Abstract
Background
Radiofrequency ablation (RFA) is a minimally invasive treatment for hepatocellular carcinoma (HCC). There is increasing evidence of an association between increasing hospital volume and lower postoperative mortality for many surgical procedures, but this is difficult to establish with minimally invasive treatments, where postoperative mortality is low. The aim of this study was to investigate the relationship between hospital volume and in‐hospital mortality following RFA using a Japanese nationwide database.
Methods
Data from the Diagnostic Procedure Combination database were analysed from 1 July 2010 to 31 March 2012. Multivariable logistic regression was used to analyse the relationship between hospital volume and in‐hospital mortality following RFA, with adjustment for patient background.
Results
Some 36 675 patients with HCC were identified in the database. The overall in‐hospital mortality rate from RFA was 0·31 per cent. In‐hospital mortality was significantly higher in low‐volume than high‐volume hospitals (odds ratio 2·57, 95 per cent c.i. 1·61 to 4·09; P < 0·001). Higher in‐hospital mortality was significantly associated with older age and a higher Charlson Co‐morbidity Index score.
Conclusion
RFA for HCC was associated with acceptably low mortality in Japan, but in‐hospital mortality following RFA was affected by hospital procedural volume.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most frequently diagnosed cancer and the third most common cause of cancer‐related death worldwide1. Only 20 per cent of patients with HCC are candidates for resection2. Image‐guided minimally invasive techniques have been used widely for the treatment of HCC in the past two decades and radiofrequency ablation (RFA) has yielded promising clinical results, with survival rates comparable to those of hepatectomy3. During the past decade, there has been growing interest in the use of RFA in patients with liver tumours considered to be unresectable because of impaired hepatic function or associated co‐morbidities4, 5, 6, 7. Surveillance programmes have reduced the proportion of patients presenting with advanced disease and this has also led to an increase in the use of RFA2 8.
There has been increasing evidence of an association between hospital volume and postoperative mortality and morbidity following surgical procedures9, 10, 11, although it is difficult to demonstrate such a relationship following minimally invasive treatments such as RFA, largely as a result of very low in‐hospital mortality rates3 12.
The aim of this study was to investigate the relationship between hospital volume and in‐hospital mortality following RFA for HCC in a large patient sample identified from a Japanese national inpatient database.
Methods
The Diagnosis Procedure Combination (DPC) database is a discharge abstract and administrative claims database of inpatient admissions to secondary‐ and tertiary‐care hospitals in Japan13, 14, 15. The database includes data on approximately 7 million inpatients annually, representing approximately 50 per cent of inpatient admissions to such hospitals. The database contains the following information: patient demographics; diagnoses, co‐morbidities at admission and complications after admission (recorded with Japanese text and ICD‐10 codes); therapeutic procedures encoded by original Japanese codes; duration of hospital stay; discharge status including in‐hospital death; and total costs. All 82 academic hospitals in Japan are required to participate in the database, but participation is optional for community hospitals.
The requirement for informed consent was waived for this study because of the anonymous nature of the data. Study approval was obtained from the institutional review board of the University of Tokyo.
Inpatient data for a total of 21 months (1 July 2010 to 31 March 2012) were obtained. Among 12 million inpatients included in the database, patients with a diagnosis of HCC (ICD‐10 code C220) were identified, including those who had undergone RFA. Patients who had undergone transcatheter arterial embolization ahead of RFA were excluded to avoid any influence of embolization‐related liver damage16 on in‐hospital mortality following RFA. The Charlson Co‐morbidity Index (CCI), a validated, weighted co‐morbidity score derived from unselected hospital admissions that predicts 1‐year mortality after hospital discharge17, was used to adjust for multiple co‐morbidities18. Hospital volume was defined as the annual number of RFA procedures performed for HCC. Hospital volume during the survey period was determined using the unique identifier for each hospital and categorized into tertiles (low, intermediate and high volume), so that the numbers of patients in each group were approximately equal.
Statistical analysis
Continuous variables are presented as median (i.q.r.), and categorical variables as numbers and frequencies. Univariable associations among groups were assessed using the χ2 test. Relationships between in‐hospital mortality and various factors, including patient characteristics and hospital volume, were assessed. Stepwise logistic regression analysis was used to model the concurrent effects of procedures and other factors on in‐hospital mortality. Statistical analyses were performed using SPSS® software version 23.0 (IBM, Armonk, New York, USA). The threshold for significance was set at P < 0·050.
Results
A total of 36 675 HCC patients underwent RFA at 811 hospitals during the study period. Clinical characteristics are summarized in Table 1. Some 66·4 per cent were men and the median age was 73 years; 62·7 per cent of all patients were aged years 70 or more, and 18·4 were aged at least 80 years.
Table 1.
Patient characteristics
| No of patients (n = 36 675)* | |
|---|---|
| Age (years)† | 73 (66–78) |
| ≤ 59 | 3399 (9·3) |
| 60–69 | 10 268 (28·0) |
| 70–79 | 16 256 (44·3) |
| ≥ 80 | 6752 (18·4) |
| Sex ratio (F : M) | 12 331 : 24 344 |
| Charlson Co‐morbidity Index | |
| ≤ 3 | 20 398 (55·6) |
| ≥ 4 | 16 277 (44·4) |
| Aetiology | |
| Hepatitis B virus | 3514 (9·6) |
| Hepatitis C virus | 17 654 (48·1) |
| Hepatitis B + C virus | 107 (0·3) |
| Alcohol | 1400 (3·8) |
| Alcohol + hepatitis C virus | 299 (0·8) |
| Alcohol + hepatitis B virus | 39 (0·1) |
| Alcohol + hepatitis B + C virus | 7 (0·0) |
| Others or data not provided | 13 655 (37·2) |
Values in parentheses are percentages unless indicated otherwise;
values are median (range).
Table 2 shows in‐hospital mortality rates following RFA. Hospitals with 83 or more, 33–82 and 32 or fewer RFA procedures were categorized as high‐, intermediate‐ and low‐volume hospitals respectively. The overall in‐hospital mortality rate following RFA was 0·31 per cent (115 of 36 675). In‐hospital mortality rates in high‐, intermediate‐ and low‐volume hospitals were 0·20 per cent (25 of 12 368), 0·23 per cent (29 of 12 405) and 0·51 per cent (61 of 11 902) respectively. This mortality rate was significantly higher in low‐volume hospitals (P < 0·001), older patients (P = 0·002) and those with higher CCI scores (P < 0·001). In multivariable analysis, in‐hospital mortality was significantly higher in low‐volume than in high‐volume hospitals (odds ratio 2·57, 95 per cent c.i. 1·61 to 4·09; P < 0·001). Higher in‐hospital mortality was also significantly associated with older age and higher CCI score (Fig. 1).
Table 2.
Univariable analysis of factors influencing in‐hospital mortality after radiofrequency ablation
| Proportion who died | Mortality rate (%) | P † | |
|---|---|---|---|
| Overall | 115 of 36 675 | 0·31 (0·26, 0·38) | |
| Hospital volume* | < 0·001 | ||
| High (≥ 83) | 25 of 12 368 | 0·20 (0·13, 0·30) | |
| Intermediate (33–82) | 29 of 12 405 | 0·23 (0·16, 0·34) | |
| Low (≤ 32) | 61 of 11 902 | 0·51 (0·39, 0·66) | |
| Sex | 0·95 | ||
| F | 39 of 12 331 | 0·32 (0·23, 0·43) | |
| M | 76 of 24 344 | 0·31 (0·25, 0·39) | |
| Age (years) | 0·002 | ||
| ≤ 59 | 9 of 3399 | 0·27 (0·12, 0·50) | |
| 60–69 | 23 of 10 268 | 0·22 (0·14, 0·34) | |
| 70–79 | 46 of 16 256 | 0·28 (0·21, 0·38) | |
| ≥ 80 | 37 of 6752 | 0·55 (0·39, 0·76) | |
| Charlson Co‐morbidity Index | < 0·001 | ||
| ≤ 3 | 40 of 20 398 | 0·20 (0·14, 0·27) | |
| ≥ 4 | 75 of 16 277 | 0·46 (0·36, 0·58) |
Values in parentheses are 95 per cent confidence intervals.
Defined according to the annual number of radiofrequency ablation procedures for hepatocellular carcinoma.
χ2 test.
Figure 1.
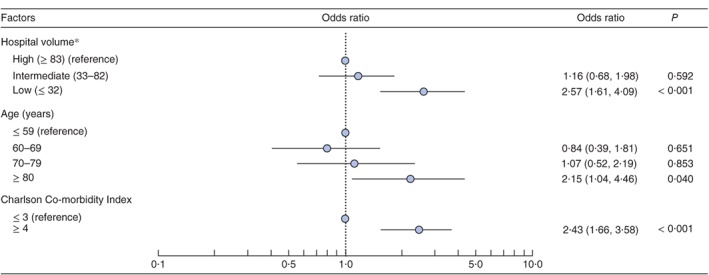
Multivariable logistic regression analysis of in‐hospital mortality following radiofrequency ablation (RFA). Odds ratios are shown with 95 per cent confidence intervals. *Defined according to the annual number of RFA procedures for hepatocellular carcinoma
Discussion
RFA is a safe and effective treatment for HCC, with an in‐hospital mortality rate ranging from 0 to 1·8 per cent3 7, 19, 20, 21, 22, 23, 24. Hospital volume is thought to influence various outcomes including in‐hospital mortality, even with minimally invasive treatments. A previous Japanese nationwide study of 11 688 patients25, however, failed to show a significant association between increasing hospital volume and decreasing mortality. It was felt that the sample size was too small to identify any association between hospital volume and RFA‐related mortality, as death occurred so rarely. Although a large number of patients was included in a meta‐analysis12, significant publication bias was identified26. The present study investigated the influence of hospital volume on in‐hospital mortality using a sample of 36 675 patients from a nationwide Japanese database.
The in‐hospital mortality rate was about 2·5‐fold higher in low‐volume hospitals than intermediate‐ and high‐volume hospitals. Volume–outcome relationships have been observed for many procedures and are often attributed to learning curve effects and standardization15 25, 27, 28, 29. A previous study30 with more than 130 000 patients from the DPC database showed that better physician and nurse staffing were associated with lower rates of ‘failure to rescue’, independent of hospital volume. This implies that it may not be the single operator that makes the difference; rather all healthcare providers involved in the care of patients undergoing RFA may be implicated. In another DPC study25, increasing mortality following hepatectomy across high‐, intermediate‐ and low‐volume hospitals was observed, but the mortality difference between high‐ and intermediate‐volume hospitals was quite small. The present study had similar findings, suggesting a threshold for a minimum number of procedures.
A study from Taiwan31 also showed that patients who received first‐line RFA for HCC in a high‐volume hospital had better overall and cancer‐specific survival than those receiving RFA in low‐volume hospital. Taking this into account with the present findings, hospital volume may be associated with therapeutic performance as well as being safer in terms of procedure‐related mortality.
This study has several limitations. Although the database covers a large number of inpatient admissions, the study population may not be representative of all patients undergoing RFA in Japan because most participating hospitals were secondary and tertiary centres. Some important clinical data that may influence mortality, such as the size and location of tumours, were not available, although this might be offset by the likelihood that patients with more difficult problems tend to be referred to high‐volume hospitals. The database included only inpatient data, so mortality after leaving hospital could not be investigated. Many hospitals in Japan, however, provide both early postoperative care and subsequent rehabilitation during a single hospital stay. According to the Organization for Economic Co‐operation and Development's Health Statistics32, in 2014 the national average length of a hospital stay in Japan was 17·2 days, which is much longer than in other countries. It seems unlikely that there would be a difference between high‐ and low‐volume hospitals in terms of mortality after discharge. Finally, cause of death was not recorded in the database. Some in‐hospital deaths may not have been directly related to RFA, but all‐cause in‐hospital mortality is more robust in terms of objectivity than treatment‐related mortality assessed by operators. It is also seems unlikely that cause of death would differ according to hospital volume.
This study has confirmed that RFA for HCC is associated with an acceptably low mortality rate in Japan, but procedure‐related mortality is affected by patient background and hospital volume. Treatment of patients with complex problems and technically difficult lesions should be concentrated at high‐volume centres.
Acknowledgements
This work was supported by Grants for Research on Policy Planning and Evaluation from the Ministry of Health, Labour and Welfare, Japan (grant numbers H28‐Policy‐Designated‐009 and H27‐Policy‐Strategy‐011), and the Research Programme on Hepatitis from the Japan Agency for Medical Research and Development.
Disclosure: The authors declare no conflict of interest.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Bruix J, Sherman M; Practice Guidelines Committee; American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma. Hepatology 2005; 42: 1208–1236. [DOI] [PubMed] [Google Scholar]
- 3. Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H et al Radiofrequency ablation for hepatocellular carcinoma: 10‐year outcome and prognostic factors. Am J Gastroenterol 2012; 107: 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L et al Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol 1996; 167: 759–768. [DOI] [PubMed] [Google Scholar]
- 5. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio‐frequency ablation versus ethanol injection. Radiology 1999; 210: 655–661. [DOI] [PubMed] [Google Scholar]
- 6. Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P et al Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg 1999; 230: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y et al Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 2005; 103: 1201–1209. [DOI] [PubMed] [Google Scholar]
- 8. Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM et al; Taiwan Hepatoma Study Group . Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20‐year follow‐up study. J Natl Cancer Inst 2009; 101: 1348–1355. [DOI] [PubMed] [Google Scholar]
- 9. Ogura K, Yasunaga H, Horiguchi H, Ohe K, Shinoda Y, Tanaka S et al Impact of hospital volume on postoperative complications and in‐hospital mortality after musculoskeletal tumor surgery: analysis of a national administrative database. J Bone Joint Surg Am 2013; 95: 1684–1691. [DOI] [PubMed] [Google Scholar]
- 10. Yasunaga H, Horiguchi H, Matsuda S, Fushimi K, Hashimoto H, Ohe K et al Relationship between hospital volume and operative mortality for liver resection: data from the Japanese Diagnosis Procedure Combination database. Hepatology Res 2012; 42: 1073–1080. [DOI] [PubMed] [Google Scholar]
- 11. Otake H, Yasunaga H, Horiguchi H, Matsutani N, Matsuda S, Ohe K. Impact of hospital volume on chest tube duration, length of stay, and mortality after lobectomy. Ann Thorac Surg 2011; 92: 1069–1074. [DOI] [PubMed] [Google Scholar]
- 12. Bertot LC, Sato M, Tateishi R, Yoshida H, Koike K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol 2011; 21: 2584–2596. [DOI] [PubMed] [Google Scholar]
- 13. Sumitani M, Uchida K, Yasunaga H, Horiguchi H, Kusakabe Y, Matsuda S et al Prevalence of malignant hyperthermia and relationship with anesthetics in Japan: data from the diagnosis procedure combination database. Anesthesiology 2011; 114: 84–90. [DOI] [PubMed] [Google Scholar]
- 14. Yasunaga H, Shi Y, Takeuchi M, Horiguchi H, Hashimoto H, Matsuda S et al Measles‐related hospitalizations and complications in Japan, 2007–2008. Intern Med 2010; 49: 1965–1970. [DOI] [PubMed] [Google Scholar]
- 15. Yasunaga H, Yanaihara H, Fuji K, Horiguchi H, Hashimoto H, Matsuda S. Impact of hospital volume on postoperative complications and in‐hospital mortality after renal surgery: data from the Japanese Diagnosis Procedure Combination Database. Urology 2010; 76: 548–552. [DOI] [PubMed] [Google Scholar]
- 16. Lu W, Li YH, Yu ZJ, He XF, Chen Y, Zhao JB et al A comparative study of damage to liver function after TACE with use of low‐dose versus conventional‐dose of anticancer drugs in hepatocellular carcinoma. Hepatogastroenterology 2007; 54: 1499–1502. [PubMed] [Google Scholar]
- 17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 18. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC et al Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care 2005; 43: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 19. Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C et al Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology 2008; 47: 82–89. [DOI] [PubMed] [Google Scholar]
- 20. Solmi L, Nigro G, Roda E. Therapeutic effectiveness of echo‐guided percutaneous radiofrequency ablation therapy with a LeVeen needle electrode in hepatocellular carcinoma. World J Gastroenterol 2006; 12: 1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Casaril A, Abu Hilal M, Harb A, Campagnaro T, Mansueto G, Nicoli N. The safety of radiofrequency thermal ablation in the treatment of liver malignancies. Eur J Surg Oncol 2008; 34: 668–672. [DOI] [PubMed] [Google Scholar]
- 22. Bilchik AJ, Wood TF, Allegra DP. Radiofrequency ablation of unresectable hepatic malignancies: lessons learned. Oncologist 2001; 6: 24–33. [DOI] [PubMed] [Google Scholar]
- 23. Curley SA, Izzo F, Ellis LM, Vauthey JN, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg 2000; 232: 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Baere T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T et al Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol 2003; 181: 695–700. [DOI] [PubMed] [Google Scholar]
- 25. Sato M, Tateishi R, Yasunaga H, Horiguchi H, Yoshida H, Matsuda S et al Mortality and morbidity of hepatectomy, radiofrequency ablation, and embolization for hepatocellular carcinoma: a national survey of 54 145 patients. J Gastroenterol 2012; 47: 1125–1133. [DOI] [PubMed] [Google Scholar]
- 26. Flather MD, Farkouh ME, Pogue JM, Yusuf S. Strengths and limitations of meta‐analysis: larger studies may be more reliable. Control Clin Trials 1997; 18: 568–579. [DOI] [PubMed] [Google Scholar]
- 27. Yamamoto H, Hashimoto H, Nakamura M, Horiguchi H, Yasunaga H. Relationship between hospital volume and hemorrhagic complication after percutaneous renal biopsy: results from the Japanese diagnosis procedure combination database. Clin Exp Nephrol 2015; 19: 271–277. [DOI] [PubMed] [Google Scholar]
- 28. Yasunaga H, Tsuchiya K, Matsuyama Y, Ohe K. High‐volume surgeons in regard to reductions in operating time, blood loss, and postoperative complications for total hip arthroplasty. J Orthop Sci 2009; 14: 3–9. [DOI] [PubMed] [Google Scholar]
- 29. Sugihara T, Yasunaga H, Horiguchi H, Nishimatsu H, Kume H, Matsuda S et al Impact of hospital volume and laser use on postoperative complications and in‐hospital mortality in cases of benign prostate hyperplasia. J Urol 2011; 185: 2248–2253. [DOI] [PubMed] [Google Scholar]
- 30. Yasunaga H, Hashimoto H, Horiguchi H, Miyata H, Matsuda S. Variation in cancer surgical outcomes associated with physician and nurse staffing: a retrospective observational study using the Japanese Diagnosis Procedure Combination Database. BMC Health Serv Res 2012; 12: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu LC, Shao YY, Kuo RN, Lin ZZ, Yeh YC, Shau WY et al Hospital volume of percutaneous radiofrequency ablation is closely associated with treatment outcomes for patients with hepatocellular carcinoma. Cancer 2013; 119: 1210–1216. [DOI] [PubMed] [Google Scholar]
- 32. Organization for Economics Cooperation and Development (OECD) . Health Statistics; 2016. http://www.oecd.org/els/health-systems/health-data.htm [accessed 2 December 2016].


