Abstract
Background
Frailty is associated with poor prognosis, but the multitude of definitions and scales of assessment makes the impact on outcomes difficult to assess. The aim of this study was to quantify the effect of frailty on postoperative morbidity and mortality, and long‐term mortality after major abdominal surgery, and to evaluate the performance of different frailty metrics.
Methods
An extended literature search was performed to retrieve all original articles investigating whether frailty could affect outcomes after elective major abdominal surgery in adult populations. All possible definitions of frailty were considered. A random‐effects meta‐analysis was carried out for all outcomes of interest. For postoperative morbidity and mortality, overall effect sizes were estimated as odds ratios (OR), whereas the hazard ratio (HR) was calculated for long‐term mortality. The potential effect of the number of domains of the frailty indices was explored through meta‐regression at moderator analysis.
Results
A total of 35 studies with 1 153 684 patients were analysed. Frailty was associated with a significantly increased risk of postoperative major morbidity (OR 2·56, 95 per cent c.i. 2·08 to 3·16), short‐term mortality (OR 5·77, 4·41 to 7·55) and long‐term mortality (HR 2·71, 1·63 to 4·49). All domains were significantly associated with the occurrence of postoperative major morbidity, with ORs ranging from 1·09 (1·00 to 1·18) for co‐morbidity to 2·52 (1·32 to 4·80) for sarcopenia. No moderator effect was observed according to the number of frailty components.
Conclusion
Regardless of the definition and combination of domains, frailty was significantly associated with an increased risk of postoperative morbidity and mortality after major abdominal surgery.
Introduction
One of the most challenging areas of surgery is accurate patient selection. Treatment decisions based on individual clinical judgement are subject to bias, and may result in inappropriate surgery and consequent adverse outcomes.
In the general population, there is a constant and growing demand for cure, with often unrealistic expectations. Strong patient motivation for surgery and a lack of standardized risk assessment may expose patients to excessive risk of major postoperative morbidity and mortality or poor long‐term prognosis. Conversely, failure to offer surgery with curative intent to patients who are judged unfit based on generic and imprecise risk variables is unacceptable1 2.
Despite technical improvements and advances in perioperative care, major abdominal operations are still associated with a high rate of severe complications, long‐term disability, and health and social costs3, 4, 5, 6, 7. Moreover, the likelihood of successfully rescuing patients from surgery‐related morbidity is still unpredictable. Failure to rescue is defined as the probability of death after a major complication8 9. Whether a patient is salvaged after a complication is a function of the care delivered by the hospital, and its resources and facilities, but mostly of patient resilience10. Failure to rescue frequently occurs in frail patients lacking the physiological reserve to survive major postoperative complications, even when treated with best available care. Frailty is a state of vulnerability to poor resolution of homeostasis following a stressor event. It develops as a consequence of cumulative decline across multiple physiological systems, and increases the risk of adverse events11.
Recently, it has been suggested that chronological age and co‐morbidity are inappropriate parameters to decide whether a patient should undergo a surgical procedure12. On the contrary, frailty may reflect a more accurate and individualized parameter of ‘biological age’13. Thus, frailty should not be considered as an exclusive state of ageing and may be detected in any person with limited functional reserve for several different reasons.
Different frailty scales have been applied to surgical cohorts, regardless of age, as a predictor of surgery‐related morbidity and mortality, with consistent results14, 15, 16. The multitude of definitions and scoring systems and the metric complexity that have been proposed in the surgical scenario, may limit routine assessment, and make it difficult to understand and decide whether it is valuable to incorporate frailty estimates into daily clinical practice.
The purpose of this study was to review the scoring methods used to evaluate frailty in surgical patients, and to assess their ability to predict adverse clinical outcomes. In particular, the aim was to assess the global impact of frailty on postoperative morbidity and mortality, and long‐term mortality in patients undergoing major abdominal operations, and to assess whether frailty metric predictive performance may differ based on the number of domains considered in the definition of frailty.
Methods
Study selection
An extended web search of the literature was performed in January 2017 by two authors. MEDLINE, Embase, PubMed, Cochrane and Scopus libraries were queried, and all papers analysing the potential impact of frailty among surgical patients, written in English and published from 1990, were considered for inclusion (Table S1, supporting information). The related articles function and the reference lists of the studies retrieved for full‐text review were used to broaden the search. In the event of overlap of institutions, authors or patients, the most recent article was considered.
Inclusion and exclusion criteria
All original articles investigating whether frailty could affect outcomes after elective major abdominal surgery in adult populations were included. Given the lack of a standard definition or consensus on the ideal frailty metric, all possible author descriptions for inclusion were considered, with no limitations on the number of items and domains used for frailty assessment.
Allocation to the frail or not‐frail group reflected the definition provided by each author. Patients of intermediate frailty were included in the frail group.
Major abdominal surgery was defined as all gastrointestinal (colorectal, gastric, small bowel, hepatic, pancreatic resection), urological (nephrectomy, cystectomy, prostatectomy) and gynaecological (uterus and ovary resection, pelvic floor reconstruction) operations, undertaken for any indication. Studies focusing on vascular, cardiac, thoracic and transplant operations were excluded. Open and laparoscopic procedures were included. Emergency surgery was defined as any operation performed within 48 h of unplanned admission from the emergency department. Any study reporting both elective and emergency abdominal operations was included if at least 80 per cent of patients had an elective procedure.
Four authors evaluated the eligibility of the studies, which were included if they provided information on at least one of the three primary outcomes (postoperative morbidity, short‐term and long‐term mortality). Where studies reported a frailty metric tested in different cohorts (separate data sets for types of surgery), or tested more than one frailty metric in the same cohort of patients, the two groups were analysed as separate series. Review articles, opinion letters and case reports were not considered.
Outcomes of interest
The primary outcomes were 30‐day major morbidity, defined according to the Clavien–Dindo classification17, or the National Surgical Quality Improvement Program (NSQIP)18 or the Veterans Affairs Surgical Quality Improvement Program (VASQIP)19 classification; short‐term mortality, defined as death within 90 days after operation; and long‐term mortality, defined as any death occurring before 1 year after surgery. Secondary outcomes were rates of hospital readmission and discharge to a location other than home.
Data collection
Data were extracted independently by four investigators; if there was disagreement, two impartial raters cross‐checked the data. Data collected included: first author, country of origin, year of publication, type of surgery, rate of operations for cancer disease and/or emergency surgery, cohort samples, number and type of screening tools used to assess frailty, and outcome measures.
Statistical analysis
A random‐effects meta‐analysis was performed for all outcomes of interest. Odds ratios (OR) were calculated for postoperative morbidity and mortality, and hazard ratios (HRs) for long‐term mortality. P < 0·050 was considered statistically significant. The weights assigned to each study were computed according to the inverse of the variance. Heterogeneity was quantified using I 2 and τ2 indices, and testing the null hypothesis that all studies shared a common effect size. Publication bias was assessed with Egger's test and funnel plots20 21.
Subgroup analyses were carried out according to the type of surgery. The effects of age and the number of domains of the frailty indices on morbidity were explored through meta‐regression and moderator analysis.
Given the high variability in frailty assessment, the aim was to explore the predictive ability of each frailty domain on the primary outcomes, so random‐effects meta‐analyses were performed for each frailty item used in the scores. The effect sizes used were those reported for each specific score item in each study. If separate data for each item comprising the frailty score were not provided, the combined‐effect score was used. Two different meta‐analyses were performed with the first including all studies, and the second including only those for which the effect sizes were reported for each item individually.
Results
Study selection
Some 5033 titles were identified and 4903 were excluded. Some 130 full‐text articles were examined and, after exclusions based on abstract review, 35 studies were included in the analysis (Fig. 1).
Figure 1.
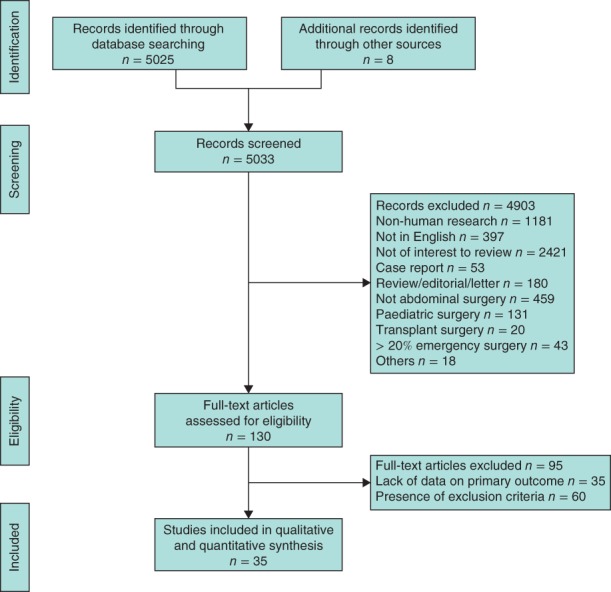
PRISMA flow chart showing selection of articles for review
Study characteristics and frailty assessment
No randomized trials were retrieved. Most studies were observational (23 of 35) with a total of 1 153 684 patients available for the analysis. Cohorts were composed of patients undergoing lower gastrointestinal (GI) surgery (10 studies), upper GI surgery (6), mixed GI surgery (4), gynaecological surgery (6), urological surgery (4) and mixed abdominal surgery (6) (Table 1)1 12, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54.
Table 1.
Characteristics of studies included in the systematic review and meta‐analysis
| Reference | Country | No. of patients |
Age (years)* |
Frail (%) |
Type of operation |
No. of items | Domains† |
Morbidity definiton |
Mortality definition |
|---|---|---|---|---|---|---|---|---|---|
| Amrock et al. 22 (1) | USA | 76 106 | 74·4 | n.r. | Lower GI | 5 | RDA; CO; NS; CF; A | NSQIP | 30 days |
| Amrock et al. 22 (2) | USA | 76 106 | 74·4 | n.r. | Lower GI | 3 | CO; NS; A | NSQIP | 30 days |
| Buettner et al. 23 (1) | USA | 1326 | 65 | n.r. | Mixed GI | 12 | RDA; CO (10); CA | CDC III–IV | 1 year |
| Buettner et al. 23 (2) | USA | 1326 | 65 | 30·0 | Mixed GI | 1 | S | CDC III–IV | 1 year |
| Choi et al. 24 | Korea | 281 | 74·8 | 26·3 | Mixed abdominal | 9 | S; RDA (2); CO; NS (2); CF (2); CA | NSQIP | n.r. |
| Cohan et al. 25 | USA | 2493 | n.r. | 21·3 | Lower GI | 6 | RDA; CO (4); NS | NSQIP | n.r. |
| Courtney‐Brooks et al. 26, ‡ | USA | 37 | 73 | 16 | Gynaecological | 5 | PF; NS; DE; GS; W | NSQIP | n.r. |
| Dale et al. 27, ‡ | USA | 76 | 67·3 | n.r. | Upper GI | 4 | NS; DE; GS; W | CDC III–IV | n.r. |
| Erekson et al. 28 | USA | 22 214 | n.r. | 0·54 | Gynaecological | 1 | NS | Overall | n.r. |
| George et al. 29 | USA | 66 105 | n.r. | 15·5 | Gynaecological | 11 | RDA; CO (9); CF | CDC IV | 30 days |
| Hodari et al. 30 | USA | 2095 | n.r. | n.r. | Upper GI | 11 | RDA; CO (10) | n.r. | 30 days |
| Jones et al. 31 | UK | 100 | 68·6 | 15·0 | Lower GI | 1 | S | n.r. | n.r. |
| Kenig et al. 32, ‡ | Poland | 75 | 75 | 8 | Mixed GI | 8 | RDA (2); M; CO; NS; CF; DE; W | CDC III–IV | n.r. |
| Kim et al. 33, ‡ | Korea | 275 | 75·4 | 35·6 | Mixed abdominal | 9 | S; RDA (2); CO; NS (2); CF (2); CA | NSQIP | 1 year |
| Kristjansson et al. 34, ‡ | Norway | 178 | 76·6 | 42·7 | Lower GI | 7 | RDA (2); M; CO; NS; CF; DE | CDC III–IV | n.r. |
| Kuroki et al. 35 | USA | 122 | 65·9 | 50·0 | Gynaecological | 1 | S | n.r. | n.r. |
| Lascano et al. 36 | USA | 18 384 | 57·7 | n.r. | Urological | 15 | RDA; CO (10); NS; CF (2); CA | CDC IV | 30 days |
| Levy et al. 37 | USA | 23 104 | 61·9 | 54·8 | Urological | 15 | RDA; CO (10); NS; CF (2); CA | CDC IV | 30 days |
| Makary et al. 38, ‡ | USA | 594 | 72·8 | 10·4 | Mixed GI | 5 | RDA; NS; DE; GS; W | NSQIP | n.r. |
| Mogal et al. 39 | USA | 9986 | 64·1 | 6·4 | Upper GI | 11 | RDA; CO (10); | CDC III–IV | 30 days |
| Neuman et al. 40 (1) | USA | 12 979 | 84·4 | 4·3 | Lower GI | 5 | CO; NS; W; F; O | n.r. | 90 days |
| Neuman et al. 40 (2) | USA | 12 979 | 84·4 | 4·3 | Lower GI | 5 | CO; NS; W; F; O | n.r. | 1 year |
| Obeid et al. 41 | USA | 58 448 | n.r. | 12·8 | Lower GI | 11 | RDA; CO (10) | CDC IV | 30 days |
| Ommundsen et al. 42 | Norway | 178 | 80 | 42·7 | Lower GI | 6 | RDA; M; CO; NS; CF; DE | n.r. | 1 year |
| Pearl et al. 43 | USA | 4329 | n.r. | 67·2 | Urological | 11 | RDA; CO (10) | n.r. | n.r. |
| Reisinger et al. 44, ‡ | The Netherlands | 159 | n.r. | 25·8 | Lower GI | 7 | RDA; PF; M; NS; CF; VH; DE | Sepsis | 30 days |
| Revenig et al. 45, ‡ | USA | 214 | 62 | 16 | Mixed abdominal | 5 | PF; NS; DE; GS; W | Overall | n.r. |
| Revenig et al. 46, ‡ | USA | 80 | 60·0 | 23·4 | Mixed abdominal | 5 | PF; NS; DE; GS; W | CDC II–III–IV | n.r. |
| Revenig et al. 1, ‡ | USA | 351 | 63 | 27·3 | Mixed abdominal | 5 | RDA; NS; DE; GS; W | n.r. | 30 days |
| Robinson et al. 12, ‡ | USA | 72 | 74 | 33 | Lower GI | 8 | RDA; CO; NS; CF; W; A; F; O | VASQIP | n.r. |
| Saxton and Velanovich47 (1) | USA | 226 | 61 | n.r. | Mixed GI | 70 | CSHA | Overall | 30 days |
| Saxton and Velanovich47 (2) | USA | 226 | 61 | n.r. | Mixed GI | 70 | CSHA | CDC II–III–IV | n.r. |
| Sur et al. 48 | USA | 100 | 65·6 | 31·0 | Upper GI | 1 | DE | NSQIP | n.r. |
| Suskind et al. 49 | USA | 95 108 | n.r. | 21·5 | Urological | 11 | RDA; CO (10) | NSQIP | n.r. |
| Tan et al. 50, ‡ | Japan | 83 | 81·2 | 28 | Lower GI | 5 | RDA; NS; DE; GS; W | CDC II–III–IV | n.r. |
| Tegels et al. 51 (1) | The Netherlands | 127 | 69·8 | 23·6 | Upper GI | 7 | RDA; PF; M; NS; CF; VH; DE | CDC III–IV | In hospital |
| Tegels et al. 51 (2) | The Netherlands | 127 | 69·8 | n.r. | Upper GI | 7 | RDA; PF; M; NS; CF; VH; DE | CDC III–IV | 6 months |
| Uppal et al. 52 (1) and (2)§ | USA | 6551 | n.r. | n.r. | Gynaecological | 11 | RDA; CO (10) | CDC III–IV | n.r. |
| Velanovich et al. 53 (1) | USA | 727 041 | n.r. | n.r. | Mixed abdominal | 11 | RDA; CO (10) | Overall | 30 days |
| Velanovich et al. 53 (2) | USA | 23 569 | n.r. | n.r. | Gynaecological | 11 | RDA; CO (10) | Overall | 30 days |
| Wagner et al. 54 | USA | 518 | 72 | 25·1 | Upper GI | 1 | S | n.r. | 1 year |
Values are mean or median.
Values in parentheses are number of items used to create the domain.
Prospective studies; the others were retrospective.
Uppal and colleagues52 considered two different scores for the same metric system, on the same population; morbidity outcomes are reported separately for the two scores. n.r., Not reported; GI, gastrointestinal; RDA, reduced daily activities; CO, co‐morbidity; NS, nutritional status; CF, cognitive function; A, anaemia; NSQIP, National Surgical Quality Improvement Program; CA, cancer; CDC, Clavien–Dindo classification; S, sarcopenia; PF, physical fitness; DE, depression/exhaustion; GS, grip strength; W, walking test; M, medication; F, falls; O, others; VH, visual and hearing deficit; VASQIP, Veterans Affairs Surgical Quality Improvement Program; CSHA, Canadian Study of Health and Aging 70 Item Frailty Score.
Frailty was assessed through many combinations of different components, ranging from one to 70 items. The prevalence of frail patients ranged from 0·5 to 67·2 per cent. Most surgical procedures were performed for cancer; only four studies25 28, 29 41 had fewer than half of the patients without malignancy.
Outcomes of interest
In analyses of all the included studies, frailty was associated with an increased risk of postoperative major morbidity (OR 2·56, 95 per cent c.i. 2·08 to 3·16); the I 2 value for heterogeneity was 98·4 per cent (Fig. 2). The OR for short‐term mortality was 5·77 (4·41 to 7·55) (Fig. 3 a) and the HR for long‐term mortality was 2·71 (1·63 to 4·49) (Fig. 3 b). Heterogeneity was high (I 2 = 94·3 per cent for short‐term mortality and I 2 = 88·3 per cent for long‐term mortality).
Figure 2.
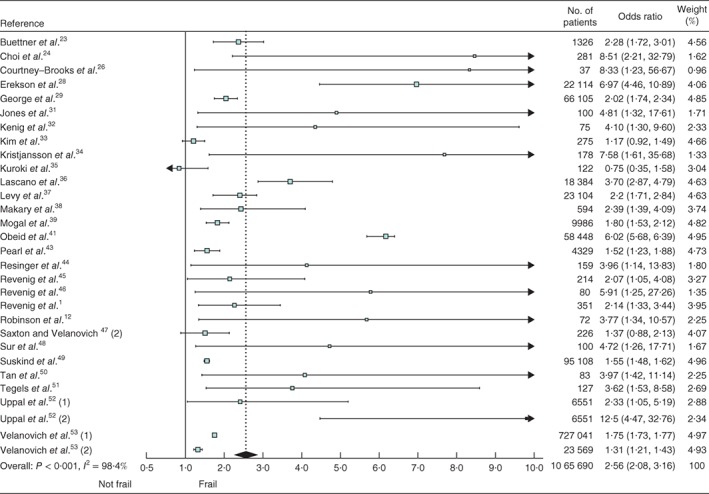
Forest plot of the effect of frailty on major postoperative morbidity. Odds ratios are shown with 95 per cent confidence intervals
Figure 3.
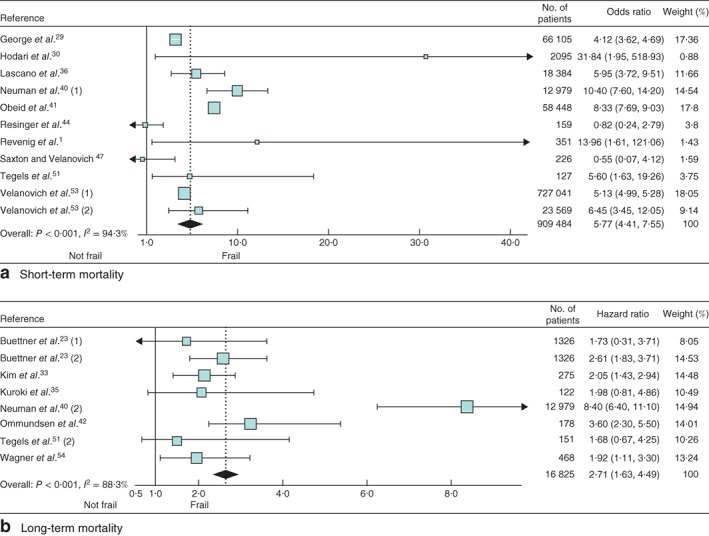
Forest plots of the effect of frailty on a short‐term and b long‐term mortality. Odds ratios and hazard ratios are shown with 95 per cent confidence intervals
Only for major morbidity was the distribution of studies asymmetrical, although no significant publication bias was detected by Egger's linear regression test (P = 0·211, P = 0·666 and P = 0·143 for major morbidity, and short‐ and long‐term mortality respectively) (Fig. S1, supporting information).
Subgroup and moderator analyses
To lower the potential bias related to different operations, a subgroup analysis was undertaken according to the type of surgery. The effect of frailty on major morbidity was confirmed across all specialties. Similarly, the association between frailty and the likelihood of death was confirmed for all types of surgery, except for mixed elective surgery (727 267 patients), where the effect on short‐term mortality was no longer observed (OR 2·14, 95 per cent c.i. 0·25 to 18·12; P = 0·485) (Table S2, supporting information).
Because frailty may be related to ageing, moderator analysis was performed to adjust for potential differences in population ageing across the studies. No moderator effect of age on postoperative morbidity (β = –0·08, α = 0·01, P = 0·503) or short‐term mortality (β = –0·29, α = 0·03, P = 0·426) was detected. On meta‐regression, age modulated the effect of frailty on long‐term mortality (β = –3·38, α = 0·06, P = 0·021) (Fig. S2, supporting information).
No moderator effect on the primary outcomes was observed according to the number of frailty index components (β = 1·06, α = –0·01, P = 0·215 for postoperative morbidity; β = 2·17, α = –0·04, P = 0·172 for short‐term mortality; β = 0·75, α = 0·04, P = 0·419 for long‐term mortality) (Fig. S3, supporting information).
Secondary outcomes
The cumulative risk of readmission was significantly increased in frail patients (OR 3·78, 95 per cent c.i. 1·77 to 8·05; P = 0·001), whereas frailty was not significantly associated with discharge to a location other than home (OR 3·74, 0·81 to 17·30; P = 0·091) (Fig. S4, supporting information).
Frailty scores and domains
Ten studies reported data on the risk of morbidity for a single frailty domain. To analyse potential different effects on outcome prediction, several different meta‐analyses were carried out for each frailty domain considered. All domains, except cognitive function and walking test, were significantly associated with the occurrence of major postoperative morbidity, with ORs ranging from 1·09 (95 per cent c.i. 1·00 to 1·18) for the presence of co‐morbidities, to 2·52 (1·32 to 4·80) for sarcopenia (Table 2).
Table 2.
Analysis of studies reporting the effect size for each item of the score in predicting major postoperative morbidity
| Reference |
Reduced daily activity |
Sarcopenia | Co‐morbidities |
Nutritional status |
Cognitive function |
Depression/ exhaustion |
Walking test |
No. of patients |
|---|---|---|---|---|---|---|---|---|
| Odds ratio | ||||||||
| Amrock et al.22 (1) | 2·08 (1·89, 2·32) | – | – | 1·34 (1·28, 1·40) | 1·21 (1·10, 1·43) | – | – | 76 106 |
| Amrock et al.22 (2) | – | – | 1·09 (1·00, 1·18) | 1·45 (1·43, 1·58) | – | – | – | 76 106 |
| Buettner et al.23 (2) | – | 2·28 (1·72, 3·01) | – | – | – | – | – | 1326 |
| Choi et al.24 | 3·66 (0·94, 14·20) | 4·57 (1·98, 10·50) | 1·27 (0·55, 2·89) | 3·25 (1·42, 7·46) | 3·01 (1·31, 6·90) | – | – | 281 |
| Dale et al.27 | – | – | – | 0·81 (0·29, 2·26) | – | 4·04 (1·40, 11·80) | 1·02 (0·50, 2·06) | 76 |
| Jones et al.31 | – | 4·81 (1·32, 17·60) | – | – | – | – | – | 100 |
| Erekson et al.28 | – | – | – | 2·49 (1·48, 4·17) | – | – | – | 22 214 |
| Kenig et al.32 | 1·70 (0·50, 5·80) | – | 1·20 (0·40, 3·50) | 1·10 (0·40, 2·90) | 1·70 (0·50, 5·80) | 1·10 (0·20, 2·40) | 3·60 (1·10, 13·40) | 75 |
| Kuroki et al.35 | – | 0·74 (0·35, 1·58) | – | – | – | – | – | 122 |
| Revenig et al.1 | 1·11 (0·59, 2·10) | – | – | 1·90 (1·22, 2·96) | – | 1·49 (0·94, 2·36) | 1·63 (0·69, 3·86) | 351 |
| Sur et al.48 | – | 4·72 (1·26, 17·7) | – | – | – | 3·70 (1·21, 1·71) | – | 100 |
| Overall | 1·85 (1·29, 2·66) | 2·52 (1·32, 4·80) | 1·09 (1·00, 1·18) | 1·45 (1·31, 1·62) | 1·65 (0·89, 3·07) | 2·13 (1·12, 4·06) | 1·56 (0·82, 2·97) | |
| P (effect) | 0·001 | 0·005 | 0·041 | < 0·001 | 0·112 | 0·022 | 0·174 | |
| I 2 (%) | 31·8 | 70·7 | 0 | 65·6 | 58·5 | 45·6 | 34·5 | |
| P (heterogeneity) | 0·220 | 0·008 | 0·923 | 0·008 | 0·090 | 0·028 | 0·217 | |
| No. of patients | 76 813 | 1929 | 76 462 | 175 209 | 76 462 | 602 | 502 |
Values in parentheses are 95 per cent confidence intervals.
Comparable results were observed after adding studies to the meta‐analyses that did not provide separate ORs for each frailty domain (Fig. S5, supporting information).
Discussion
This meta‐analysis included data from 35 studies reporting over one million patients. Preoperative existence of a frailty condition was associated with more than double the risk of developing major postoperative morbidity, a six times higher risk of early postoperative mortality, and a threefold increase in long‐term mortality compared with non‐frail patients. This suggests that, in patients who are scheduled for major surgical interventions, frailty should always be assessed before deciding whether to, and how to, proceed.
Even more worrisome is the discrepancy between the rate of major morbidity and short‐term mortality after surgery. Early deaths after elective operations are expected to be a consequence of major morbidity, related directly to the procedure, rather than as a consequence of the primary disease. A similar risk of short‐term mortality and major morbidity would therefore be expected. It can be hypothesized that an underlying frailty condition may be responsible for failure to rescue after the occurrence of a major surgical complication8, 9, 10. This issue should not be underestimated in the decision‐making process when assessing possible alternatives to surgery.
A limitation of the present analysis is the high degree of heterogeneity of the studies for all primary outcomes. A possible explanation lies in the inclusion criteria applied to select studies, incorporating all studies reporting major abdominal operations, including gastrointestinal, urological and gynaecological or mixed procedures. However, on subgroup analysis frailty remained a risk factor for adverse outcomes across different surgical procedures. An additional potential source of heterogeneity was the variability in the definition of major postoperative morbidity, although all of the scores of complication severity have been validated extensively and are commonly accepted in the surgical community17, 18, 19.
Another potential source of bias was ageing. In non‐surgical cohorts, a clear correlation between prevalence of frailty and age has been reported55. The meta‐regression showed that age per se did not increase the risk of major postoperative morbidity and short‐term mortality. This supports frailty as a marker of ‘biological age’ with more value than chronological age13. Conversely, ageing modulated the effect size of long‐term mortality in meta‐regression, suggesting that other factors contribute to long‐term mortality.
The results of this meta‐analysis should be interpreted with caution because of the variability in the definition of frailty across studies and the number of domains used to measure this condition. Frailty was assessed using 12 different definitions, which incorporated from one to 70 domains in different combinations. Nevertheless, the subgroup analysis of different domains, and the meta‐regression on the number of items, showed that the risk estimates for each outcome remained similar after stratification. This suggests that complex methods to assess frailty are not superior to simple ones, and that each domain may have an independent weight in composing the overall risk. In this context, the present data do not support the superiority of one frailty definition nor the superiority of one domain over the others in the creation of frailty scales.
The ultimate risk metrics should be easy to measure, accurate, objective, reproducible, transferable, quick and cheap. Even the most accurate score may become unusable if too complex and time‐consuming, thereby reducing its practicality. Feasibility is a function of the time, expertise and resources available in daily clinical practice; whether to apply comprehensive and inclusive frailty assessments or instead to use quick and easy screening tools may depend on many local variables, but should be taken into consideration in each healthcare organization.
A recent study56 demonstrated that frail surgical patients consume significantly more healthcare resources after hospital discharge, including 30‐day readmission, than non‐frail patients. These results further corroborate the importance of providing a preoperative frailty evaluation in patients undergoing major surgery, as it is possible that the cost of readmissions and additional treatment may exceed the cost of frailty assessments.
The secondary endpoints of this study fully confirmed the above results. There was a higher rate of discharge to a location other than home and hospital readmission in frail patients.
Choosing the right treatment for the right patient is essential in achieving the best outcome57. A question raised is how to use the finding that frailty is a risk factor for poor surgical outcome. It could be used to restrict access of frail patients to major surgery, although this is somewhat constraining given the increasing proportion of older and frail patients58. It could enable more individual risk assessment, discussion and consent to take place, or indeed allow targeted preoperative optimization of patients. A recent commentary by Wick and Finlayson59 challenges medical research to ‘move from measurement to action’, with the need to demonstrate that outcomes may be truly improved by modifying frailty components. Integrated care delivery models, such as enhanced recovery after surgery programmes, have already confirmed the possibility of significantly improving clinical and functional outcomes in elderly and high‐risk patients60, 61, 62. In this situation, despite limited evidence, prehabilitation programmes, including preoperative optimization of coexisting chronic disease therapy, nutritional status, physical function and physiological support63, 64, 65, may represent a more comprehensive and effective opportunity.
Regardless of the tools and combinations of domains used to create a frailty index, this condition is significantly associated with an increased risk of developing major complications, and of short‐ and long‐term mortality after abdominal operations.
Disclosure
The authors declare no conflict of interest.
Supporting information.
Additional supporting information may be found online in the supporting information tab for this article.
Supporting information
Table S1 Search strategy performed on January 12, 2017.
Table S2 Subgroup analysis for target organ or apparatus of operation.
Fig. S1 Funnel plots of major morbidity (A), short‐term mortality (B) and long‐term mortality (C).
Fig. S2 Meta‐regression using patient age as moderator.
Fig. S3 Meta‐regression using number of items as moderator.
Fig. S4 Forest plots of secondary outcomes.
Fig. S5 Forest plot of major morbidity in a subgroup analysis for different domains.
Funding information
No funding information is provided
References
- 1. Revenig LM, Canter DJ, Henderson MA, Ogan K, Kooby DA, Maithel SK et al Preoperative quantification of perceptions of surgical frailty. J Surg Res 2015; 193: 583–589. [DOI] [PubMed] [Google Scholar]
- 2. Symeonidis D, Christodoulidis G, Koukoulis G, Spyridakis M, Tepetes K. Colorectal cancer surgery in the elderly: limitations and drawbacks. Tech Coloproctol 2011; 15(Suppl 1): S47–S50. [DOI] [PubMed] [Google Scholar]
- 3. Segelman J, Nygren J. Evidence or eminence in abdominal surgery: recent improvements in perioperative care. World J Gastroenterol 2014; 20: 16615–16619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferreyra G, Long Y, Ranieri VM. Respiratory complications after major surgery. Curr Opin Crit Care 2009; 15: 342–348. [DOI] [PubMed] [Google Scholar]
- 5. Macellari F, Paciaroni M, Agnelli G, Caso V. Perioperative stroke risk in nonvascular surgery. Cerebrovasc Dis 2012; 34: 175–181. [DOI] [PubMed] [Google Scholar]
- 6. Romagnoli S, Zagli G, Tuccinardi G, Tofani L, Chelazzi C, Villa G et al Postoperative acute kidney injury in high‐risk patients undergoing major abdominal surgery. J Crit Care 2016; 35: 120–125. [DOI] [PubMed] [Google Scholar]
- 7. Gianotti L, Braga M, Frei A, Greiner R, Di Carlo V. Health care resources consumed to treat postoperative infections: cost saving by perioperative immunonutrition. Shock 2000; 14: 325–330. [DOI] [PubMed] [Google Scholar]
- 8. Silber JH, Romano PS, Rosen AK, Wang Y, Even‐Shoshan O, Volpp KG. Failure‐to‐rescue: comparing definitions to measure quality of care. Med Care 2007; 45: 918–925. [DOI] [PubMed] [Google Scholar]
- 9. Tamirisa NP, Parmar AD, Vargas GM, Mehta HB, Kilbane EM, Hall BL et al Relative contributions of complications and failure to rescue on mortality in older patients undergoing pancreatectomy. Ann Surg 2016; 263: 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med 2009; 361: 1368–1375. [DOI] [PubMed] [Google Scholar]
- 11. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robinson TN, DS Wu, Pointer L, Dunn CL, Cleveland JC Jr, Moss M. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg 2013; 206: 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cesari M, Prince M, Thiyagarajan JA, De Carvalho IA, Bernabei R, Chan P et al Frailty: an emerging public health priority. J Am Med Dir Assoc 2016; 17: 188–192. [DOI] [PubMed] [Google Scholar]
- 14. Fagard K, Leonard S, Deschodt M, Devriendt E, Wolthuis A, Prenen H et al The impact of frailty on postoperative outcomes in individuals aged 65 and over undergoing elective surgery for colorectal cancer: a systematic review. J Geriatr Oncol 2016; 7: 479–491. [DOI] [PubMed] [Google Scholar]
- 15. Beggs T, Sepehri A, Szwajcer A, Tangri N, Arora RC. Frailty and perioperative outcomes: a narrative review. Can J Anaesth 2015; 62: 143–157. [DOI] [PubMed] [Google Scholar]
- 16. Handforth C, Clegg A, Young C, Simpkins S, Seymour MT, Selby PJ et al The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol 2015; 26: 1091–1101. [DOI] [PubMed] [Google Scholar]
- 17. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fink AS, Campbell DA Jr, Mentzer RM Jr, Henderson WG, Daley J, Bannister J et al The National Surgical Quality Improvement Program in non‐veterans administration hospitals: initial demonstration of feasibility. Ann Surg 2002; 236: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khuri SF, Daley J, Henderson W, Hur K, Demakis J, Aust JB et al The Department of Veterans Affairs' NSQIP: the first national, validated, outcome‐based, risk‐adjusted, and peer‐controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg 1998; 228: 491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. J Clin Epidemiol 2001; 54: 1046–1055. [DOI] [PubMed] [Google Scholar]
- 21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amrock LG, Neuman MD, Lin HM, Deiner S. Can routine preoperative data predict adverse outcomes in the elderly? Development and validation of a simple risk model incorporating a chart‐derived frailty score. J Am Coll Surg 2014; 219: 684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buettner S, Wagner D, Kim Y, Margonis GA, Makary MA, Wilson A et al Inclusion of sarcopenia outperforms the modified frailty index in predicting 1‐year mortality among 1326 patients undergoing gastrointestinal surgery for a malignant indication. J Am Coll Surg 2016; 222: 397–407.e2. [DOI] [PubMed] [Google Scholar]
- 24. Choi JY, Yoon SJ, Kim SW, Jung HW, Kim KI, Kang E et al Prediction of postoperative complications using multidimensional frailty score in older female cancer patients with American Society of Anesthesiologists physical status class 1 or 2. J Am Coll Surg 2015; 221: 652–660.e2. [DOI] [PubMed] [Google Scholar]
- 25. Cohan JN, Bacchetti P, Varma MG, Finlayson E. Outcomes after ileoanal pouch surgery in frail and older adults. J Surg Res 2015; 198: 327–333. [DOI] [PubMed] [Google Scholar]
- 26. Courtney‐Brooks M, Tellawi AR, Scalici J, Duska LR, Jazaeri AA, Modesitt SC et al Frailty: an outcome predictor for elderly gynecologic oncology patients. Gynecol Oncol 2012; 126: 20–24. [DOI] [PubMed] [Google Scholar]
- 27. Dale W, Hemmerich J, Kamm A, Posner MC, Matthews JB, Rothman R et al Geriatric assessment improves prediction of surgical outcomes in older adults undergoing pancreaticoduodenectomy: a prospective cohort study. Ann Surg 2014; 259: 960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Erekson EA, Yip SO, Ciarleglio MM, Fried TR. Postoperative complications after gynecologic surgery. Obstet Gynecol 2011; 118: 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. George EM, Burke WM, Hou JY, Tergas AI, Chen L, Neugut AI et al Measurement and validation of frailty as a predictor of outcomes in women undergoing major gynaecological surgery. BJOG 2016; 123: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hodari A, Hammoud ZT, Borgi JF, Tsiouris A, Rubinfeld IS. Assessment of morbidity and mortality after esophagectomy using a modified frailty index. Ann Thorac Surg 2013; 96: 1240–1245. [DOI] [PubMed] [Google Scholar]
- 31. Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross‐sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis 2015; 17: O20–O26. [DOI] [PubMed] [Google Scholar]
- 32. Kenig J, Olszewska U, Zychiewicz B, Barczynski M, Mituś‐Kenig M. Cumulative deficit model of geriatric assessment to predict the postoperative outcomes of older patients with solid abdominal cancer. J Geriatr Oncol 2015; 6: 370–379. [DOI] [PubMed] [Google Scholar]
- 33. Kim SW, Han HS, Jung HW, Kim KI, Hwang DW, Kang SB et al Multidimensional frailty score for the prediction of postoperative mortality risk. JAMA Surg 2014; 149: 633–640. [DOI] [PubMed] [Google Scholar]
- 34. Kristjansson SR, Nesbakken A, Jordhøy MS, Skovlund E, Audisio RA, Johannessen HO et al Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Crit Rev Oncol Hematol 2010; 76: 208–217. [DOI] [PubMed] [Google Scholar]
- 35. Kuroki LM, Mangano M, Allsworth JE, Menias CO, Massad LS, Powell MA et al Pre‐operative assessment of muscle mass to predict surgical complications and prognosis in patients with endometrial cancer. Ann Surg Oncol 2015; 22: 972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lascano D, Pak JS, Kates M, Finkelstein JB, Silva M, Hagen E et al Validation of a frailty index in patients undergoing curative surgery for urologic malignancy and comparison with other risk stratification tools. Urol Oncol 2015; 33: 426e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levy I, Finkelstein M, Bilal KH, Palese M. Modified frailty index associated with Clavien–Dindo IV complications in robot‐assisted radical prostatectomies: a retrospective study. Urol Oncol 2017; 35: 425–431. [DOI] [PubMed] [Google Scholar]
- 38. Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen‐Roche K, Patel P et al Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210: 901–908. [DOI] [PubMed] [Google Scholar]
- 39. Mogal H, Vermilion SA, Dodson R, Hsu FC, Howerton R, Shen P et al Modified frailty index predicts morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol 2017; 24: 1714–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neuman HB, Weiss JM, Leverson G, O'Connor ES, Greenblatt DY, Loconte NK et al Predictors of short‐term postoperative survival after elective colectomy in colon cancer patients >/= 80 years of age. Ann Surg Oncol 2013; 20: 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Obeid NM, Azuh O, Reddy S, Webb S, Reickert C, Velanovich V et al Predictors of critical care‐related complications in colectomy patients using the National Surgical Quality Improvement Program: exploring frailty and aggressive laparoscopic approaches. J Trauma Acute Care Surg 2012; 72: 878–883. [DOI] [PubMed] [Google Scholar]
- 42. Ommundsen N, Wyller TB, Nesbakken A, Jordhøy MS, Bakka A, Skovlund E et al Frailty is an independent predictor of survival in older patients with colorectal cancer. Oncologist 2014; 19: 1268–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pearl JA, Patil D, Filson CP, Arya S, Alemozaffar M, Master VA et al Patient frailty and discharge disposition following radical cystectomy. Clin Genitourin Cancer 2017; 15: e615–e621. [DOI] [PubMed] [Google Scholar]
- 44. Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewé KW, Hoofwijk AG et al Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg 2015; 261: 345–352. [DOI] [PubMed] [Google Scholar]
- 45. Revenig LM, Canter DJ, Taylor MD, Tai C, Sweeney JF, Sarmiento JM et al Too frail for surgery? Initial results of a large multidisciplinary prospective study examining preoperative variables predictive of poor surgical outcomes. J Am Coll Surg 2013; 217: 665–670.e1. [DOI] [PubMed] [Google Scholar]
- 46. Revenig LM, Canter DJ, Master VA, Maithel SK, Kooby DA, Pattaras JG et al A prospective study examining the association between preoperative frailty and postoperative complications in patients undergoing minimally invasive surgery. J Endourol 2014; 28: 476–480. [DOI] [PubMed] [Google Scholar]
- 47. Saxton A, Velanovich V. Preoperative frailty and quality of life as predictors of postoperative complications. Ann Surg 2011; 253: 1223–1229. [DOI] [PubMed] [Google Scholar]
- 48. Sur MD, Namm JP, Hemmerich JA, Buschmann MM, Roggin KK, Dale W. Radiographic sarcopenia and self‐reported exhaustion independently predict NSQIP serious complications after pancreaticoduodenectomy in older adults. Ann Surg Oncol 2015; 22: 3897–3904. [DOI] [PubMed] [Google Scholar]
- 49. Suskind AM, Walter LC, Jin C, Boscardin J, Sen S, Cooperberg MR et al Impact of frailty on complications in patients undergoing common urological procedures: a study from the American College of Surgeons National Surgical Quality Improvement database. BJU Int 2016; 117: 836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tan KY, Kawamura YJ, Tokomitsu A, Tang T. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am J Surg 2012; 204: 139–143. [DOI] [PubMed] [Google Scholar]
- 51. Tegels JJ, de Maat MF, Hulsewé KW, Hoofwijk AG, Stoot JH. Value of geriatric frailty and nutritional status assessment in predicting postoperative mortality in gastric cancer surgery. J Gastrointest Surg 2014; 18: 439–445. [DOI] [PubMed] [Google Scholar]
- 52. Uppal S, Igwe E, Rice LW, Spencer RJ, Rose SL. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol 2015; 137: 98–101. [DOI] [PubMed] [Google Scholar]
- 53. Velanovich V, Antoine H, Swartz A, Peters D, Rubinfeld I. Accumulating deficits model of frailty and postoperative mortality and morbidity: its application to a national database. J Surg Res 2013; 183: 104–110. [DOI] [PubMed] [Google Scholar]
- 54. Wagner D, Buttner S, Kim Y, Gani F, Xu L, Margonis GA et al Clinical and morphometric parameters of frailty for prediction of mortality following hepatopancreaticobiliary surgery in the elderly. Br J Surg 2016; 103: e83–e92. [DOI] [PubMed] [Google Scholar]
- 55. Ruiz M, Cefalu C, Reske T. Frailty syndrome in geriatric medicine. Am J Med Sci 2012; 344: 395–398. [DOI] [PubMed] [Google Scholar]
- 56. Wahl TS, Graham LA, Hawn MT, Richman J, Hollis RH, Jones CE et al Association of the modified frailty index with 30‐day surgical readmission. JAMA Surg 2017; 152: 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Saaty TL. Decision making with the analytic hierarchy process. Int J Serv Sci 2008; 1: 83–98 [Google Scholar]
- 58. Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA et al Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006; 54: 991–1001. [DOI] [PubMed] [Google Scholar]
- 59. Wick EC, Finlayson E. Frailty – going from measurement to action. JAMA Surg 2017; 152: 757–758. [DOI] [PubMed] [Google Scholar]
- 60. Braga M, Pecorelli N, Scatizzi M, Borghi F, Missana G, Radrizzani D et al; PeriOperative Italian Society . Enhanced recovery program in high‐risk patients undergoing colorectal surgery: results from the PeriOperative Italian Society Registry. World J Surg 2017; 41: 860–867. [DOI] [PubMed] [Google Scholar]
- 61. Zouros E, Liakakos T, Machairas A, Patapis P, Tzerbinis H, Manatakis DK et al Fast‐track pancreaticoduodenectomy in the elderly. Am Surg 2017; 83: 239–249. [PubMed] [Google Scholar]
- 62. Slieker J, Frauche P, Jurt J, Addor V, Blanc C, Demartines N et al Enhanced recovery ERAS for elderly: a safe and beneficial pathway in colorectal surgery. Int J Colorectal Dis 2017; 32: 215–221. [DOI] [PubMed] [Google Scholar]
- 63. Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS et al Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc 2013; 27: 1072–1082. [DOI] [PubMed] [Google Scholar]
- 64. Partridge JS, Harari D, Martin FC, Peacock JL, Bell R, Mohammed A et al Randomized clinical trial of comprehensive geriatric assessment and optimization in vascular surgery. Br J Surg 2017; 104: 679–687. [DOI] [PubMed] [Google Scholar]
- 65. West MA, Loughney L, Lythgoe D, Barben CP, Sripadam R, Kemp GJ et al Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: a blinded interventional pilot study. Br J Anaesth 2015; 114: 244–251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Search strategy performed on January 12, 2017.
Table S2 Subgroup analysis for target organ or apparatus of operation.
Fig. S1 Funnel plots of major morbidity (A), short‐term mortality (B) and long‐term mortality (C).
Fig. S2 Meta‐regression using patient age as moderator.
Fig. S3 Meta‐regression using number of items as moderator.
Fig. S4 Forest plots of secondary outcomes.
Fig. S5 Forest plot of major morbidity in a subgroup analysis for different domains.


