Abstract
Dietary fiber may increase calcium absorption, but its role in bone mineralization is unclear. Furthermore, the health effect of dietary fiber may be different between genders. We examined the association between dietary fiber (total fiber and fiber from cereal, fruits, vegetables, nuts and legumes) and bone loss at the femoral neck, trochanter and lumbar spine (L2–4) in older men and women. In the Framingham Offspring Study, at baseline (1996–2001), diet was assessed using the Willett food frequency questionnaire and bone mineral density (BMD) was measured using dual-energy X-ray absorptiometry. Follow-up BMD was measured in 2001–2005 and 2005–2008 among 792 men (mean age, 58.1yr; BMI, 28.6kg/m2) and 1,065 women (57.3yr; 27.2kg/m2). We used sex-specific generalized estimating equations in multivariable regressions to estimate the difference (β) of annualized BMD change in percent (%ΔBMD) at each skeletal site per 5 g/d increase in dietary fiber. We further estimated the adjusted mean for bone loss (annualized %ΔBMD) among participants in each higher quartile (Q2, Q3 or Q4) compared with those in the lowest quartile (Q1) of fiber intake. Higher dietary total fiber (β=0.06, p=0.003) and fruit fiber (β=0.04, p=0.008) was protective against bone loss at the femoral neck in men but not in women. When examined in quartiles, men in Q2–Q4 of total fiber had significantly less bone loss at the femoral neck versus those in Q1 (all p<0.04). Fiber from vegetables appeared to be protective against spine bone loss in women but not men. There were no associations with cereal fiber or nut and legume fiber and bone loss in men or women. Our findings suggest that higher dietary fiber may modestly reduce bone loss in men at the hip.
Keywords: Dietary fiber, Bone mineral density, bone loss, Sex-specific difference, Framingham Study
Introduction
Osteoporosis is a common skeletal disorder leading to increasing bone fragility and fracture risk that poses a substantial healthcare burden and affects physical function, comorbidity and mortality in both men and women with advancing age(1,2). Dietary contributors to osteoporosis have focused on vitamin D and calcium, but nutrients beyond these(3,4) may also affect bone health. Intake of fruits and vegetables(5) and a fruit, vegetable and cereal pattern(6) in the Framingham cohorts have been suggested to be positively related to change in hip BMD in men but not in women. Even though these food sources are major contributors of dietary fiber, studies on dietary fiber per se are limited.
Recent experimental studies suggest that dietary fiber may benefit mineral absorption, BMD, and bone turnover(7–9). According to the Institute of Medicine, dietary fiber is defined as non-digestible carbohydrates and lignin that are intrinsic and intact in plants(10). Studies have shown increased calcium absorption in rodent models treated with various fermentable dietary fiber(11–15). Some(15–17) but not all(12,13,18) of these studies reported a positive effect on BMD. Data from human controlled trials have demonstrated that dietary fiber increased calcium absorption(19,20) and bone mineralization(20) in children/adolescents. The only cross-sectional observational study(21), to our knowledge, reported that dietary fiber was positively related to forearm BMD in children but not in adolescents. However, results from clinical trials in postmenopausal women are inconsistent: Although a positive association has been shown between fiber intake and calcium absorption(22–25), this does not translate to change in BMD(26,27). Different directions of bone turnover markers were also reported(24,25,27). To our knowledge, no study has examined the associations of fiber intake with fractures.
Ultimately, the effect of dietary fiber on bone health in older adults is unclear, which may involve various factors including calcium intake and hormones. Previous studies suggested that higher dietary fiber was related to lower estrogen levels in women(28–30) and that the duration of menopause affected calcium absorption with fiber intake(23). Further, dietary fiber may exert its health effects via change of gut microbiome(8), which is different between sexes(31). Additionally, sex-specific differences are known in genetics in bone physiology, bone geometry and bone gonadal hormone response. These sex differences point to the importance of conducting sex-specific analysis for genes and environmental factors in association studies on osteoporosis(32). Together, we hypothesized a sex difference in the inverse association between dietary fiber and bone loss.
In this study, we examined the longitudinal association between intake of dietary fiber and change in BMD in the Framingham Offspring Study. We further examined fiber sub-types including cereal, fruits, vegetables, nuts and legumes because the type of fiber may have different bone health effects such that cereal grain fiber was suggested to be more beneficial in cardiovascular diseases and type 2 diabetes(33–35).
Subjects and Methods
Study population
Participants from the Framingham Offspring cohort, which was assembled in 1971 consisting of adult children of the Original Framingham Heart Study and spouses of the offspring participants, were enrolled and subsequently examined approximately every 4 years to investigate familial risk factors for cardiovascular diseases(36). In 1996–2001, as part of the Framingham Osteoporosis Study, BMD measures of the hip and lumbar spine (LS; L2–L4) were obtained in 3,072 Offspring participants. Follow-up BMD measurements were obtained in 2002–2005 (n=1,311) and in 2005–2008 (n=1,830), of whom 786 persons had three BMD measures at the hip and 778 persons had three BMD measures at the LS. The current study comprised 792 men and 1,065 women who had at least one follow-up BMD measure and valid dietary assessments completed in either 1995–1998 or 1998–2001 (Figure 1). All participants provided informed written consent and the study was approved by the Institutional Review Boards at Boston University and Hebrew SeniorLife.
Figure 1.
Flow chart for the Framingham Offspring Study participants who were included in the analyses of dietary fiber and annualized percent change in bone mineral density (%∆BMD)
BMD, bone mineral density; Lumbar spine; LS2–4, location 2 to 4.
Baseline dietary assessment
At baseline, habitual dietary intake was assessed using the semi-quantitative Willett validated food frequency questionnaire (FFQ)(37–39). The FFQ listed 126 food items with frequency of consumption and standard serving sizes. Participants were also allowed to add up to three additional foods, types of breakfast cereal and cooking oil that are not listed in the FFQ. The nutrient values were estimated primarily based on the USDA food composition database and supplemented by other published data(39). Estimates from the FFQ reflected a long-term average dietary fiber intake based on several weeks of formal diet records with a correlation coefficient of 0.68 for dietary fiber(37–39). Total fiber intake in grams/day was derived as the sum of fiber from cereal, fruits, vegetables, and nuts and legumes. We only included participants whose FFQs had less than 12 food items left blank, those with no missing values for dietary total fiber and calorie intakes ≥500 kcal and <4,200 kcal for men and <4,000 kcal for women.
Measurements of BMD
BMD (g/cm2) of the femoral neck (FN) and trochanter (TR) and of the LS, L2–L4 was measured at the baseline exam and at least one follow-up exam. BMD measurements completed at baseline in 1996–2001 were obtained using a LUNAR DPX-L dual-energy X-ray absorptiometry (Lunar Corp., Madison, WI, USA) and were repeated using a GE Lunar Prodigy (GE Lunar, Madison, WI, USA) in 2002–2005 and in 2005–2008. For Lunar DPX-L, the precision was 1.7% at the femoral neck, 2.5% at the trochanter, and 0.9% at the spine, which is similar to the range of 1.8% to 1.9% reported by others(40,41). For Lunar Prodigy, the % coefficient of variances were 1.8, 2.3, 1.2 and 1.1 for the femoral neck, trochanter, total hip and L2–4 regions, respectively(42). The right hip was scanned unless there was a history of fracture or hip replacement (there were 4 hip fracture prevalent cases in total with 2 fractures per sex), in which case the left hip was scanned. Percentage change in BMD over the follow-up was calculated as: %ΔBMD = [(BMD at follow-up – BMD at baseline) / BMD at baseline] × 100 (1) as described earlier(43) with adjustment for change in technology from DPX-L to Lunar Prodigy based on the previous calibration study(42). Annualized %ΔBMD was then calculated using formula (1) divided by time difference (years) between the baseline and follow-up BMD measures. Alternatively, we used rate of bone loss per year (g/cm2/year) estimated by [(BMD at follow-up – BMD at baseline)] divided by time difference described as above.
Other covariates
Body weight (pounds) and height (inches) were measured at baseline and every four years without shoes. Weight was measured in light-weight clothing and height was measured using a wall mounted stadiometer. Body mass index (BMI) (kg/m2) was calculated as weight (kg) divided by height (m) squared. Cigarette smoking was assessed via questionnaire as current cigarette smoker (smoked regularly in the past year), former smoker or never smoker; we combined former and never smokers into a group of current non-smokers. For estrogen use, women were divided into current estrogen users at the time of the exam and non-current users including those who had never used or formerly used estrogen. Physical activity was measured with use of the Physical Activity Scale in the Elderly (PASE), which has been previously validated in older adults(44,45). If PASE was missing at baseline (n=16), we used values at the previous exam (1991–1995) as an estimate. Furthermore, menopause status (yes versus no) and use of estrogen (yes versus no) were determined at each visit as the BMD measurement. The Dietary Guidelines Adherence Index (DGAI-2010) score was applied to the baseline FFQ to determine participants’ diet quality according to the Dietary Guidelines for Americans 2010(46,47) and ranged from 0 (lowest adherence) to 100 (highest adherence). If the DGAI-2010 was missing at baseline (n=377), values at the previous exam (1991–1995) were used. Because dietary fiber is one of the 24 components in the DGAI-2010, we modified the DGAI-2010 by subtracting the fiber component.
Statistical analysis
Descriptive statistics were generated using means and standard deviations for normal distribution otherwise median (interquartile range) of continuous measures and frequencies and percentages for categorical measures. For continuous variables, Shapiro-Wilk W test was used to test normality and ANOVA was tested for differences among quartiles of total fiber. Dietary fiber, including total fiber and sub-group fiber was modeled as both a continuous variable as well as a categorical variable in quartiles. We first calculated the residuals of dietary fiber for men and women separately by regressing fiber intake on total calories(48) to obtain sex-specific quartiles of dietary fiber. We further calculated average T score based on the BMD of women aged 20–29 years in the NHANES(49) and had standardized the BMD values(50).
Dietary fiber may interact with sex hormones to impact bone loss, therefore we first assessed whether there sex modified this association by including a cross product term of each dietary fiber variable and sex in separate regression models. Because dietary total fiber and femoral bone loss was suggested to be modified by sex (p for interaction=0.05), we performed the analyses using a linear regression model in men and women separately. In the unadjusted model, only exam period was adjusted as part of the GEE regression for repeated measure(51) (model 1). In the base model (model 2), we controlled for age (years), BMI (kg/m2), height (m) and exam period for repeated BMD measures. In the full model (Model 3), we further adjusted for current cigarette smoking (yes, no), physical activity (PASE, continuous), modified DGAI 2010 (continuous), calcium supplement intake (yes, no), vitamin D supplement intake (yes, no), caffeine intake (mg/day), dietary calcium (in quartile, mg/day), dietary vitamin D (in quartile, I.U./day), menopausal status (yes, no, in women only), and estrogen therapy use (yes, no, in women only). These covariates have been suggested as significant factors for BMD in the Framingham Osteoporosis Study(6,43,52). As fiber may be related to diet quality, we included a dietary index to reduce possible confounding by a potential healthy diet effect in those who consume higher dietary fiber. No evidence suggested that any of the covariates were mediators. We used the Generalized Estimating Equations in the regression models to account for the correlation between the repeated BMD measurements. The difference (β) in %ΔBMD at each bone site per 5 g/day increase in dietary fiber on a continuous scale and the least squares adjusted mean (β) comparing a higher quartile (Q2, Q3 or Q4) with the lowest quartile Q1 (reference) were obtained from the regression models. We took the same approach to examine dietary fiber intake in relation to annual rate of bone loss (g/cm2/year).
Menopause and hormone therapy are known risk factors for osteoporosis(53,54). A previous study reported that women who experienced menopause for >6 years had higher calcium absorption in the fiber group than the placebo group, but no difference was observed in women who experienced menopause for 2–6 years(23). Therefore, we conducted stratified analyses by i) menopausal status (premenopausal versus postmenopausal, defined as menstrual periods stopped at least one year), and among postmenopausal women; ii) by estrogen users (yes/no); and iii) by stage of menopause (early postmenopausal, i.e. menstrual periods stopped less or equal to 5 years versus late postmenopausal, i.e. menstrual periods stopped more than 5 years).
All statistical analyses were conducted using SAS Version 9.3 (SAS Institute, Inc., Cary, North Carolina). Based on the scientific evidence from the literature, we had a priori hypothesis that higher versus lower intake of the same type of dietary fiber (total or sub-group) was associated with greater change in BMD. We also limited the BMD measurements to three skeletal sites. Hence, the p values in this study were not adjusted for multiple testing.(55) A two-sided p value less than 0.05 was considered statistically significant.
Results
Among the 3,072 participants with baseline BMD, the 1,008 participants who were dead or lost of contact were not included in this study. The distribution of deaths or loss of contact did not vary across the quartiles of dietary total fiber with chi-square tests ranged from 0.08 to 0.20. Baseline characteristics are described in Table 1 across quartiles of dietary total fiber by sex. The ratio of males and females in each follow-up group in 2002–2005 or in 2005–2008 was similar (p=0.30) with 44.1% and 46.3 % as men, respectively. The mean (standard deviation) for age was 58.1 (8.9) years and for BMI was 28.6 (4.2) kg/m2 in men and 57.3 (9.0) years and 27.2 (5.6) kg/m2 in women. Dietary fiber intake was similar in both genders with 19.7 (7.9) g/day for men and 19.5 (8.1) g/day for women. BMI, height, PASE, vitamin D supplementation use did not differ across the quartiles of total fiber intake in either gender.
Table 1.
Baseline characteristics of men and women by quartiles of dietary total fiber (g/d) at the baseline examination (1996–2001) in the Framingham Offspring Study.
| Men (n=792) | Women (n=1,065) | |||||||
|---|---|---|---|---|---|---|---|---|
| Quartiles of dietary total fiber | Q1 (low) | Q2 | Q3 | Q4 (high) | Q1 (low) | Q2 | Q3 | Q4 (high) |
| N | 198 | 198 | 198 | 198 | 266 | 266 | 267 | 266 |
| Age (year)1,* | 56.2 (8.9) | 58.2 (8.2) | 58.2 (9.0) | 59.9 (9.0) | 55.8 (9.3) | 57.1 (8.4) | 57.6 (8.9) | 58.8 (9.0) |
| BMI (kg/m2)1 | 28.9 (4.2) | 28.6 (4.3) | 28.7 (4.2) | 28.1 (4.1) | 27.6 (5.8) | 27.1 (5.7) | 27.0 (5.6) | 27.0 (5.2) |
| Height (cm)1 | 175.7 (7.0) | 174.6 (6.4) | 174.9 (6.4) | 175.6 (6.7) | 161.4 (6.6) | 161.9 (6.4) | 161.5 (5.9) | 161.5 (6.4) |
| Current smokers, n (%)* | 29 (14.7) | 22 (11.1) | 13 (6.6) | 8.0 (4.0) | 48 (18.1) | 14 (5.3) | 15 (5.6) | 12 (4.5) |
| Physical activity (PASE)1 | 166.6 (88.1) | 171.3 (84.5) | 160.3 (81.7) | 152.4 (79.9) | 135.1 (69.4) | 138.5 (66.0) | 149.0 (77.0) | 142.8 (69.5) |
| Total energy intake (kcal)2,* | 2029 (999) | 1719 (773.7) | 1817 (788) | 2031 (688) | 1825 (828) | 1589 (763) | 1610 (713) | 1812 (664) |
| Dietary total fiber(g/d)2,* | 13.4 (7.9) | 15.8 (7.1) | 19.9 (6.2) | 27.3 (8.3) | 13.6 (6.3) | 15.5 (7.5) | 19 (7.2) | 26.1 (8.9) |
| Cereal fiber (g/d)2,* | 4.9 (3.8) | 5.4 (3.5) | 6.9 (3.7) | 8.5 (4.6) | 5.1 (3.2) | 5.2 (3.4) | 6 (3.7) | 7.4 (4.1) |
| Fruit fiber (g/d)2,* | 1.4 (1.7) | 2.3 (2.4) | 3.8 (2.7) | 5.4 (4.4) | 1.7 (2.1) | 2.5 (2.6) | 3.7 (2.7) | 5.6 (3.9) |
| Vegetable fiber (g/d)2,* | 2.7 (2.1) | 3.3 (2.1) | 4.2 (2.1) | 5.8 (3.7) | 2.9 (1.9) | 3.7 (2.1) | 4.7 (2.5) | 6.7 (3.3) |
| *Nut and legume fiber (g/d)2,* | 1.7 (1.6) | 1.9 (1.7) | 2.2 (1.5) | 3.0 (2.8) | 1.3 (1.4) | 1.7 (1.4) | 2.1 (1.6) | 2.7 (3.2) |
| Dietary vitamin D (IU/d)2,* | 183 (195) | 185 (135) | 192 (122) | 251 (180) | 202(192) | 189 (158) | 202 (160) | 253 (132 |
| Vitamin D supplement use, n (%) | 2 (1.0) | 2 (1.0) | 4 (2.0) | 2 (1.0) | 7 (2.6) | 9 (3.4) | 15 (5.6) | 12 (4.5) |
| Dietary calcium (mg/d)2,* | 652.4 (546.7) | 625.8 (467.2) | 670.5 (368.9) | 806.5 (464.1) | 677.5 (573.5) | 644.2 (440.9) | 669.9 (406) | 804.9 (422.6) |
| Calcium supplement use, n (%)‡ | 9 (4.6) | 5 (2.5) | 10 (5.1) | 14 (7.1) | 70 (26.3) | 80 (30.1) | 111 (41.6) | 111 (41.7) |
| Caffeine intake (units/d)2,* | 347.6 (277.7) | 343.6 (237.0) | 221.7 (270.0) | 158.4 (307.6) | 251.6 (244.4) | 183.7 (305.7) | 167.8 (291.9) | 165.0 (305.6) |
| Modified DGAI 2010 score3,* | 50.1 (11.9) | 53.5 (12.1) | 58.3 (11.7) | 63.1 (13.5) | 54.3 (13.5) | 59.9 (12.9) | 63.2 (12.2) | 68.3 (10.2) |
| Post-menopausal (yes), n (%) | - | - | - | - | 184 (69.2) | 197 (74.1) | 202 (75.7) | 211 (79.3) |
| Current estrogen use, n (%) | - | - | - | - | 90 (33.8) | 81 (30.5) | 88 (33.0) | 85 (32.0) |
Values for mean (standard deviation);
Values for median (Interquartile range)
Represents differences of characteristics among quartiles of total fiber intake within each gender are statistically significant at p<0.05
Represents differences of characteristics among quartiles of total fiber intake within women only are statistically significant at p<0.05.
The annual %ΔBMD between the baseline and each follow-up BMD measures is described by quartile intake of total fiber in Supplemental Table 1. The average time between the baseline BMD and the first follow-up was 4.7 (range 1.8 to 7.9) years, and that between the baseline BMD and the second follow-up was 8.1 (range 4.6 to 11.5) years. In addition, the T-scores at the baseline and the follow-ups (in 2002–2005 and in 2005–2008) were as follows: −0.50, −0.51, −0.57 for men and −1.17, −1.17, −1.26 for women, respectively.
As shown in Table 2, the results from three different models are similar. For men, per 5 g/day increase in dietary total fiber was associated with less bone loss by 0.06% in annual %ΔBMD (p=0.003) at the femoral neck in the fully adjusted model. This corresponds to 0.0005g/cm2 BMD in lower annual rate of bone loss (p=0.0015) at the femoral neck. A positive association was also observed for fruit fiber (β =0.10, p=0.008), which corresponds to 0.001g/cm2 in lower annual rate of bone loss at the femoral neck (p=0.004). The positive association of dietary total fiber with change in trochanter BMD had a tendency towards significance (β =0.04, p=0.08). For LS (L2–4) change of BMD, a weak positive association was observed with fruit fiber for men (β =0.07, p=0.10) and a significant association was found with vegetable fiber for women (β =0.12, p=0.01). Fiber from nuts and legumes was not related to change of BMD. However, no protective association between dietary fiber and hip bone loss was observed in women.
Table 2.
Association of dietary total fiber and its sub-types per 5 g/day increase with annualized percent change in BMD (%ΔBMD, g/cm2)1 in men (n=792) and women (n=1,065) in the Framingham Offspring Study
| %Δ Femoral neck BMD | %Δ Trochanter BMD | %Δ Lumbar spine 2–4 BMD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β2 | SE | p | β | SE | p | β | SE | p | |
| Dietary total fiber (g/d) | |||||||||
| Men | |||||||||
| Model 11 | 0.04 | 0.02 | 0.02 | 0.04 | 0.02 | 0.03 | 0.02 | 0.02 | 0.31 |
| Model 23 | 0.04 | 0.02 | 0.02 | 0.04 | 0.02 | 0.02 | 0.018 | 0.02 | 0.36 |
| Model 34 | 0.06 | 0.02 | 0.003 | 0.04 | 0.02 | 0.08 | −0.008 | 0.02 | 0.69 |
| Women | |||||||||
| Model 1 | 0.011 | 0.02 | 0.48 | 0.004 | 0.02 | 0.83 | 0.03 | 0.02 | 0.02 |
| Model 2 | 0.001 | 0.02 | 0.94 | 0.003 | 0.02 | 0.90 | 0.02 | 0.01 | 0.21 |
| Model 3 | 0.009 | 0.02 | 0.58 | −0.008 | 0.03 | 0.75 | 0.02 | 0.02 | 0.24 |
|
| |||||||||
| Cereal fiber (g/d) | |||||||||
| Men | |||||||||
| Model 1 | 0.02 | 0.03 | 0.44 | 0.02 | 0.03 | 0.63 | −0.017 | 0.04 | 0.64 |
| Model 2 | 0.02 | 0.03 | 0.47 | 0.01 | 0.03 | 0.71 | −0.02 | 0.034 | 0.60 |
| Model 3 | 0.04 | 0.03 | 0.22 | 0.02 | 0.03 | 0.56 | −0.025 | 0.04 | 0.50 |
| Women | |||||||||
| Model 1 | 0.02 | 0.03 | 0.40 | 0.04 | 0.04 | 0.29 | −0.01 | 0.03 | 0.80 |
| Model 2 | 0.01 | 0.03 | 0.63 | 0.03 | 0.04 | 0.41 | −0.02 | 0.03 | 0.48 |
| Model 3 | 0.01 | 0.03 | 0.66 | 0.01 | 0.04 | 0.79 | −0.02 | 0.03 | 0.40 |
|
| |||||||||
| Fruit fiber (g/d) | |||||||||
| Men | |||||||||
| Model 1 | 0.09 | 0.03 | 0.007 | 0.07 | 0.04 | 0.09 | 0.10 | 0.04 | 0.01 |
| Model 2 | 0.09 | 0.03 | 0.009 | 0.07 | 0.04 | 0.07 | 0.10 | 0.04 | 0.02 |
| Model 3 | 0.10 | 0.04 | 0.008 | 0.04 | 0.04 | 0.31 | 0.07 | 0.04 | 0.10 |
| Women | |||||||||
| Model 1 | 0.04 | 0.04 | 0.34 | −0.01 | 0.06 | 0.83 | 0.09 | 0.04 | 0.04 |
| Model 2 | 0.01 | 0.04 | 0.78 | −0.006 | 0.06 | 0.91 | 0.03 | 0.04 | 0.41 |
| Model 3 | 0.02 | 0.05 | 0.67 | −0.03 | 0.07 | 0.61 | 0.04 | 0.05 | 0.36 |
|
| |||||||||
| Vegetable fiber (g/d) | |||||||||
| Men | |||||||||
| Model 1 | 0.05 | 0.04 | 0.18 | 0.09 | 0.04 | 0.02 | 0.007 | 0.04 | 0.87 |
| Model 2 | 0.05 | 0.04 | 0.16 | 0.10 | 0.04 | 0.01 | 0.008 | 0.04 | 0.84 |
| Model 3 | 0.06 | 0.04 | 0.13 | 0.07 | 0.04 | 0.10 | −0.057 | 0.04 | 0.16 |
| Women | |||||||||
| Model 1 | −0.0003 | 0.04 | 0.99 | −0.006 | 0.05 | 0.90 | 0.13 | 0.04 | 0.002 |
| Model 2 | −0.01 | 0.04 | 0.79 | −0.004 | 0.05 | 0.93 | 0.10 | 0.04 | 0.006 |
| Model 3 | 0.01 | 0.04 | 0.76 | −0.003 | 0.06 | 0.96 | 0.12 | 0.04 | 0.01 |
|
| |||||||||
| Nut and legume fiber (g/d) | |||||||||
| Men | |||||||||
| Model 1 | −0.04 | 0.06 | 0.49 | 0.01 | 0.05 | 0.91 | −0.055 | 0.06 | 0.33 |
| Model 2 | −0.04 | 0.06 | 0.51 | 0.02 | 0.05 | 0.65 | −0.05 | 0.06 | 0.35 |
| Model 3 | −0.02 | 0.06 | 0.71 | 0.014 | 0.06 | 0.80 | −0.085 | 0.06 | 0.15 |
| Women | |||||||||
| Model 1 | −0.0004 | 0.05 | 0.99 | −0.06 | 0.07 | 0.42 | 0.0007 | 0.05 | 0.99 |
| Model 2 | −0.02 | 0.05 | 0.74 | −0.07 | 0.07 | 0.31 | −0.006 | 0.05 | 0.91 |
| Model 3 | 0.03 | 0.05 | 0.58 | −0.04 | 0.07 | 0.52 | −0.007 | 0.05 | 0.90 |
Percent change in BMD was measured from BMD at the baseline in 1996–2001 and the follow-up exams in 2002–2005 and 2005–2008.
Based on exchangeable correlation matrix using Generalized Estimating Equations; SE, standard error; p, p value; β represent difference of %ΔBMDassociated with per 5 g/d increase in dietary fiber.
Model 1 was unadjusted except exam period as part of the GEE model;
Model 2 was further adjusted for total energy intake (kcal/day), age (year), BMI (kg/m2), height (m);
Model 3 was further adjusted for current cigarette smoking (yes/no), physical activity (PASE), modified DGAI 2010 excluding fiber component, calcium supplement intake (yes/no), vitamin D supplement intake (yes/no), caffeine intake (mg/d), dietary calcium (in quartiles, mg/d), dietary vitamin D (in quartiles, I.U./d), menopausal status (yes, no, in women only), and current estrogen use (yes, no, in women only).
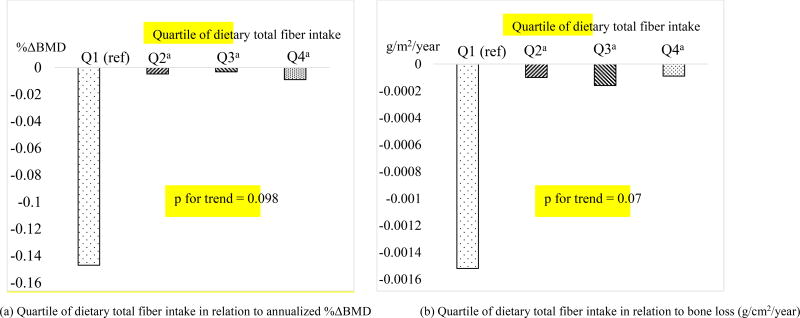
When dietary total fiber was analyzed as a categorical variable in quartiles, in men, there was significantly less femoral neck bone loss, represented by either annual %ΔBMD or annual rate of bone loss (g/cm2/year) comparing a higher quartile (Q2, Q3 or Q4) with Q1 of dietary total fiber (Figure 2). Men in the higher quartiles of dietary total fiber had significantly less bone loss with Least Squared Means ranged −0.009 to −0.003% for annualized change of BMD at the femoral neck versus −0.15% in men in Q1 (all p<0.04). No statistical difference was found for specific types of fiber or at other skeletal sites comparing a higher with the lowest quartile. The results in women were in general null and similar to those in dietary fiber on a continuous scale (See Supplemental Table 2). The β estimates for dietary fiber with annual rate of bone loss were materially the same as for annual %ΔBMD. Hence, we only presented the results in the form of annual %ΔBMD.
Figure 2.
Adjusted least square means of annualized percent change in bone mineral density
(%∆BMD)(Panel a) and annual rate of bone loss (g/cm2/year,
panel b) at the femoral neck by quartiles (Q1,  ; Q2,
; Q2,  ; Q3,
; Q3,  ; Q4,
; Q4,  ) of
dietary total fiber intake in men (n=792).
) of
dietary total fiber intake in men (n=792).
aindicates statistical significance when compared with Q1 (all p<0.04);
Models adjusted for total energy intake (kcal/day), age (year), BMI (kg/m2), height (m), exam period for repeated measures, current cigarette smoking (yes, no), physical activity (PASE, continuous), modified DGAI 2010 score (continuous), calcium supplement intake (yes, no), vitamin D supplement intake (yes, no), caffeine intake (mg/day), dietary calcium (in quartiles, mg/day), and dietary vitamin D (in quartiles, I.U./day).
In addition, no significant associations were seen between any of the dietary fiber variables and bone loss at femoral neck, trochanter and LS examined (p range: 0.25 to 0.98) in the following sub-groups of women: premenopausal (n=271), late postmenopausal (n=578) and early postmenopausal (n=216); and postmenopausal women who used estrogen therapy (n=297) and those who did not (n=497).
Discussion
The present study reported a longitudinal association between dietary fiber and bone loss among community-dwelling older adults. Our results suggest a possible sex-specific difference such that the observed associations were driven primarily by men in whom higher fiber intake was associated with less bone loss at the hip during an 8-year follow-up. For every 5 g per day increase of dietary total fiber and fruit fiber, we found that there was a decrease of 0.06% and 0.04% in annualized change of femoral neck BMD, respectively. For women, we did not observe associations regardless status of menopausal or estrogen use. Such sex differences could be attributed to a hormonal effect that may override a small benefit of dietary fiber against bone loss.
Recent evidence from fiber feeding studies in animals or in children/adolescents and postmenopausal women has suggested prominent results in improved calcium absorption(11–15,19,20,22), but data in bone turnover biomarkers suggested either increase(24), decrease(27) or no changes(25) of bone formation and resorption markers. No long-term studies reported difference in change in BMD in the fiber group versus the placebo group in postmenopausal women(26,27). One possible biological mechanism for the beneficial effect of dietary fiber on the skeleton is the prebiotic properties of fiber in modulating the microbial composition in the gut to improve calcium absorption due to the release of short-chain fatty acids during fermentation(18,56). Further, dietary fiber may promote desirable gut microbiota composition to reduce inflammation and stimulate hormones to regulate bone density and signal bone turnover.(8) Other mechanisms include that dietary fiber may enlarge the absorption surface in the intestine lumen to increase mineral absorption(7).
Our data suggested a possible sex difference in dietary fiber in relation to bone loss. The β coefficient per 5 g dietary total fiber with femoral neck BMD change suggested a multiplicative interaction between men and women (the β was 0.025 if adjustment for sex in the model). Earlier studies in women have suggested that dietary fiber reduces estrogen concentration in both postmenopausal women(28) and premenopausal women(29,30), possibly through lowering β-glucuronidase activity in the feces and therefore decreasing reabsorption of estrogen in the colon(29). In addition, studies have shown a sex difference in the microbiome composition due to the influence of sex hormones(31,57). The distribution of dietary fiber intake was similar between men and women in this study, although the recommended dietary fiber intake is higher in men than women(10). It is unclear what may explain the sex difference between dietary fiber and bone loss in our study, it is plausible that dietary fiber may affect bone loss, at least, through the impact of sex-hormones on microbiome.
The protective association of dietary fiber with bone loss in older men as seen in the current study is in line with male rodents treated with dietary fiber that were found to have enhanced calcium absorption(15,16,58), higher bone mineral content(16) and bone strength(59) as compared to the controls, although animal data may not be translated to humans directly. Furthermore, in our analyses of quartiles of dietary total fiber in men, we observed a potential threshold effect in lower bone loss at the second quartile and above comparing with the lowest quartile. That is men who consumed dietary total fiber in Q2 and above had comparable less bone loss at the femoral neck as compared with men in Q1 (all p<0.04). This observation is in agreement with an earlier study in male rodents(16), where the maximum effect of inulin fiber on BMD was found at the concentration from 0 to 5 g/100 g diet (p <0.001), but no further BMD increase at a higher dosage from 5 to 10 g/100 g diet (p= 0.11)(16).
The relationship of dietary fiber with bone loss in women in our study was generally null particularly in the hip. In a 2-year randomized clinical trial, postmenopausal women supplemented with prebiotic fiber did not show a difference in change in BMD at 24 months at the femur or LS compared to the group supplemented with calcium alone or with placebo(27), although it is possible that a 2 year duration is not sufficient to detect the effect of dietary fiber on change in BMD. When we stratified women by menopausal status, we did not see associations between dietary fiber and change in BMD, which may indicate that the dominant effects of estrogen deficiency in women may have obscured the smaller effects of fiber on bone loss. In our sample of the Framingham Offspring Study, 94% of the women were over 45 years.
Because our analysis hinted a threshold effect for low dietary total fiber with greater bone loss in men in the quartile analysis (Figure 2), we should be cautious in interpreting the results of test for trend. Due to the relatively small number of participants and a modest protective effect observed in this study, future studies with a larger sample size should evaluate whether the association between dietary fiber and bone loss is better characterized as dose-dependent or as threshold based.
Strengths of this study include the use of a population-based cohort, repeated measures of BMD over 3 time points, and examination of dietary fiber from specific food sources in both men and women to examine the relationship between dietary fiber and change of BMD. One of the limitations of this study is that dietary data is self-reported and may prone to biases, resulting in the potential to underestimate the observed associations. Despite our attempts to control for potential confounders, residual confounding may still occur in observational studies. However, generalizability of our results may be limited to non-Hispanic White populations in the US because Framingham study participants are Caucasian, whose fiber intake and BMD change may be different from other racial ethnic groups.
In conclusion, our data suggest that dietary total fiber and fiber from fruits may protect against bone loss at the hip in older men but not in older women. This protective association in men was modest even though the results reached statistical significance. As femoral neck BMD is the primary BMD measure to predict risk of hip fracture, findings from this study deserve further investigation to elucidate the mechanisms in microbiota composition induced by dietary fiber and sex hormone concentration in relation to bone health.
Supplementary Material
Acknowledgments
This study was supported by NIH grants T32 AR 7598, AR47785, AR 051568, R01 AR041398 and P60AR047785 and in part by the U.S. Department of Agriculture Agricultural Research Service, under Agreement No. 58-1950-4-003. The Framingham Offspring Study was supported by NHLBI, Framingham Heart Study (NHLBI/NIH contract #N01-HC-25195) and the Boston University School of Medicine.
Funding: This study was supported by NIH grants T32AR7598, AR47785, AR051568 and P60AR047785. The Framingham Offspring Study was supported by NHLBI, Framingham Heart Study (NHLBI/NIH contract #N01-HC-25195) and the Boston University School of Medicine.
Footnotes
Supplemental material: A supplemental table has been included in the submission.
Disclosure: All authors state that they have no conflict of interest.
Authors’ roles: Study design: Z Dai, D.T. Felson and S Sahni. Data analysis: Z Dai, Y Zhang and N Lu. Data interpretation: Z Dai, Y Zhang, D.T. Felson, D.P. Kiel, and S Sahni. Drafting manuscript: Z Dai. Revising manuscript content and approving final version of manuscript: all authors. Z Dai and N Lu take responsibility for the integrity of the data analysis.
References
- 1.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–56. [PMC free article] [PubMed] [Google Scholar]
- 2.Ensrud KE. Epidemiology of fracture risk with advancing age. J Gerontol A Biol Sci Med Sci. 2013 Oct;68(10):1236–42. doi: 10.1093/gerona/glt092. [DOI] [PubMed] [Google Scholar]
- 3.Morgan SL. Nutrition and bone: it is more than calcium and vitamin D. Womens Health (Lond) 2009 Nov;5(6):727–37. doi: 10.2217/whe.09.64. Epub 2009/10/30. [DOI] [PubMed] [Google Scholar]
- 4.Sahni S, Mangano KM, McLean RR, Hannan MT, Kiel DP. Dietary Approaches for Bone Health: Lessons from the Framingham Osteoporosis Study. Curr Osteoporos Rep. 2015 Aug;13(4):245–55. doi: 10.1007/s11914-015-0272-1. Epub 2015/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr. 1999 Apr;69(4):727–36. doi: 10.1093/ajcn/69.4.727. Epub 1999/04/10. [DOI] [PubMed] [Google Scholar]
- 6.Tucker KL, Chen H, Hannan MT, Cupples LA, Wilson PW, Felson D, et al. Bone mineral density and dietary patterns in older adults: the Framingham Osteoporosis Study. Am J Clin Nutr. 2002 Jul;76(1):245–52. doi: 10.1093/ajcn/76.1.245. Epub 2002/06/26. [DOI] [PubMed] [Google Scholar]
- 7.Scholz-Ahrens KE, Schrezenmeir J. Inulin and oligofructose and mineral metabolism: the evidence from animal trials. J Nutr. 2007 Nov;137(11 Suppl):2513S–23S. doi: 10.1093/jn/137.11.2513S. Epub 2007/10/24. [DOI] [PubMed] [Google Scholar]
- 8.McCabe L, Britton RA, Parameswaran N. Prebiotic and Probiotic Regulation of Bone Health: Role of the Intestine and its Microbiome. Curr Osteoporos Rep. 2015 Dec;13(6):363–71. doi: 10.1007/s11914-015-0292-x. Epub 2015/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporos Rep. 2015 Apr;13(2):125–30. doi: 10.1007/s11914-015-0257-0. Epub 2015/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Board FaN. In: Dietary reference intakes for energy, carbohydrate, fiber, fat fatty acids, cholesterol, protein, and amino acids. Academies IoMotN, editor. Washington, D.C.: The National Academies Press; 2001. p. 340. [Google Scholar]
- 11.Takahara S, Morohashi T, Sano T, Ohta A, Yamada S, Sasa R. Fructooligosaccharide consumption enhances femoral bone volume and mineral concentrations in rats. J Nutr. 2000 Jul;130(7):1792–5. doi: 10.1093/jn/130.7.1792. Epub 2000/06/27. [DOI] [PubMed] [Google Scholar]
- 12.Zafar TA, Weaver CM, Zhao Y, Martin BR, Wastney ME. Nondigestible oligosaccharides increase calcium absorption and suppress bone resorption in ovariectomized rats. J Nutr. 2004 Feb;134(2):399–402. doi: 10.1093/jn/134.2.399. Epub 2004/01/30. [DOI] [PubMed] [Google Scholar]
- 13.Mitamura R, Hara H. Ingestion of difructose anhydride III partially restores calcium absorption impaired by vitamin D and estrogen deficiency in rats. Eur J Nutr. 2006 Jun;45(4):242–9. doi: 10.1007/s00394-006-0592-0. Epub 2006/02/16. [DOI] [PubMed] [Google Scholar]
- 14.Legette LL, Lee W, Martin BR, Story JA, Campbell JK, Weaver CM. Prebiotics enhance magnesium absorption and inulin-based fibers exert chronic effects on calcium utilization in a postmenopausal rodent model. J Food Sci. 2012 Apr;77(4):H88–94. doi: 10.1111/j.1750-3841.2011.02612.x. Epub 2012/03/08. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Vieyra MI, Del Real A, Lopez MG. Agave fructans: their effect on mineral absorption and bone mineral content. J Med Food. 2014 Nov;17(11):1247–55. doi: 10.1089/jmf.2013.0137. Epub 2014/07/30. [DOI] [PubMed] [Google Scholar]
- 16.Roberfroid MB, Cumps J, Devogelaer JP. Dietary chicory inulin increases whole-body bone mineral density in growing male rats. J Nutr. 2002 Dec;132(12):3599–602. doi: 10.1093/jn/132.12.3599. Epub 2002/12/07. [DOI] [PubMed] [Google Scholar]
- 17.Nzeusseu A, Dienst D, Haufroid V, Depresseux G, Devogelaer JP, Manicourt DH. Inulin and fructo-oligosaccharides differ in their ability to enhance the density of cancellous and cortical bone in the axial and peripheral skeleton of growing rats. Bone. 2006 Mar;38(3):394–9. doi: 10.1016/j.bone.2005.09.006. Epub 2005/10/27. [DOI] [PubMed] [Google Scholar]
- 18.Albarracin M, Weisstaub AR, Zuleta A, Drago SR. Extruded whole grain diets based on brown, soaked and germinated rice. Effects on cecum health, calcium absorption and bone parameters of growing Wistar rats. Part I. Food Funct. 2016 Jun 15;7(6):2722–8. doi: 10.1039/c6fo00441e. Epub 2016/05/21. [DOI] [PubMed] [Google Scholar]
- 19.Griffin IJ, Davila PM, Abrams SA. Non-digestible oligosaccharides and calcium absorption in girls with adequate calcium intakes. Br J Nutr. 2002 May;87(Suppl 2):S187–91. doi: 10.1079/BJNBJN/2002536. Epub 2002/06/29. [DOI] [PubMed] [Google Scholar]
- 20.Abrams SA, Griffin IJ, Hawthorne KM, Liang L, Gunn SK, Darlington G, et al. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am J Clin Nutr. 2005 Aug;82(2):471–6. doi: 10.1093/ajcn.82.2.471. Epub 2005/08/10. [DOI] [PubMed] [Google Scholar]
- 21.Gunnes M, Lehmann EH. Dietary calcium, saturated fat, fiber and vitamin C as predictors of forearm cortical and trabecular bone mineral density in healthy children and adolescents. Acta Paediatr. 1995 Apr;84(4):388–92. doi: 10.1111/j.1651-2227.1995.tb13656.x. Epub 1995/04/01. [DOI] [PubMed] [Google Scholar]
- 22.van den Heuvel EG, Schoterman MH, Muijs T. Transgalactooligosaccharides stimulate calcium absorption in postmenopausal women. J Nutr. 2000 Dec;130(12):2938–42. doi: 10.1093/jn/130.12.2938. Epub 2000/12/09. [DOI] [PubMed] [Google Scholar]
- 23.Tahiri M, Tressol JC, Arnaud J, Bornet FR, Bouteloup-Demange C, Feillet-Coudray C, et al. Effect of short-chain fructooligosaccharides on intestinal calcium absorption and calcium status in postmenopausal women: a stable-isotope study. Am J Clin Nutr. 2003 Feb;77(2):449–57. doi: 10.1093/ajcn/77.2.449. Epub 2003/01/24. [DOI] [PubMed] [Google Scholar]
- 24.Holloway L, Moynihan S, Abrams SA, Kent K, Hsu AR, Friedlander AL. Effects of oligofructose-enriched inulin on intestinal absorption of calcium and magnesium and bone turnover markers in postmenopausal women. Br J Nutr. 2007 Feb;97(2):365–72. doi: 10.1017/S000711450733674X. Epub 2007/02/15. [DOI] [PubMed] [Google Scholar]
- 25.Jakeman SA, Henry CN, Martin BR, McCabe GP, McCabe LD, Jackson GS, et al. Soluble corn fiber increases bone calcium retention in postmenopausal women in a dose-dependent manner: a randomized crossover trial. Am J Clin Nutr. 2016 Sep;104(3):837–43. doi: 10.3945/ajcn.116.132761. Epub 2016/07/29. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z, Stini WA, Marshall JR, Martinez ME, Guillen-Rodriguez JM, Roe D, et al. Wheat bran fiber supplementation and bone loss among older people. Nutrition. 2004 Sep;20(9):747–51. doi: 10.1016/j.nut.2004.05.015. Epub 2004/08/25. [DOI] [PubMed] [Google Scholar]
- 27.Slevin MM, Allsopp PJ, Magee PJ, Bonham MP, Naughton VR, Strain JJ, et al. Supplementation with calcium and short-chain fructo-oligosaccharides affects markers of bone turnover but not bone mineral density in postmenopausal women. J Nutr. 2014 Mar;144(3):297–304. doi: 10.3945/jn.113.188144. Epub 2014/01/24. [DOI] [PubMed] [Google Scholar]
- 28.Heber D, Ashley JM, Leaf DA, Barnard RJ. Reduction of serum estradiol in postmenopausal women given free access to low-fat high-carbohydrate diet. Nutrition. 1991 Mar-Apr;7(2):137–9. discussion 9–40. Epub 1991/03/01. [PubMed] [Google Scholar]
- 29.Goldin BR, Adlercreutz H, Gorbach SL, Warram JH, Dwyer JT, Swenson L, et al. Estrogen excretion patterns and plasma levels in vegetarian and omnivorous women. N Engl J Med. 1982 Dec 16;307(25):1542–7. doi: 10.1056/NEJM198212163072502. Epub 1982/12/16. [DOI] [PubMed] [Google Scholar]
- 30.Gaskins AJ, Mumford SL, Zhang C, Wactawski-Wende J, Hovey KM, Whitcomb BW, et al. Effect of daily fiber intake on reproductive function: the BioCycle Study. Am J Clin Nutr. 2009 Oct;90(4):1061–9. doi: 10.3945/ajcn.2009.27990. Epub 2009/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dominianni C, Sinha R, Goedert JJ, Pei Z, Yang L, Hayes RB, et al. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One. 2015;10(4):e0124599. doi: 10.1371/journal.pone.0124599. Epub 2015/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karasik D, Ferrari SL. Contribution of gender-specific genetic factors to osteoporosis risk. Ann Hum Genet. 2008 Sep;72(Pt 5):696–714. doi: 10.1111/j.1469-1809.2008.00447.x. Epub 2008/05/20. [DOI] [PubMed] [Google Scholar]
- 33.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. 2010 Jun;23(1):65–134. doi: 10.1017/S0954422410000041. Epub 2010/06/23. [DOI] [PubMed] [Google Scholar]
- 34.Threapleton DE, Greenwood DC, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C, et al. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. Bmj. 2013;347:f6879. doi: 10.1136/bmj.f6879. Epub 2013/12/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Consortium TI. Dietary fibre and incidence of type 2 diabetes in eight European countries: the EPIC-InterAct Study and a meta-analysis of prospective studies. Diabetologia. 2015 Jul;58(7):1394–408. doi: 10.1007/s00125-015-3585-9. Epub 2015/05/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. Dec. 1975;4(4):518–25. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 37.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985 Jul;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. Epub 1985/07/01. [DOI] [PubMed] [Google Scholar]
- 38.Willett WC, Sampson L, Browne ML, Stampfer MJ, Rosner B, Hennekens CH, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988 Jan;127(1):188–99. doi: 10.1093/oxfordjournals.aje.a114780. Epub 1988/01/01. [DOI] [PubMed] [Google Scholar]
- 39.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992 May 15;135(10):1114–26. doi: 10.1093/oxfordjournals.aje.a116211. discussion 27–36. [DOI] [PubMed] [Google Scholar]
- 40.Mazess RB, Barden HS, Ettinger M, Johnston C, Dawson-Hughes B, Baran D, et al. Spine and femur density using dual-photon absorptiometry in US white women. Bone Miner. 1987 May;2(3):211–9. Epub 1987/05/01. [PubMed] [Google Scholar]
- 41.Nilas L, Christiansen C. Rates of bone loss in normal women: evidence of accelerated trabecular bone loss after the menopause. Eur J Clin Invest. 1988 Oct;18(5):529–34. doi: 10.1111/j.1365-2362.1988.tb01052.x. Epub 1988/10/01. [DOI] [PubMed] [Google Scholar]
- 42.Gagnon DR, McLean RR, Hannan MT, Cupples LA, Hogan M, Kiel DP. Cross-calibration and comparison of variability in 2 bone densitometers in a research setting: the framingham experience. J Clin Densitom. 2010 Apr-Jun;13(2):210–8. doi: 10.1016/j.jocd.2010.01.003. Epub 2010/03/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PW, et al. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000 Apr;15(4):710–20. doi: 10.1359/jbmr.2000.15.4.710. Epub 2000/04/26. [DOI] [PubMed] [Google Scholar]
- 44.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993 Feb;46(2):153–62. doi: 10.1016/0895-4356(93)90053-4. Epub 1993/02/01. [DOI] [PubMed] [Google Scholar]
- 45.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999 Jul;52(7):643–51. doi: 10.1016/s0895-4356(99)00049-9. Epub 1999/07/03. [DOI] [PubMed] [Google Scholar]
- 46.Services USDoAaUSDoHaH. Dietary Guidelines for Americans 2010. Washington, DC: 2010. p. 112. [Google Scholar]
- 47.Sauder KA, Proctor DN, Chow M, Troy LM, Wang N, Vita JA, et al. Endothelial function, arterial stiffness and adherence to the 2010 Dietary Guidelines for Americans: a cross-sectional analysis. Br J Nutr. 2015 Jun 14;113(11):1773–81. doi: 10.1017/S0007114515000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997 Apr;65(4 Suppl):1220S–8S. doi: 10.1093/ajcn/65.4.1220S. discussion 9S–31S. [DOI] [PubMed] [Google Scholar]
- 49.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468–89. doi: 10.1007/s001980050093. Epub 1998/12/16. [DOI] [PubMed] [Google Scholar]
- 50.Lu Y, Genant HK, Shepherd J, Zhao S, Mathur A, Fuerst TP, et al. Classification of osteoporosis based on bone mineral densities. J Bone Miner Res. 2001 May;16(5):901–10. doi: 10.1359/jbmr.2001.16.5.901. Epub 2001/05/09. [DOI] [PubMed] [Google Scholar]
- 51.Smith TaS. B. PROC GENMOD with GEE to Analyze Correlated Outcomes Data Using SAS. 2006 [Google Scholar]
- 52.Sahni S, Broe KE, Tucker KL, McLean RR, Kiel DP, Cupples LA, et al. Association of total protein intake with bone mineral density and bone loss in men and women from the Framingham Offspring Study. Public Health Nutr. 2014 Nov;17(11):2570–6. doi: 10.1017/S1368980013002875. Epub 2013/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002 Jun;23(3):279–302. doi: 10.1210/edrv.23.3.0465. Epub 2002/06/07. [DOI] [PubMed] [Google Scholar]
- 54.Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002 May 25;359(9320):1841–50. doi: 10.1016/S0140-6736(02)08706-8. Epub 2002/06/05. [DOI] [PubMed] [Google Scholar]
- 55.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990 Jan;1(1):43–6. Epub 1990/01/01. [PubMed] [Google Scholar]
- 56.Weaver CM, Martin BR, Story JA, Hutchinson I, Sanders L. Novel fibers increase bone calcium content and strength beyond efficiency of large intestine fermentation. J Agric Food Chem. 2010 Aug 25;58(16):8952–7. doi: 10.1021/jf904086d. Epub 2010/08/04. [DOI] [PubMed] [Google Scholar]
- 57.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013 Mar 01;339(6123):1084–8. doi: 10.1126/science.1233521. Epub 2013/01/19. [DOI] [PubMed] [Google Scholar]
- 58.Ohta A, Motohashi Y, Sakai K, Hirayama M, Adachi T, Sakuma K. Dietary fructooligosaccharides increase calcium absorption and levels of mucosal calbindin-D9k in the large intestine of gastrectomized rats. Scand J Gastroenterol. 1998 Oct;33(10):1062–8. doi: 10.1080/003655298750026769. Epub 1998/11/26. [DOI] [PubMed] [Google Scholar]
- 59.Lobo AR, Colli C, Filisetti TMCC. Fructooligosaccharides improve bone mass and biomechanical properties in rats. Nutrition Research. 2006;26(8):413–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.