Abstract
Background
Surgical‐site infections (SSIs) increase the length of hospital admission and costs. SSI prevention guidelines include preoperative antibiotic prophylaxis. This review assessed the reporting quality and cost‐effectiveness of preoperative antibiotics used to prevent SSI.
Methods
PubMed, Web of Science, Cumulative Index to Nursing and Allied Health Literature, Index of Economic Articles (EconLit), Database of Abstracts of Reviews of Effect (including the National Health Service Economic Evaluation Database) and Cochrane Central databases were searched systematically from 1970 to 2017 for articles that included costs, preoperative antibiotic prophylaxis and SSI. Included were RCTs and quasi‐experimental studies conducted in Organisation for Economic Co‐operation and Development countries with participants aged at least 18 years and published in English. Two reviewers assessed eligibility, with inter‐rater reliability determined by Cohen's κ statistic. The Consolidated Health Economic Evaluation and Reporting Standards (CHEERS) and modified Drummond checklists were used to assess reporting and economic quality. Study outcomes and characteristics were extracted, and incremental cost‐effectiveness ratios were calculated, with costs adjusted to euros (2016) (€1 = US $1·25; £1 sterling = €1·28).
Results
Twelve studies published between 1988 and 2014 were included from 646 records identified; nine were RCTs, two were nested within RCTs and one was a retrospective chart review. Study quality was highest in the nested studies. Cephalosporins (first, second and third generation) were the most frequent prophylactic interventions. Eleven studies demonstrated clinically effective interventions; ten were cost‐effective (the intervention was dominant); in one the intervention was dominated by the control; and in one the intervention was more effective and more expensive than the control.
Conclusion
Preoperative antibiotic prophylaxis does reduce SSI, costs to hospitals and health providers, but the reporting of economic methods in RCTs is not standardized. Routinely nesting economic methods in RCTs would improve economic evaluations and ensure appropriate selection of prophylactic antibiotics.
Introduction
Surgical‐site infections (SSIs) occur in 1–25 per cent of surgical patients, although the occurrence and severity vary1, 2, 3. These variations depend on the type, duration and time of day of the operation, and the time from infection onset to detection and successful treatment1 3, 4, 5, 6. SSI leads to longer hospital stays and higher costs to patients, hospitals and health systems7, 8, 9, 10, 11. In Europe, a minimum estimate of increased health cost due to SSI in 2004 was €1·47–19·1 billion12, and more recently in the USA (2014) SSI was associated with double the costs compared with those for a patient without SSI13.
Jointly, the Centers for Disease Control and Prevention (CDC) in the USA, the National Institute for Health and Care Excellence in the UK and the World Health Organization developed SSI prevention guidelines4. These include several prevention measures: preoperative screening of patients and decolonization of nasal cavities, showering, hair removal, intraoperative skin preparation using chlorhexidine, preoperative prophylactic antibiotic administration (within 1 h before surgery), normothermia and body temperature regulation, use of incision drapes, administration of supplemental oxygen throughout the operation, control of the patient's glucose level, and postoperative use of surgical dressings and appropriate hand hygiene. The prevention measures may be implemented individually or as a bundle (3–5 interventions are grouped together).
Several systematic reviews have reported on aseptic skin preparation (including surgical hand asepsis, intraoperative skin antisepsis and skin preparation with chlorhexidine)14, 15, 16, dressings including wound edge protection devices16 17, increased oxygen supplementation18, glucose control19 and thermoregulation20. Two reviews have reported on the cost‐effectiveness of the interventions14 16 and the quality of health economic reporting16.
Despite the routine use of antibiotic prophylaxis, which is inexpensive21, 22, 23, SSIs continue to occur. This suggests that implementation of SSI prevention is suboptimal – that more can be done, and done cost‐effectively. To date, no cost‐effectiveness review of preoperative antibiotic prophylaxis has been performed, despite the existence of clinical guidelines for antibiotic prophylaxis in surgery21, 22, 23.
The aim of this review was to evaluate the cost‐effectiveness of preoperative antibiotic prophylaxis used to prevent SSIs, and to assess the reporting quality of clinical effectiveness and cost‐effectiveness for each study.
Methods
Data sources
Published studies were identified by following the Cochrane Review Group search strategy24, the University of York Centre for Reviews and Dissemination25 and the PRISMA statement26. Six databases were searched: the Cochrane Library (Cochrane Central), PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL via EBSCO), Web of Science core collection, Journal of Economic Literature and the Index of Economic Articles (EconLit via EBSCO), and Database of Abstracts of Reviews of Effect (DARE, via the University of York Centre for Reviews and Dissemination, which incorporates the National Health Service Economic Evaluation Database (NHS EED)). Earlier databases were searched from 1970 (PubMed, EconLit) and others from 1994 (DARE and NHS EED), 1996 (Cochrane Central) and 1982 (CINAHL). The search of all databases was concluded on 28 June 2017.
Search strategy
Keywords and search terms were matched with database‐specific medical subject heading (MeSH) terms or title fields. Keywords for four different themes were linked with AND (cost AND prophylaxis AND prevention AND surgical‐site infection). Full search strategies can be found in Table S1 (supporting information). Search results were exported into EndNote® version X7 (Thomson Reuters, New York, USA) and duplicates were removed. Manual screening of references from included articles was performed to identify additional publications not identified by the search.
Selection criteria
Systematic reviews, guidelines, conference proceedings and letters were excluded. Only articles published in English and in peer‐reviewed journals were included. The studies had to define a SSI, even if it did not conform to the CDC definition4: an infection related to an operative procedure that occurs at or near the surgical incision within 30 days of the procedure or within 1 year if an implant is left in place. PICO (population, intervention, comparison and outcomes) were used to evaluate study eligibility. Studies were included if they were economic evaluations in RCTs or quasi‐experimental studies that compared the efficacy between different antibiotic prophylaxis regimens or placebo. Economic evaluations were defined as the comparative analysis of the costs and consequences of alternative programmes27. Studies were excluded if they were performed in non‐OECD (Organisation for Economic Co‐operation and Development) countries. OECD countries were defined as high‐income‐earning economies28, and included 31 OECD members (Table S2, supporting information). Other exclusion criteria were: study participants younger than 18 years of age and surgery that did not require a general anaesthetic.
Data extraction
Data from outcomes and resource use studies were used to construct and judge the cost‐effectiveness. Two reviewers independently applied the inclusion and exclusion criteria to the eligible studies. They first screened the titles, then abstracts and finally the full text. At each step their agreement was assessed using Cohen's κ statistic with a 95 per cent c.i.29. Cohen's κ statistic adjusts the proportion of articles for which there is agreement by the amount of agreement expected by chance alone29 30. Agreement strengths for Cohen's κ are defined29 30 as: poor, κ < 0·00; slight, κ = 0·00–0·20; fair, κ = 0·21–0·40; moderate, κ = 0·41–0·60; substantial, κ = 0·61–0·80; and almost perfect, κ = 0·81–1·00.
Disagreements were resolved by discussion, and when consensus could not be reached a third reviewer acted as referee. Reasons for exclusion were documented. All eligible articles that passed the full‐text screening were included in the review.
Extracted study data were recorded in a data collection form; they included year and country of study, study design, definition of SSI, population demographics, surgical procedures, antibiotic prophylaxis (costs, dosage and mode of administration), mean hospital and patient costs, and outcome data (duration of hospital stay, mortality, incidence of SSI, bacteria identified and antimicrobial resistance).
Reporting quality assessment
The 24‐item Consolidated Health Economic Evaluation and Reporting Standards (CHEERS) checklist31 was used to assess comprehensively the quality of the clinical and methodological reporting relating to title, structured abstract, methods, results, discussion, conclusion, funding and conflicts of interest. Two of the checklist items (choice of a model and assumptions) were not included as they were not applicable to any of the studies. Each of the remaining 22 items were assigned a weighted rating16: 0, did not report; 1, reported poorly; 2, reported well. The overall quality rating is the proportion of items reported well: high quality, 17 or more of 22 (77 per cent or above); medium/acceptable quality, 11 or more and fewer than 17 of 22 (50 per cent or above and less than 77 per cent); and low/unacceptable quality, fewer than 11 of 22 (less than 50 per cent). There is methodological reporting overlap between the CHEERS checklist and the economic quality checklist described below.
Economic quality assessment
A modified version of the Drummond et al. checklist27 was used to assess the quality of the economic and methodological reporting. The checklist includes ten questions, of which two have subquestions. These 12 questions enabled assessment of the following elements for each study: methods used (appropriate and accurate measurement of costs and outcomes), clinical effectiveness, limitations, uncertainty, relevance, generalizability and conclusions. Answers assigned to each question could be: ‘yes’, ‘no’ or ‘not applicable’. The overall quality ratings are based on the number of questions answered as ‘yes’: high quality, nine or more of 12 (75 per cent or above); medium/acceptable quality, six or more and fewer than nine of 12 (50 per cent or more and less than 75 per cent); and low/unacceptable quality, fewer than six of 12 (less than 50 per cent).
Incremental cost‐effectiveness ratio
When treatment effect (TE) and incremental cost‐effectiveness ratios (ICERs) were not reported, they were calculated using the study data. Treatment effect is defined as the difference between the control and intervention effect (TEc − TEi). To determine the incremental cost saving of SSIs averted, the difference in mean total cost between the intervention and control prophylaxis was divided by the treatment effect. Calculated ICER costs were then adjusted to British pounds (2016) in a two‐step process, using the Campbell and Cochrane Economics Methods Group–Evidence for Policy and Practice Information and Coordinating Centre cost converter web‐based tool32 33. Step 1 inflates the cost from the original price year to April 2016, using a Gross Domestic Product deflator index (GDPD values), obtained from the International Monetary Fund World Economic Outlook Database GDP deflator index data set34. Step 2 converts the original currency to British pounds, using conversion rates based on Purchasing Power Parities for GDP (PPP values)32 33. Using a web‐based tool, the 2016 British pound to euro conversion factor for £1 sterling is €1·28. When not stated, accepted standard practice to infer price year and/or currency33 was used. The price year was assumed to be either the year the study ended or the year of publication, and the original currency to be the same as that in the study setting.
Results
The search yielded 628 articles; 508 remained once duplicates had been removed. The remaining articles were subjected to a systematic review by two independent reviewers who applied the inclusion criteria. A further 18 articles were identified by hand‐searching. The inclusion criteria were first applied to the article titles, then abstracts and finally the full text. Cohen's κ statistic calculated for each step showed almost perfect (κ = 0·89, 95 per cent c.i. 0·80 to 0·98), substantial (κ = 0·64, 0·53 to 0·75) and moderate (κ = 0·55, 0·45 to 0·65) agreement respectively. Five full‐text articles required review by a third reviewer, and one was included. The five main reasons for full‐text exclusion were: age restriction (81 articles), inadequate or no cost data (34), discussion or symposium paper (16), systematic review (14) and studies performed in non‐OECD country (13). Twelve articles met the inclusion criteria (Fig. 1).
Figure 1.
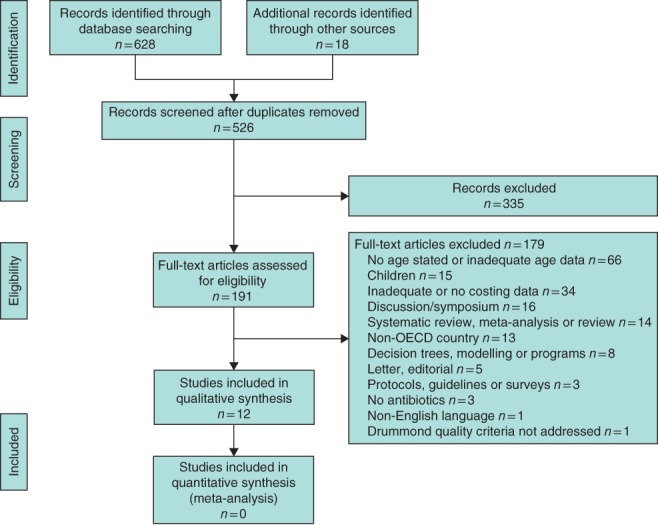
PRISMA flow diagram for the review. OECD, Organisation for Economic Co‐operation and Development
Table 1 provides detailed characteristics of the 12 included studies35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46. These were published between 1988 and 2014 with four published after 200038 39, 44 46. Nine36–38,40–45 were RCTs, two39 46 were nested within an RCT and one35 was a retrospective chart review. Eight were conducted in Europe (Greece35 43, Scotland37, UK38, Spain40, Italy42, Finland45 and the Netherlands39), three in the USA36 41, 46 and one in Japan44. The studies encompassed head and neck, gynaecological, vascular, cardiothoracic, general (breast and endocrine, intestinal and colorectal, and hepatopancreatobiliary) and orthopaedic surgery. Eleven studies35–38,40–46 evaluated the effectiveness of preoperative prophylaxis of the antibiotic cephalosporin (either first, second or third generation). These included ‘clean’ surgery (neck dissection35, axillary lymph node dissection36, coronary artery bypass graft (CABG)38 45, abdominal aortic or lower limb prosthetic vascular surgery42) and ‘clean‐contaminated’ surgery (abdominal or vaginal hysterectomy37 41, 43, digestive tract resection with anastomosis39, colonic resection and colorectal surgery41 46, biliary40 and gallbladder surgery44). One study39 evaluated selective decontamination of the digestive tract in clean‐contaminated surgery of the digestive tract with anastomosis.
Table 1.
Characteristics of included studies
| Preoperative prophylaxis | Preoperative prophylaxis outcome measures | ||||||
|---|---|---|---|---|---|---|---|
| Reference | Population | Follow‐up | Control | Intervention | Primary (efficacy) | Secondary (cost analysis) | Conclusion |
| Blair et al.35 (1995) | ‘Clean’ neck dissection: 192 | n.s. | No prophylaxis | Cefazolin 600 mg* | First‐generation cephalosporin; clindamycin and penicillin versus no antibiotic to prevent postoperative wound infection | Cost‐benefit analysis (hospital stay and cost) | No significant difference in infections. Preoperative antibiotic prophylaxis advocated. Cost‐effective |
| No prophylaxis | Clindamycin 2 g* | ||||||
| No prophylaxis | Penicillin* | ||||||
| No prophylaxis | Drug name n.s.*, † | ||||||
| Bold et al.36 (1998) | Axillary lymph node dissection: 178 | 4 weeks after surgery | Placebo (normal saline) | Cefonicid 1 g (single dose) | Second‐generation cephalosporin versus placebo to decrease postoperative wound complications | Cost‐benefit analysis | No significant difference in infections. Preoperative antibiotic prophylaxis advocated |
| Davey et al.37 (1988) | Abdominal or vaginal hysterectomy: 400 | Every 3 days, then after discharge (visit week 2, phone call week 6) | Placebo (normal saline) | Cephradine 2 g (single dose) | First‐generation cephalosporin versus broad‐spectrum penicillin to prevent wound infection | Cost‐benefit analysis (patient, hospital and community services) | Cephradine antibiotic prophylaxis advocated in abdominal hysterectomy. Antibiotic prophylaxis questionable in vaginal hysterectomy |
| Mezlocillin 5 g (single dose) | |||||||
| Dhadwal et al.38 (2007) | Median sternotomy for primary CABG of at least 1 thoracic artery and at least 1 of 4 defined risk factors: 201‡ and 186§ | Daily until discharge, then after discharge (week 6 and 90 days) | Cefuroxime 1·5 g (single dose), then cefuroxime 750 mg at reversal of anticoagulation, 8 and 16 h after surgery | Rifampicin 600 mg (single dose), then gentamicin 2 mg/kg + vancomycin 15 mg/kg on induction of anaesthesia. Postoperative vancomycin 7·5 mg/kg at 12, 24 and 36 h | Second‐generation¶ cephalosporin versus gentamicin combined with rifampicin and vancomycin to prevent sternal wound infection | Cost‐benefit analysis | Longer and broader‐ spectrum preoperative antibiotic prophylaxis advocated. Cost‐effective |
| Dijksman et al.39 (2012) | Intestinal resection with primary anastomosis, with or without a diverting ileostomy or closure of a temporary colostomy: 289 | 1 year | Placebo for 2 days before surgery, then parenteral perioperative cefuroxime 1500 mg + metronidazole 500 mg 30 min before surgery. Cefuroxime 1500 mg + metronidazole 500 mg continued 8‐hourly for 24 h | SDD (polymyxin B sulphate100 mg + tobramycin 80 mg + amphotericin B 500 mg) for 2 days before surgery and continued for at least 3 days after surgery or until normal bowel function. Parenteral perioperative antibiotic cefuroxime 1500 mg + metronidazole 500 mg 30 min before surgery. Cefuroxime 1500 mg + metronidazole 500 mg continued 8‐hourly for 24 h | Perioperative selective decontamination of digestive tract (polymyxin B sulphate with tobramycin and amphotericin B) versus placebo to reduce infection | Cost‐effectiveness analysis | Selective decontamination of digestive tract advocated. Cost‐effective |
| Garcia‐Rodriguez et al.40 (1989) | Gastroduodenal or biliary surgery with at least 1 of 11 defined risk factors: 1451 | 16 days | Cefoxitin 2 g (single i.v. dose), then cefoxitin 2 g 6, 12 and 18 h after surgery | Cefotaxime 1 g (single dose) | Second‐ and third‐generation cephalosporin¶ to prevent postoperative infection | Cost‐benefit analysis | Cefotaxime antibiotic prophylaxis advocated. Cost‐effective |
| Jones et al.41 (1987) | Obstetrics and gynaecology, gastrointestinal; orthopaedics and other (total joint replacement and open reduction of fractures) surgical procedures: 812 | 30 days | Cefotaxime 1·0 g (slow i.v. bolus after anaesthesia but 30 min before incision). Additional cefotaxime 1·0 g given during surgery if procedure duration 2 h or more. For bowel surgery, standard bowel preparation before prophylaxis | Cefoperazone 1·0 g (slow i.v. bolus after anaesthesia but 30 min before incision). For bowel surgery, standard bowel preparation before prophylaxis | Two third‐generation cephalosporins to prevent perioperative infection | Cost containment | Both cefoperazone and cefotaxime antibiotic prophylaxis advocated. Both cost‐effective |
| Marroni et al.42 (1999) | Abdominal aortic or lower limb prosthetic vascular surgery: 238 | Daily until discharge, then after discharge (3 monthly for 1 year, then at 24 months) | Cefazolin 2 g (single i.v. dose) | Teicoplanin 400 mg (single dose) | Efficacy and tolerability of first‐generation cephalosporin and a glycopeptide to prevent postoperative infection | Cost‐benefit analysis | Cefazolin antibiotic prophylaxis advocated. Cost‐effective |
| Matkaris et al.43 (1991) | Abdominal hysterectomy: 200 | 4–5 days if no SSI, otherwise kept in hospital until infection resolved | No prophylaxis | Ceftriaxone 2 g (single dose). Additional dose if postoperative infection | Efficacy and safety of three third‐generation cephalosporins to prevent postoperative infection | Cost‐benefit analysis | Single dose of any of the three antibiotic prophylaxes advocated. Cefotaxime was most cost‐effective |
| Cefotaxime 2 g (single dose). Additional dose if postoperative infection | |||||||
| Ceftazidime 2 g (single dose). Additional dose if postoperative infection | |||||||
| Matsui et al.44 (2014) | Laparoscopic cholecystectomy for gallbladder stones or polyps: 437 | 8 days after surgery in outpatient setting | No prophylaxis | Cefazolin 1 g (3 doses before skin incision, then 12 and 24 h after surgery). Additional cefazolin 1 g in theatre if duration of surgery more than 3 h | First‐generation† cephalosporin to reduce postoperative complications, including SSI and distant infection | Cost‐ effectiveness analysis | Antibiotic prophylaxis advocated. Cost‐effective |
| Sisto et al.45 (1994) | CABG: 551 | Daily until discharge (10–12 days) or to another hospital (6–7 days) | Ceftriaxone 2 g (single dose) | Cefuroxime 1·5 g (single dose), then cefuroxime 1·5 g (8‐hourly to end of postoperative day 2) | Efficacy and side‐effects of single‐dose third‐generation cephalosporin versus multiple doses of second‐generation cephalosporin to prevent postoperative infection | Cost‐benefit analysis | Efficacy of ceftriaxone and cefuroxime equivalent. Ceftriaxone cheaper and simpler to use |
| Wilson et al.46 (2008) | Colorectal surgery: 672# | 4 weeks after surgery | Ertapenem 1 g (single dose) | Cefotetan 2 g (single dose) | Preoperative prophylaxis of second‐generation cephalosporin and a β‐lactam to reduce postoperative infectious complications | Cost‐benefit analysis | Ertapenem antibiotic prophylaxis advocated. Cost‐effective |
Prophylactic antibiotic dose not stated;
antibiotic trade name or generation of the cephalosporin not stated;
intention‐to‐treat data for antibiotic efficacy;
per‐protocol data for costs38;
blinding not stated;
per‐protocol data. n.s., Not stated; CABG, coronary artery bypass graft; SDD, selective decontamination of digestive tract; i.v., intravenous; SSI, surgical‐site infection. A more detailed version of this table is available as Table S3, supporting information47,48.
Quality assessment of reporting
The reporting quality of most of the studies was low to moderate using the CHEERS statement checklist31 (Table 2; Table S4, supporting information). Only one study39 had a high reporting quality for 18 of the 22 items. Three studies37, 38, 39 reported economic evaluations in their titles. In most studies the objectives, methods (settings, populations and comparators) were well reported35, 36, 37, 38, 39 41, 43, 44, 45, 46, although time horizons and discounting were poorly reported35 37, 38 40, 41, 42, 43, 44 46. Overall the results were poorly reported, including study parameters, incremental costs and characterization of uncertainty and heterogeneity36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46. Discussion around the individual study findings, their limitations and generalizability was also of poor quality37 40, 41, 42, 43, 44, 45, 46. Source of funding and conflict of interest was poorly reported: four35 36, 41 44 reported funding and two38 44 reported conflict of interest. Only one44 of these studies reported on both funding and conflict of interest.
Table 2.
CHEERS checklist summary of reporting quality
| No. of studies reporting (n = 12) | ||||
|---|---|---|---|---|
| Questions | Not reported | Poorly reported | Well reported | |
| Title and abstract | Title | 6 | 3 | 3 |
| Abstract | 0 | 6 | 6 | |
| Introduction | Background and objectives | 0 | 2 | 10 |
| Methods | Target population and subgroups | 0 | 3 | 9 |
| Setting and location | 0 | 4 | 8 | |
| Study perspective | 0 | 5 | 7 | |
| Comparators | 0 | 5 | 7 | |
| Time horizon | 3 | 6 | 3 | |
| Discount rate | 12 n.a. | 0 | 0 | |
| Choice of health outcomes | 2 | 7 | 3 | |
| Measurement of effectiveness | 2 | 7 | 3 | |
| Measurement and valuation of preference‐based outcomes | 1 n.a. | 7 | 4 | |
| Estimating resources and costs | 1 n.a.; 1 | 7 | 3 | |
| Currency, price date and conversion | 5 | 6 | 1 | |
| Choice of model | 12 n.a. | 0 | 0 | |
| Assumptions | 12 n.a. | 0 | 0 | |
| Analytical methods | 0 | 11 | 1 | |
| Results | Study parameters | 12 | 0 | 0 |
| Incremental costs and outcomes | 10 | 0 | 2 | |
| Characterizing uncertainty | 9 | 1 | 2 | |
| Characterizing heterogeneity | 3 | 8 | 1 | |
| Discussion | Study findings, limitations, generalizability and current knowledge | 0 | 9 | 3 |
| Other | Source of funding | 8 | 0 | 4 |
| Conflict of interest | 10 | 0 | 2 | |
n.a., Not applicable.
Clinical effectiveness of antibiotic prophylaxis, length of hospital stay and mortality
All studies included a definition for postoperative SSI (Table 3). Four studies38 40, 42 46 used several variations of recognized definitions: the National Nosocomial Infections Surveillance54, 55, 56, variations of the CDC definition50 53 and the National Research Council definition50 52. The definition used by Blair and colleagues35 was developed by Johnson and co‐workers49 in 1984, and the definition reported by Dijksman et al.39 was that of Rommes et al.51, used in the nested study of Roos and colleagues47.
Table 3.
Evidence of efficacy of preoperative prophylactic antibiotics
| Preoperative prophylaxis | Sample size | Postoperative infections | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Surgical procedure | Definition of postoperative infection | Control | Intervention | Total (M : F) | Control* | Intervention* | Control* | Intervention* | P |
| Blair et al.35 † | Neck dissection | Wound infection: based on wound grading scale developed by Johnson et al.49 | No prophylaxis | Cefazolin 600 mg | 192 (139 : 53) | 99 (51·6) | 58 (30·2) | 10 (10·0) | 3 (3·3) | 0·08 |
| Clindamycin 2 g | 13 (6·8) | |||||||||
| Penicillin | 17 (8·6) | |||||||||
| Drug n.s. | 5 (2·6) | |||||||||
| Bold et al.36 | Axillary lymph node dissection | Infection of surgical wound in the absence of any other site of infection | Placebo (normal saline) | Cefonicid 1 g | 178 (24 : 154) | 90 (50·6) | 88 (49·4) | 12 (13·0) | 5 (6·0) | 0·08‡ |
| Davey et al.37 § | AH or VH | Infected wound; pelvic infection | Placebo (normal saline) | Cephradine 2 g | 400 (0 : 400) | AH 102 (25·5) | AH 97 (24·3) | Hospital wound | ||
| Pelvic | ||||||||||
| VH 29 (7·2) | VH 34 (8·5) | AH 20 (19·6) | AH 6 (6) | < 0·05 | ||||||
| VH 6 (21) | VH 1 (3) | < 0·05 | ||||||||
| Hospital total | ||||||||||
| AH 42 (41·2) | AH 16 (16) | < 0·01 | ||||||||
| VH 10 (34) | VH 8 (24) | 0·41 | ||||||||
| Home wound | ||||||||||
| Pelvic | ||||||||||
| AH 9 (8·8) | AH 10 (10) | 0·81 | ||||||||
| VH 2 (7) | VH 1 (3) | VH 0·59 | ||||||||
| Home total | ||||||||||
| AH 15 (14·7) | AH 25 (26) | AH 0·05 | ||||||||
| VH 7 (24) | VH 10 (29) | VH 0·02 | ||||||||
| Mezlocillin 5 g | AH 102 (25·5) | AH 101 (25·3) | Hospital wound | |||||||
| Pelvic | ||||||||||
| VH 29 (7·2) | VH 37 (9·2) | AH 20 (19·6) | AH 18 (17·8) | 0·86 | ||||||
| VH 6 (21) | VH 0 (0) | < 0·01 | ||||||||
| Hospital total | ||||||||||
| AH 42 (41·2) | AH 30 (29·7) | 0·11 | ||||||||
| VH 10 (34) | VH 6 (16) | 0·15 | ||||||||
| Home wound | ||||||||||
| Pelvic | ||||||||||
| AH 9 (8·8) | AH 4 (4·0) | 0·25 | ||||||||
| VH 2 (7) | VH 0 (0) | 0·19 | ||||||||
| Home total | ||||||||||
| AH 15 (14·7) | AH 14 (13·9) | 1·000 | ||||||||
| VH 7 (24) | VH 2 (5) | 0·04 | ||||||||
| Dhadwal et al.38 | CABG | NNIS infection risk score35 CDC sternal wound50 | Cefuroxime 1·5 g | Rifampicin 600 mg; gentamicin 2 mg/kg; vancomycin 15 mg/kg | 201 (165 : 36) | 106 (52·8) | 95 (47·2) | NNIS 30‐day infection | ||
| 12 (11·3) | 4 (4) | 0·063 | ||||||||
| Sternal wound (90 days) | ||||||||||
| 25 (23·6) | 8 (8) | 0·004¶ | ||||||||
| Superficial | ||||||||||
| 11 (10·4) | 4 (4) | 0·097 | ||||||||
| Deep | ||||||||||
| 8 (7·5) | 2 (2) | 0·15# | ||||||||
| Organ space | ||||||||||
| 6 (5·7) | 2 (2) | 0·36# | ||||||||
| Deep + organ space | ||||||||||
| 14 (13·2) | 4 (4) | 0·03 | ||||||||
| Sternal debridement | ||||||||||
| 19 (17·9) | 4 (4) | 0·002 | ||||||||
| Harvest site infection | ||||||||||
| 7 (6·6) | 45 (5) | 0·69 | ||||||||
| Dijksman et al.39 | Digestive tract surgery | Wound infection, intra‐abdominal abscess and anastomotic leak47,51. Calculated event rate was percentage of patients who suffered at least 1 infectious complication | Placebo. Parenteral perioperative antibiotic cefuroxime 1500 mg + metronidazole 500 mg | SDD (polymyxin B sulphate100 mg + tobramycin 80 mg + amphotericin B 500 mg). Parenteral perioperative antibiotic cefuroxime 1500 mg + metronidazole 500 mg | 289 (156 : 133) | 146 (50·5) | 143 (49·5) | 45 (30·8) | 28 (19·6) | 0·03¶ |
| Garcia‐Rodriguez et al.40 ** | Gastroduodenal or biliary surgery | Surgical wound infection: cellulitis with purulent secretion, with or without dehiscence (NRC52) | Cefoxitin 2 g | Cefotaxime 1 g | 1451 (624 : 827) | 716 (50·2) | 722 (49·8) | Wound infection | ||
| 54 (7·5) | 24 (3·3) | < 0·002 | ||||||||
| Jones et al.41 †† | Gastrointestinal; gynaecological, orthopaedic (total joint replacement and open reduction of fractures) and other surgery | Postoperative surgical incision or peritoneal cavity infection | Cefotaxime 1 g | Cefoperazone 1 g | 812 (42 : 770) | 401 (49·4) | 411 (50·6) | Wound infection | ||
| 12 (3·0) | 9 (2·2) | > 0·05 | ||||||||
| Total general | 96 | 89 | 1 (1) | 2 (2) | 1·000 | |||||
| UGIT | 72 | 66 | 0 (0) | 0 (0) | ||||||
| Colorectal | 24 | 23 | 1 (4) | 2 (9) | 1·000 | |||||
| Total O+G | 168 | 168 | 9 (5·4) | 6 (3·6) | 0·60 | |||||
| Hysterectomy | 119 | 125 | 8 (6·7) | 6 (4·8) | 0·59 | |||||
| C‐section | 19 | 18 | 1 (5) | 0 (0) | 1·000 | |||||
| Other O+G | 30 | 25 | 0 (0) | 0 (0) | ||||||
| Total orthopaedic | 74 | 77 | 1 (1) | 0 (0) | 0·49 | |||||
| Total joints | 51 | 59 | 0 (0) | 0 (0) | ||||||
| Other orthopaedic | 23 | 18 | 1 (4) | 0 (0) | 1·000 | |||||
| Other surgery | 61 | 77 | 1 (2) | 1 (1) | 1·000 | |||||
| Marroni et al.42 ‡‡ | Abdominal aortic or lower limb prosthetic vascular surgery | Surgical wound infection; deep wound infection (CDC53) | Cefazolin 2 g | Teicoplanin 400 mg | 238 (220 : 18) | 119 (50·0) | 119 (50·0) | SSI | ||
| 2 (1·7) | 7 (5·9) | 0·19 | ||||||||
| Graft | ||||||||||
| 2 (1·7) | 0 (0·0) | 0·49 | ||||||||
| Wound | ||||||||||
| 5 (4·2) | 2 (1·7) | 0·46 | ||||||||
| Matkaris et al.43 | AH | Fever > 38°C for 24 h, blood analysis, urine analysis, clinical evaluation | No prophylaxis | Ceftriaxone 2 g | 200 (0 : 200) | 50 (25·0) | 50 (25·0) | 15 (30) | 3 (6) | < 0·01§§ |
| Cefotaxime 2 g | 50 (25·0) | 4 (8) | ||||||||
| Ceftazidime 2 g | 50 (25·0) | 4 (8) | ||||||||
| Matsui et al.44 ¶¶ | Laparoscopic cholecystectomy for removal of gallbladder stones or polyps | SSI (surgical wound and subhepatic abscess) | No prophylaxis | Cefazolin 1 g | 1037 (490 : 547) | 519 (50·0) | 518 (50·0) | SSI | ||
| 19 (3·7) | 4 (0·8) | 0·001 | ||||||||
| Wound | ||||||||||
| 16 (3·1) | 4 (0·8) | 0·005 | ||||||||
| Subhepatic | ||||||||||
| 3 (0·6) | 0 (0·0) | 0·249 | ||||||||
| All infections | ||||||||||
| 35 (6·7) | 6 (1·2) | < 0·001 | ||||||||
| Sisto et al.45 ## | CABG | Superficial and deep sternal wound infection; donor‐site infection | Ceftriaxone 2 g | Cefuroxime 1·5 g, then cefuroxime 1·5 g 8‐hourly until end of day 2 after surgery | 551 (437 : 114) | 274 (49·7) | 277 (50·3) | Superficial | ||
| 4 (1·5) | 7 (2·5) | 0·56 | ||||||||
| Deep | ||||||||||
| 8 (2·9) | 8 (2·9) | 1·00 | ||||||||
| Donor site | ||||||||||
| 3 (1·1) | 4 (1·4) | 1·00 | ||||||||
| Wilson et al.46 *** | Colorectal surgery | SSI (organ space; deep incisional; either superficial infection or anastomotic leak) (NNIS54,55) | Ertapenem 1 g | Cefotetan 2 g | 672 (365 : 307) | 338 (50·3) | 334 (49·7) | SSI | ||
| 62 (18·3) | 104 (31·1) | < 0·001 | ||||||||
| Organ/space | ||||||||||
| 4 (1·2) | 12 (3·6) | 0·05 | ||||||||
| Deep | ||||||||||
| 13 (3·8) | 17 (5·1) | 0·46 | ||||||||
| Superficial | ||||||||||
| 45 (13·3) | 75 (22·5) | 0·002 | ||||||||
| Anastomotic leak | ||||||||||
| 10 (3·0) | 14 (4·2) | 0·41 | ||||||||
Values in parentheses are percentages.
Intervention failure results for cefazolin, clindamycin and cefoperazone were pooled as individual results were not stated; statistical method was not stated, but assumed to be Fisher's exact test.
Fisher's exact test (P < 0·050 was considered significant with 80 per cent confidence level).
Analysis of significance in fourfold tables was done with the χ2 test with Yates' correction unless the total number of observations was less than 60 or the number in any cell was zero, when Fisher's exact test was used; threefold or greater tables were analysed with the χ2 test.
χ2 or Fisher's exact test with two‐sided significance level of 0·05.
χ2 test with Yates' correction.
Intention‐to‐treat data; statistical analysis with Fisher's exact test; infection data were missing for six patients in the control group and seven in the intervention group.
Per‐protocol data; statistical analysis with Fisher's exact test or χ2 test; P < 0·050 considered significant;
χ2 test with a two‐sided significance level of 0·05 when expected frequencies were less than 5.
Statistical method not stated.
χ2 test with significance level of 0·05; Fisher's exact test used for subhepatic comparison as expected frequencies in cells were less than 5.
Student's t test for parametric data and Mann–Whitney or χ2 test for non‐parametric data; significance level of 0·05.
Per‐protocol data; absolute difference and 95 per cent c.i. for percentage prophylactic failure were determined in a statistical model adjusting for surgical procedure; 95 per cent c.i. that did not overlap zero indicated significant difference between groups at P < 0·050. n.s., Not stated; AH, abdominal hysterectomy; VH, vaginal hysterectomy; CABG, coronary artery bypass graft; NNIS, National Nosocomial Infections Surveillance; CDC, Centers for Disease Control and Prevention; SDD, selective decontamination of digestive tract; NRC, National Research Council; UGIT, upper gastrointestinal tract; O+G, obstetrics and gynaecology; C‐section, caesarean section; SSI, surgical‐site infection.
All studies reported SSI rates and the effectiveness of the preoperative antibiotic prophylaxis. Prophylactic effectiveness was demonstrated in 11 studies35–44,46, although effectiveness was statistically significant in only seven37, 38, 39, 40 43, 44 46. Blair and colleagues35 demonstrated effectiveness of the intervention compared with placebo, but failed to stipulate which of the three interventions was effective (cefazolin, clindamycin or cefoperazone). Effectiveness was therefore calculated for the pooled interventions. Matkaris et al.43 demonstrated significant effectiveness of three prophylactic antibiotics versus the no‐antibiotic control, and also reported comparable differences between the three prophylactic antibiotics. The study that did not demonstrate prophylactic effectiveness for the intervention compared a single dose of ceftriaxone (third‐generation cephalosporin) with three doses of cefuroxime (second generation) given three times daily, in patients undergoing CABG45.
Eleven studies35, 36, 37, 38, 39, 40, 41, 42, 43, 44 46 reported length of hospital stay (LOS), although the reporting was inconsistent between treatment groups as well as between infected and non‐infected patients (Table 4). Overall LOS was reduced in the intervention group for all of the studies, although this was significant in only one study44. LOS was increased in the presence of infection compared with no infection in two studies35 40. Five studies38, 39, 40 42, 45 reported on mortality, although none stated the day of admission when the death occurred; there was no significant difference in mortality rates between intervention and control groups in the five studies38, 39, 40 42, 45. There was one death from infection in each arm of the Marroni study42, whereas in the Sisto study45 no death was from infection. Mortality was not reported in the paper by Wilson et al.46, but was reported in the nested study of Itani and co‐workers48; the difference was not statistically significant and was not directly related to the prophylaxis.
Table 4.
Length of hospital stay and mortality associated with preoperative prophylactic antibiotics
| Population | Length of hospital stay* | Mortality‡ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Surgical procedure | Preoperative prophylaxis | C | I | C | I | No infection | Infection | P | C | I | P |
| Blair et al.35 § | Neck dissection | No prophylaxis versus cefazolin, clindamycin and cefoperazone | 99 | 93 | 8 (4–22) | 23 (10–73) | n.c. | n.s. | n.s. | |||
| Bold et al.36 ¶ | Axillary lymph node dissection | Placebo (normal saline) versus cefonicid | 90 | 88 | 5·9 (2–15) | 3 | n.c. | n.s. | n.s. | |||
| Davey et al.37 | AH or VH | AH: placebo (normal saline) versus cephradine | 102 | 97 | 8·7 (8·2–9·2) | 8·0 (7·7–8·3) | n.c | n.s. | n.s. | |||
| AH: placebo (normal saline) versus mezlocillin | 101 | 8·7 (8·2–9·2) | 7·9 (7·6–8·2) | n.c. | n.s. | n.s. | ||||||
| VH: placebo (normal saline) versus cephradine | 29 | 34 | 7·2 (6·7–7·7) | 8·1 (7·2–9·0) | n.c. | n.s. | n.s. | |||||
| VH: placebo (normal saline) versus mezlocillin | 37 | 7·2 (6·7–7·7) | 7·3 (7·0–7·6) | n.c. | n.s. | n.s. | ||||||
| Dhadwal et al.38 # | CABG | Cefuroxime versus rifampicin + gentamicin + vancomycin | 106 | 95 | 11·7 (4–69) | 9·5 (4–73) | 0·063 | 4 (4) | 1 (1) | 0·630 | ||
| Dijksman et al.39 | Digestive tract surgery | Placebo, cefuroxime and metronidazole versus SDD, cefuroxime and metronidazole | 146 | 143 | (12, 9–18) | (11, 9–14) | 0·055 | 5 (3·4) | 6 (4·2) | 0·732 | ||
| Garcia‐Rodriguez et al.40 ** | Gastroduodenal or biliary surgery | Cefoxitin versus cefotaxime | 716 | 722 | 11·7 (4–69) | 9·5 (4–73) | 10·2 (9·9–10·5) | 13·7 (12·4–15·0) | < 0·001 | 7 (0·6) | 4 (0·6) | n.s. |
| Jones et al.41 †† | Hysterectomy, genitourinary, gastrointestinal and other (mainly orthopaedic total joint replacement and open reduction of fractures) surgery | Cefotaxime versus cefoperazone | 401 | 411 | 11·5 (12–30) | 14·3 (12–30) | n.c. | n.s. | n.s. | |||
| Marroni et al.42 | Abdominal aortic or lower limb prosthetic vascular surgery | Cefazolin versus teicoplanin | 119 | 119 | 14·8 | 16·2 | n.c. | 3 (2·5) | 4 (3·4) | 1·000 | ||
| Matkaris et al.43 | AH | No antibiotic prophylaxis versus ceftriaxone | 50 | 50 | 5·46 | 4·32 | < 0·001 | n.s. | n.s. | |||
| No antibiotic prophylaxis versus cefotaxime | 50 | 5·46 | 4·36 | < 0·001 | n.s. | n.s. | ||||||
| No antibiotic prophylaxis versus ceftazidime | 50 | 5·46 | 4·50 | < 0·001 | n.s. | n.s. | ||||||
| Matsui et al.44 ‡‡ | Laparoscopic cholecystectomy for removal of gallbladder stones or polyps | No antibiotic prophylaxis versus cefazolin | 519 | 518 | 4·07(3·00)† | 3·69(5·26)† | 0·010 | 0 (0) | 0 (0) | |||
| Sisto et al.45 | CABG | Ceftriaxone versus cefuroxime | 274 | 277 | n.s. | n.s. | n.c. | 3 (1·1) | 4 (1·4) | 1·000 | ||
| Wilson et al.46 §§ | Colorectal surgery | Ertapenem versus cefotetan | 338 | 334 | 7·6 (6·8–8·2) | 8·7 (7·7–9·7) | n.c. | 3 of 451 (0·7) | 7 of 450 (1·6) | 0·340 | ||
Values are mean (median, range) unless indicated otherwise;
values are mean(s.d.).
Values in parentheses are percentages.
Infection rate and length of stay (LOS) for cefazolin, clindamycin and cefoperazone were pooled as individual results were not stated; mean cost per patient was based on length of hospital stay (LOS).
Patients with infection were admitted to hospital (7 placebo, 1 intervention).
Mann–Whitney U test for LOS and χ2 test with Yates' correction for mortality.
Intention‐to‐treat data; infection data were missing for six patients in the control group and seven in the intervention group.
Per‐protocol data.
Intention‐to‐treat data.
Per‐protocol data; intention‐to‐treat data used for mortality reported in the nested study of Itani et al.48. C, control; I, intervention; n.c., not calculated (insufficient data in article); n.s., not stated; AH, abdominal hysterectomy; VH, vaginal hysterectomy; CABG, coronary artery bypass graft; SDD, selective decontamination of the digestive tract. P values are those reported in the article.
Bacterial isolates and antimicrobial resistance
Six studies38 40, 41, 42 45, 46 reported and identified the bacterial pathogens responsible for SSIs; the pathogens were similar across the studies (Table 5). Clostridium difficile, a toxic organism found in the intestine causing colitis, was identified in one study45 after surgery following a second dose of cefuroxime. Wilson et al.46 also reported C. difficile colitis (in 2 patients who received ertapenem) and antimicrobial resistance of the pathogens to ertapenem versus cefotetan in the nested study48. Resistance of pathogens to ertapenem was much lower (16 per cent) than that to cefotetan (67 per cent). Only two other studies38 41 reported antimicrobial resistance. Dhadwal and colleagues38 found no increase in vancomycin‐resistant Enterococcus or methicillin‐resistant Staphylococcus aureus (MRSA) in CABG, although Gram‐positive bacteria resistant to rifampicin were identified in both control (cefuroxime) and investigation (rifampicin, vancomycin and gentamicin) groups. Jones and co‐workers41 found few pathogens (8 per cent) resistant to cefoperazone and, although no pathogens were resistant to cefotaxime, 72 per cent were inhibited by cefotaxime in several surgical procedures.
Table 5.
Evidence of preoperative prophylactic antibiotics in bacterial isolates and resistance patterns
| Preoperative prophylaxis | Bacterial isolates | |||||
|---|---|---|---|---|---|---|
| Reference | Population | Control | Intervention | Control | Intervention | Bacterial resistance patterns |
| Dhadwal et al.38 * | Median sternotomy for primary CABG of at least one thoracic artery and at least one of four defined risk factors: 201 | Cefuroxime 1·5 g (single dose), then cefuroxime 750 mg at reversal of anticoagulation 8 and 16 h after surgery | Rifampicin 600 mg (single dose), then gentamicin 2 mg/kg + vancomycin 15 mg/kg on induction of anaesthesia. Postoperative vancomycin 7·5 mg/kg at 12, 24 and 36 h | 19 of 99 | 7 of 87 | No increase in vancomycin‐ resistant Enterococcus or MRSA |
| GNB: 15 | GNB: 7 | |||||
| GPB: 10 | GPB: 4 | |||||
| Rifampicin‐resistant GPB: 4 | Rifampicin‐ resistant GPB: 1 | |||||
| Vancomycin‐ resistant GPB: 0 | Vancomycin‐ resistant GPB: 0 | |||||
| Anaerobic: 2 | Anaerobic: 1 | |||||
| Yeast: 1 | Yeast: 1 | |||||
| Garcia‐Rodriguez et al.40 † | Gastroduodenal or biliary surgery with at least one of the 11 defined risk factors: 1451 | Cefoxitin 2 g (single i.v. dose), then cefoxitin 2 g 6,12 and 18 h after surgery | Cefotaxime 1 g (single dose) | Escherichia coli and Staphylococcus aureus most common; frequency and study group not mentioned | Not stated | |
| Jones et al.41 | Hysterectomy, genitourinary, gastrointestinal or other (total joint replacement and open reduction of fractures) surgical procedures: 812 | Cefotaxime 1·0 g (slow i.v. bolus after anaesthesia but 30 min before incision). Additional cefotaxime 1·0 g given during surgery if procedure duration 2 h or more. For bowel surgery, standard bowel preparation before prophylaxis | Cefoperazone 1·0 g (slow i.v. bolus after anaesthesia but 30 min before incision). For bowel surgery, standard bowel preparation before prophylaxis | 12 of 21 | 18 of 21 | Aerobic organisms 92% susceptible to cefoperazone and 72% inhibited by cefotaxime |
| GNB: 2 | GNB: 2 | |||||
| GPB: 5 | GPB: 3 | |||||
| Anaerobic: 3 | Anaerobic: 2 | |||||
| Marroni et al.42 | Abdominal aortic or lower limb prosthetic vascular surgery: 238 | Cefazolin 2 g (single i.v. dose) | Teicoplanin 400 mg (single dose) | Graft | n.s. | |
| MRSA: 0 | MRSA: 0 | |||||
| SWI | ||||||
| GNB: 1 | GNB: 2 | |||||
| GPB: 1 | GPB: 1 | |||||
| UTI | ||||||
| GNB: 3 | GNB: 4 | |||||
| Bloodstream | ||||||
| GNB: 2 | GNB: 0 | |||||
| Sisto et al.45 | CABG: 551 | Ceftriaxone 2 g (single dose) | Cefuroxime 1·5 g (single dose), then cefuroxime 1·5 g 8‐hourly until end of postoperative day 2 | Mediastinitis | n.s. | |
| GNB: 1 | GNB: 1 | |||||
| GPB: 6 | GPB: 4 | |||||
| Anaerobic: 0 | Anaerobic: 1 | |||||
| Clostridium difficile: 0 | C. difficile: 1 | |||||
| Wilson et al.46 ‡ | Colorectal surgery: 672) | Ertapenem 1 g (single dose) | Cefotetan 2 g (single dose) | GPB: 42 | GPB: 51 | 67% resistant to cefotetan; 16% resistant to ertapenem |
| Anaerobic: 36 | Anaerobic: 44 | |||||
| GNB: 17 | GNB: 23 | |||||
| C. difficile: 2 | ||||||
Intention‐to‐treat data for antibiotic efficacy.
Infection data were missing for six patients in the control group and seven in the intervention group.
Per‐protocol data; bacterial isolates and susceptibility data from nested study by Itani et al.48. GNB, Gram‐negative bacteria; GPB, Gram‐positive bacteria; MRSA, methicillin‐resistant Staphylococcus aureus; SWI, surgical wound infection; UTI, urinary tract infection; CABG, coronary artery bypass graft.
Quality assessment of economic evaluation
A modified Drummond checklist27 was used to assess economic methodological quality for each study (Table 6; Table S5, supporting information). Overall four studies39, 40, 41 46 were evaluated as being of high quality, six36–38,43–45 as moderate/acceptable quality, and two35 42 as low/unacceptable quality. All studies defined an answerable question and included an alternative treatment. Eight studies37–41,44–46 accurately measured their outcomes and costs, which were both reported in the appropriate units. No study performed sensitivity analysis or discounted cost, although discounting was not applicable in six studies37 39, 41 44, 45, 46. Only one study39 performed an ICER analysis.
Table 6.
Summary of quality assessment checklist for assessing economic evaluations of included studies
| No. of studies reporting (n = 12) | ||||
|---|---|---|---|---|
| Question | Yes | No | Unsure | Not applicable |
| Well defined question stated? | 12 | 0 | 0 | 0 |
| Description of alternatives? | 12 | 0 | 0 | 0 |
| Evidence of clinical effectiveness established? | 10 | 1 | 1 | 0 |
| Relevant costs and outcomes identified? | 7 | 5 | 0 | 0 |
| Costs measured accurately in appropriate units? | 8 | 4 | 0 | 0 |
| Outcomes measured accurately in appropriate units | 8 | 4 | 0 | 0 |
| Costs valued credibly? | 10 | 2 | 0 | 0 |
| Outcomes valued credibly? | 10 | 2 | 0 | 0 |
| Costs discounted? (n = 6) | 0 | 6 | 0 | 6 |
| Was incremental analysis performed? | 1 | 11 | 0 | 0 |
| Was sensitivity analysis performed? | 1 | 11 | 0 | 0 |
| Was generalizability discussed? | 2 | 10 | 0 | 0 |
Cost analysis of antibiotic prophylaxis
Of the included studies, nine35, 36, 37, 38 40, 42 43, 45 46 were cost‐benefit studies, two were cost‐effectiveness studies39 44 and one41 was a cost containment study (Table 1; Table S3, supporting information). These were all from the perspective of the healthcare provider, with costs reported as mean cost per patient or per patient episode. Sources for the cost data were reported in all studies, and costs included prophylactic antibiotic, daily hospital charge, nursing/staff time, hospital care, care after discharge, and treatment of the SSIs (Table 7). The currencies reported were: euros39, British pounds37, US dollars35 36, 38 40, 41, 42, 43, 44, 45, 46, drachma43 and pesetas40; both drachma and pesetas were converted to US dollars, which was the currency used in all cost analyses. Only four studies39 40, 42 46 reported the price year for the currency conversion. Nine studies35,36,38–40,43–46 reported cost savings favouring the use of the preoperative prophylaxis intervention and two37 42 reported cost savings favouring the control prophylaxis. Davey and colleagues37 showed significant clinical effectiveness for cephradine and mezlocillin in abdominal and vaginal hysterectomy, but neither intervention was considered cost‐effective. One study39 reported an ICER when using selective decontamination of the digestive tract versus placebo in gastrointestinal surgery, with the prevention of at least one infection leading to a reported saving of €23 164 per patient. No study discounted costs, although Dijksman et al.39 stated that the reason for not discounting costs included a 1‐year time horizon, and they did perform a sensitivity analysis. One study45 considered only the acquisition and delivery cost of the antibiotic prophylaxis and not the treatment failures.
Table 7.
Summary of reported costs and incremental cost‐effectiveness ratio calculated from study data
| Reference | Intervention versus control | Intervention failure* | Control failure* | Treatment effect (TEc − TEi) | Mean cost of intervention (includes treatment cost) | Mean cost of control (includes treatment cost) | Incremental cost per patient | Incremental cost per patient (2016 €)† | ICER (2016 €)† |
|---|---|---|---|---|---|---|---|---|---|
| Blair et al.35 ‡ | Cefazolin, clindamycin and cefoperazone versus placebo | 3 of 93 (3) | 10 of 99 (10) | 7 | $36 240·00 | $36 030·00 | $210·00 | 293·79 | Dominant |
| Bold et al.36 § | Cefonicid versus placebo | 5 of 88 (6) | 12 of 90 (13) | 7 | $149·80 | $364·87 | −$215·07 | −269·26 | Dominant |
| Davey et al.37 ¶ | AH: cephradine versus placebo | 40 of 97 (41) | 53 of 102 (52·0) | 11 | £18·26 | £31·34 | −£13·08 | −37·92 | Dominant |
| AH: mezlocillin versus placebo | 40 of 101 (39·6) | 53 of 102 (52·0) | 12·4 | £17·61 | £31·34 | −£13·73 | −37·92 | Dominant | |
| VH: cephradine versus placebo | 14 of 34 (41) | 15 of 29 (52) | 11 | £40·60 | £41·20 | −£0·60 | −1·65 | Dominant | |
| VH: mezlocillin versus placebo | 7 of 37 (19) | 15 of 29 (52) | 33 | £8·80 | £41·20 | −£32·40 | −89·50 | Dominant | |
| Dhadwal et al.38 # | Rifampicin +gentamicin +vancomycin versus cefuroxime | 8 of 87 (9) | 25 of 99 (25) | 16 | $15 158·00 | $19 054·00 | −$3896·00 | −4315·99 | Dominant |
| Dijksman et al.39 ** | SDD (amphotericin B, polymyxin B sulphate + tobramycin) versus placebo | 28 of 143 (19·6) | 45 of 146 (30·8) | 11·2 | €12 031·00 | €14 635·00 | −€2604·00 | −2731·28 | Dominant |
| Garcia‐Rodriguez et al.40 †† | Cefotaxime versus cefoxitin | 22 of 722 (3·3) | 54 of 716 (7·7) | 4·4 | $28·64 | $104·43 | −$75·79 | −120·72 | Dominant |
| Jones et al.41 ‡‡ | Cefoperazone versus cefotaxime | 9 of 411 (2·2) | 12 of 401 (3·0) | 0·8 | $14·50 | $12·90 | $1·60 | 2·64 | 5·12 |
| Marroni et al.42 §§ | Cefazolin versus teicoplanin | 7 of 119 (5·9) | 2 of 119 (1·7) | −4·2 | $4803·13 | $4361·86 | $441·27 | 552·45 | Dominated by control |
| Matkaris et al.43 ¶¶ | Ceftriaxone versus no antibiotic | 3 of 50 (6) | 15 of 50 (30) | 24 | $150·12 | $248·03 | −$97·91 | −140·10 | Dominant |
| Cefotaxime versus no antibiotic | 4 of 50 (8) | 15 of 50 (30) | 22 | $128·06 | $248·03 | −$119·97 | −171·67 | Dominant | |
| Ceftazidime versus no antibiotic | 4 of 50 (8) | 15 of 50 (30) | 22 | $137·81 | $248·03 | −$110·22 | −157·71 | Dominant | |
| Matsui et al.44 ## | Cefazolin versus no antibiotic | 6 of 518 (1·2) | 35 of 519 (6·7) | 5·5 | $766·10 | $831·90 | −$65·80 | −60·75 | Dominant |
| Sisto et al.45 *** | Ceftriaxone versus cefuroxime | 21 of 274 (7·7) | 23 of 277 (8·3) | 0·6 | $36·11 | $107·82 | −$71·71 | −95·95 | Dominant |
| Wilson et al.46 ††† | Ertapenen versus cefotetan | 143 of 334 (42·8) | 95 of 338 (28·1) | −14·7 | $15 230·00 | $17 411·00 | −$2181·00 | −2340·81 | Dominant |
Values in parentheses are percentages.
‘Discounted’ cost per patient and incremental cost‐effectiveness ratio (ICER) calculated by means of a two‐step discounting process using the Campbell and Cochrane Economics Methods Group–Evidence for Policy and Practice Information and Coordinating Centre cost converter web‐based tool32,33. The 2016 implied conversion factor is US $1 = £0·70 sterling; the 2016 euro conversion factor is £1 sterling = €1·28.
Treatment effects of cefazolin, clindamycin and cefoperazone were pooled, and costs were pooled and averaged; cost inferred from study setting to be US$; for conversion of 1992 US dollars to 2016 British pounds, the implied inflation factor for US $1 in 1992 to 2016 value is 1·57.
Price year inferred from publication date; for conversion of 1998 US dollars to 2016 British pounds, the implied inflation factor for US $1 in 1998 to 2016 is 1·41.
Price year inferred from publication date; for conversion of 1988 British pounds to 2016 British pounds, the implied inflation factor for £1 sterling in 1988 to 2016 is 2·16.
Price year inferred from study end date; cost data based on per‐protocol analysis; for conversion of 2004 US dollars to 2016 British pounds, the implied inflation factor for US $1 in 2004 to 2016 is 1·24.
For conversion of 2008 euros to 2016 euros, the implied inflation factor for €1 in 2008 to 2016 is 1·05.
Cost inferred from study setting to be US$; for conversion of 1988 US dollars to 2016 British pounds, the implied inflation factor for US $1 in 1988 to 2016 is 1·79; infection data were missing for six patients in the control group and seven in the intervention group.
Price year inferred from publication date; all treatment failures; for conversion of 1987 US dollars to 2016 British pounds, the implied inflation factor for US $1 in 1987 to 2016 is 1·87.
Price year inferred from study end date; for conversion of 1998 US dollars to 2016 British pounds, the implied inflation factor for US $1 in 1998 to 2016 is 1·41.
Price year inferred from publication date; for conversion of 1991 US dollars to 2016 British pounds, the implied inflation factor for US $1 in 1991 to 2016 is 1·61.
Price year inferred from publication date; for conversion of 2013 US dollars to 2016 British pounds, the implied inflation factor for US $1 in 2013 to 2016 is 1·04.
Price year inferred from study end date; for conversion of 1994 US dollars to 2016 British pounds, the implied inflation factor for US $1 in 1994 to 2016 is 1·50.
Cost inferred from study setting to be US$; cost data based on per‐protocol analysis; for conversion of 2005 US dollars to 2016 British pounds, the implied inflation factor for US $1 in 2005 to 2016 is 1·21. TEc, treatment effect for control; TEi, treatment effect for intervention; AH, abdominal hysterectomy; VH, vaginal hysterectomy; SDD, selective decontamination of digestive tract. A more detailed version of this table is available as Table S6, supporting information.
Calculated incremental cost‐effectiveness ratio
The calculated ICER was based on the results of each study, their reported currency and euros (2016) (Table 7; Table S6, supporting information). Eight studies did not clearly state the price year for the cost calculations, so the year in which the study ended38 42, 45 and date of publication36 37, 41 43, 44 were used. The calculated treatment effect showing the proportion of infections averted ranged from 0·06 per cent in clean CABG surgery45 to 0·33 per cent in clean‐contaminated vaginal hysterectomy37, with one study42 showing a negative effect in vascular prosthetic surgery. The intervention in ten studies35–40,43–46 was dominant (more effective and cheaper than the control) and in one study42 the intervention was dominated by the control (it was less effective and more expensive). In the remaining study41, the intervention was more effective and more expensive than the control. This resulted in an incremental increase of €2·64 per patient and a resultant ICER of €5·12 for the year 2016.
Discussion
This review aimed to evaluate the cost‐effectiveness of preoperative antibiotic prophylaxis in preventing SSIs, including assessment of the reporting quality of the clinical and cost‐effectiveness. Twelve studies published between 1988 and 2014 were identified, and included preoperative antibiotic prophylaxis as well as costs. Most of the studies had a large sample size: five had more than 500 participants, four had between 200 and 500 participants and three had fewer than 200 participants. All studies reported some measure of costs, but only two reported on incremental cost‐effectiveness and none included any of the recommended economic checklists27 31. All identified studies reported on prophylactic effectiveness, although few included antibiotic resistance and none addressed the appropriateness of antibiotic stewardship.
Prophylactic effectiveness was achieved in ten studies. The size of these effects is considered clinically important, particularly in contaminated and clean‐contaminated surgery37 39, 40, 41 44, 46, which has a higher risk of baseline SSI compared with clean procedures57. Five35 36, 38 42, 45 of the included studies involved clean surgical procedures, so clinical effectiveness in four35 36, 38 42 of these studies was not unexpected. Prophylactic effectiveness was also achieved even when the comparator was another antibiotic38 40, 41 46. Most of the prophylactic interventions involved first‐, second‐ or third‐generation cephalosporins compared with either placebo or a control. Cephalosporins are safe and have a long half‐life, ensuring penetration of tissues21. They offer cover against most S. aureus strains and some Gram‐negative organisms, but not coagulase‐negative staphylococci or MRSA22. Only two studies mentioned screening for C. difficile. Cephalosporins, especially third‐generation drugs, have been linked to patients having an increased risk of colonization with C. difficile, causing toxic C. difficile colitis22, even when administered as a single dose58 59. The size and dosage of antibiotic prophylaxis is important, as single‐dose administration may precipitate resistance unless the prophylactic drug has a sufficient half‐life and tissue penetration. One study showed that a single dose of the intervention (cefoperazone) was less effective clinically and cost more than control prophylaxis (cefotaxime). Both of these antibiotics are third‐generation cephalosporins, and both were administered as a single bolus 30 min after anaesthesia but before incision. Cefotaxime was administered again during surgery if the duration of the procedure exceeded 2 h.
Teicoplanin, a glycopeptide, may also be administered as a single dose. Its use as an intervention, however, was less effective and more expensive compared with cefazolin (a first‐generation cephalosporin). Cefazolin remains the prophylactic choice in vascular surgery as it is effective against S. aureus (the most frequently isolated organism in infected vascular wounds). Cefazolin has been shown to be as effective as cefamandole and cefuroxime in prosthetic vascular surgery60. With the increase in MRSA, vancomycin is an alternative, but it is toxic. Teicoplanin is similar to vancomycin, but is less toxic and has a longer half‐life, so may be administered once daily. Teicoplanin lacks activity against Gram‐negative bacteria, however, and most infections in the teicoplanin study were caused by Gram‐negative bacteria; this may have contributed to the increased costs per patient.
Combining the findings of economic evaluations with those of clinical‐effectiveness trials provides healthcare policy‐makers with evidence‐based options for healthcare decision‐making. The methodology of economic evaluations needs to be defined clearly at the study outset. This review identified low to acceptable reporting of the economic evaluations, but with great variation, whereas the reporting of clinical effectiveness was more standardized. The most recent studies were more consistent in terminology and reporting of costs and their units. Some of the studies did not include treatment failures in their cost analysis, and this may result in an intervention that is cost‐saving but not necessarily cost‐effective. In addition, cost‐effectiveness may be more favourable in procedures that carry a higher baseline risk of SSI when the cost of prophylaxis is the same. Length of hospital stay is a recognized factor contributing to costs7, 8, 9 11, and all studies reported a reduced length of stay compared with the control regimen; however, it was difficult to determine the exact costs of the stay. It is also recognized that mean daily costs decrease with extended length of stay, with the most intensive costs incurred in the period shortly after admission9; this may be perceived as a disincentive for hospitals to eliminate all SSIs9 10. None of the included studies reported decreasing costs of the hospital admission; all reported a daily hospital charge. Mortality also has an associated cost, and in cost‐effectiveness studies is considered a permanent sequela. Only five studies and a nested study reported mortality, and none included deaths in the cost analysis.
The methodological quality of the included studies was not well reported, as evidenced by low scores on the CHEERS checklist31, whereas economic reporting was moderate to high, with seven studies ranking 75 per cent or above on the modified Drummond quality checklist27. Two of the highest‐quality studies were among the most recent ones, published in 2008 and 2012. There was, however, no standard method of reporting costs, and some cost components were not always reported; discounting was not reported in any study. Consistent inclusion of standardized economic studies in clinical trials and quasi‐experimental studies would allow evidence‐based decision‐making with respect to antibiotic efficacy and cost‐effectiveness.
This review has five main limitations. First, the search terms used may not have identified all articles, as a wide variety of terms exist to describe economic evaluations, prophylaxis and infection. Second, the review was restricted to studies performed in OECD countries. The purpose of the restriction was to reduce the effect of differences in operating theatre conditions and surgical procedures on the incidence of SSI. Third, the ICER analysis is based on the published study data and, because there was heterogeneity between the studies and sensitivity analysis was not always reported, it was limited to point estimates. Fourth, in this review, an ICER was not sensitive enough to rank cost‐effectiveness, as most of the interventions were dominant. For the dominant interventions using an ICER the range of difference could not be determined, and possibly a quality‐adjusted life‐year framework would be more suitable; however, this would require standardized reporting. Fifth, despite the importance of preventing primary antibiotic resistance, the review did not attempt to address the development of resistance or antibiotic stewardship, because no study reported on either. This also implies that the results of these studies have limited generalizability; if resistance patterns differ, a drug that is (cost‐)effective in one context may not be in another. The specific findings of the studies reviewed here should therefore be treated with caution.
The strengths of this review are several. It is the first to include both clinical and economic effectiveness of preoperative prophylaxis; it included five databases, and the numerous keywords were matched with indexed terms specific to the databases. This review summarized large data sets that encompassed many surgical specialties and procedures. It is recommended29 30, 61 that more than one reviewer should screen for papers to be included in a systematic review. This review used two independent reviewers, and the κ statistic for each level of screening was at the higher end of the scale (from substantial to almost perfect).
This review of the cost‐effectiveness of preoperative antibiotic prophylaxis found that most interventions were cost‐effective. To ensure that preoperative prophylaxis continues to prevent SSI, there needs to be increased awareness of the prevalence of resistance within each facility and improved antibiotic stewardship to reduce the development of resistance. Antibiotic stewardship includes use of the appropriate recommended antibiotic prophylaxis based on the most common pathogens likely to cause SSI for a specific surgical procedure, following recommended timing of administration before incision to ensure maximum tissue concentration, adjusting the prophylaxis dose according to the patient's bodyweight, redosing the prophylaxis at intervals of two half‐lives, and discontinuing prophylaxis after surgery within recommended time frames. New antibiotic prophylaxis regimens may be implemented when they are less effective or more expensive if economic methods are not included routinely in RCTs and quasi‐experimental studies. Economic methods would improve the understanding and true economic benefit of these new regimens. The economic methods need to be standardized against recommended guidelines and incorporate sensitivity analysis, discount rates, year and date of the study, unit costs, mortality, treatment effects, antibiotic resistance and quality‐of‐life costs.
Disclosure
The authors declare no conflict of interest.
Supporting information
Appendix S1
Table S1 Database search terms including complete searches for Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Web of Science (WOS)
Table S2 List of OECD countries*
Table S3 CHEERS checklist of reporting quality
Table S4 Quality assessment checklist for assessing economic evaluations of included studies
Funding information
No funding
References
- 1. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 2009; 37: 387–397. [DOI] [PubMed] [Google Scholar]
- 2. Anderson DJ. Surgical site infections. Infect Dis Clin North Am 2011; 25: 135–153. [DOI] [PubMed] [Google Scholar]
- 3. Ozdemir S, Gulpinar K, Ozis SE, Sahli Z, Kesikli SA, Korkmaz A et al The effects of preoperative oral antibiotic use on the development of surgical site infection after elective colorectal resections: a retrospective cohort analysis in consecutively operated 90 patients. Int J Surg 2016; 33: 102–108. [DOI] [PubMed] [Google Scholar]
- 4. Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 1992; 13: 606–608. [PubMed] [Google Scholar]
- 5. Lankiewicz JD, Yokoe DS, Olsen MA, Onufrak F, Fraser VJ, Stevenson K et al Beyond 30 days: does limiting the duration of surgical site infection follow‐up limit detection? Infect Control Hosp Epidemiol 2012; 33: 202–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boltz MM, Hollenbeak CS, Julian KG, Ortenzi G, Dillon PW. Hospital costs associated with surgical site infections in general and vascular surgery patients. Surgery 2011; 150: 934–942. [DOI] [PubMed] [Google Scholar]
- 7. Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA. Hospital costs associated with surgical complications: a report from the private‐sector National Surgical Quality Improvement Program. J Am Coll Surg 2004; 199: 531–537. [DOI] [PubMed] [Google Scholar]
- 8. Broex EC, van Asselt AD, Bruggeman CA, van Tiel FH. Surgical site infections: how high are the costs? J Hosp Infect 2009; 72: 193–201. [DOI] [PubMed] [Google Scholar]
- 9. Shepard J, Ward W, Milstone A, Carlson T, Frederick J, Hadhazy E et al Financial impact of surgical site infections on hospitals: the hospital management perspective. JAMA Surg 2013; 148: 907–914. [DOI] [PubMed] [Google Scholar]
- 10. Jenks PJ, Laurent M, McQuarry S, Watkins R. Clinical and economic burden of surgical site infection (SSI) and predicted financial consequences of elimination of SSI from an English hospital. J Hosp Infect 2014; 86: 24–33. [DOI] [PubMed] [Google Scholar]
- 11. Plowman R, Graves N, Griffin MA, Roberts JA, Swan AV, Cookson B et al The rate and cost of hospital‐acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed. J Hosp Infect 2001; 47: 198–209. [DOI] [PubMed] [Google Scholar]
- 12. Leaper DJ, Van Goor H, Reilly J, Petrosillo N, Geiss HK, Torres AJ et al Surgical site infection – a European perspective of incidence and economic burden. Int Wound J 2004; 1: 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schweizer ML, Cullen JJ, Perencevich EN, Vaughan Sarrazin MS. Costs associated with surgical site infections in Veterans Affairs hospitals. JAMA Surg 2014; 149: 575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee I, Agarwal RK, Lee BY, Fishman NO, Umscheid CA. Systematic review and cost analysis comparing use of chlorhexidine with use of iodine for preoperative skin antisepsis to prevent surgical site infection. Infect Control Hosp Epidemiol 2010; 31: 1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Noorani A, Rabey N, Walsh SR, Davies RJ. Systematic review and meta‐analysis of preoperative antisepsis with chlorhexidine versus povidone–iodine in clean‐contaminated surgery. Br J Surg 2010; 97: 1614–1620. [DOI] [PubMed] [Google Scholar]
- 16. Gillespie BM, Chaboyer W, Erichsen‐Andersson A, Hettiarachchi RM, Kularatna S. Economic case for intraoperative interventions to prevent surgical‐site infection. Br J Surg 2017; 104: e55–e64. [DOI] [PubMed] [Google Scholar]
- 17. Gheorghe A, Roberts TE, Pinkney TD, Bartlett DC, Morton D, Calvert M. The cost‐effectiveness of wound‐edge protection devices compared to standard care in reducing surgical site infection after laparotomy: an economic evaluation alongside the ROSSINI trial. PLoS One 2014; 9: e95595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel A, Bergman A, Moore B, Haglund U. The economic burden of complications occurring in major surgical procedures: a systematic review. Appl Health Econ Health Policy 2013; 11: 577–592. [DOI] [PubMed] [Google Scholar]
- 19. Kao LS, Meeks D, Moyer VA, Lally KP. Peri‐operative glycaemic control regimens for preventing surgical site infections in adults. Cochrane Database Syst Rev 2009; (3)CD006806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beltramini AM, Salata RA, Ray AJ. Thermoregulation and risk of surgical site infection. Infect Control Hosp Epidemiol 2011; 32: 603–610. [DOI] [PubMed] [Google Scholar]
- 21. Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK et al; American Society of Health-System Pharmacists; Infectious Disease Society of America; Surgical Infection Society; Society for Healthcare Epidemiology of America. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 2013; 70: 195–283. [DOI] [PubMed] [Google Scholar]
- 22. Scottish Intercollegiate Guidelines Network (SIGN) . Antibiotic Prophylaxis in Surgery; SIGN Publication number 104; July 2008 [updated April 2014]. http://www.sign.ac.uk/assets/sign104.pdf%20%5baccessed%2016%20June%202017%5d].
- 23. Anderson DJ, Podgorny K, Berrios‐Torres SI, Bratzler DW, Dellinger EP, Greene L et al Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35: 605–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0; updated March 2011. http://handbook.cochrane.org [accessed 30 May 2014]. [Google Scholar]
- 25. Centre for Reviews and Dissemination (CRD), University of York . Systematic Reviews: CRD's Guidance for Undertaking Reviews in Health Care; 2009. https://www.crd.york.ac.uk/CRDWeb/GuideToSearching.asp [accessed 30 May 2014]. [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009; 151: 264–269. [PMC free article] [PubMed] [Google Scholar]
- 27. Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes (3rd ed.). Oxford University Press: New York, 2005. [Google Scholar]
- 28. World Bank Group . Data: Country and Lending Groups 2014 http://data.worldbank.org/about/country-and-lending-groups%20%5baccessed%2016%20July%202014%5d].
- 29. Watson PF, Petrie A. Method agreement analysis: a review of correct methodology. Theriogenology 2010; 73: 1167–1179. [DOI] [PubMed] [Google Scholar]
- 30. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–174. [PubMed] [Google Scholar]
- 31. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D et al; CHEERS Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013; 346: f1049. [DOI] [PubMed] [Google Scholar]
- 32. Campbell and Cochrane Economics Methods Group (CCEMG) and Evidence for Policy and Practice Information and Coordinating Centre (EPPI‐Centre) . CCEMG – EPPI‐Center Cost Converter, v.1.5; April 2016. http://eppi.ioe.ac.uk/costconversion/default.aspx [accessed 20 May 2017].
- 33. Shemilt I, Thomas J, Morciano M. A web‐based tool for adjusting costs to a specific target currency and price year. Evid Policy 2010; 6: 51–59. [Google Scholar]
- 34. International Monetary Fund . World Economic Outlook Database; April 2016. https://www.imf.org/external/pubs/ft/weo/2016/01/weodata/index.aspx [accessed 20 May 2017].
- 35. Blair EA, Johnson JT, Wagner RL, Carrau RL, Bizakis JG. Cost analysis of antibiotic prophylaxis in clean head and neck surgery. Arch Otolaryngol Head Neck Surg 1995; 121: 269–271. [DOI] [PubMed] [Google Scholar]
- 36. Bold RJ, Mansfield PF, Berger DH, Pollock RE, Singletary SE, Ames FC et al Prospective, randomized, double‐blind study of prophylactic antibiotics in axillary lymph node dissection. Am J Surg 1998; 176: 239–243. [DOI] [PubMed] [Google Scholar]
- 37. Davey PG, Duncan ID, Edward D, Scott AC. Cost‐benefit analysis of cephradine and mezlocillin prophylaxis for abdominal and vaginal hysterectomy. Br J Obstet Gynaecol 1988; 95: 1170–1177. [DOI] [PubMed] [Google Scholar]
- 38. Dhadwal K, Al‐Ruzzeh S, Athanasiou T, Choudhury M, Tekkis P, Vuddamalay P et al Comparison of clinical and economic outcomes of two antibiotic prophylaxis regimens for sternal wound infection in high‐risk patients following coronary artery bypass grafting surgery: a prospective randomised double‐blind controlled trial. Heart 2007; 93: 1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dijksman LM, Roos D, Gerhards MF, Tijssen JG, Gouma DJ, Dijkgraaf MG. Cost‐effectiveness of perioperative selective decontamination of the digestive tract versus placebo in elective gastrointestinal surgery. Dig Surg 2012; 29: 384–390. [DOI] [PubMed] [Google Scholar]
- 40. Garcia‐Rodriguez JA, Puig‐LaCalle J, Arnau C, Porta M, Vallve C. Antibiotic prophylaxis with cefotaxime in gastroduodenal and biliary surgery. Am J Surg 1989; 158: 428–432. [DOI] [PubMed] [Google Scholar]
- 41. Jones RN, Wojeski WV. Single‐dose cephalosporin prophylaxis of 929 surgical procedures in a prepaid group practice: a prospective, randomized comparison of cefoperazone and cefotaxime. Diagn Microbiol Infect Dis 1987; 6: 323–334. [DOI] [PubMed] [Google Scholar]
- 42. Marroni M, Cao P, Fiorio M, Maghini M, Lenti M, Repetto A et al Prospective, randomized, double‐blind trial comparing teicoplanin and cefazolin as antibiotic prophylaxis in prosthetic vascular surgery. Eur J Clin Microbiol Infect Dis 1999; 18: 175–178. [DOI] [PubMed] [Google Scholar]
- 43. Matkaris M, Markantes K, Stayannis K, Iatrakis G, Kourounis G, Tzingounis V. Reduction of hospital cost and administration of prophylactic antibiotherapy in gynecological surgery. Isr J Med Sci 1991; 27: 134–136. [PubMed] [Google Scholar]
- 44. Matsui Y, Satoi S, Kaibori M, Toyokawa H, Yanagimoto H, Matsui K et al Antibiotic prophylaxis in laparoscopic cholecystectomy: a randomized controlled trial. PLoS One 2014; 9: e106702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sisto T, Laurikka J, Tarkka MR. Ceftriaxone vs cefuroxime for infection prophylaxis in coronary bypass surgery. Scand J Thorac Cardiovasc Surg 1994; 28: 143–148. [DOI] [PubMed] [Google Scholar]
- 46. Wilson SE, Turpin RS, Kumar RN, Itani KM, Jensen EH, Pellissier JM et al Comparative costs of ertapenem and cefotetan as prophylaxis for elective colorectal surgery. Surg Infect (Larchmt) 2008; 9: 349–356. [DOI] [PubMed] [Google Scholar]
- 47. Roos D, Dijksman LM, Oudemans‐van Straaten HM, de Wit LT, Gouma DJ, Gerhards MF. Randomized clinical trial of perioperative selective decontamination of the digestive tract versus placebo in elective gastrointestinal surgery. Br J Surg 2011; 98: 1365–1372. [DOI] [PubMed] [Google Scholar]
- 48. Itani KMF, Wilson SE, Awad SS, Jensen EH, Finn TS, Abramson MA. Ertapenem versus cefotetan prophylaxis in elective colorectal surgery. N Engl J Med 2006; 355: 2640–2651. [DOI] [PubMed] [Google Scholar]
- 49. Johnson JT, Myers EN, Thearle PB, Sigler BA, Schramm VL Jr. Antimicrobial prophylaxis for contaminated head and neck surgery. Laryngoscope 1984; 94: 46–51. [DOI] [PubMed] [Google Scholar]
- 50. Jonkers D, Elenbaas T, Terporten P, Nieman F, Stobberingh E. Prevalence of 90‐days postoperative wound infections after cardiac surgery. Eur J Cardiothorac Surg 2003; 23: 97–102. [DOI] [PubMed] [Google Scholar]
- 51. Rommes JH, Rios G, Zandstra DF. Therapy of infection. Trends Anaesth Crit Care 2001; 12: 25–33. [Google Scholar]
- 52. Berard F, Gandon J. Postoperative wound infections: the influence of ultraviolet irradiation of the operating room and of various other factors. Ann Surg 1964; 160(Suppl 2): 1–192. [PubMed] [Google Scholar]
- 53. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988; 16: 128–140. [DOI] [PubMed] [Google Scholar]
- 54. Horan TC, Culver DH, Gaynes RP, Jarvis WR, Edwards JR, Reid CR. Nosocomial infections in surgical patients in the United States, January 1986–June 1992. National Nosocomial Infections Surveillance (NNIS) System. Infect Control Hosp Epidemiol 1993; 14: 73–80. [DOI] [PubMed] [Google Scholar]
- 55. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 1999; 20: 250–278. [DOI] [PubMed] [Google Scholar]
- 56. Jarvis WR. Benchmarking for prevention: the Centers for Disease Control and Prevention's National Nosocomial Infections Surveillance (NNIS) system experience. Infection 2003; 31 ( Suppl 2): 44–48. [PubMed] [Google Scholar]
- 57. Bowater RJ, Stirling SA, Lilford RJ. Is antibiotic prophylaxis in surgery a generally effective intervention? Testing a generic hypothesis over a set of meta‐analyses. Ann Surg 2009; 249: 551–556. [DOI] [PubMed] [Google Scholar]
- 58. Privitera G, Scarpellini P, Ortisi G, Nicastro G, Nicolin R, de Lalla F. Prospective study of Clostridium difficile intestinal colonization and disease following single‐dose antibiotic prophylaxis in surgery. Antimicrob Agents Chemother 1991; 35: 208–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ambrose NS, Johnson M, Burdon DW, Keighley MR. The influence of single dose intravenous antibiotics on faecal flora and emergence of Clostridium difficile . J Antimicrob Chemother 1985; 15: 319–326. [DOI] [PubMed] [Google Scholar]
- 60. Edwards WH Jr, Kaiser AB, Tapper S, Edwards WH Sr, Martin RS III, Mulherin JL Jr et al Cefamandole versus cefazolin in vascular surgical wound infection prophylaxis: cost‐effectiveness and risk factors. J Vasc Surg 1993; 18: 470–476. [DOI] [PubMed] [Google Scholar]
- 61. Edwards P, Clarke M, DiGuiseppi C, Pratap S, Roberts I, Wentz R. Identification of randomized controlled trials in systematic reviews: accuracy and reliability of screening records. Stat Med 2002; 21: 1635–1640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Table S1 Database search terms including complete searches for Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Web of Science (WOS)
Table S2 List of OECD countries*
Table S3 CHEERS checklist of reporting quality
Table S4 Quality assessment checklist for assessing economic evaluations of included studies


