Abstract
Artificial systems for controlled membrane fusion applicable for drug delivery would ideally use triggers that are orthogonal to biology. To apply the strain-promoted alkyneazide cycloaddition (SPAAC) to drive membrane fusion, ODIBO lipid 1 was designed, synthesized and studied alongside ADIBO-lipids 2–4 to assess fusion with liposomes containing azido-lipid 5. Lipids 1–2 were first shown to be effective for liposome derivatization. Next, fusion was evaluated using liposomes containing 1 and varying ratios of PC and PE via a FRET dilution fusion assay, and a 1/1 PC/PE ratio yielded the greatest signal change attributed to fusion. Finally, lipids 1–4 were compared, and 1 yielded the greatest triggering of fusion, while 2–4 yielded varying efficacies depending on the structural features of each lipid. Fusion was further validated through STEM studies showing larger multilamellar assemblies after liposome mixing. This work provides a platform for triggered fusion towards drug delivery applications and an understanding of the effects of lipid structure and membrane composition on fusion.
TOC image
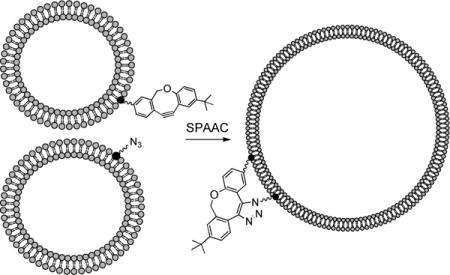
Introduction
Vesicle fusion is a critical biological process controlled by lipids and proteins that regulates numerous physiological and pathophysiological events. In natural systems, membrane fusion is directed by SNARE proteins embedded within membrane bilayers that control the association and fusion of specific membranes.1,2 The ability to mimic this action and selectively trigger the fusion of target membranes is invaluable for advancing drug targeting. As a result, the development of artificial platforms for driving membrane fusion has gained significant interest over the years. Thus, a variety of targeting groups have been used to instigate fusion,3,4 including small molecule based molecular recognition,5–13 complementary DNA strands,14–24 peptides,25–33 metal complex formation,34–37 and complementary reactive functional groups.38–46 This process typically involves the incorporation of separate interacting functional groups into different vesicles to drive association as well as manipulation of the lipid content to dictate the stability of membrane bilayer and drive fusion.
While these methods have been successful for triggering membrane fusion in vitro, the development of platforms that can translate into living systems presents significant challenges due to the diversity of functional groups present within biomolecules, and thus the difficulty in achieving selectivity in membrane targeting. Only recently have initial examples of in vivo targeted membrane fusion been reported using coiled coil peptides attached onto membrane surfaces.47,48 Bioorthogonal chemistry presents a promising strategy for attaining this goal due to the selectivity of these reactions in complex biological environments.49,50 We and others have shown that the derivatization of intact liposomes can be conveniently achieved using bioorthogonal reactions, such as the copper-catalyzed azide-alkyne cycloaddition (CuAAC),51–56 the strain promoted alkyne-azide cycloaddition (SPAAC),57–59 and the Staudinger ligation.60,61 This has been extended to the development of click chemistry-based membrane fusion systems using oxyamine-ketone38,40,44–46 as well as CuAAC39,42 conjugations. The latter system suffers due to the delivery requirements and the toxicity and side reactions associated with the copper catalyst,62 and since liposomes have been shown to decompose under CuAAC conditions.22 In addition, prospects for targeted drug delivery will be advanced by using functional groups that are effective for metabolic labeling of biological molecules in living systems.49
Herein, we describe the development of membrane fusion driven by SPAAC63 to circumvent these issues. In doing so, we have evaluated lipid analogs presenting azide64–66 and dibenzocyclooctyne (DIBO)67,68 functional groups as bioorthogonal reactive pairs to promote membrane fusion. These compounds were incorporated into unilamellar liposomes to investigate fusogenic properties as a function of the specific clickable lipids tested and the lipid composition of the vesicles. Membrane derivatization and fusion were assessed using Förster Resonance Energy Transfer (FRET) assays. These results provide insights into the optimal reactants and membrane properties for vesicle fusion driven by SPAAC.
Results and Discussion
We initiated this work with the design and synthesis of copper free clickable lipids to drive membrane fusion. For the azido-lipid, preliminary experiments led us to settle upon compound 5, which we have previously reported.59 For the cyclooctyne−lipid partner, we sought to incorporate an oxo-dibenzocyclooctyne (ODIBO) reactive partner, which has been shown to possess very fast kinetic properties in reacting with azide.69 In doing so, we designed ODIBO−lipid 1, in which this group is attached at the head group of a lipid scaffold via a tetraethylene glycol (TEG) spacer to provide distance from the membrane surface. The synthesis of lipid 1 is depicted in Scheme 1 and commences with (S)-glycerol acetonide (6). This compound was tosylated to 7, acetonide deprotected to 8, substituted with azide to 9 and two stearyl chains were introduced through coupling to 5, as we previously described.59 Next, t-butoxycarbonyl (Boc)-protected propargylamine (10) was introduced via click chemistry to 11.
Scheme 1.

Synthetic route to azido−lipid 5 and ODIBO−lipid 1.
We next needed to synthesize a tetherable ODIBO moiety to introduce this functional group onto the lipid backbone of 1. We also chose to exploit this portion of the synthesis to introduce the TEG linker that spaces the ODIBO from the membrane surface. To do so, an N-acetyl-TEG group was first introduced onto previously reported ODIBO 12 using Mitsunobu conditions to produce 13. The acetamide group was then deprotected to 14, after which the resulting amine was used for ring opening of succinic anhydride to produce ODIBO-TEG-carboxylic acid 15. Finally, Boc deprotection of 11 was followed by amide bond forming coupling reaction with 15 to produce ODIBO-lipid 1.
During the development of ODIBO−lipid 1 additional cyclooctyne containing lipid constructs became available for analysis of fusinogenic properties, particularly those containing aza-dibenzocyclooctyne ADIBO reactive groups.67,68 We concurrently developed the photocleavable and clickable analog 2 (Figure 1). In addition, both ADIBO−phosphatidylethanolamine (PE) conjugates 3 and 4, the latter of which contains a poly(ethylene glycol) (PEG) spacer between the lipid and ADIBO regions, became commercially available through Avanti Polar Lipids, Inc.
Figure 1.

Additional cyclooctyne-lipids analyzed for fusinogenic properties.
Thus, these compounds were investigated as we proceeded through the project and these compounds became accessible. Before testing the fusogenic capabilities of these cyclooctyne-labeled lipids, we first sought to evaluate their efficacy for in situ membrane derivatization. This was assessed using a FRET conjugation assay depicted in Figure 2. Here, liposomes primarily composed of phosphatidylcholine (PC, 90–91%) containing either ODIBO−lipid 1 or ADIBO−lipid 2 (9%) along with 7-nitro-2-1,3-benzoxadiazol-4-yl-PE (NBD-PE, 0–1%) as a FRET donor were treated with rhodamine-azide 1670 as a FRET acceptor. The SPAAC reaction should thus enforce proximity between the NBD and rhodamine, thereby activating FRET. All liposomes described herein were freshly prepared through mixing of chloroform solutions, drying, hydration, freeze/thaw cycles, and extrusion using either a 200 nm (for derivatization studies) or 100 nm (for fusion studies) polycarbonate filter, as further described in the experimental section.
Figure 2.

FRET conjugation assay for detection of membrane derivatization by SPAAC using ODIBO-lipid 1 or ADIBO-lipid 2.
As can be seen from the resulting data in Figure 3, liposomes containing 1 or 2 both led to a significant decrease in the NBD/rhodamine emission ratio, indicating the activation of FRET. ODIBO−lipid 1 led to a greater change in emission properties, which is in agreement with the improved reactivity of ODIBO for SPAAC compared to ADIBO. Control liposomes lacking any cyclooctyne led to a smaller but observable change in the FRET ratio. We have observed a similar result previously in studying membrane labeling, and attribute this to non-covalent interactions of 16 with the liposomes.59 Finally, we will note that since azido- and cyclooctynyl-lipids were used for liposome formation, the reactive moieties should be present in both the inner and outer leaflets of the liposomes, and these studies do not indicate whether the inner leaflet cyclooctyne reacts or not.
Figure 3.

Change in FRET (NDB/rhodamine emission ratio) as a function of time upon treatment of liposomes containing ODIBO-lipid 1, ADIBO-lipid 2 or control lacking cyclooctyne with rhodamine-azide 16. Error bars denote standard errors calculated from at least three replicate experiments.
After confirming the efficacy of liposome derivatization, we next moved to investigate membrane fusion driven by SPAAC using cyclooctynyl-lipids. Here, it was expected that SPAAC reaction between liposomes containing cyclooctynyl-lipid and those containing azide-lipid 5 would enforce close proximity between the covalently fused liposomes (Figure 4). Depending on the membrane composition, this proximity may then drive fusion, resulting in the formation of larger liposomes. This process was once again tracked by FRET, in this case using a FRET dilution assay.8,14,71,72 To do so, liposomes containing azido lipid 5 as well as both NBD-PE and rhodamine-PE were treated with liposomes containing cyclooctynyl-lipids of type 1–4 lacking any fluorescent labels. Fusion of the vesicles would thus lead to dilution of the FRET-tagged lipids within the membrane, which can be detected via an increase in the NBD/rhodamine emission ratio. Following prior procedures, we utilized a three-fold excess of unlabeled liposomes containing azido-lipid 5 to enhance the FRET dilution driven by fusion.73
Figure 4.

FRET assay for detecting membrane fusion based on the dilution of FRET pairs in original liposomes, leading to an increase in the NBD-PE/rhodamine-PE emission ratio.
The composition of membranes is an important aspect in the triggering of fusion, and partial destabilization of the bilayer is often necessary to overcome the activation barrier for merging two separate membranes. A common route to promoting fusion is to incorporate a non-bilayer lipid such as PE, which does not form stable membrane bilayers on its own due to the cone angle formed by the relative sizes of the lipid headgroup and acyl chains.74,75 Such lipids can be incorporated into liposomes formed by bilayer lipids such as PC, although they disrupt lipid packing and thus destabilize the membrane. Therefore, in our investigation of membrane fusion driven by SPAAC using ODIBO-lipid 1, we initially evaluated the effect of PE incorporation within PC liposomes on fusion properties. To study fusion, we performed FRET dilution assays as described in Figure 4 with liposomes composed of varying percentages of PC (45%, 50% or 60%), and PE (45%, 35% or 30%, respectively), ODIBO−lipid 1 (8%), NBD-PE (1%), and rhodamine-PE (1%). These were treated with partner liposomes composed of fixed percentages of PC (46%), PE (46%), and azido-lipid 5 (8%), and fluorescence was detected over time.
In these studies, at best minimal signal changes were observed in the absence of sample heating using a variety of liposomal compositions, and thus all fusion experiments described were heated to 40 °C in between fluorescence readings. In the results from these experiments shown in Figure 5, the greatest change in signal was the increase in NBD/rhodamine emission ratio observed with an equal mixture of PC and PE (45% each). Either higher or lower percentages of PE led to significant decreases in signal change that extended beyond the range reported in figure 5A. Control liposomes were also evaluated for each percentage of PE studied, and in each case less fusion was observed than the sample containing 1. The background fusion observed with the control sample containing 45% each of PC and PE was greater than the other two controls, suggesting that this ratio does lead to some non-specific fusion. Nevertheless, fusion was enhanced dramatically using ODIBO−lipid 1. A representative overlay of spectra showing the change in emission properties over time for the optimized sample is shown in Figure 5B.
Figure 5.

A. FRET assay results for fusion using ODIBO-lipid 1 containing liposomes at various PC/PE percentages (60/30 (yellow triangle), 50/40 (red circle), 45/45 (blue circle)) and their corresponding controls (black square, green square, light blue triangle, respectively) mixed with azido-lipid 5 labeled containing liposomes. The PC/PE 45/45 ratio yielded by far the greatest signal change. Error bars denote standard errors calculated from at least three replicate experiments. B. Representative overlay of spectra showing the change in emission properties over time for the PC/PE/1/NBD-PE/rhodamine-PE 45/45/8/1/1 samples.
With this evidence supporting fusion using ODIBO−lipid 2, and an effective lipid composition identified, we next set out to evaluate the triggering of fusion using the other available cyclooctyne-lipids 2–4. All samples employed liposomes composed of 45% PC, 45% PE, 8% of the appropriate cyclooctyne-lipid (1–4) and 1% each of NBD-PE and rhodamine-PE, and were treated with the same azide-containing liposomes described previously. From the results shown in Figure 6, ODIBO−lipid 1 was found to exhibit the greatest change. We attribute this to the enhanced reactivity of ODIBO, and therefore the ability of this group to covalently link a greater number of liposomes, bringing them into close proximity to promote fusion. ADIBO-PE conjugates 3 and 4 were also effective for triggering fusion compared to the control. The latter compound, which contains a PEG linker between the lipid and ADIBO, yielded greater signal change. The effect of the PEG linker is of interest since this extended tether may result in better presentation of the ADIBO group for reaction, but the resulting covalently tethered liposomes would be separated by a greater distance, which could hinder fusion70,76. The enhanced fusion observed with 3 containing a PEG linker compared to 4 indicates that the longer linker is favorable. It should also be noted that only one of the reactive partners has a long linker as the azide group of 5 is very close to the hydrophobic portion of the lipid. Compounds 3 and 4 are also negatively charged, differentiating them from the neutral compounds 1 and 2. Finally, ADIBO-lipid 2 did not show enhanced reactivity compared to the control samples. This compound has the same reactive group and a comparable linker to lipid 3, but lacks the phosphodiester moiety of the latter. It also possesses a fairly rigid and hydrophobic linker. It is possible that these properties lead to less optimal presentation of the ADIBO group, thereby diminishing fusion.
Figure 6.

FRET fusion assay results as a function of cyclooctyne-lipid using ODIBO (1, blue circle), ADIBO 2 (green circle), 3 (red triangle) or 4 (yellow square)) or control liposomes lacking cyclooctyne-lipid. Compound 1 exhibited the greatest change, and lipids 3–4 were also effective, while 2 was comparable to control. Error bars denote standard errors calculated from at least three replicate experiments.
To provide further evidence for fusion and understanding of the types of structures formed from this process, we performed scanning transmission electron microscopy (STEM) experiments using both original liposome samples and those following fusion. As can be seen in Figure 7A, images of the azido-liposomes used for fusion studies (46/46/8 PC/PE/5) show the expected liposomes of ~200 nm size formed by the extrusion process. In contrast, upon treatment with ODIBO-lipid containing liposomes (46/46/8 PC/PE/5) much larger assemblies are observed (Figure 7B), which appear to be divided into compartments due to multilamellar formations. In these fusion studies, we gravitated towards higher percentages of clickable lipids (8%) inside the liposomes to maximize FRET dilution signal in our assays. At these percentages, each liposome could undergo multiple fusion events leading to significantly larger fusion products. The assemblies provide evidence for fusion and mixing of lipid contents, while the presence of multilamellar compartments indicates that fusion is not complete for all membranes.
Figure 7.

STEM images of liposomes before and after fusion driven by SPAAC. A. Images of azido-liposomes (46/46/8 PC/PE/5) show ~200 nm liposomes. B. Images after mixing of azido- (46/46/8 PC/PE/5) and ODIBO- (46/46/8 PC/PE/1) containing liposomes indicate much larger and multilamellar assemblies, indicating fusion and incomplete lipid mixing. All scale bars correlate with 200 nm.
Finally, we sought to determine whether the aqueous contents of liposomes were undergoing mixing during fusion driven by these clickable lipids. To do so, we utilized the mixing assay in which separate liposomes encapsulating either terbium (Tb+3) or dipicolinic acid (DPA) are incubated.71,77 Mixing of encapsulated contents using this assay generates a new fluorescence signal caused by DPA-Tb+3 complexation. In our study, two samples of liposomes composed of 46% PC, 46% PE and either 8% of ODIBO-lipid 1 or azido-lipid 5, and encapsulating Tb or DPA, respectively, were prepared. When these liposomes were mixed at 40 °C, a new fluorescence signal emerged at 489 nm indicating DPA-Tb+3 binding, as seen in Figure 8. Additionally, a control study was run using two sets of liposomes containing 54% PC and 46% PE encapsulating Tb or DPA. The mixing of these liposomes under the same conditions led to a lesser increase in fluorescence, providing evidence for enhanced aqueous mixing driven by the presence of clickable lipids. The signal generated in the control can be attributed to processes including non-specific fusion (as seen in Figure 5A), and/or leakage of contents from the liposomes. Taken together, the enhanced fluorescence signal generated in the study samples provide evidence of the mixing of aqueous contents encapsulated within the liposomes using this platform.
Figure 8.

Fluorescence signal of the DPA-Tb complex (489 nm) detected over time upon mixing of study samples (46/46/8 PC/PE/1 encapsulating DPA + 46/46/8 PC/PE/5 encapsulating Tb) and control samples (54/46 PC/PE encapsulating DPA + 54/46 PC/PE encapsulating Tb). Error bars denote standard errors calculated from at least three replicate experiments.
Conclusions
In conclusion, we designed and synthesized novel cyclooctyne-lipid 1 containing the highly reactive ODIBO moiety for SPAAC. This compound was shown to be more effective for membrane derivatization and triggering of fusion compared to multiple ADIBO-lipids, and that many of the latter lipids were also effective. Fusinogenic properties using this platform were greatest using a 1/1 ratio of PC and PE, the latter of which is a non-bilayer forming lipid that is conducive for promoting fusion. STEM and aqueous mixing studies provided further evidence for the membrane fusion and the mixing of lipid and encapsulated aqueous contents. This work acts as a milestone towards the development of click chemistry-driven fusion processes as a means for selective targeting of liposomal drug delivery vehicles.
General Experimental
Reagents and solvents were generally purchased from Acros, Aldrich or Fisher Scientific and used as received. PC (L-α-Phosphatidylcholine, mixed isomers from chicken egg), PE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) (NBD-PE), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (rhodamine-PE), and ADIBO-lipids 3 and 4 were purchased from Avanti Polar Lipids, Inc. Compounds 2, 5, 7–9, and 16 were synthesized as previously reported.59,78,79 Dry solvents were obtained from a Pure solvent delivery system purchased from Innovative Technology, Inc. Column chromatography was performed using 230–400 mesh silica gel purchased from Sorbent Technologies. NMR spectra were obtained using Varian Mercury 300 and 500 MHz spectrometers. Mass spectra were obtained with JEOL DART-AccuTOF and ABI Voyager DE Pro MALDI mass spectrometers with high-resolution capabilities. Liposome extruder and polycarbonate membranes were obtained from Avestin (Ottawa, Canada). Ultrapure water was purified via a Millipore water system (≥ 18 MΩ·cm triple water purification system). Small quantities (< 5 mg) were weighed on a OHRUS analytical-grade mass balance. Fluorescence studies were performed using a Perkin Elmer LS55 fluorimeter. Plots were generated using SigmaPlot 13, and curves in Figure 3 were fitted using an exponential decay function, while those in Figures 5A and 6 were fitted using an exponential growth to maximum function. All errors bars in plots include error bars depicting the standard errors of at least three experimental replicates.
(S)-3-(4-(((tert-butoxycarbonyl)amino)methyl)-1H-1,2,3-triazol-1-yl)propane-1,2-diyl distearate (Boc-Triazole Lipid) (11)
Azido-lipid 5 (57 mg, 0.088 mmol) and Boc-protected propargylamine 10 (11.3 mg, 0.0730 mmol) were both dissolved in a 2:1 v/v mixture of tetrahydrofuran/t-butanol (3 mL).80 Copper (II) sulfate pentahydrate (72.9 mg, 0.292 mmol) and sodium ascorbate (115 mg, 0.584 mmol) were each dissolved in 0.5 mL of water in separate vials. The ascorbate solution was added to the tetrahydrofuran/t-butanol mixture, and after 5 min of stirring, the copper (II) sulfate solution was added slowly, which caused the reaction mixture to change color from clear yellow to dark brown. After stirring for 16 h, the solvent was evaporated to yield a greenish blue solid. The crude solid was purified via column chromatography (gradient elution from 1–2.5% methanol/dichloromethane) to yield boc-triazole lipid 11 in the form of a white solid (58 mg, 60%). 1H NMR (300 MHz, CDCl3): δ 7.55 ppm (s, 1H), 5.37 ppm (m, 1H), 5.07 ppm (bs, 1H), 4.59 ppm (s, 2H), 4.38 ppm (d, J = 5.9 Hz, 2H), 4.30 (dd, J = 12.0, 4.4 Hz, 1H), 4.00 ppm (dd, J = 11.1, 6.2, 1H), 2.33 ppm (m, 4H), 1.62 ppm (s, 4H), 1.44 ppm (s, 9H), 1.26 ppm (bs, 56H), 0.88 ppm (t, J = 6.5 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 173.06, 172.50, 157.81, 122.74, 109.99, 77.18, 69.30, 61.93, 50.08, 36.02, 34.01, 31.91, 29.69, 29.62, 29.47, 29.35, 29.24, 29.12, 29.02, 28.35, 24.83, 22.68, 14.10. MS-DART-(+): calc. [M+H]: 805.6782; found: 805.7267.
2-tert-butyl-11,12-didehydro-8-(2-(2-(2-(2-aminoethoxy)ethoxy)ethoxy)ethoxy)-6H-dibenzo [b,f]oxocine (ODIBO-amine, 14)
Diisopropyl azodicarboxylate (DIAD, 1.06 g, 5.23 mmol) was added to a solution of ODIBO-OH (12, 1.46 g, 5.23 mmol), CH3CONH-(CH2CH2O)4H (1.51 g, 5.23 mmol), and triphenylphosphine (PPh3, 1.65 g, 6.28 mmol) in tetrahydrofuran (50 mL) at 0 °C. The reaction mixture was allowed to stir for 5 min at 0 °C, warmed to rt, stirred for an additional 30 min, and concentrated in vacuum. The residue was purified by flash chromatography (dichloromethane: methanol, 40:1) to afford crude amide 13 (3.45 g). The crude product (3.45 g) was then dissolved into methanol (24 mL), a solution of potassium carbonate (K2CO3, 0.722 g, 5.22 mmol) in water (11.60 mL) was added, and the reaction mixture was then stirred overnight and concentrated in vacuum. The residue was re-dissolved into dichloromethane (300 mL), and washed with water (75 mL), and then brine (75 mL). The organic layer was dried over magnesium sulfate, filtered, concentrated in vacuum, and purified by flash chromatography (gradient elution with dichloromethane: methanol 40:1 to 10:1) to afford ODIBO-TEG-amine 14 (1.50 g, 63 % over two steps) as a yellow oil. 1H-NMR: δ 7.20–7.25 (m,3H), 7.09–7.11 (d, J = 9.2 Hz, 1H), 7.03 (d, J = 2.2 Hz, 1H), 6.89–6.92 (dd, J = 8.4, 2.3 Hz, 1H), 5.16–5.19 (d, J = 12 Hz, 1H), 4.51–4.54 (d, J = 12 Hz, 1H), 4.15–4.17 (t, J = 4.7 Hz, 2H), 3.85–3.88 (t, J =4.7 Hz, 2H), 3.72–3.73 (m, 2H), 3.62–3.69 (m, 6H), 3.51–3.54 (t, J = 5.0 Hz, 2H), 2.88–2.90 (t, J = 5.1 Hz, 2H), 1.31 (s, 9H). 13C-NMR: δ 167.31, 158.71, 149.13, 146.95, 126.89, 125.57, 123.69, 121.37, 118.37, 117.89, 117.65, 114.65, 114.17, 110.75, 78.02, 72.47, 70.98, 70.75, 70.38, 69.78, 67.93, 41.59, 34.55, 31.56. ESI HRMS: calcd. (M+H+): C27H36NO5 454.2593, found 454.2572.
1-((2-(tert-butyl)-11,12-didehydro-6H-dibenzo[b,f]oxocin-8-yl)oxy)-13-oxo-3,6,9-trioxa-12-azahexadecan-16-oic acid (ODIBO-TEG-COOH, 15)
Succinic anhydride (0.111 g, 1.106 mmol) was added to a solution of ODIBO amine (14, 0.386 g, 0.851 mmol) and triethylamine (0.172 g, 1.702 mmol) in chloroform (9 mL). The reaction mixture was allowed to stir for 4h, concentrated in vacuum, and purified by flash chromatography to afford ODIBO-TEG-COOH (15, 0.187 g, 40 % yield) as a light yellow oil. 1H-NMR: δ 7.22–7.27 (m, 3H), 7.10–7.112 (d, J = 9.3 Hz, 1H), 7.02–7.03 (d, J = 2.1 Hz, 1H), 6.89–6.91 (dd, J = 8.5, 2.0 Hz, 1H), 5.17–5.20 (d, J = 12.0 Hz, 1H), 4.52–4.55 (d, J = 12.0 Hz, 1H), 4.16–4.18 (t, J = 4.5 Hz, 2H), 3.86–3.89 (t, J = 4.4 Hz, 2H), 3.76–3.73 (m, 2H), 3.66–3.71 (m, 4H), 3.62–3.63 (m, 2H), 3.53–3.55 (m, 2H), 3.42–3.45 (m, 2H), 2.61–2.65 (m, 2H), 2.48–2.52 (m, 2H), 1.32 (s,9H). 13C-NMR: δ 174.85, 172.93, 167.25, 158.56, 149.13, 147.07, 126.95, 125.67, 123.76, 121.41, 118.58, 117.86, 117.60, 114.64, 114.05, 110.89, 78.02, 70.93, 70.84, 70.61, 70.32, 69.76, 67.82, 39.69, 34.59, 31.58, 31.18, 30.58. ESI HRMS: calcd. (M+H): C31H40NO8 + 554.2754, found 554.2754.
ODIBO-lipid (1)
In a flame-dried flask, lipid 11 (12 mg, 0.015 mmol) was dissolved in freshly distilled dichloromethane (1 mL). Next, 1 mL of trifluoroacetic acid (TFA) was slowly added at rt. The solution was stirred for 2 h or until TLC showed complete removal of the starting material. The solvent was next evaporated, after which the crude was immediately re-dissolved in 1 mL of dry N,N-dimethylformamide. To this was then added diisopropylethylamine (DIEA, 100 μL, 0.574 mmol), after which the reaction mixture was allowed to stir until bubbling subsided. This was then added to a flame-dried vial containing ODIBO-TEG-COOH 15 (8 mg, 0.0144 mmol) Next, the solution was suspended in an ice bath, and then 4-dimethylaminopyridine (DMAP, 1.99 mg, 0163 mmol) was added. After 10 min of stirring in an ice bath, EDC•HCl (3.12 mg, 0163 mmol) was added. The solution was allowed to stir for 10 min, and was then removed from the ice bath and stirring was continued overnight. Following this, the solvent was removed using a rotary evaporator, and then the crude was re-dissolved in 5 mL of chloroform. The organic solution was washed with saturated sodium bicarbonate (2 × 5 mL), water (1 × 5mL), and brine (1 × 5 mL). The organic layer was next dried with magnesium sulfate, filtered, and then concentrated under N2 gas. The crude was purified via column chromatography (gradient elution with 2.5–3% methanol/dichloromethane) to produce ODIBO-lipid 1 as a yellow-white solid (10 mg, 55%). 1H-NMR (500 MHz, CDCl3): δ 7.55 ppm (s, 1H), 7.27 (s, 1H), 7.22–7.25 (dd, J = 8.8, 2.4 Hz, 2H), 7.07–7.11 (dd, J = 9.2, 2.3 Hz, 1H), 7.01–7.02 (d, J = 2.5 Hz, 1H), 6.87–6.92 (m, 1H), 6.66 (bs, 1H), 5.35 (s, 1H), 5.17 (d, J = 12.0 Hz, 1H), 5.11 (dd, J = 6.6, 3.1 Hz, 1H), 4.52–4.55 (d, J = 12.0 Hz, 1H), 4.45–4.47 (m, 2H), 4.30 (dd, J = 12.0, 4.4 Hz, 1H) 4.17 (t, J = 4.5 Hz, 2H), 4.04 (dd, J = 11.1, 6.2, 2H), 3.88 (t, J = 4.4 Hz, 2H), 3.51–3.76 (m, 10H), 3.42–3.45 (m, 2H),, 2.49 (s, 4H), 2.32 ppm (m, 4H), 1.62 ppm (m, 4H), 1.31 ppm (s, 9H), 1.26 (bs, 56H), 0.88 ppm (t, J = 6.5 Hz, 6H). 13C NMR (125 MHz): δ183.13, 173.08, 172.55, 172.28, 167.07, 158.44, 148.92, 145.12, 126.70, 125.42, 123.53, 122.97, 121.18, 119.43, 117.66, 117.42, 114.42, 113.87, 110.64, 109.99, 77.81, 70.77, 70.50, 70.13, 69.89, 69.62, 69.29, 67.67, 61.97, 50.01, 39.27, 35.38, 35.07, 34.36, 34.00, 33.98, 31.91, 31.36, 29.69, 29.65, 29.48, 29.35, 29.26, 29.13, 29.03, 28.13, 24.83, 24.73, 22.68, 14.10. calculated exact mass [M+H] 1240.8828 MS-ESI-(+): found 1240.8828.
Liposome preparation
Stock solutions of lipids 1 and 2, PC, NBD PE, and rhodamine-azide 16 (Rhd-N3) were made in chloroform for fluorimeter studies. Examples of these stock solutions were prepared as follows: 6 mg of ODIBO-lipid 1, 1.2 mg of ADIBO-lipid 2, 100 mg of PC, 1 mg of NBD-PE, and 1.8 mg of Rhd-N3 16 were dissolved in 1 mL, 1 mL, 4 mL, 1.5 mL, and 2 mL of chloroform, respectively, to form stock solutions of 4.83 mM lipid 1, 0.97 mM lipid 2, 32.5 mM PC, 0.72 mM NBD-PE, and 1.18 mM Rhd-N3 16. In three separate separate vials, we used these stock solutions to add the following: 11.8 μL PC (90%), 8 μL lipid 1 (9%), and 5.8 μL NBD-PE (1%) in vial A; 11.9 μL PC (90%), 40 μL lipid 2 (9%), and 5.9 μL NBD-PE (1%) in vial B; and 11.9 μL PC (99%) and 5.4 μL (1%) in vial C. Each lipid solution was evaporated using argon gas to generate a solid lipid film. All vials were placed under vacuum for 4 h and then dissolved in Tris-HCl buffer (10 mM)/NaCl (90 mM)/EDTA (1 mM) to produce 2 mM liposome solutions. The solutions were hydrated for 1 h at 50 °C, and then subjected to 10 freeze/thaw cycles to form unilamellar vesicles. Next, the liposome solutions were extruded 15 times using a 200 nm polycarbonate membrane. Other liposomes used for studies were produced in the same manner using different percentages of the appropriate lipids.
Membrane derivatization studies
Rhodamine-azide 16 (48.96 μL, 58.0 nmol) was added to the two separate microcuvettes, and then evaporated with Ar gas to leave solid Rhd-N3. Using the ODIBO 1 liposome solution (22 μL) was added to one of the cuvettes, and then diluted with Tris buffer (300 μL) to give a final total concentration of 220 μM. An initial reading was collected; the resulting solution was mixed thoroughly with a pipette for 5 minutes, and the cuvette was then placed in the fluorimeter. The fluorimeter was set to scan an excitation wavelength of 460 nm and with emission and excitation slits of 9.5 nm and 7.5 nm, respectively. Fluorescent readings were collected for ~40 min. This procedure was repeated for the study of ADIBO lipid 2 in the second microcuvette containing Rhd-N3 16.
Membrane fusion FRET dilution studies using ODIBO-lipid 1 by varying PC/PE percentages
PE (5 mg), azido-lipid 5 (2.3 mg), and rhodamine-PE (2 mg) were dissolved in 1.5 mL, 2 mL, and 675 μL of chloroform, respectively, to form stock solutions of 4.48 mM PE, 5.24 mM lipid 5, and 0.77 mM rhodamine-PE. These stock solutions along with the stock solutions of PC (32.46 mM), ODIBO lipid 1 (4.83 mM), and NBD-PE (0.72 mM) were used to make lipid solutions of 60:30:8:1:1 (PC:PE:1:NBD-PE:Rhd-PE), 50:40:8:1:1 (PC:PE:1:NBD-PE:Rhd-PE), 45:45:8:1:1 (PC:PE:1:NBD-PE:Rhd-PE), 46:46:8 (PC:PE:5), and 50:50 (PC:PE). Each lipid solution was converted into a 2 mM liposome solution by employing a similar protocol outlined in the liposome preparation section using 100 nm polycarbonate membranes for extrusion. Cyclooctyne-labeled liposome solution (20 μL) was added to a microcuvette and diluted with Tris buffer (420 μL), and an initial reading was taken on the fluorimeter (0 min). Next, azido-labeled liposome solution (60 μL) was added to the cuvette to give a final total concentration of 250 μM. The solution was mixed thoroughly with a pipette for ~ 2 min while the cuvette was submerged in a warm water bath (~ 40 °C). Fluorescence measurements were taken at 5, 15, 25, 35, 60, and 90 minutes after mixing, with heating in between each fluorescence scan. The ratio of fluorescence intensities at NBD emission (520 nm) and Rhd emission (588 nm) was calculated, and each ratio was normalized by subtracting the initial NBD/Rhd ratio from each measurement.
FRET dilution studies using cyclooctyne-lipids 1–4
ADIBO-PEG lipid 4 (5 mg), and ADIBO-lipid 3 (5 mg) were dissolved in 400 μL and 1 mL of chloroform, respectively, to form stock solutions of 4.44 mM of ADIBO 4 and 5.02 mM of ADIBO 3. These stock solutions and the stock solutions of PC (32.46 mM), PE (4.48 mM), lipids 1, 2, and 5 (4.83 mM, 0.97 mM, and 5.24 mM, respectively), NBD-PE (0.72 mM), and Rhd-PE (0.77 mM) were used to make lipid solutions of 45:45:8:1:1 (PC:PE:cyclooctyne-lipid:NBD-PE:Rhd-PE), 46:46:8 (PC:PE:azide 5), and 49:49:1:1 (PC:PE:NBD-PE:Rhd-PE). One lipid solution was made for each cyclooctyne lipid (1 4). Each lipid solution was converted into a 2 mM liposome solution by employing a similar protocol outlined in the previous liposome preparation protocol using 100 nm polycarbonate membranes for extrusion. Cyclooctyne-labeled liposome solution (20 μL) was added to a microcuvette and diluted with Tris buffer (420 μL), and an initial reading was taken on the fluorimeter (0 min). Next, azido-labeled liposome solution (60 μL) was added to the cuvette to give a final total concentration of 250 μM. The solution was mixed thoroughly with a pipette for ~ 2 min while the cuvette was submerged in a warm water bath (40 °C). Fluorescence measurements were taken at 5, 15, 25, 35, 60, and 90 minutes after mixing, with heating in between each fluorescence scan. The ratio of fluorescence intensities at NBD emission (520 nm) and Rhd emission (588 nm) was calculated, and each ratio was normalized by subtracting the initial NBD/Rhd ratio from each measurement.
Scanning transmission electron microscopy (STEM) experiments
Liposomes consisting of azido-lipid (46/46/8 PC/PE/5), ODIBO-lipid (46/46/8 PC/PE/5) were prepared using the procedure listed above, each of which was prepared to a total concentration of 400 μM. One portion of azido-lipid liposomes was saved for STEM studies, while another aliquot was mixed with the ODIBO-lipid solution, heated to 35 °C in a water bath for 5 min, and then allowed to sit at rt for 1 h. A drop (5–10 μL) from each solution was immobilized onto separate carbon filters. Each filter was stained using a 5% (w/v) solution of phosphotungstic acid, and the filter was stored in a desiccator overnight prior to experiment. Images were collected using a Zeiss Auriga Scanning Electron Microscope. The electron beam was set at 25 eV, and images were detected using an Everhardt-Thornley SE2 detector.
Membrane fusion content mixing assay
Stock chloroform solutions of PC (65 mM), PE (13.9 mM) ODIBO lipid 1 (1.3 mM), and azido lipid 5 were prepared and used to make four lipid solutions with three different compositions: O: 46:46:8 (PC:PE:1), A: 46:46:8 (PC:PE:5), and C: 54:46 (PC:PE). The solvent was removed using a rotary evaporator and the films were dried under vacuum overnight. Hydration of the lipid films was done using one of two buffers both containing 2 mM L-(–)-histidine and 2 mM N-[Tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid sodium salt (TES), with buffer T containing 15 mM TbCl3 and buffer D containing 50 mM DPA. O was hydrated in buffer D, A was hydrated in buffer D, and C liposomes were prepared both ways (CT and CD). From here, liposomes were formed following the protocol laid out in the liposome preparation section such that 1 mL of each liposome type was formed, each with a 4 mM lipid concentration. After extrusion using 100 nM polycarbonate filters, unencapsulated TbCl3 and DPA was removed using size exclusion chromatography (SEC). SEC was adapted from a previously reported protocol.77 60 mL of SEC buffer was prepared with ultrapure water with 2 mM TES, 2 mM histidine, 100 mM NaCl and 1 mM EDTA to which 1.2 g of Sephadex™ G-50 Medium (GE Healthcare) was added and allowed to hydrate for 3 hours with occasional mixing. Glass microcolumns with 8 mm diameters were gravity-packed with 5 cm of the hydrated SEC beads. Each liposome solution was eluded by gravity through its own column with SEC buffer. 1 mL fractions were collected, kept 4 °C, and used within 24 hours. Liposomes were observed in the second fractions and aliquots of these were used for fluorescence studies. Fluorescence experiments were done on a PerkinElmer LS-55 luminescence spectrometer using a microcuvette with a 100 uL observation chamber and a scan rate of 100 nm/min. Excitation was at 278 nm with a 5.0 nm slit. Emission slit was 7.5 nm and was measured using an average of 3 scans. Max emission was observed at 489 nm. For the experimental mixing group: 300 uL of liposomes O were added first and a baseline scan was taken, 300 uL of liposomes A were mixed in and another scan was taken before beginning to heat and mix between measurements. Heating was done by placing the sealed cuvette in a 40 °C water bath followed by 5 s of vortexing and the heating times between measurements increased incrementally as follows: 30 s, 30 s, 2 min, 2 min, 5 min, 5 min, 10 min, 15 min, 20 min for a total of 1 hour. For the control mixing group, the study began with a baseline measurement of CD followed by addition of CT with all other aspects being the same as the experimental group.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute Of General Medical Sciences of the National Institutes of Health under Award Number R15GM120705 (MDB) and the National Science Foundation under Award Number CHE-1565646. We additionally acknowledge Dr. John Dunlap for assistance with STEM studies.
Footnotes
Supporting Information
Supporting Information Available: Spectra for characterizing synthetic compounds. This material is available free of charge via the Internet athttp://pubs.acs.org.Experimental Section
References Cited
- 1.Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 2.Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 3.Voskuhl J, Ravoo BJ. Molecular recognition of bilayer vesicles. Chem Soc Rev. 2009;38:495–505. doi: 10.1039/b803782p. [DOI] [PubMed] [Google Scholar]
- 4.Paleos CM, Tsiourvas D, Sideratou Z. Interaction of Vesicles: Adhesion, Fusion and Multicompartment Systems Formation. ChemBioChem. 2011;12:510–521. doi: 10.1002/cbic.201000614. [DOI] [PubMed] [Google Scholar]
- 5.Marchi-Artzner V, Gulik-Krzywicki T, Guedeau-Boudeville MA, Gosse C, Sanderson JM, Dedieu JC, Lehn JM. Selective adhesion, lipid exchange and membrane-fusion processes between vesicles of various sizes bearing complementary molecular recognition groups. ChemPhysChem. 2001;2:367–376. doi: 10.1002/1439-7641(20010618)2:6<367::AID-CPHC367>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Gong Y, Luo YM, Bong D. Membrane activation: Selective vesicle fusion via small molecule recognition. J Am Chem Soc. 2006;128:14430–14431. doi: 10.1021/ja0644576. [DOI] [PubMed] [Google Scholar]
- 7.Gong Y, Ma M, Luo Y, Bong D. Functional determinants of a synthetic vesicle fusion system. J Am Chem Soc. 2008;130:6196–6205. doi: 10.1021/ja711184u. [DOI] [PubMed] [Google Scholar]
- 8.Ma MM, Gong Y, Bong D. Lipid membrane adhesion and fusion driven by designed, minimally multivalent hydrogen-bonding lipids. J Am Chem Soc. 2009;131:16919–16926. doi: 10.1021/ja9072657. [DOI] [PubMed] [Google Scholar]
- 9.MarchiArtzner V, Jullien L, GulikKrzywicki T, Lehn JM. Molecular recognition induced aggregation and fusion between vesicles containing lipids bearing complementary hydrogen bonding head groups. Chem Commun. 1997:117–118. [Google Scholar]
- 10.Jin HB, Liu Y, Zheng YL, Huang W, Zhou YF, Yan DY. Cytomimetic large-scale vesicle aggregation and fusion based on host-guest interaction. Langmuir. 2012;28:2066–2072. doi: 10.1021/la203857s. [DOI] [PubMed] [Google Scholar]
- 11.Mansfeld FM, Feng GQ, Otto S. Photo induced molecular-recognition-mediated adhesion of giant vesicles. Org Biomol Chem. 2009;7:4289–4295. doi: 10.1039/b910197g. [DOI] [PubMed] [Google Scholar]
- 12.Sawayama J, Sakaino H, Kabashima S, Yoshikawa I, Araki K. Hydrogen-bond-directed giant unilamellar vesicles of guanosine derivative: Preparation, properties, and fusion. Langmuir. 2011;27:8653–8658. doi: 10.1021/la201350r. [DOI] [PubMed] [Google Scholar]
- 13.Voskuhl J, Fenske T, Stuart MCA, Wibbeling B, Schmuck C, Ravoo BJ. Molecular recognition of vesicles: Host-guest Interactions combined with specific dimerization of zwitterions. Chem Eur J. 2010;16:8300–8306. doi: 10.1002/chem.201000623. [DOI] [PubMed] [Google Scholar]
- 14.Stengel G, Simonsson L, Campbell RA, Hook F. Determinants for membrane fusion induced by cholesterol-modified DNA zippers. J Phys Chem B. 2008;112:8264–8274. doi: 10.1021/jp802005b. [DOI] [PubMed] [Google Scholar]
- 15.Maru N, Shohda KI, Sugawara T. Successive fusion of vesicles aggregated by DNA duplex formation in the presence of triton X-100. Chem Lett. 2008;37:340–341. [Google Scholar]
- 16.Stengel G, Zahn R, Hook F. DNA-induced programmable fusion of phospholipid vesicles. J Am Chem Soc. 2007;129:9584–+. doi: 10.1021/ja073200k. [DOI] [PubMed] [Google Scholar]
- 17.Simonsson L, Jonsson P, Stengel G, Hook F. Site-Specific DNA-Controlled Fusion of Single Lipid Vesicles to Supported Lipid Bilayers. ChemPhysChem. 2010;11:1011–1017. doi: 10.1002/cphc.200901010. [DOI] [PubMed] [Google Scholar]
- 18.Chan YHM, van Lengerich B, Boxer SG. Lipid-anchored DNA mediates vesicle fusion as observed by lipid and content mixing. Biointerphases. 2008;3:FA17–FA21. doi: 10.1116/1.2889062. [DOI] [PubMed] [Google Scholar]
- 19.Chan YHM, van Lengerich B, Boxer SG. Effects of linker sequences on vesicle fusion mediated by lipid-anchored DNA oligonucleotides. Proc Natl Acad Sci U S A. 2009;106:979–984. doi: 10.1073/pnas.0812356106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan YHM, Yoshina-Ishii C, Johnson JM, Kung LA, Boxer SG. Application of a DNA tethered-vesicle system to study vesicle-vesicle interactions and protein-mediated vesicle fusion. Biophys J. 2005;88:68A–68A. [Google Scholar]
- 21.Rawle RJ, van Lengerich B, Chung M, Bendix PM, Boxer SG. Vesicle Fusion Observed by Content Transfer across a Tethered Lipid Bilayer. Biophys J. 2011;101:L37–L39. doi: 10.1016/j.bpj.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noonan PS, Mohan P, Goodwin AP, Schwartz DK. DNA hybridization-mediated liposome fusion at the aqueous liquid crystal interface. Adv Funct Mat. 2014;24:3206–3212. doi: 10.1002/adfm.201303885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu WM, Nathwani B, Lin CX, Wan J, Karatekin E, Pincet F, Shih W, Rothman JE. A Programmable DNA Origami Platform to Organize SNAREs for Membrane Fusion. J Am Chem Soc. 2016;138:4439–4447. doi: 10.1021/jacs.5b13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selden NS, Todhunter ME, Jee NY, Liu JS, Broaders KE, Gartner ZJ. Chemically programmed cell adhesion with membrane-anchored oligonucleotides. J Am Chem Soc. 2012;134:765–768. doi: 10.1021/ja2080949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kashiwada A, Tsuboi M, Takamura N, Brandenburg E, Matsuda K, Koksch B. Design and characterization of endosomal-pH-responsive coiled coils for constructing an artificial membrane fusion system. Chem Eur J. 2011;17:6179–6186. doi: 10.1002/chem.201003392. [DOI] [PubMed] [Google Scholar]
- 26.Kashiwada A, Tsuboi M, Matsuda K. Target-selective one-way membrane fusion system based on a pH-responsive coiled coil assembly at the interface of liposomal vesicles. Langmuir. 2011;27:1403–1408. doi: 10.1021/la103908u. [DOI] [PubMed] [Google Scholar]
- 27.Lygina AS, Meyenberg K, Jahn R, Diederichsen U. Transmembrane domain peptide/peptide nucleic acid hybrid as a model of a SNARE protein in vesicle fusion. Angew Chem Int Ed. 2011;50:8597–8601. doi: 10.1002/anie.201101951. [DOI] [PubMed] [Google Scholar]
- 28.Marsden HR, Korobko AV, Zheng TT, Voskuhl J, Kros A. Controlled liposome fusion mediated by SNARE protein mimics. Biomat Sci. 2013;1:1046–1054. doi: 10.1039/c3bm60040h. [DOI] [PubMed] [Google Scholar]
- 29.Pahler G, Panse C, Diederichsen U, Janshoff A. Coiled-coil formation on lipid bilayers-Implications for docking and fusion efficiency. Biophys J. 2012;103:2295–2303. doi: 10.1016/j.bpj.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabe M, Schwieger C, Zope HR, Versluis F, Kros A. Membrane interactions of fusogenic coiled-coil peptides: Implications for lipopeptide mediated vesicle fusion. Langmuir. 2014;30:7724–7735. doi: 10.1021/la500987c. [DOI] [PubMed] [Google Scholar]
- 31.Sadek M, Berndt D, Milovanovic D, Jahn R, Diederichsen U. Distance regulated vesicle fusion and docking mediated by peptide nucleic acid SNARE protein analogues. ChemBioChem. 2016;17:479–485. doi: 10.1002/cbic.201500517. [DOI] [PubMed] [Google Scholar]
- 32.Versluis F, Voskuhl J, Vos J, Friedrich H, Ravoo BJ, Bomans PHH, Stuart MCA, Sommerdijk N, Kros A. Coiled coil driven membrane fusion between cyclodextrin vesicles and liposomes. Soft Matter. 2014;10:9746–9751. doi: 10.1039/c4sm01801j. [DOI] [PubMed] [Google Scholar]
- 33.Zheng TT, Bulacu M, Daudey G, Versluis F, Voskuhl J, Martelli G, Raap J, Sevink GJA, Kros A, Boyle AL. A non-zipper-like tetrameric coiled coil promotes membrane fusion. RSC Adv. 2016;6:7990–7998. [Google Scholar]
- 34.Richard A, Marchi-Artzner V, Lalloz MN, Brienne MJ, Artzner F, Gulik-Krzywicki T, Guedeau-Boudeville MA, Lehn JM. Fusogenic supramolecular vesicle systems induced by metal ion binding to amphiphilic ligands. Proc Natl Acad Sci USA. 2004;101:15279–15284. doi: 10.1073/pnas.0406625101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paleos CM, Tsiourvas D. Interaction between complementary liposomes: a process leading to multicompartment systems formation. J Mol Recog. 2006;19:60–67. doi: 10.1002/jmr.758. [DOI] [PubMed] [Google Scholar]
- 36.Nalluri SKM, Bultema JB, Boekema EJ, Ravoo BJ. Metal ion responsive adhesion of vesicles by conformational switching of a non-covalent linker. Chem Sci. 2011;2:2383–2391. [Google Scholar]
- 37.Qu F, Liu NJ, Bu WF. Vesicle fusion intermediates obtained from the self assembly of a cationic platinum(II) complex with sulfonate terminated polystyrenes. RSC Adv. 2014;4:9750–9755. [Google Scholar]
- 38.Dutta D, Pulsipher A, Luo W, Mak H, Yousaf MN. Engineering cell surfaces via liposome fusion. Bioconjugate Chem. 2011;22:2423–2433. doi: 10.1021/bc200236m. [DOI] [PubMed] [Google Scholar]
- 39.Loosli F, Doval DA, Grassi D, Zaffalon P-L, Favarger F, Zumbuehl A. Clickosomes—using triazole linked phospholipid connectors to fuse vesicles. Chem Commun. 2012;48:1604–1606. doi: 10.1039/c2cc16827h. [DOI] [PubMed] [Google Scholar]
- 40.Elahipanah S, Radmanesh P, Luo W, O’Brien PJ, Rogozhnikov D, Yousaf MN. Rewiring gram-negative bacteria cell surfaces with bio-orthogonal chemistry via liposome fusion. Bioconj Chem. 2016;27:1082–1089. doi: 10.1021/acs.bioconjchem.6b00073. [DOI] [PubMed] [Google Scholar]
- 41.Harano K, Narita A, Nakamura E. Photocrosslinking of the exterior of a fullerene bilayer that prevents vesicle aggregation. Chem Lett. 2014;43:877–879. [Google Scholar]
- 42.Jin HB, Huang W, Zheng YL, Zhou YF, Yan DY. Construction of macroscopic cytomimetic vesicle aggregates based on click chemistry: Controllable vesicle fusion and phase separation. Chem Eur J. 2012;18:8641–8646. doi: 10.1002/chem.201201401. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Zhao Y. Tunable fusion and aggregation of liposomes triggered by multifunctional surface cross linked micelles. Bioconjugate Chem. 2012;23:1721–1725. doi: 10.1021/bc300082b. [DOI] [PubMed] [Google Scholar]
- 44.Luo W, Pulsipher A, Dutta D, Lamb BM, Yousaf MN. Remote control of tissue interactions via engineered photo switchable cell surfaces. Sci Rep. 2014;4 doi: 10.1038/srep06313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Brien PJ, Luo W, Rogozhnikov D, Chen J, Yousaf MN. Spheroid and tissue assembly via click chemistry in microfluidic flow. Bioconjugate Chem. 2015;26:1939–1949. doi: 10.1021/acs.bioconjchem.5b00376. [DOI] [PubMed] [Google Scholar]
- 46.Pulsipher A, Dutta D, Luo W, Yousaf MN. Cell-surface engineering by a conjugation-and-release approach based on the formation and cleavage of oxime linkages upon mild electrochemical oxidation and reduction. Angew Chem Int Ed. 2014;53:9487–9492. doi: 10.1002/anie.201404099. [DOI] [PubMed] [Google Scholar]
- 47.Yang J, Bahreman A, Daudey G, Bussmann J, Olsthoorn RCL, Kros A. Drug delivery via cell membrane fusion using lipopeptide modified liposomes. ACS Cent Sci. 2016;2:621–630. doi: 10.1021/acscentsci.6b00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang J, Shimada Y, Olsthoorn RCL, Snaar-Jagalska BE, Spaink HP, Kros A. Application of coiled coil peptides in liposomal anticancer drug delivery using a zebrafish xenograft model. ACS Nano. 2016;10:7428–7435. doi: 10.1021/acsnano.6b01410. [DOI] [PubMed] [Google Scholar]
- 49.Sletten EM, Bertozzi CR. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew Chem Int Edit. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Best MD. Click chemistry and bioorthogonal reactions: unprecedented selectivity in the labeling of biological molecules. Biochemistry. 2009;48:6571–6584. doi: 10.1021/bi9007726. [DOI] [PubMed] [Google Scholar]
- 51.Hassane FS, Frisch B, Schuber F. Targeted liposomes: Convenient coupling of ligands to preformed vesicles using “click chemistry”. Bioconjugate Chem. 2006;17:849–854. doi: 10.1021/bc050308l. [DOI] [PubMed] [Google Scholar]
- 52.Cavalli S, Tipton AR, Overhand M, Kros A. The chemical modification of liposome surfaces via a copper-mediated 3+2 azide-alkyne cycloaddition monitored by a colorimetric assay. Chem Commun. 2006;30:3193–3195. doi: 10.1039/b606930d. [DOI] [PubMed] [Google Scholar]
- 53.van Lengerich B, Rawle RJ, Boxer SG. Covalent attachment of lipid vesicles to a fluid-supported bilayer allows observation of DNA-mediated vesicle interactions. Langmuir. 2010;26:8666–8672. doi: 10.1021/la904822f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar A, Erasquin UJ, Qin GT, Li K, Cai CZ. “Clickable”, polymerized liposomes as a versatile and stable platform for rapid optimization of their peripheral compositions. Chem Commun. 2010;46:5746–5748. doi: 10.1039/c0cc00784f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tarallo R, Accardo A, Falanga A, Guarnieri D, Vitiello G, Netti P, D’Errico G, Morelli G, Galdiero S. Clickable functionalization of liposomes with the gH625 peptide from Herpes simplex virus type I for intracellular drug delivery. Chem Eur J. 2011;17:12659–12668. doi: 10.1002/chem.201101425. [DOI] [PubMed] [Google Scholar]
- 56.Salome C, Spanedda MV, Hilbold B, Berner E, Heurtault B, Fournel S, Frisch B, Bourel-Bonnet L. Smart tools and orthogonal click-like reactions onto small unilamellar vesicles. Chem Phys Lipids. 2015;188:27–36. doi: 10.1016/j.chemphyslip.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Bostic HE, Smith MD, Poloukhtine AA, Popik VV, Best MD. Membrane labeling and immobilization via copper free click chemistry. Chem Commun. 2012;48:1431–1433. doi: 10.1039/c1cc14415d. [DOI] [PubMed] [Google Scholar]
- 58.Blenke EO, Klaasse G, Merten H, Pluckthun A, Mastrobattista E. Liposome functionalization with copper-free “click chemistry”. J Cont Rel. 2015;202:14–20. doi: 10.1016/j.jconrel.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 59.Alam S, Alves DS, Whitehead SA, Bayer AM, McNitt CD, Popik VV, Barrera FN, Best MD. A clickable and photocleavable lipid analogue for cell membrane delivery and release. Bioconjugate Chem. 2015;26:1021–1031. doi: 10.1021/acs.bioconjchem.5b00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang HL, Ma Y, Sun XL. Chemically-selective surface glycol-functionalization of liposomes through Staudinger ligation. Chem Commun. 2009:3032–3034. doi: 10.1039/b822420j. [DOI] [PubMed] [Google Scholar]
- 61.Zhang HL, Weingart J, Jiang R, Peng JH, Wu QY, Sun XL. Bio-inspired liposomal thrombomodulin conjugate through bio-orthogonal chemistry. Bioconjugate Chem. 2013;24:550–559. doi: 10.1021/bc300399f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong V, Presolski SI, Ma C, Finn MG. Analysis and optimization of copper-catalyzed azide-akyne cycloaddition for bioconjugation. Angew Chem Int Edit. 2009;48:9879–9883. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agard NJ, Prescher JA, Bertozzi CR. A strain-promoted [3+2] azide-alkyne cycloaddition for covalent modification of blomolecules in living systems. J Am Chem Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 64.Kolb HC, Finn MG, Sharpless KB. Click chemistry: Diverse chemical function from a few good reactions. Angew Chem Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 65.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 66.Torne CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 67.Kuzmin A, Poloukhtine A, Wolfert MA, Popik VV. Surface functionalization using catalyst-free azide-alkyne cycloaddition. Bioconjugate Chem. 2010;21:2076–2085. doi: 10.1021/bc100306u. [DOI] [PubMed] [Google Scholar]
- 68.Debets MF, van Berkel SS, Schoffelen S, Rutjes F, van Hest JCM, van Delft FL. Aza-dibenzocyclooctynes for fast and efficient enzyme PEGylation via copper-free (3+2) cycloaddition. Chem Commun. 2010;46:97–99. doi: 10.1039/b917797c. [DOI] [PubMed] [Google Scholar]
- 69.McNitt CD, Popik VV. Photochemical generation of oxa-dibenzocyclooctyne (ODIBO) for metal-free click ligations. Org Biomol Chem. 2012;10:8200–8202. doi: 10.1039/c2ob26581h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tomatsu I, Marsden HR, Rabe M, Versluis F, Zheng TT, Zope H, Kros A. Influence of pegylation on peptide mediated liposome fusion. J Mat Chem. 2011;21:18927–18933. [Google Scholar]
- 71.Duzgunes N, Allen TM, Fedor J, Papahadjopoulos D. Lipid mixing during membrane aggregation and fusion - Why fusion assays disagree. Biochemistry. 1987;26:8435–8442. doi: 10.1021/bi00399a061. [DOI] [PubMed] [Google Scholar]
- 72.Struck DK, Hoekstra D, Pagano RE. Use of resonance energy-transfer to monitor membrane-fusion. Biochemistry. 1981;20:4093–4099. doi: 10.1021/bi00517a023. [DOI] [PubMed] [Google Scholar]
- 73.Stengel G, Simonsson L, Campbell RA, Hook F. Determinants for Membrane Fusion Induced by Cholesterol-Modified DNA Zippers. Journal of Physical Chemistry. 2008;112:8264–8274. doi: 10.1021/jp802005b. [DOI] [PubMed] [Google Scholar]
- 74.van den Brink-van der Laan E, Dalbey RE, Demel RA, Killian JA, de Kruijff B. Effect of nonbilayer lipids on membrane binding and insertion of the catalytic domain of leader peptidase. Biochemistry. 2001;40:9677–9684. doi: 10.1021/bi002903a. [DOI] [PubMed] [Google Scholar]
- 75.van den Brink-van der Laan EV, Killian JA, de Kruijff B. Nonbilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. Biochim Biophys Acta. 2004;1666:275–288. doi: 10.1016/j.bbamem.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 76.Vanic Z, Barnert S, Suss R, Schubert R. Fusogenic activity of PEGylated pH sensitive liposomes. J Liposome Res. 2012;22:148–157. doi: 10.3109/08982104.2011.633267. [DOI] [PubMed] [Google Scholar]
- 77.Wilschut J, Duzgunes N, Fraley R, Papahadjopoulos D. Studies on the mechanism of membrane-fusion - Kinetics of calcium-ion induced fusion of phosphatidylserine vesicles followed by a new assay for mixing aqueous vesicle contents. Biochemistry. 1980;19:6011–6021. doi: 10.1021/bi00567a011. [DOI] [PubMed] [Google Scholar]
- 78.Bonnet D, Riche S, Loison S, Dagher R, Frantz MC, Boudier L, Rahmeh R, Mouillac B, Haiech J, Hibert M. Solid phase organic tagging resins for labeling biomolecules by 1,3 -dipolar cycloaddition: application to the synthesis of a fluorescent non-peptidic vasopressin receptor ligand. Chemistry. 2008;14:6247–54. doi: 10.1002/chem.200800273. [DOI] [PubMed] [Google Scholar]
- 79.Kii I, Shiraishi A, Hiramatsu T, Matsushita T, Uekusa H, Yoshida S, Yamamoto M, Kudo A, Hagiwara M, Hosoya T. Strain-promoted double-click reaction for chemical modification of azido-biomolecules. Org Biomol Chem. 2010;8:4051–5. doi: 10.1039/c0ob00003e. [DOI] [PubMed] [Google Scholar]
- 80.Irastorza A, Aizpurua JM, Correa A. Triazole-Directed Pd-Catalyzed C(sp(2))-H Oxygenation of Arenes and Alkenes. Org Lett. 2016;18:1080–3. doi: 10.1021/acs.orglett.6b00195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


