Abstract
Background
The underlying mechanism of liver regeneration after Associating Liver Partition and Portal vein ligation (PVL) for Staged hepatectomy (ALPPS) is still unclear. The aim of this study was to evaluate the relationship between future liver remnant (FLR) volume, liver regeneration characteristics and restoration of function in an experimental model of ALPPS.
Methods
An ALPPS model in rats was developed with selective PVL, parenchymal transection and partial hepatectomy (step 1), followed by resection of the liver (step 2). Three different ALPPS groups with FLR sizes of 30, 20 and 10 per cent of total liver volume were compared with sham‐operated controls and animals undergoing resection of left lateral lobe and 90 per cent PVL with respect to morbidity, mortality, liver regeneration and function.
Results
Three of 15 animals that had ALPPS with 10 per cent FLR (ALPPS10) died after step 1. Ascites developed in two of five rats that had ALPPS with 20 per cent FLR and in three of four animals in the ALPPS10 group after step 2. Although the relative increments in FLR size and growth rates were highest in the ALPPS groups, small FLR size was associated with a sustained increase in levels of serum aminotransferases and bilirubin, a lower albumin concentration, severe sinusoidal injury, increased expression of proliferation markers and increased activation of hepatic progenitor cells after step 2.
Conclusion
There is discordance between FLR volume increase and functional restoration after the ALPPS procedure.
Surgical relevance.
The exact mechanism of liver regeneration after ALPPS is unclear. A rodent model of ALPPS was developed to study the relationship between future liver remnant (FLR) size, liver regeneration and restoration of function following ALPPS.
An ALPPS model was developed in rats. ALPPS was associated with liver dysfunction when the FLR size was below a certain level despite a large net volume increase. Hepatic hypertrophy with a low FLR was associated with increased Hippo signalling and hepatic progenitor cell (HPC) activation.
Sinusoidal injury related to the FLR size results in functional impairment. Sinusoidal injury and repopulation of HPCs may account for the discordance between FLR volume increase and functional compensation.
This observation provides a better understanding of liver regeneration after ALPPS.
Introduction
Liver resection is a potentially curative treatment option for patients with primary and secondary liver tumours. Unfortunately, only about 20–30 per cent of the patients are eligible for resection. The main limiting factors reflect total tumour burden – the size and number of lesions, their distribution within the liver parenchyma, and their relationships to major vital hepatic structures such as vessels and bile ducts. These factors determine the amount of liver tissue to be removed and the size of the future liver remnant (FLR).
The FLR needs to be of adequate size and quality to avoid liver failure after hepatectomy and small‐for‐size liver syndrome with associated morbidity and mortality. Previous studies have indicated that the size of the FLR should be at least 25 per cent of total liver volume1 or a ratio greater than 0·5 between FLR and bodyweight2 3. Various strategies such as portal vein embolization (PVE)4 and two‐stage hepatectomy5 have been developed to increase FLR and enable surgical therapy in patients otherwise deemed unresectable.
Associating Liver Partition and Portal vein ligation (PVL) for Staged hepatectomy (ALPPS) is an alternative technique for expansion of the FLR6. The ALPPS procedure combines selective PVL and parenchymal transection between the part of the liver to be removed and the FLR, as well as local resections of any tumours in the FLR during the first stage (step 1), followed by resection of the deportalized liver at the second stage (step 2). The principal advantage of this approach seems to be a greater volume increase of FLR than that seen after PVE alone, implying that a greater proportion of patients might then be offered a curative resection7. ALPPS has evoked much controversy, however, owing to associated high morbidity and mortality, even in experienced centres8, related mainly to inadequate liver function and sepsis. Some reports9 10 have suggested discordance between the volume increase of the FLR and its functional capacity.
The exact mechanism underlying ALPPS‐associated liver regeneration is still unclear. A rodent model of ALPPS was therefore developed to study the relationship between FLR size, liver regeneration and restoration of function.
Methods
Animals
Male Lewis rats (LEW/OrlRj) (Janvier Labs, Saint Berthevin, France), weighing 230–280 g and aged 9–12 weeks, were used for the experiments. All animal experiments were approved by the Norwegian Animal Research Authority (FOTS project number 8085) and performed in accordance with both the Norwegian Animal Welfare Act and FOTS guidance, which is adapted to cover important issues in the ARRIVE guidelines11. Bodyweight, survival rate and complications were recorded.
Development of the ALPPS model and experimental design
Based on research on rodent liver anatomy for the partial hepatectomies12 13, a modification was made of the preliminary ALPPS model14. On day 0, the first stage of the ALPPS procedure (step 1) was performed, consisting of 70–90 per cent PVL and parenchymal transection combined with 30 per cent hepatic parenchyma resection to mimic the clinical setting and obtain a suitably small FLR (30–10 per cent). Some 72 h after step 1 surgery, animals in the ALPPS groups underwent a second laparotomy to remove the deportalized liver lobes after ligation of the lobar arteries and bile ducts (step 2).
A series of experimental arms involved a resection group with three ALPPS groups (ALPPS10, ALPPS20 and ALPPS30) with varying sizes of FLR (10, 20 and 30 per cent), a 30 per cent liver (left lateral lobe, LLL) resection group and a 90 per cent PVL group (Table 1 and Fig. 1), designed to assess and compare the magnitude of liver regeneration following the surgical procedure. Animals in a sham‐operated control group underwent laparotomy alone (Table 1) and were killed on day 0 to determine reference parameters for liver and blood.
Table 1.
Experimental design
| Group | n | Step 1 surgery | FLR | ||||
|---|---|---|---|---|---|---|---|
| PVL | LLL resection | Parenchymal transection in ML | Volume (%) | Anatomical remnant | |||
| Volume (%) | PVL lobes | ||||||
| LLL | 15 | 0 | – | Yes | No | 70 | ML+RL+CL |
| ALPPS30 | 15 | 70 | ML | Yes | Yes | 30 | RL+CL |
| ALPPS20 | 15 | 80 | ML, CL | Yes | Yes | 20 | RL |
| ALPPS10 | 15 | 90 | ML, RL | Yes | Yes | 10 | CL |
| PVL | 15 | 90 | ML, RL, LLL | No | No | 10 | CL |
| Control | 5 | 0 | – | No | No | 100 | LLL, ML, RL, CL |
FLR, future liver remnant; PVL, portal vein ligation; LLL, left lateral lobe; ML, median lobe; RL, right lobe; CL, caudate lobe; ALPPS, Associating Liver Partition and Portal vein ligation for Staged hepatectomy.
Figure 1.

Schematic anatomy of rat liver and the surgical procedure of left lateral lobe (LLL) resection, Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS) and portal vein ligation (PVL). a Schematic anatomy of rat liver lobes: LLL, median lobes (ML; left ML and right ML), right lobes (RL; superior RL and inferior RL) and caudate lobes (CL; anterior CL and posterior CL). b Illustration of surgery steps of LLL resection, ALPPS and PVL. After step 1 surgery, the LLL was resected in the LLL group; 70–90 per cent PVL was combined with 30 per cent liver parenchyma (LLL) resection and transection between the median lobes in ALPPS30, ALPPS20 and ALPPS10 groups respectively; 90 per cent portal branches were ligated in the PVL group. In step 2 surgery (on day 3 after step 1), the deportalized liver segments were removed in the ALPPS groups. On day 7 after step 1 (day 4 after step 2), the remnant liver lobes (future liver remnant) showed significant hypertrophy
Five animals in each of the ALPPS, LLL and PVL groups were killed on days 1, 3 (before step 2 surgery) and 7. The FLR weight of these animals was recorded, and the kinetic growth rate (KGR), denoting the percentage increase in FLR per day, and the ratio of remnant liver weight relative to bodyweight (LBW) and expressed as a percentage value were calculated. Blood samples and the FLR tissue were harvested at the time points indicated above.
Assessment of liver function
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin and albumin levels were measured13.
Histological examination and immunohistochemistry
Formalin‐fixed liver tissue specimens were stained with haematoxylin and eosin, and immunostained for Ki‐67, as described previously13. Histological analyses were performed in a blinded fashion, and the number of Ki‐67‐positive hepatocytes was determined in five random visual fields at ×100 magnification.
Western blotting
A standard western blot assay was used to analyse protein expression, as described previously13. Immunostaining was examined for proliferating cell nuclear antigen (PCNA), yes‐associated protein (YAP) and β‐tubulin. The immunoreactive signals were visualized by scanning densitometry with ChemiDoc™ Touch Imaging System (Bio‐Rad Laboratories, Hercules, California, USA).
Immunofluorescence assay
For identification of hepatic progenitor cells (HPCs) in the harvested samples, cryostat sections of liver tissue were processed for double immunofluorescence staining, as described previously15 for α‐fetoprotein (AFP), cytokeratin (CK) 19 and cluster of differentiation (CD) 133, as markers of HPCs.
Further details of the methods employed in the study can be found in Appendix S1 (supporting information)
Statistical analysis
Differences were analysed using ANOVA (among LLL, ALPPS30, ALPPS20 and ALPPS10 groups) and Student's t test (between PVL and ALPPS10 groups) with SPSS® version 16.0 (IBM, Armonk, New York, USA). The statistical tests for ANOVA and t test are denoted as F and t. A probability level of less than 5 per cent was considered statistically significant.
Results
Survival and complications
A total of 80 rats were used in the experiment (Table 1) and most tolerated the operative procedure well, with an overall survival rate of 96 per cent (77 of 80) after surgery. In the ALPPS10 group, three of 15 rats died from portal hypertension and liver failure within 1 day of step 1, and overall survival was significantly lower in this group compared with that in the other groups (F = 3·814, P = 0·015). No deaths occurred in any of the other groups.
Postoperative ascites was noted after step 2 in two of five rats in the ALPPS20 group and in three of four animals in the ALPPS10 group, whereas no such complications were apparent in the other groups. The difference in complication rate was statistically significant (F = 6·212, P = 0·002).
Increased size of the future liver remnant
In the ALPPS30 and ALPPS20 groups, the growth rate was rapid throughout the observation period, whereas in the ALPPS10 and PVL groups there was slower growth after step 1 surgery and PVL. There was a marked increase in growth following step 2 in the ALPPS10 group. By day 7, the greatest relative volume increase was observed for the ALPPS10 animals, with a weight gain of 318 per cent and a LBW ratio of 1·7 per cent (Table 2), which represents 42·5 per cent of normal rat liver volume.
Table 2.
Changes in liver to bodyweight ratio and kinetic growth rate after surgery
| Group | FLR volume (%) | Day 0 | Day 1 | Day 3 | Day 7 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LBW (%)*, † | LBW (%)* | Gain (%)‡ | KGR (% per day)*, § | LBW (%)* | Gain (%)‡ | KGR (% per day)*, § | LBW (%)* | Gain (%)‡ | KGR (% per day)*, § | ||
| LLL resection | 70 | 2·8(0·2) | 3·6(0·1) | 23 | 22·6(0·6) | 3·5(0·1) | 19 | 6·2(2·9) | 3·3(0·4) | 12 | 1·7(9·2) |
| ALPPS30 | 30 | 1·2(0·1) | 1·4(0·1) | 12 | 12·2(2·3) | 2·4(0·2) | 86 | 28·6(6·4) | 3·1(0·1) | 152 | 21·7(2·5) |
| ALPPS20 | 20 | 0·8(0·1) | 1·0(0·1) | 22 | 21·6(2·5) | 1·6(0·1) | 88 | 29·3(7·0) | 2·8(0·2) | 239 | 34·0(16·8) |
| ALPPS10 | 10 | 0·4(0·1) | 0·4(0·1) | 6 | 5·5(10·7) | 0·7(0·1) | 65 | 21·6(5·5) | 1·7(0·1) | 318 | 45·3(10·4) |
| PVL | 10 | 0·4(0·1) | 0·4(0·1) | 5 | 4·5(6·0) | 0·8(0·1) | 112 | 37·4(22·8) | 1·1(0·1) | 187 | 26·8(17·3) |
Values are mean(s.d.).
Mean(s.d.) liver to bodyweight ratio (LBW) of control rats on day 0 was 4·1(0·3) per cent.
Gain of LBW at specific time point versus LBW on day 0.
Kinetic growth rate (KGR) describes the percentage increase in size of the future liver remnant (FLR) per day. LLL, left lateral lobe; ALPPS, Associating Liver Partition and Portal vein ligation for Staged hepatectomy; PVL, portal vein ligation.
The differences in dynamic changes in FLR volume with time in the ALPPS groups were reflected in the KGR. Mean(s.d.) KGR between day 0 and day 7 was 21·7(2·5), 34·0(16·8) and 45·3(10·4) per cent/day in ALPPS30, ALPPS20 and ALPPS10 groups respectively, whereas KGR in LLL and PVL groups was 1·7(9·2) and 26·8(17·3) per cent/day (Table 2). In all groups except LLL, the LBW of the FLR on day 7 was significantly higher than on days 1 and 3 (F = 259·307, 253·680 and 355·965, P = 0·001) (Fig. 2 a). The LBW in the ALPPS10 group was significantly higher than that in the PVL group on day 7 (t = 11·479, P = 0·001).
Figure 2.
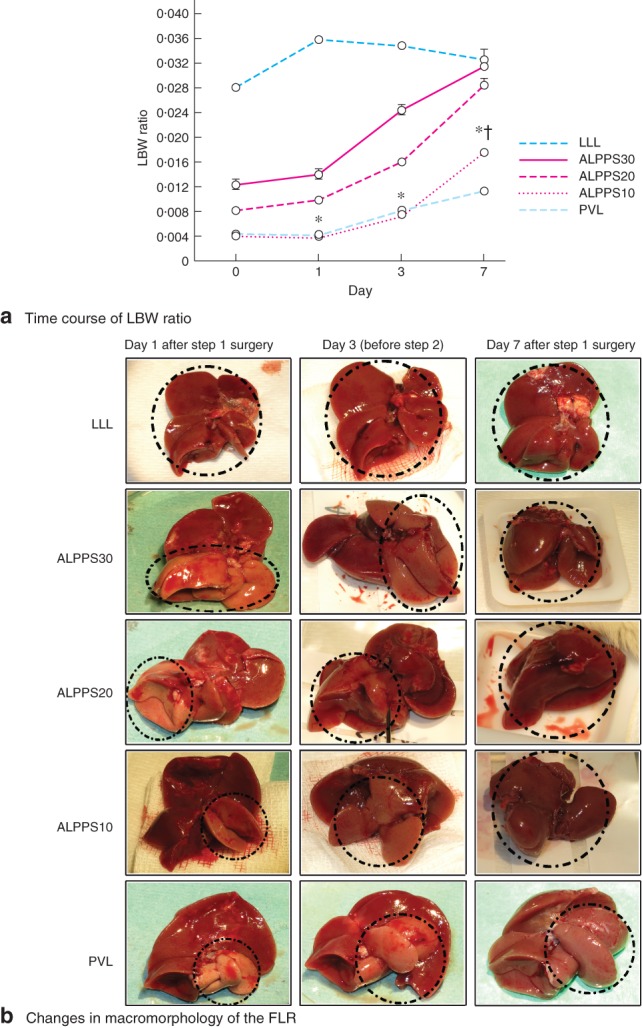
a Time course of future liver remnant (FLR) to bodyweight (LBW) ratio after left lateral lobe (LLL) resection, Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS) and portal vein ligation (PVL) on days 1, 3 and 7 after step 1 surgery. Values are mean(s.d.) (4–5 animals per group). *P < 0·050, ALPPS10 versus LLL, ALPPS30 and ALPPS20 on days 1, 3 and 7 (F = 1250, 407·06 and 37·89 respectively); †P < 0·050, ALPPS10 versus PVL on day 7 (t = 11·479). b Kinetic changes in macromorphology of the FLR on days 1, 3 and 7 after step 1 surgery of LLL resection, ALPPS and PVL. Broken black lines indicate the selected FLRs
Macromorphology of the FLR is shown in Fig. 2 b.
Histology of the future liver remnant and assessment of liver function
Histological analysis of the FLR displayed hepatocyte mitosis and hepatic sinusoidal injury characterized by sinusoidal dilatation, microvesicular steatosis, hepatocellular atrophy, and centrilobular or perisinusoidal fibrosis16. These microscopic changes were most pronounced in the ALPPS30 group on days 1–3, and in ALPPS20, ALPPS10 and PVL groups on days 1–7, but were not notable in either the LLL or the control group at any time point. Representative images from PVL and ALPPS groups are shown in Fig. 3 a–d. The sinusoidal injury score, evaluated by histological parameters of sinusoidal inflammation, dilatation and steatosis in both the periportal and centrilobular area, was visualized as highest for ALPPS10 on day 7 (F = 189·632, P = 0·001), which was a significant difference compared with LLL, ALPPS30 and ALPPS20 (F = 26·100, P = 0·001), and PVL (t = 3·363, P = 0·015) on day 7 (Fig. 3 e). Hepatocellular atrophy, necrosis and fibrosis were present but not pronounced in the ALPPS groups, and there was no difference between the groups on day 7 (F = 1·977, P = 0·168).
Figure 3.
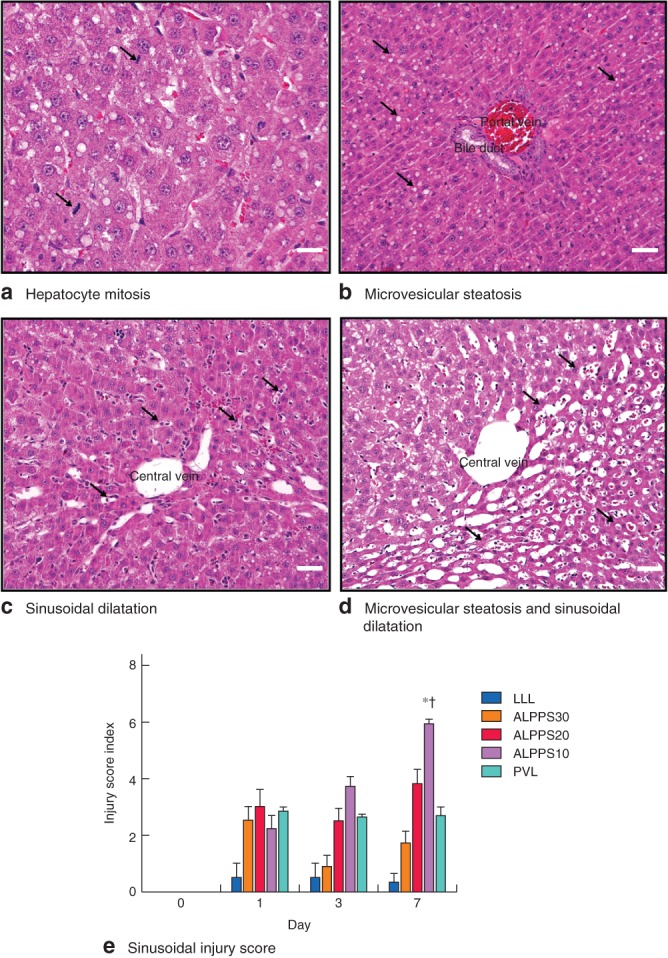
a–d Typical microscopic changes in the future liver remnant after Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS) and portal vein ligation (PVL) (haematoxylin and eosin stain, magnification ×400; scale bars 50 μm): a hepatocyte mitosis – cellular features of extreme hepatocyte hypertrophy and binuclear hepatocytes or polyploidy (arrows); b microvesicular steatosis (arrows); c marked sinusoidal dilatation (arrows); d microvesicular steatosis – atrophic hepatocytes (arrows) and sinusoidal dilatation around central vein. e Sinusoidal injury score on days 1, 3 and 7 after step 1 surgery. Values are mean(s.d.) (4–5 animals per group). *P < 0·050, ALPPS10 versus LLL, ALPPS30 and ALPPS20 (ANOVA); †P < 0·050, ALPPS10 versus PVL (Student's t test)
In all groups, apart from ALPPS10, ALT and AST levels increased significantly after step 1 surgery (F = 32·485 and 39·950 respectively, P = 0·001), returning to normal levels from day 3. Bilirubin and albumin levels were both within the normal range from day 1 to day 7 in these groups.
In the ALPPS10 group, ALT, AST and bilirubin levels increased significantly on day 1 (F = 18·890, 11·648 and 6·151 respectively, P = 0·001), normalizing on day 3. A significant increase in bilirubin (F = 22·431, P = 0·001) and a concomitant reduction in albumin (F = 38·677, P = 0·001) levels were, however, observed on day 7. This pattern was different from that in the other intervention groups (Fig. 4).
Figure 4.
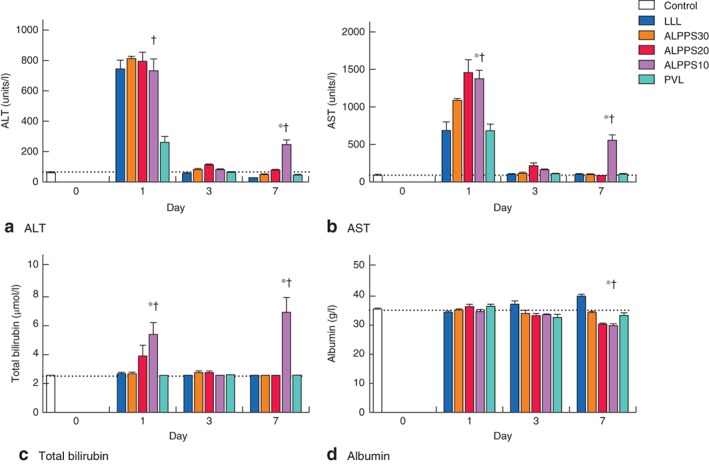
Levels of a alanine aminotransferase (ALT), b aspartate aminotransferase (AST), c total bilirubin and d albumin over the course of the experiment in control, left lateral lobe (LLL) resection, Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS) 30, ALPPS20, ALPPS10 and portal vein ligation (PVL) groups. Values are mean(s.d.) (4–5 animals per group). Horizontal dotted lines indicate the level of the parameter in the control group on day 0. *P < 0·050, ALPPS10 versus LLL, ALPPS30 and ALPPS20 (ANOVA); †P < 0·050, ALPPS10 versus PVL (Student's t test)
Characteristics of liver regeneration
Ki‐67 and PCNA evaluations in tissue samples from the FLR with immunohistochemistry or western blotting are shown in Fig. 5 a,c. Maximum expression of Ki‐67 and PCNA in LLL, PVL, ALPPS30 and ALPPS20 groups occurred after step 1, between days 1 and 3 (Fig. 5 b,d). The expression of Ki‐67 in ALPPS10 was significantly higher than that for LLL, ALPPS30 and ALPPS20 on days 3 and 7 (F = 13·875 and 26·476, P = 0·001), and PVL on day 7 (t = 9·907, P = 0·001). The maximal growth rate in ALPPS10 was observed after step 2, between days 3 and 7, and proliferation appeared to be ongoing after day 7, in contrast to growth in the other groups where the regenerative pattern was approaching a more stable and normal state by this time.
Figure 5.
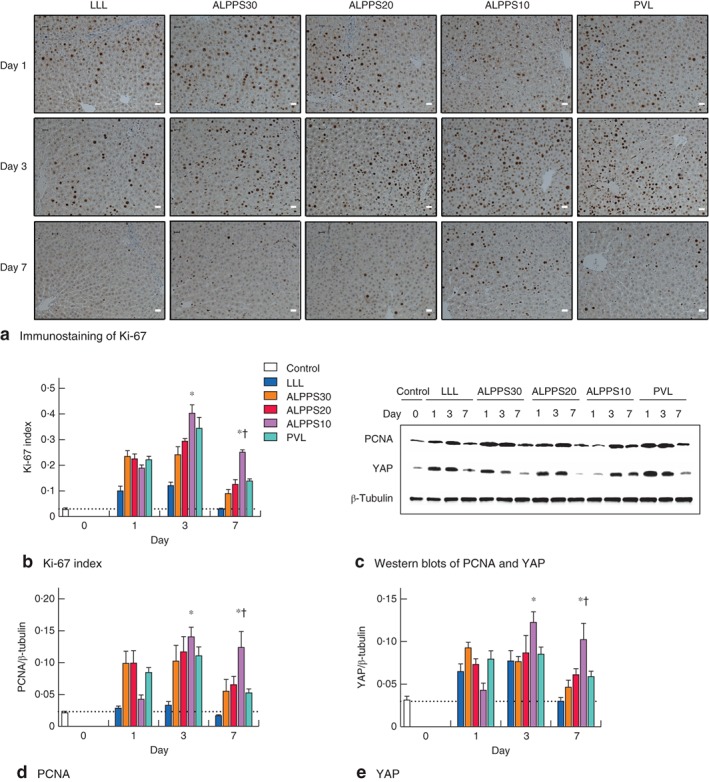
Immunostaining and analysis of a proliferation marker Ki‐67 (magnification ×400, scale bars 20 μm) and c proliferating cell nuclear antigen (PCNA) and yes‐associated protein (YAP) in the future liver remnant after step 1 of left lateral lobe (LLL) resection, Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS) and portal vein ligation (PVL) on days 1, 3 and 7. b,d,e Detection values of Ki‐67, PCNA and YAP on days 1, 3 and 7 after step 1 surgery: b quantitative data for Ki‐67 measured from immunohistochemical images; d,e quantitative analysis of PCNA and YAP expression from western blots. Values are mean(s.d.) (4–5 animals per group). Horizontal dotted lines indicate the level of the parameter in the control group on day 0. *P < 0·050, ALPPS10 versus LLL, ALPPS30 and ALPPS20 (ANOVA); †P < 0·050, ALPPS10 versus PVL (Student's t test)
The Hippo/YAP pathway is fundamental in the maintenance and restoration of liver size17, and promotes progenitor renewal, proliferation and dedifferentiation18. The expression of YAP, a major regulator of the Hippo signalling pathway, in LLL, PVL, ALPPS30 and ALPPS20 groups, showed a transient increase after step 1, followed by a return to normal levels (Fig. 5 c). YAP expression in the ALPPS10 group was significantly higher than that in LLL, ALPPS30 and ALPPS20 groups on day 3 (F = 4·133, P = 0·032) and day 7 (F = 18·703, P = 0·001), and in the PVL group on day 7 (t = 4·191, P = 0·006) (Fig. 5 e).
Activation of HPCs in the FLR using antibodies against AFP (fetal hepatoblastic marker), CK19 (epithelial marker) and CD133 (stem cell marker)15 revealed simultaneous positive staining for both CK19 and AFP/CD133 (Fig. 6 a). HPCs were distributed mainly in the periportal area (zone 1), and few HPCs were found around central veins (zone 3). No HPCs were detected in control animals. After step 1 surgery, HPCs were observed in all groups on day 1, in ALPPS10 and PVL groups on day 3, and in ALPPS30, ALPPS20, ALPPS10 and PVL groups on day 7. The mean(s.d.) number of HPCs in the ALPPS10 group on day 7 was 75·8(8·6) per 1000 liver cells, which was about four times higher than that in ALPPS30, ALPPS20 (F = 209·310, P = 0·001) and PVL (t = 13·246, P = 0·001) groups (Fig. 6 b).
Figure 6.
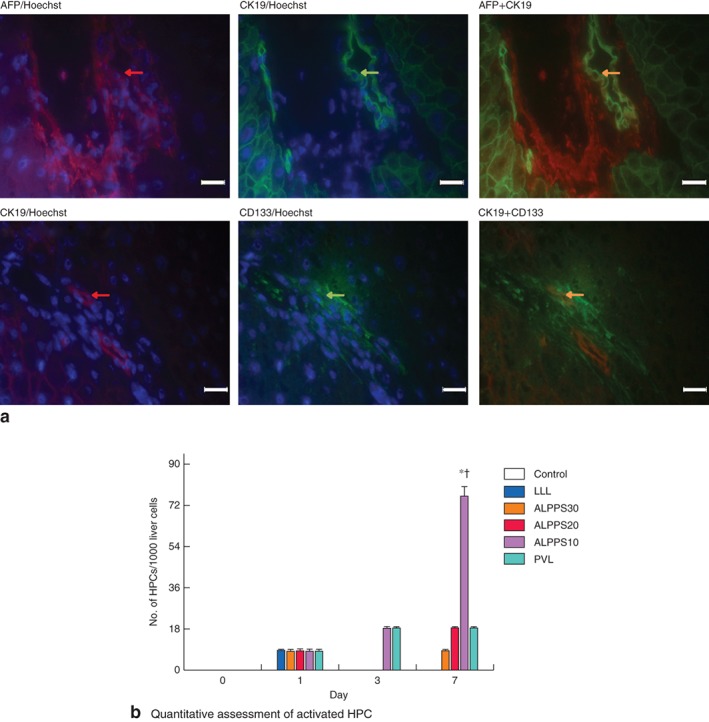
Representative expression of hepatic progenitor cell (HPC) activation in the future liver remnant after surgery. a Double immunofluorescence staining with α‐fetoprotein (AFP) (red) plus cytokeratin (CK) 19 (green) or CK19 (red) plus cluster of differentiation (CD) 133 (green), and Hoechst nuclear staining (blue) (magnification ×400, scale bars 20 μm). Expression of activated HPC is indicated by simultaneous positive staining (yellow) of both CK19 and AFP/CD133 (arrows). b Quantitative assessment of the number of HPCs per 1000 liver cells on days 1, 3 and 7 after step 1 surgery following left lateral lobe (LLL) resection, Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS) and portal vein ligation (PVL). Values are mean(s.d.) (4–5 animals per group). *P < 0·050, ALPPS10 versus LLL, ALPPS30 and ALPPS20 (ANOVA); †P < 0·050, ALPPS10 versus PVL (Student's t test)
Discussion
The development of an appropriate animal model was critical to this study. The anatomy and physiology of rat liver are well known. Precise PVL with no twisting artery branches and second‐stage hepatectomy were considered critical in creating an ALPPS model. The portal vein stem, with its accompanying artery and bile duct, divides into a left branch supplying the caudate lobe, a right branch supplying the right lobe, and a median branch supplying the right and left median lobes and the LLL. These three portal branches are easily separated from the concomitant arterial branches and formed the anatomical basis for the present ALPPS model.
Another consideration was adhesion formation between the transection surfaces after step 1, as well as the involvement of omentum and bowel that might increase the technical challenge of lobectomy in step 2. The median lobe, a separate anatomical unit close to the diaphragm, lends itself to both PVL and resection, and was considered a suitable deportalized segment for step 2.
The most widely employed rodent hepatectomy models have used right and caudate lobes (30 per cent), right lobe alone (20 per cent) and caudate lobe alone (10 per cent) as respective FLRs12, so these were used in the present model. These features may offer a comparative advantage over the other reported ALPPS models14 19.
One disadvantage of this procedure might be that the parenchymal transection did not conform to the demarcation line between deportalized segments and the FLR. This transection selection may not be critical, as the inflammatory response invoked by parenchymal transection14 20 is generally considered to be systemic rather than liver‐specific19 21.
The extent of liver resection and thus the size of the FLR have been reported as determining factors for liver failure after hepatectomy and small‐for‐size liver syndrome. A reduced parenchymal volume is insufficient to maintain normal liver function and inadequate to handle portal inflow leading to raised portal vein pressure22 23. Small‐for‐size syndrome is characterized by postoperative liver dysfunction with progressive cholestasis and coagulopathy, portal hypertension and ascites24. High portal blood flow through a relatively small liver vascular bed leading to increased portal pressure is thought to have a central role in this process22, 23, 24. It has been shown previously25 that survival rates following increasing degrees of hepatic resection in rats (75 per cent, 85 per cent or above, 90 per cent or greater hepatectomies) were 100, 18 and 0 per cent respectively at 48 h, and were linked to sinusoidal damage related to high flow and raised portal blood flow26.
With the present protocol, ALPPS with 10 per cent FLR led to increased mortality (3 of 15 animals) after step 1 and ascites (3 of 4 animals) after step 2 surgery. Furthermore, rats in the ALPPS10 group had increased levels of transaminases and bilirubin, and lowered albumin values after step 2. These changes suggest a pathophysiological setting similar to that of posthepatectomy liver failure and small‐for‐size liver syndrome, supporting clinical observations10 that FLR size in ALPPS is a critical factor affecting morbidity and mortality. From a clinical perspective, this could indicate the need for a longer time interval between step 1 and step 2 surgery when the FLR size is below a certain threshold, or the patient has raised bilirubin levels. Recent clinical evidence10 relating to risks with ALPPS supports this.
Compared with the 90 per cent PVL group, the increased mortality and morbidity in the ALPPS10 group was a striking finding. Both protocols with the same size of FLR should theoretically lead to the same level of portal hypertension. This could indicate that the ALPPS procedure incorporating parenchymal transection and local resection triggers the release of cytokines and growth factors14 21, affecting liver parenchymal integrity and function24.
The findings in all ALPPS groups in the present study confirmed clinical and experimental experience that ALPPS induces a rapid increase in the FLR volume. An inflammatory response together with altered haemodynamics are assumed to be key factors initiating rapid hypertrophy through systemic release of cytokines and growth factors14 21. These factors are essentially the same as those observed in the initiation and proliferation phase of liver regeneration after conventional liver resections27.
The marked increase in FLR volume observed in ALPPS could indicate that the regenerative process, particularly with small FLR volumes, may be different to that observed after partial hepatectomy or PVL19. Previous studies13 28 suggest that different rates of liver regeneration after major hepatectomy depend on the size of the resection, with the maximum rate of liver regeneration seen after 70 per cent hepatectomy. In the present experiments, the greatest regenerative ability was with the 10 per cent FLR. The increase of FLR weight and the maximal KGR were greater at the end of the experiment, the smaller the initial FLR. This is in agreement with a recent clinical study29 showing that KGR of the FLR in patients after ALPPS and the regenerative response in living liver donors correlated with the size of the liver remnant.
There were differences in growth patterns between the three ALPPS groups in the present study. The relative weight increase was greatest with a FLR of 10 per cent, but the maximal regenerative response was delayed in comparison with that in ALPPS20, ALPPS30 and PVL groups. The mechanism of delayed liver regeneration after step 1 in the ALPPS10 group remains unclear. As this growth pattern in ALPPS with a FLR of 10 per cent was similar to that following marginal hepatectomy (80–90 per cent) in rats28, this characteristic may be related to a FLR size below the threshold that the animals can easily tolerate.
The possible role of HPC‐mediated liver regeneration in the setting of ALPPS proved interesting in these studies. YAP is a critical regulator of liver size through expansion of undifferentiated HPCs18 30. In mature hepatocytes YAP is expressed at very low levels, whereas it is highly expressed in the progenitor cell compartments18. High levels of YAP indicate a HPC phenotype resulting in differentiation into hepatocytes and liver growth30. The present results indicated a higher expression of YAP after ALPPS compared with that following PVL and LLL, and YAP reached maximum levels on days 3–7 in ALPPS20 and ALPPS10 groups, inversely correlated to FLR size.
Although HPCs are not considered to be part of liver regeneration under most circumstances where mature hepatocytes dominate27, the present results indicated HPC activation in all of the ALPPS groups, and the number of HPCs in the ALPPS10 group on day 7 reached 75·8 per 1000 liver cells. The pattern of HPC activation seems to correlate with FLR size and extent of sinusoidal damage. Thus, as well as sinusoidal injury, the immaturity of the liver parenchyma by repopulating HPCs31 could also attribute to the discrepancy between functional capacity and volume of the FLR. The mechanism of activation and proliferation of HPCs in ALPPS requires further evaluation.
Supporting information.
Additional supporting information may be found online in the supporting information tab for this article.
Supporting information
Appendix S1 Further details of methods employed (Word document)
Acknowledgements
The authors thank H. Reims (Department of Pathology, Oslo University Hospital, Oslo, Norway) for the pathological evaluation, F. L. Jahnsen, K. Thorvaldsen Hagen, S. Chellappa and E. M. Aandahl (University of Oslo, Oslo, Norway) for technical guidance and assistance.
Disclosure: The authors declare no conflict of interest.
Funding information
No funding
References
- 1. Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C et al Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery 2000; 127: 512–519. [DOI] [PubMed] [Google Scholar]
- 2. Truant S, Oberlin O, Sergent G, Lebuffe G, Gambiez L, Ernst O et al Remnant liver volume to body weight ratio > or = 0·5%: a new cut‐off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg 2007; 204: 22–33. [DOI] [PubMed] [Google Scholar]
- 3. Breitenstein S, Apestegui C, Petrowsky H, Clavien PA. ‘State of the art’ in liver resection and living donor liver transplantation: a worldwide survey of 100 liver centers. World J Surg 2009; 33: 797–803. [DOI] [PubMed] [Google Scholar]
- 4. Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P et al Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990; 107: 521–527. [PubMed] [Google Scholar]
- 5. Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two‐stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg 2000; 232: 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA et al Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2‐staged extended right hepatic resection in small‐for‐size settings. Ann Surg 2012; 255: 405–414. [DOI] [PubMed] [Google Scholar]
- 7. Tschuor C, Croome KP, Sergeant G, Cano V, Schadde E, Ardiles V et al Salvage parenchymal liver transection for patients with insufficient volume increase after portal vein occlusion – an extension of the ALPPS approach. Eur J Surg Oncol 2013; 39: 1230–1235. [DOI] [PubMed] [Google Scholar]
- 8. Schadde E, Schnitzbauer AA, Tschuor C, Raptis DA, Bechstein WO, Clavien PA. Systematic review and meta‐analysis of feasibility, safety, and efficacy of a novel procedure: associating liver partition and portal vein ligation for staged hepatectomy. Ann Surg Oncol 2015; 22: 3109–3120. [DOI] [PubMed] [Google Scholar]
- 9. Truant S, Baillet C, Deshorgue AC, Leteurtre E, Hebbar M, Ernst O et al Drop of total liver function in the interstages of the new associating liver partition and portal vein ligation for staged hepatectomy technique: analysis of the ‘auxiliary liver’ by HIDA scintigraphy. Ann Surg 2016; 263: e33–e34. [DOI] [PubMed] [Google Scholar]
- 10. Linecker M, Stavrou GA, Oldhafer KJ, Jenner RM, Seifert B, Lurje G et al The ALPPS risk score: avoiding futile use of ALPPS. Ann Surg 2016; 264: 763–771. [DOI] [PubMed] [Google Scholar]
- 11. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010; 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martins PN, Theruvath TP, Neuhaus P. Rodent models of partial hepatectomies. Liver Int 2008; 28: 3–11. [DOI] [PubMed] [Google Scholar]
- 13. Shi JH, Huitfeldt HS, Suo ZH, Line PD. Growth of hepatocellular carcinoma in the regenerating liver. Liver Transpl 2011; 17: 866–874. [DOI] [PubMed] [Google Scholar]
- 14. Schlegel A, Lesurtel M, Melloul E, Limani P, Tschuor C, Graf R et al ALPPS: from human to mice highlighting accelerated and novel mechanisms of liver regeneration. Ann Surg 2014; 260: 839–846. [DOI] [PubMed] [Google Scholar]
- 15. Shi JH, Scholz H, Huitfeldt HS, Line PD. The effect of hepatic progenitor cells on experimental hepatocellular carcinoma in the regenerating liver. Scand J Gastroenterol 2014; 49: 99–108. [DOI] [PubMed] [Google Scholar]
- 16. Rubbia‐Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M et al Severe hepatic sinusoidal obstruction associated with oxaliplatin‐based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol 2004; 15: 460–466. [DOI] [PubMed] [Google Scholar]
- 17. Avruch J, Zhou D, Fitamant J, Bardeesy N. Mst1/2 signalling to Yap: gatekeeper for liver size and tumour development. Br J Cancer 2011; 104: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R et al YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 2007; 17: 2054–2060. [DOI] [PubMed] [Google Scholar]
- 19. Budai A, Fulop A, Hahn O, Onody P, Kovacs T, Nemeth T et al Animal models for associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): achievements and future perspectives. Eur Surg Res 2017; 58: 140–157. [DOI] [PubMed] [Google Scholar]
- 20. Yao L, Li C, Ge X, Wang H, Xu K, Zhang A et al Establishment of a rat model of portal vein ligation combined with in situ splitting. PLoS One 2014; 9: e105511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cai YL, Song PP, Tang W, Cheng NS. An updated systematic review of the evolution of ALPPS and evaluation of its advantages and disadvantages in accordance with current evidence. Medicine (Baltimore) 2016; 95: e3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Serenari M, Cescon M, Cucchetti A, Pinna AD. Liver function impairment in liver transplantation and after extended hepatectomy. World J Gastroenterol 2013; 19: 7922–7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eshkenazy R, Dreznik Y, Lahat E, Zakai BB, Zendel A, Ariche A. Small for size liver remnant following resection: prevention and management. Hepatobiliary Surg Nutr 2014; 3: 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tucker ON, Heaton N. The ‘small for size’ liver syndrome. Curr Opin Crit Care 2005; 11: 150–155. [DOI] [PubMed] [Google Scholar]
- 25. Panis Y, McMullan DM, Emond JC. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery 1997; 121: 142–149. [DOI] [PubMed] [Google Scholar]
- 26. Hanna SS, Pagliarello G, Ing A. Liver blood flow after major hepatic resection. Can J Surg 1988; 31: 363–367. [PubMed] [Google Scholar]
- 27. Shi JH, Line PD. Effect of liver regeneration on malignant hepatic tumors. World J Gastroenterol 2014; 20: 16167–16177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rabes HM. Kinetics of hepatocellular proliferation after partial resection of the liver. Prog Liver Dis 1976; 5: 83–99. [PubMed] [Google Scholar]
- 29. Croome KP, Hernandez‐Alejandro R, Parker M, Heimbach J, Rosen C, Nagorney DM. Is the liver kinetic growth rate in ALPPS unprecedented when compared with PVE and living donor liver transplant? A multicentre analysis. HPB (Oxford) 2015; 17: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe‐Mooney B, Gurung B et al Hippo pathway activity influences liver cell fate. Cell 2014; 157: 1324–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsuo K, Murakami T, Kawaguchi D, Hiroshima Y, Koda K, Yamazaki K et al Histologic features after surgery associating liver partition and portal vein ligation for staged hepatectomy versus those after hepatectomy with portal vein embolization. Surgery 2016; 159: 1289–1298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Further details of methods employed (Word document)


