Abstract
Background
Changes in medical education may limit opportunities for trainees to gain proficiency in surgical skills. Transcranial direct‐current stimulation (tDCS) can augment motor skill learning and may enhance surgical procedural skill acquisition. The aim of this study was to determine the effects of tDCS on simulation‐based laparoscopic surgical skill acquisition.
Methods
In this double‐blind, sham‐controlled randomized trial, participants were randomized to receive 20 min of anodal tDCS or sham stimulation over the dominant primary motor cortex, concurrent with Fundamentals of Laparoscopic Surgery simulation‐based training. Primary outcomes of laparoscopic pattern‐cutting and peg transfer tasks were scored at baseline, during repeated performance over 1 h, and again at 6 weeks. Intent‐to‐treat analysis examined the effects of treatment group on skill acquisition and retention.
Results
Of 40 participants, those receiving tDCS achieved higher mean(s.d.) final pattern‐cutting scores than participants in the sham group (207·6(30·0) versus 186·0(32·7) respectively; P = 0·022). Scores were unchanged at 6 weeks. Effects on peg transfer scores were not significantly different (210·2(23·5) in the tDCS group versus 201·7(18·1) in the sham group; P = 0·111); the proportion achieving predetermined proficiency levels was higher for tDCS than for sham stimulation. Procedures were well tolerated with no serious adverse events and no decreases in motor measures.
Conclusion
The addition of tDCS to laparoscopic surgical training may enhance skill acquisition. Trials of additional skills and translation to non‐simulated performance are required to determine the potential value in medical education and impact on patient outcomes. Registration number: NCT02756052 (https://clinicaltrials.gov/).
Introduction
More than 300 million surgical procedures are required to address the global burden of disease1. Surgical trainees display a wide range of abilities, but many may lack confidence in their ability to perform procedures2 3. Training programme directors believe that many senior trainees are not capable of performing major procedures independently2 3. These shortcomings may be linked to recent changes in surgical training environments, including working time restrictions that limit opportunities for trainees to gain proficiency in surgical skills4. Systematic reviews and meta‐analyses5 6 suggest that these restrictions have not improved patient safety in relation to surgical procedures, with some7, 8, 9 suggesting increased complication and morbidity rates. Reduced training opportunities and skill proficiency may be contributing factors4.
Simulation‐based task training (SBTT) provides an effective, risk‐free method of skill acquisition during surgical training and may reduce time to achieve skill proficiency10. Despite its value, SBTT suffers various limitations, including prolonged time requirements with modest effect sizes and variable long‐term retention of skills10 11. Defining methods to enhance SBTT holds the potential to accelerate training and optimize achievement of competency in complex skills such as surgery.
As a minimally invasive technique with quicker recovery and shorter hospital stays12, laparoscopic surgery has become standard for many procedures. Laparoscopic skills are difficult to acquire, with loss of depth perception, lack of tactile sensation and altered hand–eye coordination being contributory factors13. The Fundamentals of Laparoscopic Surgery (FLS) programme uses SBTT to teach laparoscopic skills14, and performance speed and accuracy may be associated with intraoperative performance15 16. Complex motor skills such as precision cutting and single‐incision laparoscopy are difficult to acquire and also prone to decay17, 18, 19, 20. Improved strategies to optimize the efficiency and effectiveness of complex surgical skill training models are required.
Transcranial direct‐current stimulation (tDCS) is a form of non‐invasive brain stimulation that delivers weak electric currents to the cortex via two scalp electrodes21. When paired with motor skill training, stimulation of the motor cortex with anodal tDCS safely enhances skill acquisition of a wide range of tasks, often with large effect sizes22 23 and long‐term skill retention22. Neuroimaging and neurophysiology studies are beginning to elucidate the possible mechanisms behind this enhancement of learning24. The application of tDCS concurrent with simulation‐based tumour resection training has been shown to enhance acquisition of the skill25. This novel finding suggests the potential value of tDCS in medical training. Applying tDCS to enhance the acquisition of complex SBTT skills, such as those required with laparoscopic surgery, has not been investigated. Establishing an ability to enhance surgical skill motor learning could benefit medical training and patient outcomes.
The present study aimed to determine whether tDCS could enhance the acquisition of laparoscopic skills. Based on the ability of tDCS to enhance simple motor26 and complex tumour resection25 skill acquisition, it was hypothesized that anodal tDCS over the dominant primary motor cortex would enhance the acquisition of two FLS skills.
Methods
This was a double‐blind, randomized, sham‐controlled, single‐centre trial (http://clinicaltrials.gov, NCT02756052, 30 March 2016). Participants provided written informed consent. The University of Calgary Research Ethics Board approved all methods, which were performed in accordance with the Declaration of Helsinki.
Recruitment e‐mails were sent to first‐ and second‐year medical students, encouraging those with an interest in surgical specialties to participate. Respondents were screened for exclusion criteria (neurological or neuropsychiatric disorders, neuropsychotropic medication, previous brain stimulation, implanted metal or electronic devices, or pregnancy). Trial objectives and possible side‐effects of tDCS were described by a standard script given to participants. Baseline characteristics including age, sex, self‐reported handedness, previous laparoscopy experience, and musical instrument and video game experience were recorded. To optimize motor learning27, competition and reward were invoked by awarding the best performer on each task an additional $50 coffee gift card (all received $20).
Outcomes
Primary outcomes were the FLS pattern‐cutting and peg transfer tasks14. These validated tasks represent unimanual and bimanual skills respectively, and are sensitive to improved performance throughout surgical training. Pattern cutting required the use of a dissector and endoscopic scissors to cut a marked circle (4 cm in diameter) from a 10 × 10‐cm two‐ply gauze. Peg transfer required the use of two dissectors to transfer six pegs to each end of a pegboard and back. A previously established scoring system14 was used. For both tasks, time score was the completion time in seconds subtracted from a cut‐off time of 300 s. Standardized error scores were calculated as the percentage area deviation from a perfectly cut circle (10 per cent deviation = 10 s penalty) or the percentage of pegs dropped outside the field of view (1 of 6 pegs lost = 17 s penalty). A total score was calculated by subtracting the error score from the time score. Proficiency scores for each task were based on previously established values28. All repetitions were scored in real‐time and video‐recorded. Two blinded assessors independently scored video recordings and the mean was used. The Purdue Pegboard Test (PPT)29 defined baseline motor function and served as a safety measure to ensure no decline in function of either hand.
Intervention
The trial design is shown in Fig. 1 a. The PPT was performed with each hand. Participants watched a 2‐min orientation video for both FLS tasks, in which the task was performed by an experienced surgeon. One repetition of each task was then performed (baseline) on a FLS Trainer System (VTI Medical, Waltham, Massachusetts, USA).
Figure 1.
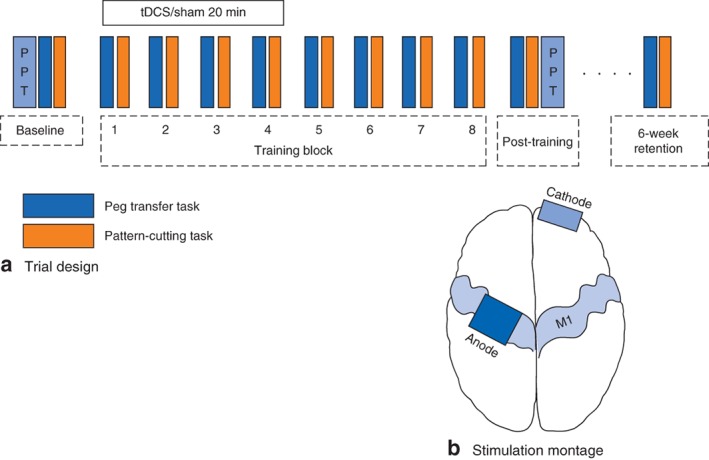
a Trial design. Participants performed the Purdue Pegboard Test (PPT) and one repetition of the peg transfer and pattern‐cutting Fundamental of Laparoscopic Surgery tasks. b Stimulation montage. The anode was positioned over the dominant primary motor cortex (M1), and the cathode over the contralateral supraorbital area
The same tDCS set‐up was performed on all participants. The anode was positioned over the dominant primary motor cortex (C3 or C4, 10–20 electroencephalography electrode system) with the cathode over the contralateral supraorbital area (Fig. 1 b; Fig. S1, supporting information). Participants blindly selected a randomization code envelope that was opened only by the investigator applying tDCS. Anodal tDCS was delivered through 25‐cm2 saline‐soaked sponge electrodes using a DC stimulator (neuroConn, Ilmenau, Germany).
Training then consisted of eight repetitions of each task performed in an interleaved manner (A‐B‐A‐B…), suggested to enhance laparoscopic skill acquisition30. Each repetition consisted of performing the task to completion. No feedback was provided during or after the task. Short breaks of approximately 60 s were permitted between repetitions. Stimulation was initiated with the first training block according to published standards31 by the same experienced investigator. In both sham and anodal tDCS conditions, current was ramped up to 1 mA over 45 s. Anodal tDCS was maintained at 1 mA for 20 min. Sham tDCS was held at 1 mA for 60 s, followed by a 45‐s ramp down to 0 mA. This sham method produces identical scalp sensations without inducing changes in cortical excitability, and provides effective blinding in tDCS trials32. The sham tDCS group acted as a control to examine the effects of laparoscopic training alone in skill acquisition.
Immediately after the last training block, a final repetition was performed to obtain post‐training scores for each task. The PPT was then repeated. Participants completed a brain stimulation safety and tolerability questionnaire, ranking sensations using a visual analogue scale (0, not present; 10, intolerable). Participants were asked to guess whether they had received anodal or sham tDCS.
Participants returned 6 weeks later and performed one repetition of each task to assess skill retention.
Sample size
Sample size calculations were based on previous work25 examining the effects of tDCS on neurosurgical skill acquisition. The application of tDCS concurrent with training of simulation‐based tumour resection resulted in a skill acquisition enhancement of approximately 10 per cent. Combined with typical mean(s.d.) changes in FLS scores of 110(10), α = 0·05 and power of 85 per cent, it was estimated that 18 participants per intervention group would be required (36 in total).
Statistical analysis
Demographics and baseline characteristics were compared using χ2 or Fisher's exact and t tests, as appropriate. Following confirmation of a normal distribution (Shapiro–Wilk test), pattern‐cutting and peg transfer scores were compared across time (baseline, after training, retention) and intervention groups using two‐way repeated‐measures ANOVA with Holm–Sidak post hoc corrections. Post‐training scores were compared between groups using t tests. Proportions reaching predetermined proficiency levels were compared using χ2 or Fisher's exact test. Paired t tests evaluated score decay between post‐training and retention, and changes in PPT from baseline to post‐training. Proportions and severity of tDCS sensations were compared (Fisher's exact test and t tests respectively). Significance was identified when P < 0·050. Hypothesis testing was one‐sided, based on the predefined hypothesis that tDCS enhances skill acquisition in a wide range of tasks. Analyses were performed using SigmaPlot® version 12·5 (Systat Software, San Jose, California, USA).
Results
From March to July 2016, 44 students expressed interest, of whom four were excluded for scheduling conflicts. One participant was unavailable for retention testing. Population characteristics are shown in Table 1. Baseline motor performance did not differ between the groups. One participant in each group had minimal laparoscopic experience (less than 10 min), one (in the sham group) spent 8 h per week playing instruments, and three (2 sham, 1 anodal) spent more than 8 h per week playing video games.
Table 1.
Demographics and baseline characteristics
| Sham tDCS (n = 19) | Anodal tDCS (n = 20) | |
|---|---|---|
| Age (years)* | 24·7(3·3) | 26·3(4·1) |
| Sex ratio (M : F) | 9 : 10 | 9 : 11 |
| Handedness | ||
| Right‐handed | 17 | 18 |
| Left‐handed | 2 | 2 |
| Year of medical school | ||
| First | 12 | 9 |
| Second | 6 | 10 |
| Third | 1 | 1 |
| PPT score* | ||
| Non‐dominant hand | 15·2(2·1) | 15·1(1·6) |
| Dominant hand | 17·0(1·8) | 16·8(1·5) |
| Peg transfer score* | 102·6(52·0) | 97·1(61·5) |
| Pattern‐cutting score* | 68·3(62·9) | 71·1(75·4) |
Values are mean(s.d.). tDCS, transcranial direct‐current stimulation; PPT, Purdue Pegboard Test.
Pattern cutting
Pattern‐cutting learning curves are shown in Fig. 2 a. Scores improved for all participants during training (F 2 = 172·99, P < 0·001). No differences were seen between stimulation groups across all time points (F 38 = 1·071, P = 0·311). After training, participants who received tDCS demonstrated higher mean(s.d.) pattern‐cutting scores than those in the sham group (207·6(30·0) versus 186·0(32·7) respectively; t = 2·143, P = 0·022). Compared with established proficiency scores (98‐s completion time, corresponding to a score of 202, assuming no error), the proportion achieving 100 per cent proficiency was 55 per cent (11 of 20) for tDCS versus 32 per cent (6 of 19) for sham (P = 0·200) (Fig. 2 b). The proportion reaching 90 per cent proficiency was 85 per cent in the tDCS group and 58 per cent in the sham group (P = 0·083). Neither group demonstrated decay in pattern cutting from post‐training to retention testing: mean(s.d.) score from 186·0(32·7) to 198·6(29·0) for sham (P = 0·132) and from 207·6(30·0) to 206·4(26·8) for tDCS (P = 0·971). Mean(s.d.) change at retention times did not differ between sham and tDCS groups (13·4(26·5) versus −0·2(29·4) respectively; t = 1·477, P = 0·154).
Figure 2.
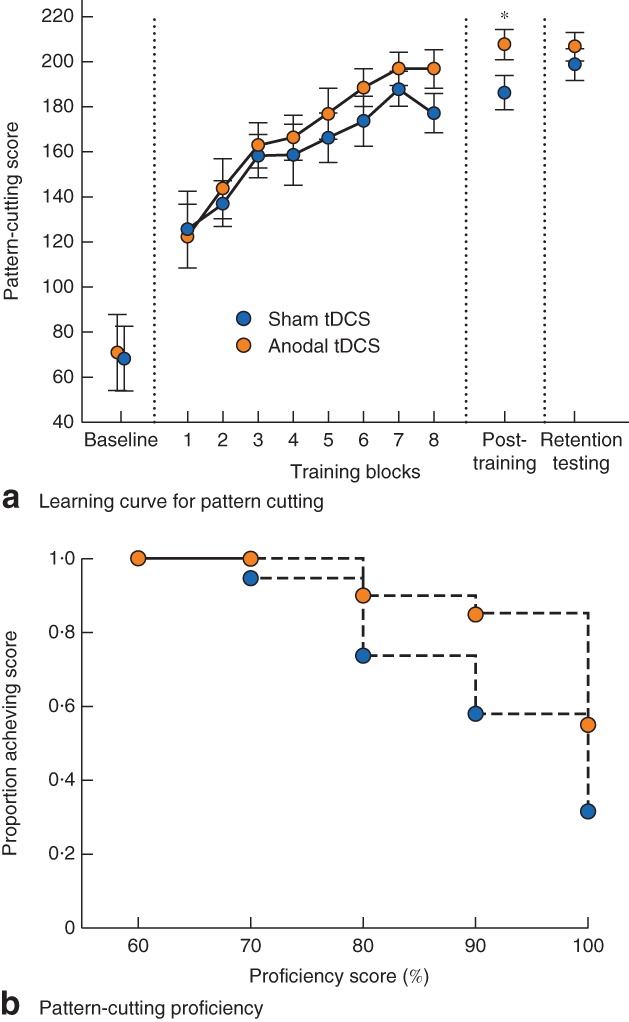
a Learning curve for Fundamentals of Laparscopic Surgery pattern cutting concurrent with sham or anodal transcranial direct‐current stimulation (tDCS). Values are mean(s.e.m.). b Proportion of participants achieving various levels of pattern‐cutting proficiency at post‐training evaluation with the application of sham stimulation or anodal tDCS. *P = 0·022 (t test)
Peg transfer
Peg transfer learning curves are shown in Fig. 3 a. Scores improved for all participants during training (F 2 = 117·18, P < 0·001). No differences were seen between stimulation groups across all time points (F 38 = 0·276, P = 0·603). At post‐training, participants receiving tDCS demonstrated slightly higher mean(s.d.) peg transfer scores than those receiving sham (210·2(23·5) versus 201·7(18·1) respectively; t = 1·262, P = 0·111). Compared with established proficiency scores (48‐s completion time, corresponding to a score of 252, assuming no errors), no participant achieved proficiency. The proportion achieving 90 per cent proficiency was 35 per cent (7 of 20) for tDCS compared with 5 per cent (1 of 19) for sham (P = 0·039) (Fig. 3 b). The proportion achieving 80 per cent proficiency was 75 per cent (15 of 20) with tDCS versus 53 per cent (10 of 19) with sham (P = 0·191). Both groups demonstrated decay in PEG TRANSFER from post‐training to retention testing: mean(s.d.) score from 201·7(18·1) to 181·9(35·1) for sham (P = 0·021) and from 210·2(23·5) to 193·4(21·6) for tDCS (P = 0·002). Mean change at retention times did not differ between sham and anodal tDCS groups (−17·2(36·9) versus −19·4(15·7) respectively; t = 0·230, P = 0·414). There were no differences in performance between left‐ and right‐hand‐dominant participants for either task.
Figure 3.
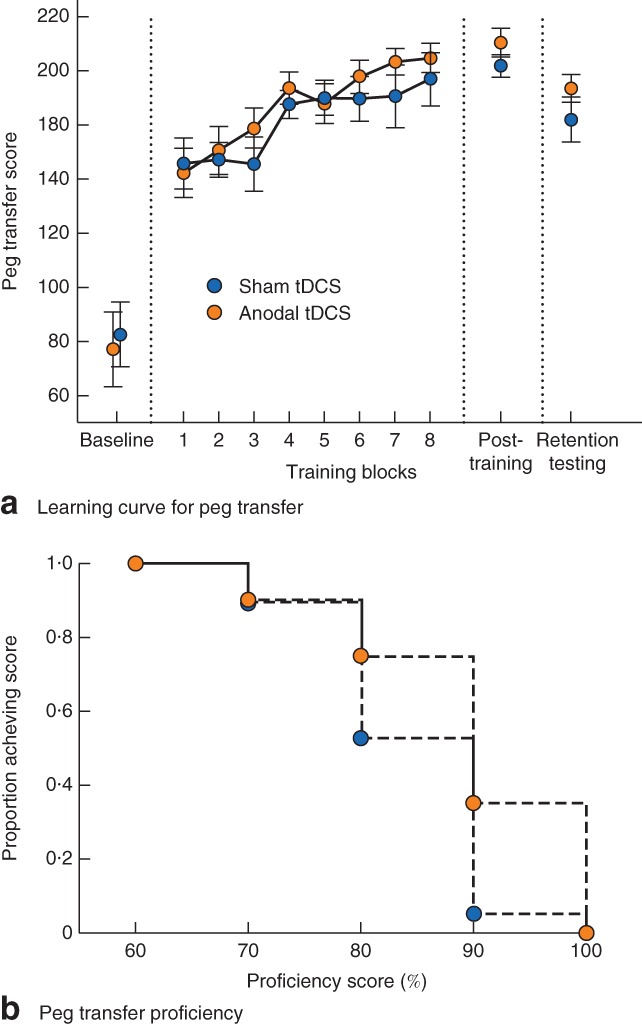
a Learning curve of Fundamentals of Laparoscopic Surgery peg transfer concurrent with sham or anodal transcranial direct‐current stimulation (tDCS). Values are mean(s.e.m.). b Proportion of participants achieving various levels of peg transfer proficiency at post‐training evaluation with the application of sham stimulation or anodal tDCS
Inter‐rater reliability
Assessor scores agreed within 1 point of each other in 94 per cent of cases, and within 2 points in more than 98 per cent of instances.
Safety and tolerability
There were no serious adverse events. Interventions were well tolerated, with mean VAS scores of 1·06 or less for all sensations (Table 2). Itching was most common, occurring in 24 (62 per cent) of the 39 participants. Sensation proportions and severity were comparable between groups with the exception of burning, which was more common (9 of 20 versus 2 of 19; P = 0·033) and less mild (VAS score 0·67 versus 0·16; P = 0·031) for tDCS compared with sham.
Table 2.
Sensations and tolerability of transcranial direct‐current stimulation
| Proportion of participants reporting sensation | VAS sensation severity ranking* | |||||
|---|---|---|---|---|---|---|
| Sham tDCS (n = 19) | Anodal tDCS (n = 20) | P † | Sham tDCS | Anodal tDCS | P ‡ | |
| Itching | 9 | 15 | 0·105 | 0·84(1·26) | 1·06(0·89) | 0·151 |
| Burning | 2 | 9 | 0·033 | 0·16(0·50) | 0·67(0·91) | 0·031 |
| Tingling | 4 | 10 | 0·096 | 0·37(0·76) | 0·71(0·85) | 0·128 |
| Discomfort | 5 | 9 | 0·320 | 0·37(0·68) | 0·33(0·48) | 0·826 |
| Pain | 2 | 2 | 0·999 | 0·05(0·23) | 0·05(0·22) | 0·971 |
Values are mean(s.d.). VAS, visual analogue scale; tDCS, transcranial direct‐current stimulation.
Fisher's exact test;
t test.
PPT scores did not decrease in either hand, regardless of intervention. The non‐dominant‐hand PPT score increased significantly in sham participants (from 15·2(2·1) at baseline to 16·3(2·1) post‐training; P = 0·002), and the dominant‐hand PPT score showed a trend towards an increase (from 17·0(1·8) to 17·5(1·8); P = 0·054). In the tDCS group, both non‐dominant (from 15·1(1·6) to 16·1(1·8); P < 0·001) and dominant (from 16·8(1·5) to 17·6(1·9); P = 0·021) PPT scores increased significantly. Change in PPT score did not differ between treatment groups. When asked to guess their intervention allocation, 30 of 39 participants responded ‘unsure’. When forced to guess, assignment was correct in 23 of 39 guesses.
Discussion
This double‐blind, randomized, sham‐controlled trial suggests that tDCS may safely enhance simulation‐based laparoscopic surgical skill acquisition over a single training session. In accordance with the hypothesis, effects of tDCS on acquisition of primarily unimanual pattern cutting were demonstrated, with possible effects on bimanual peg transfer skills. The study supports the need for additional trials combining non‐invasive brain stimulation with simulation‐based surgical skill training potentially to enhance procedural skill learning in surgical trainees.
The recent shift to competency‐based medical education has emphasized the development of structured training opportunities and enhanced skill acquisition33. Trainees must now demonstrate competency for required skills specific to their specialty in order to advance to certification. Changes in surgical training curricula involve procedural skill acquisition and the ability to demonstrate competence4 34. Working time restrictions can have major impacts on training times, estimated to be approximately 6 months over a 5‐year residency in North America35. Techniques to enhance acquisition of surgical skills are required to overcome these modern challenges in medical education.
Supplementing operating room practice with SBTT provides trainees with risk‐free opportunities to accelerate their skill learning15 16, but at the cost of additional time and risks of decay or poor translation to the operating room10. Such challenges are important for more complex skills such as laparoscopy18 20, 36. Existing training strategies including motivation, feedback techniques and redistributed practice opportunities have modest effect sizes10 37, 38. Importantly, neuromodulation is compatible, and possibly even synergistic, with such approaches.
The effects of tDCS on primarily unimanual pattern cutting, but not bimanual peg transfer tasks, were somewhat expected and may relate to anodal tDCS application over the dominant primary motor cortex with preferential effects on dominant hand function. Anodal tDCS over the dominant primary motor cortex may enhance the acquisition of purely unimanual simulation‐based tumour resection skill25. The finding of enhanced pattern‐cutting skill acquisition supports these previous findings. In contrast to unimanual skills, the peg transfer task requires approximately equal use of graspers, whereas pattern cutting clearly distinguishes a non‐dominant ‘holder’ hand with the dexterous cutting movements executed by the dominant hand. Despite this, there is evidence that unilateral motor cortex anodal tDCS can improve the function of both hands23 39. Alternate stimulation techniques such as bihemispheric anodal tDCS may yield greater effects on bimanual skills40.
The present study demonstrated significant effects of tDCS on pattern‐cutting scores. Although the reported differences appear small (an approximately 20‐point difference), these may be meaningful improvements. A previous report14 suggested that pattern‐cutting scores of 212 versus 188 distinguished the performance of laparoscopic surgeons versus fifth‐year residents. This parallels the scores of 207·6 versus 186·0 for sham versus tDCS in the present study. Smaller effects were seen for peg transfer, and no participant achieved peg transfer proficiency. It is important to consider that these effects were seen in a single short training session. Multiple days of tDCS‐paired training may lead to larger effects on skill acquisition, particularly on peg transfer, as demonstrated by previous reports of enhanced motor learning22 23. The FLS curriculum suggests a mean number of repetitions required for peg transfer proficiency of 57 (range 26–80). Here, participants performed ten repetitions of the task. Despite the low dose of training, more than one‐third of those receiving tDCS reached 90 per cent proficiency, compared with one in 19 for the sham condition. The relative lack of skill decay from post‐training is encouraging, and might be further improved with higher doses of training. Reports of tDCS‐enhanced skill retention typically apply stimulation over multiple days of training, possibly facilitating retention through consolidation22 23.
The application of anodal tDCS over the primary motor cortex is thought to modulate cortical excitability21. Increased spontaneous neuronal firing and synaptic efficacy induced by direct‐current stimulation may strengthen neuronal connectivity through long‐term potentiation‐like mechanisms41, 42, 43. These mechanisms may potentiate motor learning, which is also thought to depend on long‐term potentiation‐like plasticity occurring in the motor cortex44. Modulation of the motor network, including the primary motor cortex, premotor cortex, supplementary motor areas and basal ganglia, has been implemented in motor learning45 46. Modulated synaptic efficacy43 and cortical connectivity47 between these regions may facilitate tDCS‐enhanced motor learning. It is also important to consider that the tDCS montage applied here involved passing current through a large anode to a large cathode. Two important mechanistic implications arise from this. First, the electric fields induced by tDCS combined with large electrodes are non‐focal, and it is likely that widespread cortical and subcortical stimulation occurred. This stimulation pattern leads to difficulties in drawing conclusions of the brain regions that must be stimulated to enhance skill acquisition. Second, the prefrontal cortex may have reduced cortical excitability, implying that cognitive processing and planning regions may be influenced by the anodal tDCS montage. Error rates (dropping of pegs or off‐target cuts) did not change throughout the training session, suggesting no concerns of reduced function.
The possible integration of tDCS into training curricula and large multicentre trials is feasible. Short courses and tutorial videos48 on the safe application of tDCS are available. Current evidence49 supports the safety of tDCS. In more than 30 000 stimulation sessions of nearly 10 000 subjects there has not been a single serious adverse event, supporting the application of tDCS in training. Sensations associated with tDCS were tolerable and similar between sham and anodal stimulation paradigms, suggesting favourable tolerability and effective blinding concealment. The slight difference in burning sensation suggests cross‐over designs or application to non‐naive tDCS subjects may risk partial awareness of allocation and should be considered in future trials. Simple solutions, such as soaking electrodes in weaker concentrations of saline, have been shown50 to improve sensation tolerability, possibly providing more effective concealment.
This study has limitations. It relates only to simulation‐based training, although the same methodology has demonstrated transferability to real surgical skill15 16. Skill acquisition greatly benefits from instructor feedback, and combining tDCS with more formal feedback methods requires further study. The sample consisted of medical students rather than surgical trainees. Senior residents and fellows typically possess greater skill, and whether tDCS can enhance skill acquisition at higher levels of training remains unknown. The effects of tDCS were examined only from a single training session, whereas surgical skill training occurs over years, with repetition. tDCS motor learning studies have typically demonstrated larger effect sizes over multiple days of training. The application of tDCS throughout an intensive skill‐based curriculum may yield larger effects.
This preliminary investigation provides evidence of feasibility and possible efficacy, and future studies examining the effects of tDCS on surgical training across procedures, specialties and levels of training appear warranted.
Supporting information
Fig. S1. Example of the transcranial direct‐current stimulation setup for a right‐hand‐dominant participant. The anode (blue sponge, red cable) is positioned on the scalp overlying the approximate area of the left primary motor cortex. The cathode (yellow sponge, black cable) is positioned on the right supraorbital area. The sponges are held in place by a plastic head strap
Acknowledgements
This study was funded by the Canadian Institutes of Health Research (grant number 10012121). The funder played no role in the study, and the authors had complete access to the study data.
Disclosure: The authors declare no conflict of interest.
Funding information
Canadian Institutes of Health Research, 10012121
References
- 1. Rose J, Weiser TG, Hider P, Wilson L, Gruen RL, Bickler SW. Estimated need for surgery worldwide based on prevalence of diseases: a modelling strategy for the WHO Global Health Estimate. Lancet Glob Health 2015; 3(Suppl 2): S13−S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coleman JJ, Esposito TJ, Rozycki GS, Feliciano DV. Early subspecialization and perceived competence in surgical training: are residents ready? J Am Coll Surg 2013; 216: 764−771. [DOI] [PubMed] [Google Scholar]
- 3. Mattar SG, Alseidi AA, Jones DB, Jeyarajah DR, Swanstrom LL, Aye RW et al General surgery residency inadequately prepares trainees for fellowship: results of a survey of fellowship program directors. Ann Surg 2013; 258: 440–449. [DOI] [PubMed] [Google Scholar]
- 4. Lewis FR, Klingensmith ME. Issues in general surgery residency training – 2012. Ann Surg 2012; 256: 553–559. [DOI] [PubMed] [Google Scholar]
- 5. Ahmed N, Devitt KS, Keshet I, Spicer J, Imrie K, Feldman L et al A systematic review of the effects of resident duty hour restrictions in surgery: impact on resident wellness, training, and patient outcomes. Ann Surg 2014; 259: 1041–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jamal MH, SAR Doi, Rousseau M, Edwards M, Rao C, Barendregt JJ et al Systematic review and meta‐analysis of the effect of North American working hours restrictions on mortality and morbidity in surgical patients. Br J Surg 2012; 99: 336–344. [DOI] [PubMed] [Google Scholar]
- 7. Dumont TM, Rughani AI, Penar PL, Horgan MA, Tranmer BI, Jewell RP. Increased rate of complications on a neurological surgery service after implementation of the Accreditation Council for Graduate Medical Education work‐hour restriction. J Neurosurg 2012; 116: 483–486. [DOI] [PubMed] [Google Scholar]
- 8. Hoh BL, Neal DW, Kleinhenz DT, Hoh DJ, Mocco J, Barker FG. Higher complications and no improvement in mortality in the ACGME resident duty‐hour restriction era: an analysis of more than 107 000 neurosurgical trauma patients in the Nationwide Inpatient Sample database. Neurosurgery 2012; 70: 1369−1381. [DOI] [PubMed] [Google Scholar]
- 9. Poulose BK, Ray WA, Arbogast PG, Needleman J, Buerhaus PI, Griffin MR et al Resident work hour limits and patient safety. Ann Surg 2005; 241: 847−856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cook DA, Hatala R, Brydges R, Zendejas B, Szostek JH, Wang AT et al Technology‐enhanced simulation for health professions education: a systematic review and meta‐analysis. JAMA 2011; 306: 978–988. [DOI] [PubMed] [Google Scholar]
- 11. Cheng A, Lang TR, Starr SR, Pusic M, Cook DA. Technology‐enhanced simulation and pediatric education: a meta‐analysis. Pediatrics 2014; 133: e1313−e1323. [DOI] [PubMed] [Google Scholar]
- 12. Ding J, Xia Y, Liao G, Zhang Z, Liu S, Zhang Y et al Hand‐assisted laparoscopic surgery versus open surgery for colorectal disease: a systematic review and meta‐analysis. Am J Surg 2014; 207: 109–119. [DOI] [PubMed] [Google Scholar]
- 13. Madan AK, Frantzides CT, Park WC, Tebbit CL, Kumari NVA, O'Leary PJ. Predicting baseline laparoscopic surgery skills. Surg Endosc 2005; 19: 101–104. [DOI] [PubMed] [Google Scholar]
- 14. Derossis AM, Fried GM, Abrahamowicz M, Sigman HH, Barkun JS, Meakins JL. Development of a model for training and evaluation of laparoscopic skills. Am J Surg 1998; 175: 482–487. [DOI] [PubMed] [Google Scholar]
- 15. Sroka G, Feldman LS, Vassiliou MC, Kaneva PA, Fayez R, Fried GM. Fundamentals of laparoscopic surgery simulator training to proficiency improves laparoscopic performance in the operating room – a randomized controlled trial. Am J Surg 2010; 199: 115–120. [DOI] [PubMed] [Google Scholar]
- 16. Steigerwald SN, Park J, Hardy KM, Gillman LM, Vergis AS. Does laparoscopic simulation predict intraoperative performance? A comparison between the Fundamentals of Laparoscopic Surgery and LapVR evaluation metrics. Am J Surg 2015; 209: 34–39. [DOI] [PubMed] [Google Scholar]
- 17. Ellis SM, Varley M, Howell S, Trochsler M, Maddern G, Hewett P et al Acquisition and retention of laparoscopic skills is different comparing conventional laparoscopic and single‐incision laparoscopic surgery: a single‐centre, prospective randomized study. Surg Endosc 2016; 30: 3386–3390. [DOI] [PubMed] [Google Scholar]
- 18. Bonrath EM, Weber BK, Fritz M, Mees ST, Wolters HH, Senninger N et al Laparoscopic simulation training: testing for skill acquisition and retention. Surgery 2012; 152: 12–20. [DOI] [PubMed] [Google Scholar]
- 19. Varley M, Choi R, Kuan K, Bhardwaj N, Trochsler M, Maddern G et al Prospective randomized assessment of acquisition and retention of SILS skills after simulation training. Surg Endosc 2015; 29: 113–118. [DOI] [PubMed] [Google Scholar]
- 20. Rivard JD, Vergis AS, Unger BJ, Gillman LM, Hardy KM, Park J. The effect of blocked versus random task practice schedules on the acquisition and retention of surgical skills. Am J Surg 2015; 209: 93–100. [DOI] [PubMed] [Google Scholar]
- 21. Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000; 527: 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E et al Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A 2009; 106: 1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ciechanski P, Kirton A. Transcranial direct‐current stimulation can enhance motor learning in children. Cereb Cortex 2017; 27: 2758–2767. [DOI] [PubMed] [Google Scholar]
- 24. Dayan E, Censor N, Buch ER, Sandrini M, Cohen LG. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat Neurosci 2013; 16: 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ciechanski P, Cheng A, Lopushinsky S, Hecker K, Gan LS, Lang S et al Effects of transcranial direct‐current stimulation on neurosurgical skill acquisition: a randomized controlled trial. World Neurosurg 2017; 108: 876–884.e4. [DOI] [PubMed] [Google Scholar]
- 26. Buch ER, Santarnecchi E, Antal A, Born J, Celnik PA, Classen J et al Effects of tDCS on motor learning and memory formation: a consensus and critical position paper. Clin Neurophysiol 2017; 128: 589–603. [DOI] [PubMed] [Google Scholar]
- 27. Stanne MB, Johnson DW, Johnson RT. Does competition enhance or inhibit motor performance: a meta‐analysis. Psychol Bull 1999; 125: 133–154. [DOI] [PubMed] [Google Scholar]
- 28. Ritter EM, Scott DJ. Design of a proficiency‐based skills training curriculum for the fundamentals of laparoscopic surgery. Surg Innov 2007; 14: 107–112. [DOI] [PubMed] [Google Scholar]
- 29. Tiffin J, Asher EJ. The Purdue Pegboard: norms and studies of reliability and validity. J Appl Psychol 1948; 32: 234–247. [DOI] [PubMed] [Google Scholar]
- 30. Goldin SB, Horn GT, Schnaus MJ, Grichanik M, Ducey AJ, Nofsinger C et al FLS skill acquisition: a comparison of blocked vs interleaved practice. J Surg Educ 2014; 71: 506–512. [DOI] [PubMed] [Google Scholar]
- 31. Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P et al A technical guide to tDCS, and related non‐invasive brain stimulation tools. Clin Neurophysiol 2016; 127: 1031–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ambrus GG, Al‐Moyed H, Chaieb L, Sarp L, Antal A, Paulus W. The fade‐in – short stimulation – fade out approach to sham tDCS – reliable at 1 mA for naïve and experienced subjects, but not investigators. Brain Stimul 2012; 5: 499–504. [DOI] [PubMed] [Google Scholar]
- 33. Frank JR, Snell LS, Cate OT, Holmboe ES, Carraccio C, Swing SR et al Competency‐based medical education: theory to practice. Med Teach 2010; 32: 638–645. [DOI] [PubMed] [Google Scholar]
- 34. Klingensmith ME, Lewis FR. General surgery residency training issues. Adv Surg 2013; 47: 251–270. [DOI] [PubMed] [Google Scholar]
- 35. Nasca TJ, Day SH, Amis ES Jr; ACGME Duty Hour Task Force . The new recommendations on duty hours from the ACGME Task Force. N Engl J Med 2010; 363: e3. [DOI] [PubMed] [Google Scholar]
- 36. Akdemir A, Zeybek B, Ergenoglu AM, Yeniel AO, Sendag F. Effect of spaced training with a box trainer on the acquisition and retention of basic laparoscopic skills. Int J Gynaecol Obstet 2014; 127: 309–313. [DOI] [PubMed] [Google Scholar]
- 37. Zendejas B, Brydges R, Hamstra SJ, Cook DA. State of the evidence on simulation‐based training for laparoscopic surgery: a systematic review. Ann Surg 2013; 257: 586–593. [DOI] [PubMed] [Google Scholar]
- 38. Stefanidis D. Optimal acquisition and assessment of proficiency on simulators in surgery. Surg Clin North Am 2010; 90: 475–489. [DOI] [PubMed] [Google Scholar]
- 39. von Rein E, Hoff M, Kaminski E, Sehm B, Steele CJ, Villringer A et al Improving motor performance without training: the effect of combining mirror visual feedback with transcranial direct current stimulation. J Neurophysiol 2015; 113: 2383–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gomes‐Osman J, Field‐Fote EC. Bihemispheric anodal corticomotor stimulation using transcranial direct current stimulation improves bimanual typing task performance. J Mot Behav 2013; 45: 361–367. [DOI] [PubMed] [Google Scholar]
- 41. Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist 2011; 17: 37–53. [DOI] [PubMed] [Google Scholar]
- 42. Bindman LJ, Lippold OC, Redfearn JW. Long‐lasting changes in the level of the electrical activity of the cerebral cortex produced by polarizing currents. Nature 1962; 196: 584–585. [DOI] [PubMed] [Google Scholar]
- 43. Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG et al Direct current stimulation promotes BDNF‐dependent synaptic plasticity: potential implications for motor learning. Neuron 2010; 66: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rioult‐Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci 1998; 1: 230–234. [DOI] [PubMed] [Google Scholar]
- 45. Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem 2002; 78: 553–564. [DOI] [PubMed] [Google Scholar]
- 46. Sampaio‐Baptista C, Filippini N, Stagg CJ, Near J, Scholz J, Johansen‐Berg H. Changes in functional connectivity and GABA levels with long‐term motor learning. Neuroimage 2015; 106: 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu J, Srinivasan R, Kaur A, Cramer SC. Resting‐state cortical connectivity predicts motor skill acquisition. Neuroimage 2014; 91: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. DaSilva AF, Volz MS, Bikson M, Fregni F. Electrode positioning and montage in transcranial direct current stimulation. J Vis Exp 2011; 51: pii: 2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T et al Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul 2016; 9: 641–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dundas JE, Thickbroom GW, Mastaglia FL. Perception of comfort during transcranial DC stimulation: effect of NaCl solution concentration applied to sponge electrodes. Clin Neurophysiol 2007; 118: 1166–1170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Example of the transcranial direct‐current stimulation setup for a right‐hand‐dominant participant. The anode (blue sponge, red cable) is positioned on the scalp overlying the approximate area of the left primary motor cortex. The cathode (yellow sponge, black cable) is positioned on the right supraorbital area. The sponges are held in place by a plastic head strap


