 | 0.98 (0.56, 1.71) | 0.933 | 0.95 (0.66, 1.36) | 0.782 | 0.84 (0.58, 1.23) | 0.379 |
| P-trend | 0.841 | P-trend | 0.897 | P-trend | 0.833 | P-trend | 0.419 | |
| Carbohydrate-rich foodsc | ||||||||
| T1 (363; 248, 450) | (reference) | (reference) | (reference) | (reference) | ||||
| T2 (621; 564, 707) | 0.71 (0.40, 1.28) | 0.256 | 0.68 (0.37, 1.24) | 0.206 | 1.11 (0.76, 1.62) | 0.593 | 0.99 (0.67, 1.48) | 0.978 |
| T3 (979; 852, 1207) | 0.73 (0.40, 1.33) | 0.309 | 0.71 (0.35, 1.43) | 0.336 | 1.41 (0.96, 2.08) | 0.084 | 1.20 (0.77, 1.87) | 0.430 |
| P-trend | 0.282 | P-trend | 0.293 | P-trend | 0.093 | P-trend | 0.452 | |
CI, confidence interval; IQR, inter-quartile range; RRR, relative risk ratio; T1, tertile 1; T2, tertile 2; T3, tertile 3
Effect estimates for energy intake are per 500 kcal increment, for macronutrients are per 5% substitution for another macronutrient, and for food groups are per one tertile increase in median intake (P<0.05 with asterisk)
Model 1 adjusted for maternal energy intake only
Model 2 adjusted for maternal energy intake, age, ethnicity, education, income, smoking status, alcohol intake, physical activity, parity and gestational diabetes mellitus.
The combined sum of intakes from grains, desserts and sweet snacks, and sugar-sweetened beverages.
Associations of food group intakes with GWG z-scores and status
Individual carbohydrate-rich food groups such as grains, desserts and sweet snacks, and sugar-sweetened beverages did not show any independent association with GWG z-scores after adjustment for confounders. When combined together as a single food group – ‘Carbohydrate-rich foods’, we found an association between higher intakes of this food group and higher GWG z-scores (a 0.20 SD higher GWG in T3 compared to T1, 95% CI: 0.03, 0.37) even after adjusting for energy intake and other confounders (Table 2). Additionally, higher intakes of dairy products was associated with lower GWG (0.18 SD lower in T3 compared to T1, 95% CI: -0.34, -0.03) in the adjusted model (Table 2). No significant associations were observed for other food groups with GWG z-scores after adjustment for confounders.
When examining associations with GWG status, it was observed that higher intakes of plant-based protein foods were associated with lower likelihood of inadequate GWG (60% lower risk in T3 compared to T1, 95% CI: 0.22, 0.75) as well as lower likelihood of excessive GWG (34% lower risk in T3 compared to T1, 95% CI: 0.46, 0.94) after adjustment for confounders (Table 3). Additionally, higher fruit and vegetable intakes were associated with a lower likelihood of inadequate GWG (60% lower risk in T3 compared to T1, 95% CI: 0.22, 0.73).
Higher intakes of animal-based protein foods, and fast food and savoury snacks were independently associated with higher GWG z-scores or higher likelihood of excessive GWG in the unadjusted model (P-trends<0.05); these associations were attenuated after adjusting for confounders especially energy intake (Tables 2 and 3).
Subgroup and sensitivity analyses
Results were consistent in the subgroup analysis with GWG z-scores when employing the MSM method (Supplemental Table 2, Online Resource). We further observed higher intakes of fruit and vegetables, and sugar-sweetened beverages to be significantly associated with higher GWG z-scores after adjustment for confounders. Although significance was lost for the association between dairy products and GWG z-scores, the study estimates remained in the same direction.
Similarly, we observed consistent results in the subgroup analysis with GWG status (Supplemental Table 3, Online Resource). The significant associations of a higher-carbohydrate and lower-fat intake, and of a higher intake of plant-based protein foods, with GWG status were not statistically significant in the subgroup analysis but the study estimates remained in the same direction.
Similar study estimates and significance levels were observed when the main analyses were repeated using GWG status classified according to Asian BMI cut-offs (data not shown).
Discussion
In this study, higher energy intake during pregnancy was associated with higher GWG z-scores. When assessed under isocaloric conditions, higher-carbohydrate and lower-fat intakes were significantly associated with higher GWG z-scores and a higher likelihood of excessive GWG statistically, suggesting that dietary macronutrient composition may influence GWG. This finding is consistent with those from the food group analyses, whereby the combined sum of all carbohydrate-rich foods (desserts and sweet snacks, sugar sweetened beverages, grains, fruit and vegetables) in the diet was associated with higher GWG z-scores. Additionally, we found higher intakes of dairy products to associate with lower GWG z-scores, and plant-based protein foods to associate with achieving optimal weight gain during pregnancy.
Our findings support current literature that higher energy intake during pregnancy is associated with greater absolute weight gain or a higher risk of excessive GWG [8]. Likewise, we consistently observed energy intake to be the main determinant of GWG in our food group analyses. Although we found higher intake of fast food and savoury snacks, desserts and sweet snacks, and sugar-sweetened beverages (energy-dense foods) to be associated with higher GWG or higher excessive GWG; these were attenuated with adjustment for energy intake indicating that energy intake is the main driver of these associations. Two previous studies in pregnant women also found an attenuation in association between higher intake of fried food and higher GWG and higher odds of excessive GWG after adjustment for energy intake [9,10].
Of note in this study is the significant association of carbohydrate-fat substitution model with higher GWG z-scores. Whilst a recent systematic review showed no consistent directionality of associations between carbohydrate intake and GWG in the 18 observational studies reviewed [8], it is important to highlight that most of these studies focused on single macronutrient rather than combining different macronutrients in one statistical model, which failed to consider that the effects of one macronutrient may be explained by the other nutrient it replaces in an iso-caloric model [25]. For studies that used the substitution model, one showed increasing mono-unsaturated fat at the expense of carbohydrate intakes to be associated with lower risk of excessive GWG [10]; but another found no significant association with rate of GWG when substituting carbohydrates for fats [28] possibly due to the study population being from a developing country with different sociodemographic and nutritional status to our cohort.
Furthermore, our observation of higher fruit and vegetable intakes associating with lower risks of inadequate GWG, suggest potential role of carbohydrate quality in adequate weight gain during pregnancy. Carbohydrate sources with lower glycemic index (GI) and higher fiber content such as fruit and vegetables contribute to lower energy density [29], and increased satiety by increasing gastrointestinal transit time [29,30]; thereby producing a reduction in ad libitum energy intake. In contrast, carbohydrate foods higher in added sugars (e.g. desserts, sweet snacks and sugar-sweetened beverages) or refined grains tend to be high in energy density [31]; thus contributing to passive over-consumption. This is supported by our finding of a significant positive association between ‘carbohydrate-rich foods’ and GWG z-scores.
Similarly, we found evidence that the source of protein is important in achieving optimal weight gain during pregnancy. We observed a higher intake of plant-based protein food to be related to a lower likelihood of excessive GWG as well as a lower likelihood of inadequate GWG. The relatively low energy density [32], high fiber [29], and high micronutrients content [33] of these foods, which have been shown to play important roles in appetite regulation, metabolism and tissue maintenance, may explain the association observed with optimal weight gain. Furthermore, higher intake of dairy products was found to associate with lower GWG z-scores. The beneficial association observed may be attributable to the high content of calcium which has been shown to influence energy metabolism by stimulating lipolysis and inhibits fatty acid synthesis [34], and conjugated linoleic acid which has been shown to reduce body fat and increase lean muscle mass [35,36]. This contrasted findings from three other studies in pregnant women [10,37,38], but direct comparison of study findings was difficult due to methodological differences such as definitions of dietary exposure and GWG.
This study has several strengths. To the best of our knowledge, we are the first to use z-scores for the investigation of maternal dietary intake and GWG, which is a method of estimating weight gain independent of gestational age [20], thus allowing the use of weights measured at different time-points of pregnancy to more clearly reflect total GWG. In using macronutrient substitution model, we were able to show that GWG increases when carbohydrate intake replaces fat intake which many previous studies have not been able to elucidate [8]. The additional analysis of food groups intakes with GWG allowed findings on diet and GWG relationships to be more holistically interpreted.
Several limitations must be noted. Dietary intake was assessed mid-to-late pregnancy, while GWG captures weight gain from early to late pregnancy, thus the temporality of the associations observed remains questionable. There is, however, evidence to suggest that average intake of food and energy-adjusted nutrients did not change appreciably (<5%) from the 1st to 2nd trimester [39]. The use of the MSM approach in an attempt to improve estimation of usual dietary intake, however, was limited by the lack of consumption frequency information, and we had to make several assumptions. This may have biased the estimation of dietary intake, leading to slight discrepancies in results between the main and subgroup analysis, but it is important to note that the directions of the associations remained the same. We are limited to using IOM recommendations for GWG status because cut-off points for GWG z-scores have not been established, thus remains biased by gestational age. We therefore only used the recommendation for rate of weight gain in the second and third trimesters instead of recommendation for total weight gain. There remains the possibility of misclassification bias of GWG status using pre-pregnancy BMI category based on self-reported pre-pregnancy weight and the use of World Health Organization’s international BMI classification rather than those applicable to Asian populations [40]. However, misclassification bias is likely to be minimal as we found a strong correlation in BMI (r=0.97) and a strong agreement in BMI categories (kappa=0.72) pre-pregnancy and during first trimester, and results from our sensitivity analysis provided similar study estimates to the main analyses. Furthermore, the recommended weight gain ranges were largely based upon studies of Caucasian women and may not be applicable to Asian populations; however, the lack of Asian studies for appropriate weight gain precluded the use of a regional- or country-specific GWG guideline. Lastly, as in all observational studies, residual confounding is likely to exist.
In conclusion, we found energy intake to be the main determinant of GWG, but effective dietary strategies on how to practically achieve energy balance rather than just advising to eat less are needed to effect a change. This study presents evidence suggesting that balancing the proportion of carbohydrate and fat intakes and improving the quality of carbohydrate and protein can potentially contribute to achieving optimal GWG. These findings are in line with current recommendations to consume higher amounts of wholegrains, fruit and vegetables and lean protein, and lower amounts of foods with added sugars for better health outcomes for the mother and infant; although further evidence are needed to support recommendations to reduce carbohydrate intake on the basis of sugar, fiber and GI and increasing plant-based protein for optimal GWG. The prevalence of excessive GWG in our cohort is alarmingly high; it is thus important to provide specific dietary guidance to prevent excessive weight gain especially among overweight and obese women who were observed to have the highest prevalence of excessive weight gain in our cohort. Strategies to ensure pregnant women achieve sufficient weight gain deserves equal attention considering the associated adverse pregnancy and birth outcomes. Lastly, consistent GWG definitions and measurement methods are necessary to facilitate comparison of results across studies.
Supplementary Material
Acknowledgements
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore – NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), and Nestec. KMG is supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre and by the European Union's Seventh Framework Programme (FP7/2007-2013), projects Early Nutrition and ODIN under grant agreement numbers 289346 and 613977.
We will like to acknowledge the contribution of the GUSTO study group: Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit F.P. Broekman, Boon Long Quah, Borys Shuter, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, Fabian Yap, George Seow Heong Yeo, Helen Chen, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna D. Holbrook, Joshua J. Gooley, Keith M. Godfrey, Kenneth Kwek, Kok Hian Tan, Krishnamoorthy Niduvaje, Leher Singh, Lin Lin Su, Lourdes Mary Daniel, Lynette Pei-Chi Shek, Marielle V. Fortier, Mark Hanson, Mary Foong-Fong Chong, Mary Rauff, Mei Chien Chua, Michael Meaney, Mya Thway Tint, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Peter D. Gluckman, Pratibha Agarwal, Rob M. van Dam, Salome A. Rebello, Seang-Mei Saw, Shang Chee Chong, Shirong Cai, Shu-E Soh, Sok Bee Lim, Chin-Ying Stephen Hsu, Victor Samuel Rajadurai, Walter Stunkel, Wee Meng Han, Wei Wei Pang, Yap-Seng Chong, Yin Bun Cheung, Yiong Huak Chan and Yung Seng Lee.
Footnotes
Ethical Standards
The GUSTO study has received ethical approval from the Institutional Review Board of KKH and NUH, and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all study participants prior to their inclusion in the study.
Conflict of interest
PDG, KMG and YSC have received reimbursement for speaking at conferences sponsored by companies selling nutritional products. These authors are part of an academic consortium that has received research funding from Abbot Nutrition, Nestec, and Danone. All other authors declare that they have no conflict of interest.
Authors’ contributions
JSL and MFFC designed the research, wrote the manuscript and had primary responsibility of final content. SES, SLL, MC conducted research and contributed to data analysis. JSL performed statistical analysis. LPCS, FKPY, KHT, PDG, KMG and YSC designed and led the GUSTO study. All authors were involved in study conception and data interpretation, critically reviewed the manuscript for intellectual content, read and approved the final manuscript.
References
- 1.Rasmussen K, Yaktine A, editors. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. 2009. [PubMed] [Google Scholar]
- 2.Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VWV, Eriksson JG, Broekman BFP. Influence of maternal obesity on the long-term health of offspring. The Lancet Diabetes & Endocrinology. 2017;5(1):53–64. doi: 10.1016/S2213-8587(16)30107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deputy N, Sharma A, Kim S. Gestational Weight Gain - United States, 2012 and 2013. Morbidity and Mortality Weekly Report (MMWR) 2015;64(43):1215–1220. doi: 10.15585/mmwr.mm6443a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang S, Peng A, Wei S, Wu J, Zhao J, Zhang Y, Wang J, Lu Y, Yu Y, Zhang B. Pre-Pregnancy Body Mass Index, Gestational Weight Gain, and Birth Weight: A Cohort Study in China. PLoS One. 2015;10(6):e0130101. doi: 10.1371/journal.pone.0130101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koh H, Ee TX, Malhotra R, Allen JC, Tan TC, Østbye T. Predictors and adverse outcomes of inadequate or excessive gestational weight gain in an Asian population. J Obstet Gynaecol Res. 2013;39(5):905–913. doi: 10.1111/j.1447-0756.2012.02067.x. [DOI] [PubMed] [Google Scholar]
- 6.Westerterp KR. Diet induced thermogenesis. Nutr Metab (Lond) 2004;1(1):5. doi: 10.1186/1743-7075-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerstein DE, Woodward-Lopez G, Evans AE, Kelsey K, Drewnowski A. Clarifying concepts about macronutrients' effects on satiation and satiety. J Am Diet Assoc. 104(7):1151–1153. doi: 10.1016/j.jada.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 8.Tielemans MJ, Garcia AH, Peralta Santos A, Bramer WM, Luksa N, Luvizotto MJ, Moreira E, Topi G, de Jonge EA, Visser TL, Voortman T, et al. Macronutrient composition and gestational weight gain: a systematic review. Am J Clin Nutr. 2016;103(1):83–99. doi: 10.3945/ajcn.115.110742. [DOI] [PubMed] [Google Scholar]
- 9.Sartorelli DS, Barbieri P, Perdona GC. Fried food intake estimated by the multiple source method is associated with gestational weight gain. Nutr Res. 2014;34(8):667–673. doi: 10.1016/j.nutres.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Stuebe AM, Oken E, Gillman MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am J Obstet Gynecol. 2009;201(1):58.e51–58. doi: 10.1016/j.ajog.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guilloty NI, Soto R, Anzalota L, Rosario Z, Cordero JF, Palacios C. Diet, Pre-pregnancy BMI, and Gestational Weight Gain in Puerto Rican Women. Maternal & Child Health Journal. 2015;19(11):2453–2461. doi: 10.1007/s10995-015-1764-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park CK, Timm V, Neupane B, Beyene J, Schmidt LA, McDonald SD. Factors Associated With Women's Plans to Gain Weight Categorized as Above or Below the National Guidelines During Pregnancy. J Obstet Gynaecol Can. 2015;37(3):225–235. doi: 10.1016/S1701-2163(15)30308-X. [DOI] [PubMed] [Google Scholar]
- 13.Larsen SC, Angquist L, Laurin C, Morgen CS, Jakobsen MU, Paternoster L, Smith GD, Olsen SF, Sorensen TI, Nohr EA. Association between Maternal Fish Consumption and Gestational Weight Gain: Influence of Molecular Genetic Predisposition to Obesity. PLoS One. 2016;11(3):e0150105. doi: 10.1371/journal.pone.0150105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr. 2010;91(2):373–380. doi: 10.3945/ajcn.2009.28166. [DOI] [PubMed] [Google Scholar]
- 15.Freudenheim JL. Study design and hypothesis testing: issues in the evaluation of evidence from research in nutritional epidemiology. Am J Clin Nutr. 1999;69(6):1315s–1321s. doi: 10.1093/ajcn/69.6.1315S. [DOI] [PubMed] [Google Scholar]
- 16.Soh S-E, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, Stünkel W, Holbrook JD, Kwek K, Chong Y-S, Saw SM, et al. Cohort Profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43(5):1401–1409. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 17.Soh SE, Chong YS, Kwek K, Saw SM, Meaney MJ, Gluckman PD, Holbrook JD, Godfrey KM. Insights from the Growing Up in Singapore Towards Healthy Outcomes (GUSTO) cohort study. Ann Nutr Metab. 2014;64(3–4):218–225. doi: 10.1159/000365023. [DOI] [PubMed] [Google Scholar]
- 18.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77(5):1171–1178. doi: 10.1093/ajcn/77.5.1171. [DOI] [PubMed] [Google Scholar]
- 19.USDA National nutrient database for standard reference, release 24. 2011 https://ndb.nal.usda.gov/ndb/
- 20.Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. Am J Clin Nutr. 2013;97(5):1062–1067. doi: 10.3945/ajcn.112.051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung YB. Statistical Analysis of Human Growth and Development. CRC Press; FL, US: 2013. [Google Scholar]
- 22.Lagiou P, Tamimi RM, Mucci LA, Adami HO, Hsieh CC, Trichopoulos D. Diet during pregnancy in relation to maternal weight gain and birth size. Eur J Clin Nutr. 2004;58(2):231–237. doi: 10.1038/sj.ejcn.1601771. [DOI] [PubMed] [Google Scholar]
- 23.Maslova E, Halldorsson TI, Astrup A, Olsen SF. Dietary protein-to-carbohydrate ratio and added sugar as determinants of excessive gestational weight gain: a prospective cohort study. BMJ Open. 2015;5(2):e005839. doi: 10.1136/bmjopen-2014-005839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO Consultation. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1: diagnosis and classification of diabetes mellitus. WHO; Geneva: 1999. [Google Scholar]
- 25.Faerch K, Lau C, Tetens I, Pedersen OB, Jorgensen T, Borch-Johnsen K, Glumer C. A statistical approach based on substitution of macronutrients provides additional information to models analyzing single dietary factors in relation to type 2 diabetes in danish adults: the Inter99 study. J Nutr. 2005;135(5):1177–1182. doi: 10.1093/jn/135.5.1177. [DOI] [PubMed] [Google Scholar]
- 26.Harttig U, Haubrock J, Knuppel S, Boeing H. The MSM program: web-based statistics package for estimating usual dietary intake using the Multiple Source Method. Eur J Clin Nutr. 2011;65(Suppl 1):S87–91. doi: 10.1038/ejcn.2011.92. [DOI] [PubMed] [Google Scholar]
- 27.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley; NJ, US: 2004. [Google Scholar]
- 28.Changamire FT, Mwiru RS, Msamanga GI, Spiegelman D, Urassa W, Hertzmark E, Fawzi WW, Peterson KE. Macronutrient and sociodemographic determinants of gestational weight gain among HIV-negative women in Tanzania. Food Nutr Bull. 2014;35(1):43–50. doi: 10.1177/156482651403500106. [DOI] [PubMed] [Google Scholar]
- 29.Slavin JL. Dietary fiber and body weight. Nutrition. 21(3):411–418. doi: 10.1016/j.nut.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Roberts SB. Glycemic index and satiety. Nutr Clin Care. 2003;6(1):20–26. [PubMed] [Google Scholar]
- 31.Ambrosini GL, Johns DJ, Northstone K, Emmett PM, Jebb SA. Free Sugars and Total Fat Are Important Characteristics of a Dietary Pattern Associated with Adiposity across Childhood and Adolescence. J Nutr. 2016;146(4):778–784. doi: 10.3945/jn.115.224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drewnowski A, Almiron-Roig E, Marmonier C, Lluch A. Dietary energy density and body weight: is there a relationship? Nutr Rev. 2004;62(11):403–413. doi: 10.1111/j.1753-4887.2004.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 33.Astrup A, Bugel S. Micronutrient deficiency in the aetiology of obesity. Int J Obes. 2010;34(6):947–948. doi: 10.1038/ijo.2010.81. [DOI] [PubMed] [Google Scholar]
- 34.Zemel MB, Miller SL. Dietary calcium and dairy modulation of adiposity and obesity risk. Nutr Rev. 2004;62(4):125–131. doi: 10.1111/j.1753-4887.2004.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 35.Terpstra AHM, Beynen AC, Everts H, Kocsis S, Katan MB, Zock PL. The Decrease in Body Fat in Mice Fed Conjugated Linoleic Acid Is Due to Increases in Energy Expenditure and Energy Loss in the Excreta. J Nutr. 2002;132(5):940–945. doi: 10.1093/jn/132.5.940. [DOI] [PubMed] [Google Scholar]
- 36.Kim MR, Park Y, Albright KJ, Pariza MW. Differential responses of hamsters and rats fed high-fat or low-fat diets supplemented with conjugated linoleic acid. Nutr Res. 22(6):715–722. doi: 10.1016/S0271-5317(02)00372-X. [DOI] [Google Scholar]
- 37.Olafsdottir AS, Skuladottir GV, Thorsdottir I, Hauksson A, Steingrimsdottir L. Maternal diet in early and late pregnancy in relation to weight gain. Int J Obes (Lond) 2006;30(3):492–499. doi: 10.1038/sj.ijo.0803184. [DOI] [PubMed] [Google Scholar]
- 38.Olsen SF, Halldorsson TI, Willett WC, Knudsen VK, Gillman MW, Mikkelsen TB, Olsen J. Milk consumption during pregnancy is associated with increased infant size at birth: prospective cohort study. Am J Clin Nutr. 2007;86(4):1104–1110. doi: 10.1093/ajcn/86.4.1104. [DOI] [PubMed] [Google Scholar]
- 39.Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Kleinman KP, Oken E, Gillman MW. Changes in dietary intake from the first to the second trimester of pregnancy. Paediatr Perinat Epidemiol. 2006;20(1):35–42. doi: 10.1111/j.1365-3016.2006.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/s0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.