Abstract
Rationale
(R,S)-ketamine is a rapid and effective antidepressant drug that produces a response in two thirds of patients with treatment-resistant depression (TRD). The underlying biochemical differences between a (R,S)-ketamine responder (KET-R) and non-responder (KET-NR) have not been definitively identified but may involve serine metabolism.
Objectives
The aim of the study was to examine the relationship between baseline plasma concentrations of D-serine and its precursor L-serine and antidepressant response to (R,S)-ketamine in TRD patients.
Methods
Plasma samples were obtained from 21 TRD patients at baseline, 60 min before initiation of the (R,S)-ketamine infusion. Patients were classified as KET-Rs (n=8) or KET-NRs (n=13) based upon the difference in Montgomery–Åsberg Depression Rating Scale (MADRS) scores at baseline and 230 min after infusion, with response defined as a ≥50 % decrease in MADRS score. The plasma concentrations of D-serine and L-serine were determined using liquid chromatography-mass spectrometry.
Results
Baseline D-serine plasma concentrations were significantly lower in KET-Rs (3.02±0.21 μM) than in KET-NRs (4.68±0.81 μM), p<0.001. A significant relationship between baseline D-serine plasma concentrations and percent change in MADRS at 230 min was determined using a Pearson correlation, r=0.77, p<0.001, with baseline D-serine explaining 60 % of the variance in (R,S)-ketamine response. The baseline concentrations of L-serine (L-Ser) in KET-Rs were also significantly lower than those measured in KET-NRs (66.2 ± 9.6 μM vs 242.9 ± 5.6 μM, respectively; p<0.0001).
Conclusions
The results demonstrate that the baseline D-serine plasma concentrations were significantly lower in KET-Rs than in KET-NRs and suggest that this variable can be used to predict an antidepressant response following (R,S)-ketamine administration.
Keywords: (R,S)-ketamine; Antidepressant; Treatment-resistant depression; Bipolar depression; D-serine; N-methyl-D-aspartate receptor; Serine racemase; Plasma response marker
Introduction
A substantial number of patients with major depressive disorder (MDD) and bipolar depression (BD) do not respond to current antidepressant therapy and, for those who do respond, full antidepressant effects typically take 6 weeks or more (Machado-Vieira et al. 2008). This lag of therapeutic effects is associated with considerable morbidity and the risk of suicidal behavior. These limitations have prompted the exploration for new therapeutic targets with the hopes of developing rapid and more effective treatments for patients with treatment-resistant depression (TRD) who fail standard antidepressant therapy. One new class of antidepressant agents is glutamatergic modulators such as (R,S)-ketamine (KET) (Browne and Lucki 2013; Niciu et al. 2014a). KET was developed as an anesthetic agent based upon its non-competitive inhibition of the N-methyl-D-aspartate (NMDA) receptor. However, recent studies have demonstrated that a single subanesthetic dose of KET produces a rapid antidepressant response in two thirds of TRD and BD patients, which can last 7 days or more (Zarate et al. 2012; Murrough et al. 2013).
The underlying biochemical pathways producing the antidepressant effects as well as the basis for the differences between patients who respond to KET (KET-Rs) and those who do not respond (KET-NRs) have not been definitively identified. We have recently reported the results from a plasma metabolomics study in 22 BD patients that suggested that differences in the basal mitochondrial β-oxidation of fatty acids was a contributing factor to the antidepressant response or non-response to KET (Villaseñor et al. 2014). The results also demonstrated that the average plasma concentration of D-serine (D-Ser) was significantly greater in KET-NRs compared to that in KET-Rs (means of 3.7 μM±24.1 % vs 2.9 μM±19.0 % R.S.D., respectively; p<0.05) and that the average L-serine (L-Ser) plasma concentration in KET-NRs was higher than that in KET-Rs, although the difference did not reach significance (unpublished data). These were intriguing observations as D-Ser is an NMDA receptor co-agonist for which this synaptic receptor has a preferential affinity relative to glycine and which plays a key role in long-term potentiation and NMDA-induced neurotoxicity (Henneberger et al. 2010; Papouin et al. 2012). In addition, recent studies have demonstrated a link between endogenous D-Ser plasma levels and CNS diseases and pathological states (Jiraskova-Vanickova et al. 2011).
The data from the plasma metabolomics study could not be used to ascertain the association between plasma concentrations of D-Ser and the antidepressant response to KET because baseline plasma concentrations of D-Ser were not available and the patients were maintained on mood stabilizers. Thus, it could not be determined if the difference in D-Ser plasma concentrations between KET-Rs and KET-NRs reflected baseline concentrations, differential responses to KET and/or the mood stabilizers or a combination of these variables. The present study addresses these issues by examining the relationship between D-Ser plasma concentrations and the antidepressant response to KET in TRD patients who were drug-free at the time of the study (Zarate et al. 2012). The aims of the current study were as follows: (1) to characterize the relationship between baseline D-Ser and L-Ser plasma levels in KET-Rs and KET-NRs and the anti-depressant response produced by KET, (2) to determine the effect of KET administration on D-Ser and L-Ser plasma concentrations in KET-Rs and KET-NRs, and (3) to characterize the relationship between D-Ser plasma concentrations and changes in the Clinician Administered Dissociative States Scale (CADSS) scores in KET-Rs and KET-NRs.
Materials and methods
Clinical studies
All subjects were inpatients at the Clinical Research Center (CRC) of the National Institute of Mental Health (NIMH) in Bethesda, Maryland, throughout the entire study, and the Combined Neuroscience Institutional Review Board of the National Institutes of Health (NIH) approved the study. All subjects provided written informed consent before entry into the study and were assigned a clinical research advocate from the NIMH Human Subjects Protection Unit to monitor the consent process and research participation throughout the study. The full details of the study have been previously published (Zarate et al. 2012). In brief,
Men and women aged 18–65 years were eligible to participate if they had a diagnosis of recurrent major depressive disorder without psychotic features, as diagnosed using the Structured Clinical Interview for Axis I DSM-IV Disorders—Patient Version (Zarate et al. 2012). The subjects had previously failed at least two adequate antidepressant trials and were currently experiencing a major depressive episode of at least 4 weeks in duration. Patients with significant suicidal risk at screening were excluded from the study. All medications were tapered off for the purpose of the study, and the subjects were free of co-morbid substance abuse or dependence (excluding nicotine or caffeine) for at least 3 months and had a negative urine toxic screen on admission. Subjects were required to have a score of ≥22 on the Montgomery–Åsberg Depression Rating Scale (MADRS) at screening and on the day of KET infusion (baseline), with no greater than a 25 % decrease inMADRS total score between these two time points.
All subjects were in good physical health as determined by medical history, physical examination, blood labs, electrocardiogram (ECG), chest X-ray, urinalysis, and toxicology screen. Comorbid axis I anxiety disorder diagnoses were permitted. Exclusion criteria included any serious unstable medical disorder or condition, previous use of KET, or concomitant treatment with psychotropic medications or ECT in the 2 weeks prior to KET infusion (5 weeks for fluoxetine); in addition, female subjects could not be pregnant or nursing.
The patients received a 40-min infusion of 50 ml of a 0.5mg/kg dose of KET initiated at ~9:00 a.m. Plasma samples were collected prior to the initiation of the infusion, at 40 (end of the infusion), 80, 110, and 230 min and on days 1, 2, and 3 post-infusion, and frozen at −80 °C until analysis.
Analysis of D-Ser and L-Ser in plasma
The plasma concentrations of D-Ser and L-Ser were determined using a previously reported validated assay employing liquid chromatography with mass spectrometric detection (Xie et al. 2014). In brief,
Instrumentation
The chromatographic experiments were carried out on a Shimadzu Prominence HPLC system (Shimadzu, Columbia, MD, USA). The samples were introduced to the analytical column using Shimadzu SIL-20A autosampler and maintained at 4 °C in the autosampler tray, and injections of 20 μl were made. The samples were run on a 5500 QTRAP triple quadruple mass spectrometer equipped with a Turbo V electrospray ionization source (AB Sciex, Concord, ON, Canada).
Plasma samples
Plasma samples (100 μl) were combined with a 20-μl aliquot of the internal standard D-arginine (10 nmol/ml in acetone) and 400 μl acetone and then centrifuged at 13,000×g for 10 min at 4 °C. A 400-μl aliquot of the supernatant was subsequently derivatized with 300 μl (R)-1-Boc-2-piperidinecarbonyl chloride, the solution evaporated to dryness and the residue dissolved in 100 μl of methanol/water (10:90, v/v) and transferred to the autosampler for analysis.
Chromatographic conditions
Chromatographic separation was achieved on a Zorbax Eclipse XDB-C18 column (4.6 mm×150 mm, 5 μm; Agilent Technologies, Santa Clara, CA, USA) protected with an Agilent C18 guard column at room temperature. The mobile phase consisted of water with 0.3 % trifluoroacetic acid (TFA) (elute A) and methanol with 0.3 % TFA (elute B). The gradient eluent at a flow rate of 0.4 ml/min was programmed as follows: 0–15 min, 5–9 % B; 15–22 min, 15%B; and 22–25 min, 5%B. The total run time was 25 min and the injection volume per sample was 20 μl.
Mass spectrometry conditions
Tandem mass spectrometry (MS/MS) analysis was performed using a triple quadrupole mass spectrometer model API 5500Q system from Applied Biosystems/MDS Sciex equipped with Turbo V electrospray ionization source (TIS)® (Applied Biosystems, Foster City, CA, USA). The data was acquired and analyzed using Analyst version 1.5.1 (Applied Biosystems). Positive electrospray ionization data were acquired using multiple reaction monitoring (MRM). The TIS instrumental source settings for temperature, curtain gas, ion source gas 1 (nebulizer), ion source gas 2 (turbo ion spray), collision energy, and ion spray voltage were 550 °C, 20 psi, 45 psi, 80 psi, 15 V, and 4,500 V, respectively. The TIS compound parameter settings for declustering potential, entrance potential, and collision cell exit potential were 80, 10, and 10 V, respectively. The standards were characterized using the following MRM ion transitions: D-Ser and L-Ser derivatization products (m/z 231.5 to 106.1) and D-Arg derivatization product (m/z 300.4 to 175.0).
Statistical analysis
To compare responder and non-responder groups on demographic variables, t tests were used with continuous measures and chi-squares were used with categorical measures. A Pearson correlation was used to examine the relationship between baseline D-Ser levels and percent change in depression severity. Linear mixed models with restricted maximum likelihood estimation and compound symmetry covariance structures were used to examine D-Ser over time comparing KET-NR and KET-R groups. An initial model included baseline as a time point to compare groups at baseline and examine changes from baseline in each group. A second model included baseline as a covariate and examined response group differences. Bonferroni post hoc tests were used to make specific comparisons and two-tailed p<0.05 was used for significance.
Results
Demographics and clinical characteristics
Plasma samples from 21 TRD patients, 13 men and 8 women, who had participated in a single open-label study of the efficacy of KET were utilized in this study (Table 1) (Zarate et al. 2012). The patients were classified as KET-Rs (n=8) or KET-NRs (n=13) based upon the difference in their scores on the MADRS obtained 60 min before the initiation of the KET infusion and at 230 min after receiving a 40-min intravenous infusion of 0.5 mg/kg KET. Response was defined as a ≥50% decrease in MADRS score.
Table 1.
The demographics of the patients included in the current study and the data obtained from diagnostic tests
| Non-responders | Responders | Total | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| (n=13) | (n=8) | |||||||
| N | % | N | % | N | % | χ2 | p | |
| Gender (male) | 7 | 54 | 6 | 46 | 13 | 62 | 0.94 | .33 |
| ETOH abuse or dependence | 4 | 31 | 3 | 38 | 7 | 33 | 0.10 | .75 |
| Prior hospitalization | 9 | 69 | 5 | 63 | 14 | 67 | 0.10 | .75 |
| Smoking (current) | 2 | 15 | 2 | 25 | 4 | 19 | 0.30 | .59 |
| Prior suicide attempt | 5 | 39 | 2 | 25 | 7 | 33 | 0.40 | .53 |
| Mean | SD | Mean | SD | Mean | SD | t | p | |
| Age (years) | 44.23 | 13.69 | 52.00 | 10.77 | 46.59 | 12.96 | 1.36 | .19 |
| Age of onset (years) | 18.38 | 11.70 | 25.25 | 11.77 | 21.00 | 11.93 | 1.30 | .21 |
| BMI | 26.74 | 5.05 | 33.88 | 4.16 | 29.46 | 5.83 | 3.35 | .003 |
| Montgomery-Asberg Depression Rating Scale | 34.08 | 5.98 | 30.50 | 4.69 | 32.71 | 5.69 | 1.44 | .17 |
| Beck Depression Inventory | 26.69 | 8.25 | 26.25 | 6.99 | 26.52 | 7.61 | 0.13 | .90 |
| Hamilton Depression Rating Scale | 21.31 | 4.54 | 20.00 | 4.63 | 20.81 | 4.50 | 0.64 | .53 |
| Clinician Administered Dissociative States Scale | 2.38 | 3.04 | 3.00 | 2.00 | 2.62 | 2.66 | 0.51 | .62 |
| Scale for suicide ideation | 3.85 | 6.78 | 1.75 | 2.44 | 3.05 | 5.55 | 0.84 | 0.41 |
| Young Mania Scale | 3.85 | 1.68 | 5.50 | 2.98 | 4.48 | 2.34 | 1.64 | 0.12 |
The patients were categorized as non-responders and responders as described in the text
D-Ser and L-Ser baseline plasma concentrations
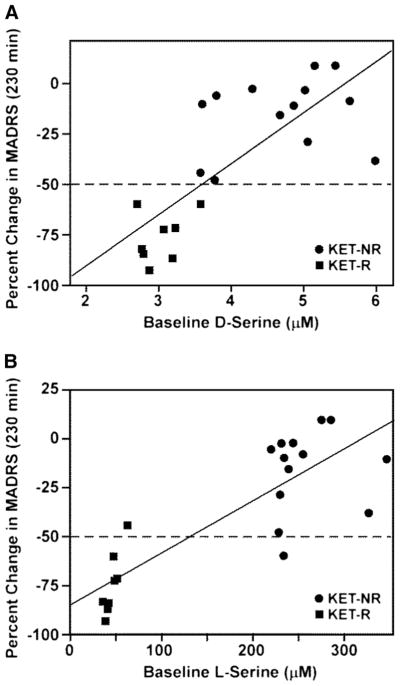
Plasma concentrations of D-Ser and L-Ser in KET-Rs and KET-NRs were determined in samples obtained 60 min before initiation of the KET infusion (baseline), and representative chromatograms are presented in Fig. 1a, b. The average baseline D-Ser plasma concentration for the KET-NRs was 4.68±0.81 μM, which was higher than the levels we determined in healthy controls using this assay, 2.61±0.74 μM (Xie et al. 2014), as well as previously reported values for healthy controls and patients with MDD, 2.06±0.05 to 2.32±0.92 μM, respectively (Hashimoto et al. 2003; Mitani et al. 2006; Alexander et al. 2013). On the other hand, the average D-Ser plasma concentration of the 8 KET-Rs was 3.02±0.21 μM, similar to previously reported values for MDD patients. The average baseline L-Ser plasma concentration for all 21 patients in the study was 175.6±109.7 μM, which was within the range of concentrations reported in other studies, 107.8±3.5 to 175.0±30.6 μM (Hashimoto et al. 2003; Mitani et al. 2006; Alexander et al. 2013). The baseline concentration of D-Ser measured in KET-Rs (3.02±0.04 μM) was significantly lower than that in KET-NRs (4.68±0.07 μM), p<0.0001 (Table 2 and Fig. 1c). The baseline concentration of L-Ser in KET-Rs (66.2±9.6 μM) was also significantly lower than that in KET-NRs (242.9±5.6 μM), p<0.0001 (Table 2).
Fig. 1.
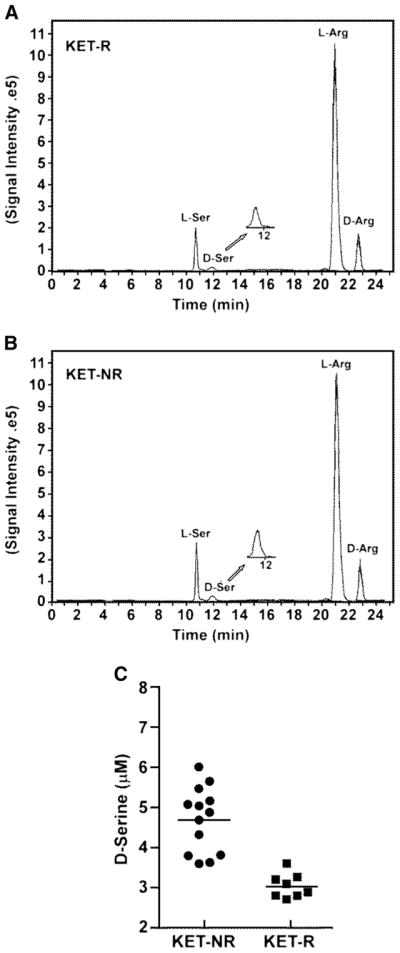
Baseline plasma levels of D-serine (D-Ser) and L-serine (L-Ser) in TRD patients prior to receiving a 40-min intravenous infusion of 0.5 mg/kg (R,S)-ketamine determined using liquid chromatography with mass spectrometric detection with D-arginine (D-Arg) as the internal standard; see text for details. Patients were classified as responding to (R,S)-ketamine (KET-Rs) or non-responders (KET-NRs) based upon the difference in Montgomery–Åsberg Depression Rating Scale (MADRS) scores at baseline and 230 min after infusion, with response defined as a ≥50 % decrease in MADRS score. a Representative chromatogram from the baseline plasma sample obtained from a KET-R in which the measured plasma concentrations of D-Ser and L-Ser are 2.76 and 34.6 μM, respectively. b Representative chromatogram from the baseline plasma sample obtained from a KET-NR in which the measured plasma concentrations of D-serine and L-serine are 5.44 and 273.6 μM, respectively. c Baseline D-Ser concentration by response group, where squares denote KET-Rs and circles denote KET-NRs
Table 2.
Baseline plasma concentrations (μM) of D-serine and L-serine in MDD patients classified as responders (Rs) or non-responders (NRs) to treatment with (R,S)-ketamine
| Patient no. | D-Ser | Rs L-Ser |
Patient no. | D-Ser | NRs L-Ser |
|---|---|---|---|---|---|
| 1 | 2.77 | 34.6 | 9 | 5.44 | 273.6 |
| 2 | 2.86 | 37.0 | 10 | 5.98 | 325.4 |
| 3 | 2.78 | 40.4 | 11 | 4.86 | 345.1 |
| 4 | 3.18 | 40.1 | 12 | 5.15 | 282.6 |
| 5 | 2.69 | 46.5 | 13 | 5.01 | 229.8 |
| 6 | 3.07 | 48.3 | 14 | 4.29 | 242.9 |
| 7 | 3.22 | 50.6 | 15 | 4.67 | 237.7 |
| 8 | 3.57 | 232.1 | 16 | 3.79 | 217.8 |
| 17 | 5.05 | 228.3 | |||
| 18 | 5.63 | 253.5 | |||
| 19 | 3.77 | 226.4 | |||
| 20 | 3.60 | 233.5 | |||
| 21 | 3.57 | 61.4 | |||
| Average | 3.02* | 66.2* | 4.68 | 242.9 | |
| SD | 0.30 | 62.8 | 0.81 | 67.2 | |
| SE | 0.04 | 9.6 | 0.07 | 5.6 |
The data are presented as average, standard deviation (SD) and standard error (SE). The data from Rs were compared to the corresponding data from NRs using an unpaired Student’s t test (two-tailed). See text for experimental details
p<0.001
Relationship between baseline D-Ser and L-Ser concentrations and KET-related changes in MADRS scores
A significant relationship between baseline D-Ser plasma concentrations and percent change in MADRS scores at 230 min was determined using a Pearson correlation, r= 0.77, p<0.001 (Fig. 2a). Prior analysis of various predictors of the KET response indicated that body mass index (BMI) was a significant factor (Niciu et al. 2014a). When BMI and baseline D-Ser plasma concentration are included in a linear regression model, D-Ser remains a significant predictor (standardized beta=0.65, p=0.002), but BMI does not (standardized beta=−0.22, p=0.24) (Table 3). Baseline D-Ser appears to explain 60 % of the variance in KET response, while the two factors together explained a total of 63 % of the variance.
Fig. 2.
Relationship between plasma levels of D-serine (μM) and L-serine (μM) at baseline and percent change in MADRS from baseline to 230 min post-(R,S)-ketamine infusion, where squares denote KET-Rs and circles denote KET-NRs. a Relationship between plasma levels of D-serine (μM) and percent change in MADRS; b relationship between plasma levels of L-serine (μM) at baseline and percent change in MADRS
Table 3.
BMI and baseline D-Ser plasma concentration as predictors of KET
| Model | Variables | R2 | R2 Change | F (for Change) | df | p (for Change) | |
|---|---|---|---|---|---|---|---|
| A | 1 | BMI | 0.353 | 0.353 | 10.35 | 1,19 | 0.005 |
| 2 | BMI, baseline D-serine | 0.626 | 0.274 | 13.18 | 1,18 | 0.002 | |
| B | 1 | Baseline D-serine | 0.596 | 0.596 | 28.01 | 1,19 | <.001 |
| 2 | Baseline D-serine, BMI | 0.626 | 0.030 | 1.47 | 1,18 | 0.242 |
A: BMI is a significant predictor of outcome (model 1). In addition, D-serinemakes a significant addition to the variance explained by BMI (model 2). B: D-serine is a significant predictor (model 1), but there is no additional variance explained by BMI (model 2)
A significant relationship between baseline L-Ser plasma concentrations and percent change in MADRS scores at 230 min was determined using a Pearson correlation, r= 0.83, p<0.001 (Fig. 2b). When BMI and baseline L-Ser plasma concentration are included in a linear regression model, L-Ser remains a significant predictor (standardized beta= 0.70, p<0.001) and BMI is also significant (standardized beta=−0.28, p=0.04) (Supplementary Table S1). Baseline L-Ser appears to explain 69 % of the variance in KET response, while the two factors together explained a total of 75 % of the variance.
Of note, baseline D-Ser and D,L-Ser plasma concentrations are strongly related when explaining variance in the antidepressant response (r=0.86, p<0.001); however, they are not additive as they explain overlapping variance.
Effect of KET on D-Ser and L-Ser plasma concentrations
Plasma concentrations of D-Ser and L-Ser in KET-Rs and KET-NRs were determined at baseline and serially up to 21 days post-administration. Samples were obtained from all of the KET-Rs during the sampling period and from all 13 KET-NR patients through day 1, but only eight samples were obtained from this subgroup on day 21. The administration of KET had no effect on the baseline plasma concentration of L-Ser, and there were no significant changes in this parameter throughout the study (data not shown).
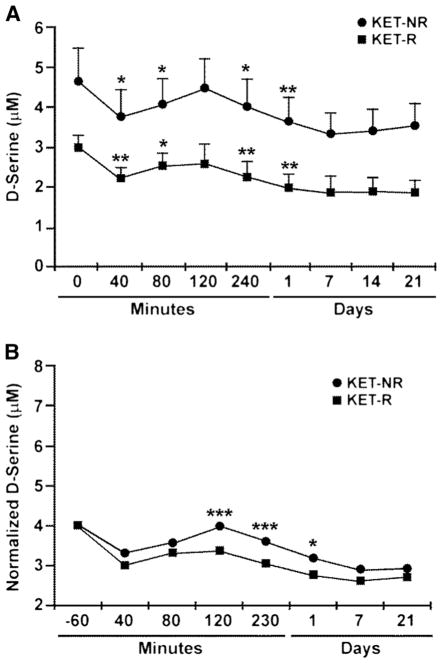
The administration of KET affected the plasma concentrations of D-Ser, which ranged from 3.02±0.21 μM (baseline) to 1.89±0.28 μM (day 21) in KET-Rs and from 4.68±0.81 μM (baseline) to 3.56±0.55 μM (day 21) in KET-NRs (Fig. 3a). D-Ser plasma concentrations were significantly decreased at the end of the KET infusion in both KET-Rs and KET-NRs by 26 and 19 %, respectively (Fig. 3a; p<0.001). The effect was attenuated from the 40-min to the 120-min sampling point, at which time the D-Ser concentrations returned to baseline levels for 25 % of the KET-Rs and 8 % of the KET-NRs and the magnitude of the effect decreased to 13 and 4 % below baseline, respectively. D-Ser concentrations declined after the 120-min post-infusion; maximum effect was observed on day 7 with relative decreases of 39 % (KET-Rs) and 28 % (KET-NRs) compared to baseline. The effect of KET on D-Ser plasma concentrations was examined using a secondary mixed model controlled for baseline D-Ser levels (Fig. 3b). This model had a significant “group×time” interaction (F=2.58, df=6.90, p=0.02) and indicated significantly lower D-Ser plasma concentrations in KET-Rs at 120 min (p<0.001), 230 min (p<0.001), and day 1 (p=0.01).
Fig. 3.
The effect of a 40-min intravenous infusion of 0.5 mg/kg (R,S)-ketamine on the plasma concentration of D-serine (μM) in MDD patients determined from baseline to 21 days post-infusion. Patients were classified as responding to (R,S)-ketamine (KET-Rs) or non-responders (KET-NRs) based upon the difference in Montgomery–Åsberg Depression Rating Scale (MADRS) scores at baseline and 230 min after infusion, with response defined as a ≥50 % decrease in MADRS score. Linear mixed models with restricted maximum likelihood estimation and compound symmetry covariance structure were used to examine D-serine levels over time by response group, where squares denote KET-Rs and circles denote KET-NRs. The initial model (a) used baseline as a separate time point and the second (b) used baseline as a covariate. Bonferroni post hoc tests were used to compare response groups at individual time points, with ***p<0.005, **p<0.01, and *p<0.05
Basal D-Ser plasma concentration and KET-induced changes in CADSS scores
The relationship between the administration of KET and changes in CADSS scores was examined using a linear mixed model. The results demonstrate that at the end of the KET infusion, i.e., the 40-min time point, CADSS scores were elevated in both KET-Rs (p<0.001) and KET-NRs (p<0.001) (Fig. 4). When baseline scores were considered, the elevation in CADSS scores observed in KET-Rs was significantly greater than the scores in KET-NRs (p<0.001), and a negative correlation was observed between baseline D-Ser levels and increased scores at 40 min, r=−0.52, p=0.02. The CADSS scores returned to baseline at the 80-min time point for both groups and remained at baseline for the remainder of the study (Fig. 4).
Fig. 4.
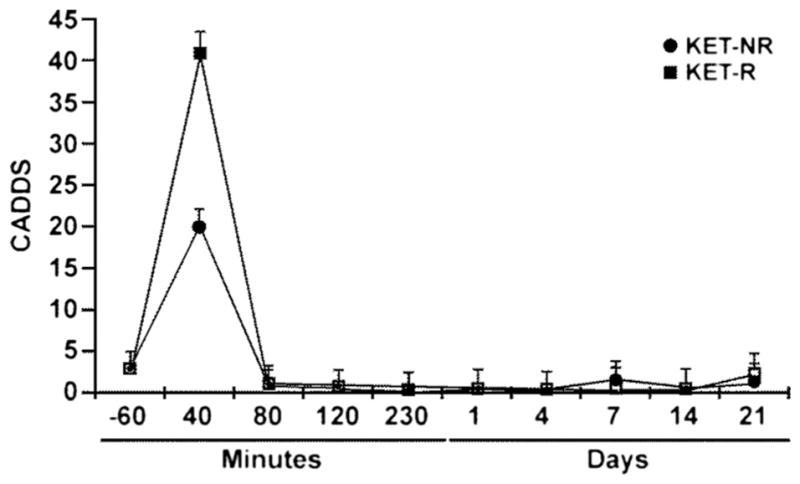
Changes in the average Clinician Administered Dissociative States Scale (CADSS) scores over time in MDD patients classified as responding to (R,S)-ketamine (squares) or non-responders (circles) measured before administration of (R,S)-ketamine (−60 min) and up to 21 days post-administration
Discussion
The data from this study demonstrate that baseline D-Ser and L-Ser plasma concentrations were significantly lower in KET-Rs relative to KET-NRs, suggesting that either D-Ser or L-Ser baseline plasma concentration can be used to predict an antidepressant response following administration of a subanesthetic dose of KET. This observation differs from the data of a previous study using trazodone, a serotonin antagonist and reuptake inhibitor (SARI), as the main antidepressant agent where non-response to antidepressant treatment was characterized by significantly lower D,L-Ser serum levels and that these levels were actually increased by a 5-week administration of the drug. (Maes et al. 1998). The study by Maes et al. (1998) also indicated that low serum levels of L-aspartate, L-asparagine, L-threonine, and taurine also indicated non-response to trazodone therapy, while plasma concentrations of L-glutamate, L-glutamine, and glycine had no association with response. A recent metabolomics-based study of response biomarkers to antidepressant therapy with citalopram and escitalopram, selective serotonin reuptake inhibitors (SSRIs), also demonstrated that non-responders to SSRI treatment had lower serum levels of L-aspartate and L-asparagine relative to responders, while responders had lower plasma levels of glycine and L-glutamate and there were no significant differences in D,L-Ser between responders and non-responders (Ji et al. 2011). In our initial metabolomics study of the antidepressant response to treatment with (R,S)-Ket, we did not observe significant differences in the plasma concentrations of glycine and L-glutamate between KET-Rs and KET-NRs (unpublished data), and the current study did not examine the relationship of these amino acids to the antidepressant response produced by KET. It would be of interest to compare the SARI and SSRI response markers to the antidepressant response to KET therapy in TRD patients and to assess a possible link between baseline plasma concentrations of D-Ser and L-Ser and the antidepressant response as a function of the mode of action of the antidepressant.
It is important to note that the approach used in this study also differed from previous studies of Ser plasma concentrations in MDD and non-depressed controls, which were designed to determine if this parameter could be used as a marker of depressive disease. The results from the previous studies demonstrated that baseline plasma concentrations of D,L-Ser, L-Ser, or D-Ser could not be used to differentiate between MDD patients and non-depressed controls (Altamura et al. 1995; Maes et al. 1998; Mitani et al. 2006). However, L-Ser plasma concentration has been proposed as a biomarker for the severity of depression in MDD patients based on the observation of a significant inverse correlation between L-Ser plasma levels and scores on the 21-item Hamilton Rating Scale for Depression (HAM-D) (Mitani et al. 2006). In the current study, no significant differences were observed between KET-Rs and KET-NRs on any of the administered tests at baseline (Table 1) even though there were significant differences in baseline L-Ser plasma concentrations between the two groups. In our study the TRD patients were drug free while in the study conducted by Mitani et al. (2006), 17 of the 23 depressed patients were on antidepressant medications, including tricyclic antidepressants, SSRIs, and an α2-adrenergic receptor antagonist. This difference in the participants’ treatment modality is significant and could certainly contribute to the apparent divergence between the two studies.
The observation that baseline plasma concentrations of either D-Ser or L-Ser can be used to predict the antidepressant response to KET is consistent with the biological connection between the two compounds, as D-Ser is produced by enantio-conversion of L-Ser by serine racemase. Previous in vitro studies have demonstrated that increased L-Ser concentrations result in D-Ser accumulation (Singh et al. 2012), and it would be expected that the significantly higher baseline plasma levels of L-Ser in KET-NRs relative to KET-Rs would be mirrored by the baseline D-Ser plasma concentrations. While this is indeed the case, the ratios of L-Ser to D-Ser at baseline were not equivalent and, instead, were found to be significantly higher in KET-NRs versus in KET-Rs, 51.8±1.1 and 20.8±2.6, respectively (p=0.0002). The data indicates that the conversion of L-Ser to D-Ser is less efficient in KET-NRs relative to KET-Rs. There are a number of potential causes of this difference, including the saturation of the enzymatic process, in which the amount of serine racemase expressed in KET-Rs and KET-NRs is similar, but the enantio-conversion of L-Ser is less efficient in KET-NRs due to difference in the expression pattern of serine racemase isoforms. Another possibility is that the degradation of D-Ser was more efficient in KET-Rs through a process mediated by both serine racemase and D-amino acid oxidase (Crow et al. 2012). Our results suggest that there may be differences in the relative expression and/or activity of these two enzymes in KET-NRs and KET-Rs. In this regard, genetic variations in both of these enzymes have been associated with schizophrenia (Labrie and Roder 2010) and lower D-Ser levels have also been associated with schizophrenia (Hashimoto et al. 2003; Calcia et al. 2012). The examination of the genetic variations of serine racemase and D-amino acid oxidase in KET-Rs and KET-NRs was outside of the design of the current study and will be addressed in future investigations.
The association between baseline L-Ser plasma concentrations and the antidepressant response to KET is an interesting observation in light of the multiple biological roles played by the molecule. L-Ser provides a large fraction of the one-carbon pool utilized in the synthesis of purine nucleotides and N5,N10-methylenetetrahydrofolate. It is also used as a precursor in the synthesis of phospholipids and is converted into the NMDA co-agonists, D-Ser, and glycine (Snell and Fell 1990; Lehninger et al. 1993; Tabatabaie et al. 2010; Ji et al. 2011). In the CNS, L-Ser is primarily synthesized in astrocytes through a pathway initiated from the glycolytic intermediate 3-phosphoglycerate, and it involves conversion of 3-phosphohydroxypyruvate to 3-phosphoserine via a coupled reaction that converts glutamate to 2-ketoglutarate (Snell and Fell 1990; Lehninger et al. 1993; Tabatabaie et al. 2010).
The relationship between the increased baseline plasma concentrations of L-Ser in KET-NRs relative to KET-Rs cannot be determined from the data obtained in this study. However, the effect is consistent with the results from the metabolomics analysis of the plasma samples obtained from BD patients, in which the majority of the signals associated with lysophosphatidylethanolamine and lysophosphatidylcholine were increased in KET-Rs relative to KET-NRs (Villaseñor et al. 2014), and with data from a recent study utilizing a metabolomics analysis of urinary patterns that identified an increase in glycine and 2-ketoglutarate signals in the samples obtained from MDD patients when compared to controls (Zheng et al. 2013). While the biological source of the difference in L-Ser levels between KET-Rs and KET-NRs is currently unknown, the recent study by Tabatabaie et al. (2010) suggests that the low L-Ser plasma levels associated with congenital neurological abnormalities are associated with deficiencies in 3-phosphoglycerate dehydrogenase and phosphoserine amino-transferase expression. A recent study in mice has also indicated that the production of L-Ser via the phosphorylation pathway involving 3-phosphoglycerate dehydrogenase is a key rate-limiting factor for maintaining D-Ser brain concentrations (Yang et al. 2010). A difference between the expression and activity of these enzymes in KET-Rs and KET-NRs would be consistent with the data from this study, and this relationship will be explored in future work.
Another potential source of the observed differences in KET-Rs and KET-NRs is the significant correlation between BMI and response, although when controlling for BMI, D-Ser and L-Ser remain significant predictors of improvement in depression. Previous studies have indicated that the antidepressant response to KET is associated with mitochondrial activity reflected in fatty acid metabolism (Villaseñor et al. 2014). However, the observed inverse correlation between BMI and the baseline plasma levels of D-Ser (r=−0.59, p= 0.005) and L-Ser (−0.44, p=0.046) is of great interest in light of the reported relationship between BMI and fatty acid metabolism (Jensen et al. 1989; Horrobin and Bennett 1999; Crous-Bou et al. 2012), and L-Ser biosynthesis and fatty acid metabolism (Snell and Fell 1990; Lehninger et al. 1993; Tabatabaie et al. 2010). Thus, the data from this study suggest that these seemingly diverse but interconnected phenomena contribute to the response to KET therapy and should be collectively investigated. This observation has been incorporated into the ongoing studies of KET and its response.
While baseline plasma concentrations of both D-Ser and L-Ser can be considered as predictors of the antidepressant response to KET, the determination of D-Ser plasma concentrations would appear to be the more relevant of these parameters. This assumption is due, in part, to the biological activity of D-Ser as an essential co-agonist of presynaptic NMDA receptors, where it has a preferential affinity relative to glycine, and the role it plays in long-term potentiation and NMDA-induced neurotoxicity (Henneberger et al. 2010; Papouin et al. 2012). In addition, the plasma concentrations of L-Ser in KET-Rs and KET-NRs were not affected by the administration of KET even though D-Ser plasma concentrations were reduced in a biphasic manner in both groups (Fig. 2a, b). The significant ~20–25 % decrease in D-Ser plasma levels observed at the end of the KET infusion in the two groups of patients suggests a rapid, concentration-dependent pharmacological effect of KET on D-Ser plasma concentrations, which is attenuated by the fast and extensive metabolism of the drug. One potential mechanism includes the KET-associated inhibition of one or more of the alanine-serine-cysteine transporters (ACST1, ASCT2, Asc-1), which are involved in the uptake and release of D-Ser (Henneberger et al. 2013). This mechanism provides a plausible explanation for the rapid decline and rebound of the D-Ser plasma concentrations, but it cannot explain the prolonged and significant decline in D-Ser plasma concentrations that occurs between the 120-min and day 21 time points in the study (Fig. 2a, b). The latter observation may be a result of the extensive metabolism of KET that yields significant plasma concentrations of several metabolites, such as (R,S)-norketamine, (R,S)-dehydronor ketamine, and (2S, 6S; 2R, 6R) - hydroxynorketamine. These metabolites are present in the 40-min plasma sample, and their concentrations increase over the course of the study, while that of KET rapidly decreases (Zarate et al. 2012). The potential importance of these metabolites was demonstrated in a recent study that associated the plasma levels of KET metabolites with clinical response in MDD and BD patients (Zarate et al. 2012). In addition, studies in Wistar rats showed that (R,S)-norketamine and (2S,6S)-hydroxynorketamine are active metabolites (Paul et al. 2014), and in vitro studies indicated that (R,S)-dehydronorketamine decreases the intracellular concentrations of D-Ser (Singh et al. 2013). Thus, while baseline D-Ser plasma levels predicted the antidepressant KET response, the data suggest that changes in plasma D-Ser levels may also be a potential post-administration biomarker of the pharmacological response to KET and KET metabolites. This possibility will be the focus of further studies.
Our data do not permit to directly establish a relationship between the antidepressant effects of KET and the temporal decrease in D-Ser plasma concentrations. However, a recent study has shown a correlation between the dissociative effects induced by KET and the antidepressant response (Luckenbaugh et al. 2014). The data from our study are consistent with the previous findings and suggest that the lower baseline D-Ser plasma concentrations found in KET-Rs and the reduction in plasma D-Ser concentrations produced by the KET infusion are pharmacological mechanisms associated with this phenomenon. The results indicate that the administration of KET produces a state closer to achieving NMDA receptor blockade (functional NMDAR antagonism) in KET-Rs relative to KET-NRs and that this effect is manifested as a behavioral readout of greater dissociation (i.e., higher CADSS scores). The proposed effect is consistent with the significantly lower baseline D-Ser plasma concentrations observed in subjects with schizophrenia relative to healthy controls and the negative association of D-Ser plasma levels with severity of the symptoms of the disease (Hashimoto et al. 2003; Calcia et al. 2012). It is also consistent with NMDA receptor hypofunction, the “hypoglutamatergic hypothesis,” and the psychosis-inducing effect of KET in healthy volunteers (Pomarol-Clotet et al. 2006).
A recent review described the quest to identify human biomarkers of antidepressant response to KET treatment (Niciu et al. 2014b). The review described studies utilizing neuroimaging, sleep architecture, genetic markers, and family histories. The ability of these techniques to predict response varied and no single effective descriptor was identified. A second study has identified baseline neurocognitive performance as a potential predictor of response to treatment with KET, with KET-Rs having poorer baseline neurocognitive performance (Murrough et al. 2014). Since D-Ser plays a key role in long-term potentiation and NMDA-related synaptic plasticity (Henneberger et al. 2010; Papouin et al. 2012; Hopkins et al. 2013), the higher D-Ser baseline concentrations found in KET-NRs is consistent with better neurocognitive performance in this group of patients.
The data from this study suggest that the pre-treatment determination of baseline D-Ser levels in MDD patients may be an effective, rapid, and simple method to predict antidepressant response to treatment with KET. These preliminary results will be tested in a prospective study incorporating this approach, which is currently in progress, and the results will be reported elsewhere. In addition, the results of this study also suggest that one must consider biomarkers of response in relationship to the pharmacological mechanism of the therapeutic agent, as plasma levels of glycine have been identified as a biomarker of response for treatment with the SSRIs citalopram and escitalopram (Ji et al. 2011), but were not identified in the initial metabolomics study of KET response. This hypothesis will also be tested in the current ongoing clinical trial.
Acknowledgments
This work was supported in part by the Intramural Research Programs of the National Institute on Aging (IWW) and National Institute of Mental Health (CAZ) of the National Institutes of Health (NIH) and the Spanish Ministry of Science and Technology (MCIT) grant CTQ2011-23562 (CB). A. V. acknowledges her fellowship provided by EADS CASA.
Contributor Information
Ruin Moaddel, Intramural Research Program, National Institute on Aging, National Institutes of Health (NIH), Baltimore, MD, USA.
David A. Luckenbaugh, Experimental Therapeutics & Pathophysiology Branch Intramural Research Program, National Institute of Mental Health (NIMH), NIH, Bethesda, MD, USA
Ying Xie, Intramural Research Program, National Institute on Aging, National Institutes of Health (NIH), Baltimore, MD, USA.
Alma Villaseñor, Center for Metabolomics and Bioanalysis (CEMBIO), Facultad de Farmacia, Universidad CEU San Pablo, Campus Montepríncipe, Madrid, Spain.
Nancy E. Brutsche, Experimental Therapeutics & Pathophysiology Branch Intramural Research Program, National Institute of Mental Health (NIMH), NIH, Bethesda, MD, USA
Rodrigo Machado-Vieira, Experimental Therapeutics & Pathophysiology Branch Intramural Research Program, National Institute of Mental Health (NIMH), NIH, Bethesda, MD, USA.
Anuradha Ramamoorthy, Intramural Research Program, National Institute on Aging, National Institutes of Health (NIH), Baltimore, MD, USA.
Maria Paz Lorenzo, Center for Metabolomics and Bioanalysis (CEMBIO), Facultad de Farmacia, Universidad CEU San Pablo, Campus Montepríncipe, Madrid, Spain.
Antonia Garcia, Center for Metabolomics and Bioanalysis (CEMBIO), Facultad de Farmacia, Universidad CEU San Pablo, Campus Montepríncipe, Madrid, Spain.
Michel Bernier, Intramural Research Program, National Institute on Aging, National Institutes of Health (NIH), Baltimore, MD, USA.
Marc C. Torjman, Department of Anesthesiology, Cooper Medical School of Rowan University, Camden, NJ, USA
Coral Barbas, Center for Metabolomics and Bioanalysis (CEMBIO), Facultad de Farmacia, Universidad CEU San Pablo, Campus Montepríncipe, Madrid, Spain.
Carlos A. Zarate, Jr., Experimental Therapeutics & Pathophysiology Branch Intramural Research Program, National Institute of Mental Health (NIMH), NIH, Bethesda, MD, USA
Irving W. Wainer, Intramural Research Program, National Institute on Aging, National Institutes of Health (NIH), Baltimore, MD, USA. Department of Anesthesiology, Cooper Medical School of Rowan University, Camden, NJ, USA. Bioanalytical and Drug Discovery Unit, Laboratory of Clinical Investigation, National Institute on Aging, National Institutes of Health, Suite 100, Biomedical Research Center, 251 Bayview Boul., Baltimore, MD 21224-6825, USA
References
- Alexander GM, Reichenberger E, Peterlin BL, Perreault MJ, Grothusen JR, Schwartzman R. Plasma amino acid changes in complex regional pain syndrome. Pain Res Treat. 2013;2013:742407. doi: 10.1155/2013/742407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamura C, Maes M, Dai J, Meltzer HY. Plasma concentration of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur Neuropsychopharmacol. 1995;5(Suppl):71–75. doi: 10.1016/0924-977x(95)00033-l. [DOI] [PubMed] [Google Scholar]
- Browne CA, Lucki I. Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol. 2013;4:161. doi: 10.3389/fphar.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcia MA, Maderia C, Alheira FV, Silva TCS, Tannos FM, Vargas-Lopes C, Goldenstein N, Brasil MC, Ferreira ST, Panizzutti R. Plasma levels of D-serine in Brazilian individuals with schizophrenia. Schizophr Res. 2012;142:83–87. doi: 10.1016/j.schres.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Crous-Bou M, RennertG SR, Rodriquez-Moranta F, Rennert HS, Lejbkowicz F, Kopelovich L, Lipkin SM, Gruber SB, Moreno V. Genetic polymorphisms in fatty acid metabolism genes and colorectal cancer. Mutagenesis. 2012;27:169–176. doi: 10.1093/mutage/ger066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow JP, Marecki JC, Thompson M. D-Serine production, degradation and transport in ALS: critical role of methodology. Neurol Res Int. 2012;2012:625245. doi: 10.1155/2012/625245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, Nakazato M, Kumakiri C, Okada S, Hasegawa H, Imai K, Iyo M. Decreased serum levels of D-serine in patients with schizophrenia. Evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry. 2003;60:572–576. doi: 10.1001/archpsyc.60.6.572. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SHR, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Bard L, King C, Jennings A, Rusahov NMDA receptor activation: two targets for two co-agonists. Neurochem Res. 2013;38:1156–1162. doi: 10.1007/s11064-013-0987-2. [DOI] [PubMed] [Google Scholar]
- Hopkins SC, Campbell UC, Heffernan MLR, Spear KL, Jeggo RD, Spanswick DC, Varney MA, Large TH. Effects of D-amino oxidase inhibition on memory performance and long-term potentiation in vivo. Pharm Res Per. 2013;1:e007. doi: 10.1002/prp2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrobin DF, Bennett CN. Depression and bipolar disorder: relationship to impaired fatty acid and phospholipid metabolism and to diabetes, cardiovascular disease, immunological abnormalities, cancer, ageing and osteoporosis. Prostaglandins Leukot Essent Fat Acids. 1999;60:217–234. doi: 10.1054/plef.1999.0037. [DOI] [PubMed] [Google Scholar]
- Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles JM. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest. 1989;83:1168–1173. doi: 10.1172/JCI113997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Hebbring S, Zhu H, Jenkins GD, Biernacka J, Snyder K, Drews M, Fiehn O, Zeng Z, Schaid D, Mrazek DA, Kaddurah-Daouk R, Weinshilboum RM. Glycine and a glycine dehydrogenase (GLDC) SNP as citalopram/escitalopram response biomarkers in depression: pharmacometabolomics-informed pharmacogenomics. Clin Pharmacol Ther. 2011;89:97–104. doi: 10.1038/clpt.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiraskova-Vanickova J, Ettrich R, Vorlova B, Hoffman HE, Lepsik M, Jansa P, Konvalenka J. Inhibition of human serine racemase, an emerging target for medicinal chemistry. Curr Drug Targets. 2011;12:1037–1055. doi: 10.2174/138945011795677755. [DOI] [PubMed] [Google Scholar]
- Labrie V, Roder JC. The involvement of NMDA receptor D-serine/glycine site in the pathophysiology and treatment of schizophrenia. Neurosci Biobehav Rev. 2010;34:351–372. doi: 10.1016/j.neubiorev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Lehninger AL, Nelson DL, Cox MM. Principles of biochemistry. 2. Worth Publishers; New York: 1993. [Google Scholar]
- Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, Guevara S, Zarate CA., Jr Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord. 2014;159:56–61. doi: 10.1016/j.jad.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, Luckenbaugh DA, Manji HK, Zarate CA., Jr Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depressive disorder. J Clin Psychiatry. 2008;69:946–958. doi: 10.4088/jcp.v69n0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Verkerk R, Vandoolaeghe E, Lin A, Scharpé S. Serum levels of excitatory amino acids, serine, glycine, histidine, threonine, taurine, alanine and arginine in treatment-resistant depression: modulation by treatment with antidepressants and prediction of clinical responsitivity. Acta Psychiatr Scand. 1998;97:302–308. doi: 10.1111/j.1600-0447.1998.tb10004.x. [DOI] [PubMed] [Google Scholar]
- Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby CR, Jr, Kawahara R. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1155–1158. doi: 10.1016/j.pnpbp.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Wan L-B, Iacovicello B, Collins KA, Solon C, Glicksberg B, Perez AM, Mathew SJ, Charney DS, Iosifescu DV, Burdick KE. Neurocognitive effects of ketamine in treatment-resistant major depression: association with antidepressant response. Psychopharmacology. 2014;231:481–488. doi: 10.1007/s00213-013-3255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Henter ID, Luckenbaugh DA, Zarate CA, Jr, Charney DS. Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Ann Rev Pharmacol Toxicol. 2014a;54:119–139. doi: 10.1146/annurev-pharmtox-011613-135950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Luckenbaugh DA, Ionescu DF, Guevara S, Machado-Vieira R, Richards EM, Brutsche NE, Nolan NM, Zarate CA., Jr Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry. 2014b;75:e417–e423. doi: 10.4088/JCP.13m08698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet J-P, Oliet SHR. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150:633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Paul RK, Singh NS, Khadeer M, Moaddel R, Sanghvi M, Green CE, O’Loughlin K, Torjman MC, Bernier M, Wainer IW. (R, S)-Norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin (mTOR) function. Anesthesiology. 2014;121:149–159. doi: 10.1097/ALN.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Honey GD, Murray GK, Corlett PR, Absalom AR, Lee M, McKenna PJ, Bullmore ET, Fletcher PC. Psychological effects of ketamine in healthy volunteers: phenomenological study. Br J Psychiatry. 2006;189:173–179. doi: 10.1192/bjp.bp.105.015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NS, Paul RK, Sichier M, Moaddel R, Bernier M, Wainer IW. Capillary electrophoresis-laser-induced fluorescence (CE-LIF) assay for measurement of intracellular D-serine and serine racemase activity. Anal Biochem. 2012;421:460–466. doi: 10.1016/j.ab.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NS, Paul RK, Ramamoorthy A, Torjman MC, Moaddel R, Bernier M, Wainer IW. Nicotinic acetylcholine receptor antagonists alter the function and expression of serine racemase in PC-12 and 1321N1 cells. Cell Signal. 2013;25:2634–2646. doi: 10.1016/j.cellsig.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K, Fell DA. Metabolic control analysis of mammalian serine metabolism. Adv Enzym Regul. 1990;30:13–32. doi: 10.1016/0065-2571(90)90006-n. [DOI] [PubMed] [Google Scholar]
- Tabatabaie L, Klomp LW, Berger R, de Koning TJ. L-Serine synthesis in the central nervous system: a review on serine deficiency disorders. Mol Genet Metab. 2010;99:256–262. doi: 10.1016/j.ymgme.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Villaseñor A, Ramamoorthy A, Silva dos Santos M, Lorenzo MP, Laje G, Zarate C, Jr, Barbas C, Wainer IW. A pilot study of plasma metabolomic patterns from patients treated with ketamine for bipolar depression: evidence for a response-related difference in mitochondrial networks. Br J Pharmacol. 2014;171:2230–2242. doi: 10.1111/bph.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Alexander GM, Schwartzman RJ, Singh N, Torjman M, Goldberg M, Wainer IW, Moaddel R. Development and validation of a sensitive LC-MS/MS method for the determination of D-serine in human plasma. J Pharm Biomed Anal. 2014;89:1–5. doi: 10.1016/j.jpba.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Wada A, Yoshida K, Miyoshi Y, Sayano T, Esaki K, Kinoshita MO, Tomonaga S, Azuma N, Watanabe M, Hamase K, Zaitsu K, Machida T, Messing A, Itochara S, Hirabayashi Y, Furuya S. Brain-specific Phgdh deletion reveals a pivotal role for L-serine biosynthesis in controlling the level of D-serine, an N-methyl-D-aspartate receptor co-agonist, in adult brain. J Biol Chem. 2010;285:41380–41390. doi: 10.1074/jbc.M110.187443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SLV, Ramamoorthy A, Moaddel R, Wainer IW. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry. 2012;72:331–338. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Wang Y, Chen L, Yang D, Meng H, Zhou D, Zhong J, Lei Y, Melgiri ND, Xie P. Identification and validation of urinary metabolite biomarkers for major depressive disorder. Mol Cell Proteomics. 2013;12:207–214. doi: 10.1074/mcp.M112.021816. [DOI] [PMC free article] [PubMed] [Google Scholar]