Abstract
Motor complications are a consequence of chronic treatment of Parkinson’s disease (PD) and include motor fluctuations (wearing-off phenomenon) and levodopa-induced dyskinesia. Both can have a significant impact on functionality and quality of life and thus proper recognition and management is essential. The phenomenology and temporal relationship of motor complications to the schedule of levodopa dosing can be helpful in characterizing them. There are several therapeutic approaches to motor complications, including pharmacological and surgical options. The current review summarizes the different types of motor complications according to phenomenology and the currently available medical treatments, including ongoing trials for management of this condition.
Keywords: Motor fluctuations, Motor complications, Levodopa-induced dyskinesia, Parkinson’s Disease
Introduction
The use of levodopa for Parkinson’s disease (PD) was first described in 1961, and it remains the most effective medication available for treatment of this condition.1 However, levodopa displays a peculiar pharmacokinetic and pharmacodynamic profile such that chronic use of oral formulations can eventually lead to treatment complications such as motor and non-motor fluctuations and levodopa-induced dyskinesia (LID). Dopaminergic-induced complications are divided into motor and non-motor complications.2 Motor complications will be reviewed in detail in this article, including wearing-off, dose failure, beginning of dose worsening, end-of-dose rebound and levodopa-induced dyskinesia. Non-motor complications such as autonomic, sensory and behavioral symptoms are covered in another article in this issue.
A combination of disease progression (loss of nigrostriatal dopamine terminals) and fluctuating levodopa levels (due to both central and peripheral mechanisms) likely give rise to the motor complications of PD. In the first years of dopaminergic therapy, there is an excellent response to treatment. Patients do not notice any fluctuation in response to individual doses of levodopa, and if they miss or are late taking a dose, they may not report any issues. This phenomenon is known as the “long-duration response” (LDR)3 of levodopa. Over time, on average after 2–5 years of chronic use of levodopa, patients begin to become more aware of the duration of action, and the “short duration response” becomes more critical, with a less effective response and oscillations in benefit duration. Fluctuating levels of levodopa are aggravated by several peripheral pharmacokinetic factors, including slow rate of gastric emptying, erratic jejunal absorption, and competition with dietary amino acids at absorption sites, as well as transmission across the blood-brain barrier.4,5 Thus as the disease progresses, PD patients often experience a decreased duration of action, slower or even failed therapeutic effect, and a need to take more frequent doses of levodopa.
Risk Factors and Causes of Dopaminergic-induced Motor Complications
Motor complications are very common and almost inevitable if a PD patient is adequately treated with levodopa. The most important risk factors (and thus etiological factors) include disease progression, disease severity, higher individual doses of levodopa, peripheral pharmacokinetic factors affecting absorption of levodopa, and possibly genetic risk factors. These factors result in abnormal intrasynaptic dopamine concentrations with loss of normal constant stimulation of postsynaptic dopamine receptors and fluctuating benefit from levodopa, as well as altered basal ganglia circuitry firing and resultant hyperkinetic movements (dyskinesia) in response to levodopa dosing.
Disease progression is a major factor in the development of motor complications. Many epidemiological studies have investigated this issue and consistently report an incidence of 10% per year. Within 2–5 years, up to 50% of patients may experience some degree of motor complications, and between 80 to 100% of PD patients will develop motor complications after 10 years of dopaminergic therapy.6,7,8 In terms of PD subtypes, patients with akinetic-rigid PD appear less likely to develop fluctuations, but also commonly have less effective anti-parkinsonian benefit of levodopa compared to tremor-dominant PD.9
Interestingly, disease severity as assessed by Unified Parkinson’s Disease Rating Score motor exam (UPDRS Part III) appears to play a lesser role, and may not correlate with dyskinesia.10 This is often seen clinically when PD patients who have very mild symptoms can often develop marked LID. However, another well recognized clinical finding is that dyskinesia is usually worse, or begins on the most affected side of the body.11
It is well known that higher levodopa doses are associated with the higher risk of developing wearing-off and dyskinesia.10 Early use of COMT inhibitors (with resultant increased dopamine levels) also increased the risk of dyskinesia, as shown by the STRIDE study.12 In a more recent cohort of 234 patients, six variables correlated with risk of developing wearing-off in a ranking order of relevance: total daily levodopa dosage, levodopa daily dosage adjusted to weight, duration of levodopa treatment, disease duration at assessment, Hoehn & Yahr (H&Y) stage, UPDRS III, and overall accuracy of prediction was 69.7 %.13 Additionally, disease duration at levodopa initiation did not relate to wearing-off development.13 In keeping with these findings, a recent paper also suggested that motor fluctuations and dyskinesia are not associated with the duration of levodopa therapy, but rather with longer disease duration and higher levodopa daily dose.14
Motor fluctuations are also a consequence of issues related to the peripheral and central pharmacokinetics of levodopa. The rate of gastric emptying in PD is one of the main determinants of levodopa bioavailability.4 Slow gastric emptying delays the delivery of levodopa to intestinal absorption sites in the small bowel, possibly increasing pre-systemic decarboxylation and resulting in reduced levodopa absorption. Other factors that may potentially interfere with gastric emptying include food (described below), low gastric pH, constipation, and anticholinergic binding drugs.4
The specific interaction of dietary protein, due to competition with amino acid transporters across the GI tract and the blood brain barrier, is another factor complicating levodopa administration. The interaction with food is often variable, with some subjects being very sensitive and others far less so. One study has suggested that clinically significant protein interaction with levodopa occurs mostly in PD patients with earlier disease onset and positive family history.15 Other peripheral factors include presence of Helicobacter pylori (H. pylori) infection, though its impact on levodopa absorption remains controversial. Previous studies have demonstrated a higher prevalence of H. pylori infection in patients with PD compared to controls, which may affect levodopa absorption, resulting in motor fluctuations. It has been suggested that H. pylori infection may reduce motor fluctuations;16 however, more recent studies have correlated its eradication with significant improvement in clinical response to levodopa and less motor complications.17,18
The peripheral factors described above that limit levodopa access to the brain further exacerbate the central fluctuations in dopamine levels that occur. The loss of nigrostriatal dopaminergic terminals and of normal dopamine recycling and turnover as the disease progresses gives rise to pulsatile dopamine receptor stimulation. Positron emission tomography (PET) molecular imaging has shown that in advanced PD patients, levodopa administration induces sharp increases in striatal dopamine levels that correlate with LID severity.19
Young-onset PD patients may develop LID and motor fluctuations earlier and more frequently than patients with an older age of onset. One study reported median intervals to the development of both dyskinesia and motor fluctuations in young-onset PD patients of 3 years, while in the older-onset patients, median intervals were 4–6 years.20 Subsequent studies have consistently shown that younger age at onset is a significant risk factor for earlier development of motor fluctuations and LID.10,21,22
Female gender has also been described as a risk factor for both wearing-off phenomenon and LID. Several studies have suggested that the prevalence of wearing-off is about one third higher among women than men.10,22,23 However, this finding has not been consistent. Another study showed that female gender was a risk factor for dyskinesia (HR 2.73 (1.49 – 4.98), p = 0.001) but was not a significant risk factor for motor fluctuations (HR females versus males 1.48 (0.94 – 2.33), p = 0.087).21 The reason for an apparent higher risk of motor complications among women is not yet known, but it has been suggested to relate to relatively lower body weights, altered pharmacokinetics and bioavailability of levodopa or the effects of low estrogen.24,25 With regard to body weight, recent studies have shown that daily levodopa dose per kilogram of body weight is more relevant than the absolute daily levodopa dose.13,26,27 This may be of clinical importance in patients with weight loss, and reducing oral levodopa doses in patients losing weight to avoid dyskinesia may be a reasonable approach.
Genetic risk factors have also been investigated for an association with the development of motor complications.28–31 The autosomal recessive parkinsonism genes, PARK2 (parkin), PARK6 (pink-1) and PARK7 (DJ-1) are all associated with young-onset PD, and the frequent appearance of early dyskinesias in these patients may suggest a role of genetic factors in development of motor complications.32 However, genetic parkinsonism in general tends to affect individuals at a younger age, and, as noted above, age itself is known to be a risk factor for developing LID.10,21,22
In the more common sporadic forms of PD, polymorphisms in dopamine receptors have also been investigated. Polymorphisms in dopaminergic D2 receptors, but not of D1 receptors, may reduce the risk of developing LID.33 Polymorphisms in the dopamine transporter have also been implicated. In one study of 352 levodopa-treated PD patients, 54.5 % participants developed LID, with a mean latency of 5.0 (±4.5) years and after adjusting for gender, age at PD onset, duration of symptoms prior to levodopa exposure, and multiple testing correction. In that study only a single nucleotide polymorphism (SNP) in SLC6A3 (with 81 % genotyping success) was significantly associated with LID latency (p = 0.000041).30 In another study, diphasic dyskinesia was exclusively linked with the D2-like dopamine 3 receptor (DRD3) gene after adjusting for gender, age at PD onset, H&Y stage, and duration of levodopa treatment.29 Polymorphisms in genes that influence dopamine metabolism have also been investigated. However, individual SNPs in BDNF, COMT and MAO-A genes appear not to consistently influence prevalence or time to onset of dyskinesias.34
Studies investigating the pathophysiology of LID have so far focused mostly on peak–dose dyskinesias, with impaired peripheral absorption of levodopa contributing to abnormal pulsatile stimulation of postsynaptic dopamine receptors in the striatum. The subsequent effects on dopamine receptors and other non-dopaminergic signaling pathways is complex and more difficult to study, with a net effect of reduced inhibition of basal ganglia outputs to motor cortical regions contributing to the development of dyskinesia. The non-dopaminergic pathways also implicated in the pathophysiology of LID include glutamatergic, cannabinoid, opioid, cholinergic, adenosine, and noradrenergic systems.35 Another non-dopaminergic system involved in LID is serotonin. As serotonergic terminals do not degenerate at the same rate as dopaminergic terminals, abnormal processing of exogenous levodopa and the release of dopamine in a dysregulated manner from serotonergic terminals may contribute to pulsatile intra synaptic levels of dopamine and development of LID.19
Motor Fluctuations (Wearing-off Phenomenon)
Motor fluctuations are changes in PD symptoms related to the time of levodopa dose. “ON” period refers to the improvement of symptoms after each dose of medication, and “OFF” periods are defined by the recurrence of symptoms or a lack of levodopa effect. Oral levodopa formulations usually take at least 20 to 30 minutes to take effect but depend on the many factors mentioned above, such as protein intake, gastric emptying rate and constipation. As the disease progresses, levodopa duration is shortened, and patients often need to reduce the time interval between doses or increase individual doses. The loss of a smooth long duration response to levodopa results in specific patterns of wearing off.
Predictable wearing-off
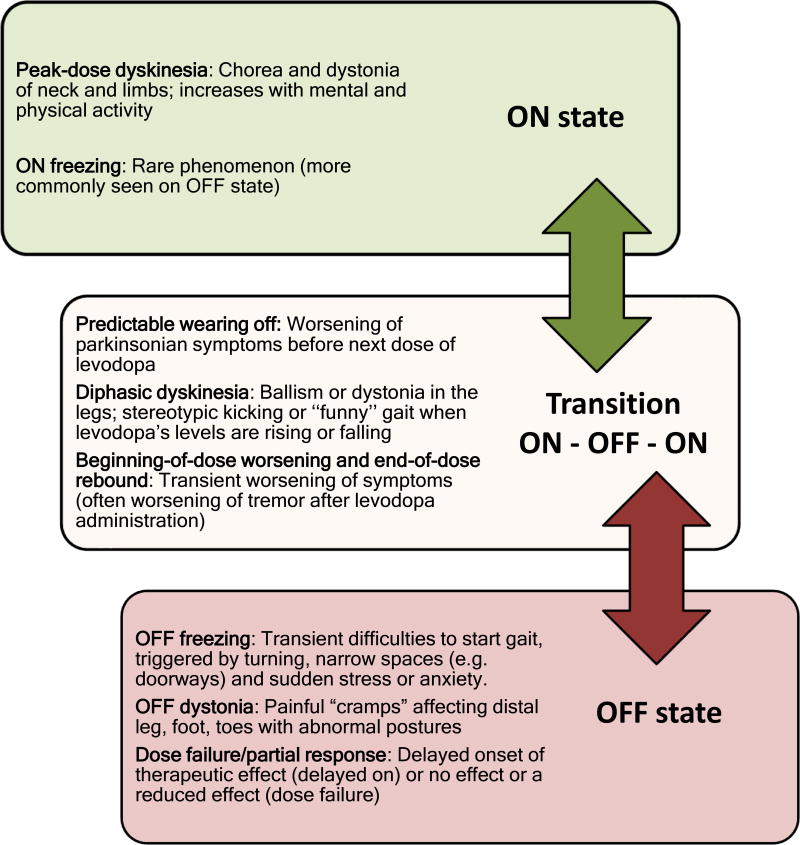
Predictable wearing-off is the regular recurrence of symptoms at the end of a dose of levodopa. This is the most frequent type of wearing-off, and is usually the earliest manifestation of motor fluctuations (Figure 1). In early PD patients, the response to a single dose of levodopa may last for several hours, and patients may not experience recurrence of symptoms even if a dose is missed; this phenomenon is named the long-duration response (LDR) to levodopa.3 As disease progresses, levodopa duration is shortened (4 hours or less), and patients need to reduce intervals between doses and/or increase individual doses. Patients may report worsening of tremor, bradykinesia, freezing of gait, difficulties in walking and/or non-motor symptoms like anxiety or panic attacks emerging before the next dose. Predictable wearing-off includes “early morning akinesia” with worsening of symptoms in the early morning due to low levels of levodopa as the last dose was the night before.11
Figure 1.
Motor fluctuations according to “ON”, “Transition ON-OFF-ON”, and "OFF" state.
Unpredictable wearing-off
Unpredictable wearing-off or “sudden OFFs” happen less frequently than predictable wearing-off, and more commonly occur in more advanced stages of PD.11 Patients report sudden recurrence of symptoms often unrelated to the timing of the next dose (it may happen anytime during the day). Because of this acute worsening of parkinsonian symptoms (which can occur within a few seconds), patients can develop sudden disabling akinesia.
On-Off fluctuations (“yo-yoing”)
Some patients may rarely experience a combination of predictable or unpredictable rapid switching from being ON to being OFF. The term “yo-yoing” infers rapid, abrupt and sometimes multiple transitions from one state to the other.11 This subtype of wearing-off is now rare due to the practice of using overall lower doses of levodopa but can be seen in more advanced PD patients.
Beginning of dose worsening/ End-of-dose rebound/ Delayed or partial response/ Dose failure
These subtypes of motor fluctuations are usually a consequence of erratic levodopa absorption as described above. Delayed onset of the effect, partial or suboptimal clinical response or even dose failure can thus occur. Some patients report worsening of symptoms just after taking levodopa at the beginning of the dose cycle, most commonly described as worsening of tremor that subsequently settles down once levodopa levels increase and stabilize (“beginning of dose worsening”). Other patients may also experience symptoms more severe than in the untreated baseline state at the end of the dose (also called “end of dose rebound”).
Spontaneous motor fluctuations
Fluctuating symptoms (both motor and non-motor) in PD are common, and many patients cannot describe a clear pattern in response to medications. Such features are usually slow, chronic changes, such that patients report ‘good days’ and ‘bad days,’ which likely have multiple etiologies. One factor, though, may relate to effects of sleep. Patients often describe improvement of symptoms in the morning and deterioration through the day.11 This phenomenon is also known as “sleep benefit.”36 Prevalence estimates range from 33 to 55% of PD patients who recognized this phenomena.37 Sleep benefit can also occur after daytime naps.
Management of the wearing off phenomenon
The first step when managing a patient with motor fluctuations is reviewing the levodopa schedule. Adjusting the timing of levodopa doses can be crucial in avoiding predictable end of dose wearing off. Overall, lower doses of levodopa given more frequently is typically the best option for managing motor complications. Although controlled-release carbidopa/levodopa was designed to provide a more smooth and longer duration effect of levodopa for treatment of wearing-off, it has not demonstrated significant benefits compared with regular-release levodopa/carbidopa formulation.38 In addition, delayed and often unpredictable responses resulting from erratic absorption have limited use, and currently is best confined to bedtime to provide more stable overnight levodopa levels.39
Gastrointestinal factors may affect levodopa absorption, and patients may report less effective dose after eating, especially after consuming meat protein, which may aggravate wearing-off in more sensitive patients. Patients should be advised to take their levodopa 30 minutes prior to eating. Additionally, constipation and use of anticholinergics may exacerbate delayed gastric emptying, which also reduces absorption of levodopa. Therefore, constipation should be promptly diagnosed and treated. Recognition and management of nonpharmacologic factors that may influence the efficacy of levodopa are also important, as anxiety and stress may lead to reduced benefit or even sudden loss of benefit. The management of a delayed response or the lack of response generally consists of interventions to optimize the duodenal absorption of levodopa, such as facilitating tablet disintegration and the gastric-emptying time.4,40 In clinical practice, patients are often advised to avoid large protein meals with levodopa doses during the day if they have experienced dose failures, switching high protein meals to dinnertime, chewing or crushing levodopa, and drinking a carbonated drink with each dose. Rescue medications such as subcutaneous apomorphine may be useful in some patients.41
Freezing of gait
“Freezing of gait” (FOG) can occur in later stages of disease as another clinical feature of motor fluctuations. It can be described as inability to move the feet, hesitation to initiate gait and a feeling that the “feet are stuck to the floor.”42 FOG occurs in up to two thirds of patients, being more common in males than females, and is associated with akinetic–rigid PD.42 FOG is a phenomenon that occurs most often during the OFF state, but it may also happen during the ON state.43 The occurrence of FOG can confer a significant risk of falls and subsequent fractures.44 Several factors can trigger FOG in PD patients, unrelated to medication timing, including anxiety and obstacles to walking including turning, initiating a step, crossing a busy road, doorways or narrow spaces.
FOG usually has a poor response to dopaminergic therapy; however, some patients may improve with levodopa dose adjustments, particularly those with FOG during OFF state. There is low level evidence that Amantadine may improve FOG and balance, and previous data suggest that amantadine is associated with self-reported improvement in FOG in PD (questionnaires), but the effect may be transient (Amantadine mean dose was 100 mg twice daily and treatment duration 20 months).45 Some patients reported reduction in benefit after four months. Potential side effects are visual hallucinations and peripheral edema. Additionally, studies have failed to show benefit of intravenous amantadine for FOG, with no significant improvement in overall scores.46,47
MAO-B inhibitors such as rasagiline have shown a positive effect on FOG. Previous large trials demonstrated improvement on the UPDRS subscores of FOG in patients with advanced PD.48,49 A recent study proposed that lower UPDRS gait scores, higher levodopa equivalent daily dose (LEDD), lower anxiety scores, lower FOG-Q scores, and higher UPDRS scores for lower extremity rigidity and rise from chair, may predict FOG-related rasagiline benefit.50
Noradrenergic mechanisms have been implicated in gait. The mixed dopamine/noradrenaline re-uptake inhibitor methylphenidate (1 mg/kg) has been evaluated as add-on in advanced PD subjects, but with conflicting benefit for FOG.51–53 There were no significant adverse events.
Levodopa-induced dyskinesia (LID)
Patients may experience involuntary movements along with motor fluctuations, including levodopa-induced dyskinesia (LID). LID can manifest as different phenomenologies, including chorea (most common), dystonia or ballism.54 LID can be classified according to time of onset in relation to levodopa dose, and include peak-dose dyskinesia (involuntary movements that coincide with the peak-action of levodopa) and diphasic dyskinesia (involuntary movements that emerge just before onset of ON period and that reappear at the end of the therapeutic benefit) and OFF period dystonia.55
Chorea is the most common movement in LID, characterized by involuntary, irregular, purposeless, non-rhythmic, abrupt and rapid movements that seem to flow from one part of the body to the other. Slower choreo-athetotic movements occur particularly as peak-dose dyskinesia. The severity of choreic movements can vary from mild (involuntary movements that may not be recognized by patients or others) to major (involuntary movements that may significantly interfere with activities of daily living). Neck and limbs are most commonly affected. Larger amplitude proximal movements (ballism) can be seen in choreo-athetotic peak-dose movements, but may also be seen in diphasic dyskinesia as involuntary leg movements. Dystonia is the second most common form of LID and can be defined as sustained contractions of agonist and antagonist muscles that may involve focal/segmental muscle groups. Dystonia can be seen in all types of LID (peak-dose dystonia, diphasic dystonia or OFF dystonia), but is most commonly associated with pain and present in OFF periods or during the transition from ON to OFF (especially affecting the lower limbs).56,57 Limb dystonia can be a presenting feature of untreated PD and especially in genetic PD (parkin - PARK-2, PINK-1 - PARK-6 and DJ-1-PARK-7).57 Blepharospasm is a focal dystonia, with increased blinking frequency and involuntary eyelid closure, and can also be seen as part of motor fluctuations. Spread of dystonia to include jaw opening, neck and hand posturing may also occur.
Myoclonus-like movements have also been described during LID, including focal and multifocal and spontaneous, action-induced, or stimulus-sensitive myoclonus.58,59 Involuntary eye movements or “ocular dyskinesia” can rarely occur as part of LID.60 Abnormal involuntary eye movements consist of repeated, stereotyped upward and/or sideways gaze deviation movements, sometimes phasic, brief and jerky, sometimes tonic and sustained for several seconds.60,61 Interestingly, the main direction of gaze deviation is often the side more affected by parkinsonism. Ocular dyskinesia can be worse in darkness and suppressed by visual fixation.62 Dyskinesia can also affect respiratory muscles in PD patients. Despite being a frequent symptom, respiratory dyskinesia is poorly recognized and often misdiagnosed.63 Respiratory dyskinesia causes tachypnea, irregular rate and depth of breathing, and is usually as a peak-dose phenomenon.64 The underlying mechanism is still unknown, but possible mechanisms posited are paradoxical choreic movements of respiratory muscles, and effects on respiratory brainstem centers.64
Interestingly, poor self-awareness is one of the hallmarks of LID in PD patients.65,66 Thus, patients often do not notice the presence of dyskinesia, unless disabling; rather, it is family members/care givers who will report it. Some authors have suggested that poor self-awareness of LID is present in a proportion of PD patients as a form of anosognosia being correlated with predominance of motor symptoms on the left body side.65 Additionally, lack of self-awareness of LID in the trunk may be due to a complex interplay involving both anosognosic mechanisms and deficits in proprioceptive axial kinesthesia.65
Peak dose dyskinesia
Dyskinesia is most common at the peak-level of levodopa action (“peak-dose dyskinesia”), and may display multiple types of movements, including chorea, ballism, and dystonia, and more rarely myoclonus. Choreiform movements in the limbs, head, neck, and trunk are the most common movements in peak-dose dyskinesia, but dystonic posturing in the limbs and cranio-cervical dystonia may also be seen. Dyskinesia frequently starts on the most affected side and may begin in the foot or lower limb.67
Reducing the development of motor complications involves recognizing those patients most at risk. The key management strategy preventing and treating LID has focused on lower doses of levodopa, with an aim at ‘continuous dopaminergic stimulation’ strategies. Overall, recent reviews suggest that current treatment strategies have been more effective, and significantly disabling dyskinesia is less common than in prior years.22
Many patients may experience mild dyskinesia that may not necessarily need to be treated. However, the presence of mild peak dose dyskinesia means that add-on therapies, as discussed above, are likely to increase dyskinesia, and so should be used cautiously. Reducing individual doses of levodopa is suggested to reduce dyskinesia, but in clinical practice, is rarely helpful as patients frequently then report worse motor OFF symptoms.
Treatment for peak dose dyskinesia should thus be started when the involuntary movements start to bother the patient or affect daily activities. The main goal is keeping dopamine levels as low as possible without compromising PD motor symptom control. Reducing concomitant COMT and MAO-B inhibitors can also reduce bothersome peak-dose dyskinesia. LCIG infusion may also improve LID by providing more steady levodopa levels, avoiding effects of peak dose with concomitant improvement of parkinsonian symptoms.68 In addition, STN DBS can also improve LID, being most effective for management of OFF dystonia, followed by diphasic dyskinesia and peak-dose dyskinesia.69
Diphasic dyskinesia
Diphasic dyskinesia happens more rarely, usually in 15% to 20% of patients, and clinically manifests as involuntary movements at the beginning and end-of-dose, when the levels of levodopa are rising and falling.70 Alternate names include “beginning-of-dose” or “end-of-dose” dyskinesia.11 Another term also used is “dystonia-improvement-dystonia” or “D-I-D” pattern. Diphasic dyskinesia tends to affect the legs predominantly, and can involve stereotypical rapid alternating leg movements, as well as unusual ballistic kicking or dystonia.58 Walking can be affected with high-stepping, so-called “funny” gait. Treatment of diphasic dyskinesia includes smoothing out daily dopamine levels by either small, fractionated more frequent levodopa doses and/or use of a dopamine agonist (if tolerated).71,41 Similar to peak dose dyskinesia, enteral levodopa infusion gel (LCIG) may help maintain more stable levodopa levels and control diphasic dyskinesia.
Adjunctive Medications to Treat Motor Complications in PD
There are several options for add-on therapy that (when combined with optimal levodopa dosing) can reduce OFF times by extending the duration of levodopa (Table 1). Clinical trials with all these agents have shown an overall average decrease in daily OFF time of between 1 or 2 hours per day.72 All adjuvant therapies have the potential risk of increasing peak-dose dyskinesia.
Table 1.
Approved medications for management of motor fluctuations and levodopa-induced dyskinesia.
| Medication | Mechanism of action | Side effects | Reference |
|---|---|---|---|
| MOTOR FLUCTUATIONS | |||
|
| |||
| IPX066 | Extended release levodopa/carbidopa formulation combining IR and ER levodopa/carbidopa. | Nausea and insomnia | 80–82 |
|
| |||
| Pramipexole, Ropinirole and Rotigotine (patch) | Dopaminergic agonists | ICD | 70–73 |
| No evidence for a significant difference in efficacy in improving OFF time between these drugs. | Sleep disturbances | ||
| Ankle edema | |||
| Skin reaction (Patch) | |||
|
| |||
| Apomorphine | Dopaminergic agonist | Nausea (and as above) | 75–76 |
| ‘Rescue’ therapy for sudden-OFFs (subcutaneous injections) or in continuous infusion for advanced PD subjects when DBS is not suitable. | |||
|
| |||
| Entacapone (Tolcapone) | COMT inhibitor (inhibition of enzymatic degradation of levodopa) | Nausea and diarrhea | 69,77 |
| Worsening of dyskinesia | |||
| Formulation in a single tablet combining levodopa/carbidopa/entacapone | |||
|
| |||
| Rasagiline and Selegiline | MAO-B inhibitor | Interaction:
|
78–79 |
|
| |||
| Levodopa/carbidopa intestinal gel (LCIG) | Suspension in carboxymethyl-cellulose gel preparation containing 20 mg/ml levodopa and 4.63 mg/ml carbidopa infused over 16 hours and delivered directly into the proximal jejunum via a PEG-J tube connected to a portable infusion pump. | Complications due to PEG or tube obstruction. | 83–85 |
| Peripheral neuropathy | |||
|
| |||
| LEVODOPA-INDUCED DYSKINESIA | |||
|
| |||
| Amantadine | NMDA glutamate receptor antagonist | Insomnia, hallucinations and peripheral edema | 87 |
IR: immediate-release; ER: extended-release; OFF state: levodopa has no effect and patient experiences worsening of parkinsonian symtpoms; LID: levodopa-induced dyskinesia; ICD: Impulsive compulsive disorder; PD: Parkinson’s disease; DBS: Deep brain stimulation; COMT: Catechol-O-methyltransferase;PEG-J: percutaneous endoscopic gastrojejunostomy; NMDA: N-methyl-D-aspartate.
Dopaminergic agonists
The most commonly used dopamine receptor agonists are ropinirole, pramipexole, and rotigotine patch (table1). There is currently no evidence for a significant difference in efficacy in improving OFF time between these drugs.73 The main limiting factor in use of all dopamine agonists is the risk of side effects including impulse control disorders (ICDs), sleep disturbances and rarely heart failure74,75 and Pisa syndrome.76 To date, there is no evidence for any difference in side effect profile between these agonists.
Several prior studies had evaluated the early use of such dopamine agonists as monotherapy to prevent motor complications. However, long-term follow-up has suggested that while effective at reducing risk of LID in first few years, once levodopa is added, subjects will still develop motor complications similar to patients consistently on levodopa.77 In addition, the clinical benefit in reducing PD motor symptoms with agonists is much less than with levodopa. Due to the higher risk of side effects, some experts no longer advocate the use of dopamine agonists as therapy for early PD. In addition to oral agonists, subcutaneous apomorphine is a very effective dopamine agonist that may be used either as a ‘rescue’ therapy for sudden-OFFs or in continuous infusion for advanced PD subjects when DBS is not suitable (table1).78,79
Catechol-O- methyltransferase (COMT) inhibitors
Inhibition of enzymatic degradation of levodopa with the COMT inhibitor, entacapone, has demonstrated a benefit on wearing-off.72 This medication has minimal side effects, mostly gastrointestinal symptoms such as nausea and diarrhea.80 Entacapone is administered with each dose of levodopa, and a special formulation combining levodopa/carbidopa and entacapone in a single tablet has been marketed to improve compliance. Tolcapone is another COMT inhibitor, which is both a peripherally and centrally acting COMT inhibitor that may have greater efficacy. Use is limited due to risk of elevated liver transaminases causing fatal hepatotoxicity.80 Tolcapone is administered 3 times daily.
Monoamine oxidase type B (MAO-B) inhibitors
Another add-on class is the MAO-B inhibitors, with two currently approved formulations: selegiline or rasagiline (table1).81 These two medications are easy to use due to once or twice daily dosing and minimal side effects. There are no comparative trials between rasagiline and selegiline. Side effects of selegiline include insomnia due to amphetamine metabolites; thus, selegiline may be helpful in patients with daytime fatigue. Interaction with serotonergic drugs can theoretically cause serotonin syndrome. However, co-use of a MAO-B inhibitor with several commonly used antidepressants has been shown to be safe from allowed use in randomized control trials.82
IPX 066
IPX 066 is a novel extended-release levodopa/carbidopa formulation combining immediate (IR) and extended release levodopa/carbidopa (ER) with a 1:4 ratio of carbidopa to levodopa (Table 1). IPX066 United States Food and Drug Administration (FDA) approval was based on three phase III double blind randomized controlled trials that showed positive results with a satisfactory safety profile.83–85 The largest double blind trial showed that IPX066 reduced daily OFF time by 1.17 h (95% CI −1.69 to −0.66; p < 0.0001).83 IPX066 was approved in January 2015 by the FDA and EU for the treatment of all stages of PD.
Levodopa/carbidopa intestinal gel
Levodopa/carbidopa intestinal gel (LCIG) is a suspension in carboxymethyl-cellulose gel preparation containing 20 mg/ml levodopa and 4.63 mg/ml carbidopa provided in cassettes containing 100 ml of LCIG equivalent to 2000 mg of LD, usually infused over 16 h. LCIG is delivered directly into the proximal jejunum via a percutaneous endoscopic gastrojejunostomy (PEG-J) tube connected to a portable infusion pump. Enteral infusion of LCIG thus bypasses gastric emptying and reduces fluctuations in drug levels. LCIG is an effective treatment for motor fluctuations in PD patients.68,86 In a double blind randomized clinical trial, average daily reduction in OFF time was −1.91 h (p = 0.0015) with increased ON time without troublesome dyskinesias when compared with IR levodopa/carbidopa (difference of 1.86 h, p = 0.0059).87 Practical challenges including cost and side effects are related to complications due to PEG or tube obstruction. LCIG has been used in Europe since 2004 and was approved by the FDA in 2015.
Amantadine
The main add-on drug to treat peak-dose dyskinesia without compromising PD motor effects is amantadine, a N-methyl-D-aspartate (NMDA) glutamate receptor antagonist.71 Potential side effects are insomnia (if administered before bedtime), hallucinations and peripheral edema. There is an ongoing study investigating early use of amantadine (200mg/d) in PD subjects after 1year of levodopa, as a means of preventing developing LID (PREMANDYSK Clinicaltrial.gov - NCT01538329).
Ongoing Clinical Trials for the Treatment of Motor Complications in PD
Several novel levodopa formulations are under investigation to bypass the GI issues related to impaired absorption that contributes to motor fluctuations. Accordion pill levodopa/carbidopa is a gastroretentive slow release levodopa formulation containing a multilayer film folded in an accordion shape.88 Studies have been reported only in abstracts to date, and a phase III study is ongoing (ClinicalTrials.gov - NCT02605434).
ND062L is a transcutaneous formulation available in a patch-pump device.88 Preliminary results are encouraging, however with limited data available (abstracts only). Minor adverse events reported so far are mostly local (transient papules) and related to subcutaneous administration. Studies at phase II and III level are ongoing (Clinicaltrials.gov - NCT02577523, NCT02726386, NCT02782481).
CVT-301 is a levodopa inhalation powder that has been evaluated for sudden-OFFs.88 A Phase II trial showed onset of effect after 10 minutes, and significant mean OFF time change from baseline after 4 weeks (− 0.9 h/day).89 Most common side effects were dizziness, cough, and nausea. A phase III trial is currently ongoing (Clinicaltrial.gov - NCT02242487).
Subcutaneous apomorphine formulation is an effective drug currently available for rescue treatment, providing quick effect onset within minutes but with a relatively short duration of action. Sublingual formulation of apomorphine as a film strip is currently in clinical development with promising results in a phase II trial, with 78.9% of patients achieving ON state within 30 minutes of administration of sublingual film, and a mean duration of ON of 50 minutes.90 A Phase III trial for the treatment of both predictable and unpredictable OFF episodes is currently active (Clinicaltrial.gov - NCT02469090).
Several non-dopaminergic targets have been investigated for motor fluctuations and levodopa-induced dyskinesia as adjunctive therapies. A recent review summarized the non-dopaminergic pathways and mechanism of action of these drugs in PD.91 Istradefylline is an adenosine A2A receptor antagonist currently approved in Japan and under review by the FDA. Potential benefits for non-motor symptoms (cognition and depression) have also been suggested.92 Reduction in OFF time with Istradefylline 20 mg/day was −0.99 h (p < 0.003) and 40 mg/day was −0.96 h (p < 0.003) compared with placebo (−0.23 h).93 Dyskinesia was the most common side effect. A phase III, 12-week, double blind, placebo-controlled, multicenter study is ongoing (Clinicaltrials.gov - NCT01968031).
Tozadenant is another adenosine A2A antagonist under development for motor fluctuations. A Phase IIb trial showed reduction of mean daily OFF time with tozadenant 120 mg (−1.1 h; p = 0.0039) and with tozadenant 180 mg group (−1.2 h; p = 0.0039).94 Common adverse events were dyskinesia, nausea and dizziness. A phase III is currently active (Clinicaltrials.gov - NCT02453386).
Safinamide is a reversible and highly selective MAOB-I, sodium channel antagonist and N-type calcium channel modulator with inhibition of excessive glutamate release that has recently been licensed in the EU for PD. Studies have shown that safinamide 50 and 100 mg/day significantly increased ON time without increasing dyskinesia.95,96,97
ADS-5102 is a long-acting, formulation of amantadine HCl extended-release administered as a capsule once daily before bedtime. A Phase II/III study showed reduction of dyskinesia compared with placebo (27% reduction in Unified Dyskinesia Rating Scale – UDysRS, p = 0.005).98 The most common side effect was constipation. Another long-acting formulation of Amantadine HCl extended-release, administered as a single dose daily in the morning, is currently under investigation in phase III trials (Clinicaltrial.gov - NCT02153632 and NCT02153645).
Serotonergic agonists have also been investigated as potential therapies for LID, including Buspirone and Eltoprazine. Buspirone is a clinically available mixed α1 adrenergic receptor and 5-HT1A agonist that was tested in a single dose study showing possible benefit in PD patients with LID.99 A Phase I study in combination with amantadine and a Phase III trial (buspirone monotherapy) are currently active (Clinicaltrial.gov - NCT02589340). Eltoprazine is a combined 5-HT1A and 5-HT1B agonist, which in a recent Phase IIa study, improved LID measured by the area under the curves of Clinical Dyskinesia Rating Scale (−1.02; p = 0.004) and Rush Dyskinesia Rating Scale (−0.15; p = 0.003).100 Another Phase II trial is currently active (Clinicaltrial.gov - NCT02439125).
Conclusion
Motor complications are extremely common in the natural history of PD, and include motor fluctuations (wearing-off phenomenon) and LID, often with significant impact in daily activities and quality of life. The main strategy for managing such patients is prevention; thus, using lower individual levodopa doses over time seems to be the best approach. The presence of LID will also often affect treatment choices for motor OFF fluctuations due to the risk of exacerbating LID. Thus, with more advanced disease, treatment options may become more difficult. Advanced therapies, such as levodopa-gel infusion or surgery, then become the options. The non-motor symptoms (covered elsewhere) clearly also affect treatment options in this population, mainly due to increasing the risk of side effects.
Acknowledgments
Maria Eliza Freitas received funding from Poul and Susan Hansen Fellowship Award. Christopher Hess receives grant support from the University of Florida Clinical and Translational Research Institute, which is supported in part by NIH award KL2 TR001429. He has served as a research committee member for the Michael J. Fox Foundation and as a speaker for the National Parkinson Foundation, the Parkinson’s Disease Foundation, and the Davis Phinney Foundation. Dr. Hess has participated in CME and educational activities on movement disorders sponsored by Allergan, Ipsen, Mertz Pharmaceuticals, Peerview Online, and QuantiaMD. Susan Fox has received fee consultancy from Adamas, Astra Zeneca; Kyowa, Teva, Novartis, Orion, Zambon; Speaker honoraria from the International Parkinson and Movement Disorder Society and American Academy of Neurology; Research funding from Michael J Fox Foundation for PD research, NIH, Parkinson Society Canada and Toronto Western Foundation.
Abbreviations
- PD
Parkinson’s disease
- LID
levodopa-induced dyskinesia
- LDR
long-duration response
- MAO-B
Monoamine oxidase type B
- COMT
catechol-O-methyl transferase
- DBS
Deep Brain Stimulation
- H&Y
Hoehn & Yahr
- H. pylori
Helicobacter pylori
- UPDRS-III
Unified Parkinson’s disease Rating Scale motor exam
- SNP
Single nucleotide polymorphism
- FOG
Freezing of gait
- LCIG
Levodopa-carbidopa intestinal gel
- PEG
Percutaneous endoscopic gastrostomy
- IR
Immediate release
- ER
Extended release
- h
hours
References
- 1.Hornykiewicz O. A brief history of levodopa. J Neurol. 2010;257(Suppl 2):S249–S252. doi: 10.1007/s00415-010-5741-y. [DOI] [PubMed] [Google Scholar]
- 2.Aquino CC, Fox SH. Clinical spectrum of levodopa-induced complications. Mov Disord. 2015;30(1):80–89. doi: 10.1002/mds.26125. [DOI] [PubMed] [Google Scholar]
- 3.Anderson E, Nutt J. The long-duration response to levodopa: phenomenology, potential mechanisms and clinical implications. Parkinsonism Relat Disord. 2011;17(8):587–592. doi: 10.1016/j.parkreldis.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Contin M, Martinelli P. Pharmacokinetics of levodopa. J Neurol. 2010;257(Suppl 2):S253–S261. doi: 10.1007/s00415-010-5728-8. [DOI] [PubMed] [Google Scholar]
- 5.Widnell K. Pathophysiology of motor fluctuations in Parkinson's disease. Mov Disord. 2005;20(Suppl 11):S17–S22. doi: 10.1002/mds.20459. [DOI] [PubMed] [Google Scholar]
- 6.Marsden CD, Parkes JD. Success and problems of long-term levodopa therapy in Parkinson's disease. The Lancet. 1977;1(8007):345–349. doi: 10.1016/s0140-6736(77)91146-1. [DOI] [PubMed] [Google Scholar]
- 7.Pahwa R, Lyons KE. Levodopa-related wearing-off in Parkinson's disease: identification and management. Curr Med Res Opin. 2009;25(4):841–849. doi: 10.1185/03007990902779319. [DOI] [PubMed] [Google Scholar]
- 8.Yoritaka A, Shimo Y, Takanashi M, et al. Motor and non-motor symptoms of 1453 patients with Parkinson's disease: prevalence and risks. Parkinsonism Relat Disord. 2013;19(8):725–731. doi: 10.1016/j.parkreldis.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Thenganatt MA, Jankovic J. Parkinson disease subtypes. JAMA Neurol. 2014;71(4):499–504. doi: 10.1001/jamaneurol.2013.6233. [DOI] [PubMed] [Google Scholar]
- 10.Warren Olanow C, Kieburtz K, Rascol O, et al. Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson's disease. Mov Disord. 2013;28(8):1064–1071. doi: 10.1002/mds.25364. [DOI] [PubMed] [Google Scholar]
- 11.Fox SH, Lang AE. Levodopa-related motor complications--phenomenology. Mov Disord. 2008;23(Suppl 3):S509–S514. doi: 10.1002/mds.22021. [DOI] [PubMed] [Google Scholar]
- 12.Stocchi F, Rascol O, Kieburtz K, et al. Initiating levodopa/carbidopa therapy with and without entacapone in early Parkinson disease: the STRIDE-PD study. Ann Neurol. 2010;68(1):18–27. doi: 10.1002/ana.22060. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Fang J, Li F, Gao L, Feng T. Risk factors and safe dosage of levodopa for wearing-off phenomenon in Chinese patients with Parkinson's disease. Neurol Sci. 2015;36(7):1217–1223. doi: 10.1007/s10072-015-2078-4. [DOI] [PubMed] [Google Scholar]
- 14.Cilia R, Akpalu A, Sarfo FS, et al. The modern pre-levodopa era of Parkinson's disease: insights into motor complications from sub-Saharan Africa. Brain. 2014;137(Pt 10):2731–2742. doi: 10.1093/brain/awu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Virmani T, Tazan S, Mazzoni P, Ford B, Greene PE. Motor fluctuations due to interaction between dietary protein and levodopa in Parkinson's disease. J Clin Mov Disord. 2016;3(1):8. doi: 10.1186/s40734-016-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahne K-E, Tagesson C, Nyholm D. Motor fluctuations and Helicobacter pylori in Parkinson's disease. J Neurol. 2013;260(12):2974–2980. doi: 10.1007/s00415-013-7089-6. [DOI] [PubMed] [Google Scholar]
- 17.Hashim H, Azmin S, Razlan H, et al. Eradication of Helicobacter pylori infection improves levodopa action, clinical symptoms and quality of life in patients with Parkinson's disease. PLoS ONE. 2014;9(11):e112330. doi: 10.1371/journal.pone.0112330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Çamcı G, Oğuz S. Association between Parkinson's Disease and Helicobacter Pylori. J Clin Neurol. 2016;12(2):147–150. doi: 10.3988/jcn.2016.12.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niccolini F, Rocchi L, Politis M. Molecular imaging of levodopa-induced dyskinesias. Cell Mol Life Sci. 2015;72(11):2107–2117. doi: 10.1007/s00018-015-1854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostic V, Przedborski S, Flaster E, Sternic N. Early development of levodopa-induced dyskinesias and response fluctuations in young-onset Parkinson's disease. Neurology. 1991;41(2 (Pt 1)):202–205. doi: 10.1212/wnl.41.2_part_1.202. [DOI] [PubMed] [Google Scholar]
- 21.Bjornestad A, Forsaa EB, Pedersen KF, Tysnes O-B, Larsen JP, Alves G. Risk and course of motor complications in a population-based incident Parkinson's disease cohort. Parkinsonism Relat Disord. 2016;22:48–53. doi: 10.1016/j.parkreldis.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Scott NW, Macleod AD, Counsell CE. Motor complications in an incident Parkinson's disease cohort. Eur J Neurol. 2016;23(2):304–312. doi: 10.1111/ene.12751. [DOI] [PubMed] [Google Scholar]
- 23.Colombo D, Abbruzzese G, Antonini A, et al. The “gender factor” in wearing-off among patients with Parkinson's disease: a post hoc analysis of DEEP study. The Scientific World JOURNAL. 2015;2015:787451. doi: 10.1155/2015/787451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumagai T, Nagayama H, Ota T, Nishiyama Y, Mishina M, Ueda M. Sex Differences in the Pharmacokinetics of Levodopa in Elderly Patients With Parkinson Disease. Clinical Neuropharmacology. 2014;37(6):173–176. doi: 10.1097/WNF.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 25.Nicoletti A, Arabia G, Pugliese P, et al. Hormonal Replacement Therapy in Women With Parkinson Disease and Levodopa-Induced Dyskinesia. Clinical Neuropharmacology. 2007;30(5):276–280. doi: 10.1097/wnf.0b013e318050c9f9. [DOI] [PubMed] [Google Scholar]
- 26.Sharma JC, Ross IN, Rascol O, Brooks D. Relationship between weight, levodopa and dyskinesia: the significance of levodopa dose per kilogram body weight. Eur J Neurol. 2008;15(5):493–496. doi: 10.1111/j.1468-1331.2008.02106.x. [DOI] [PubMed] [Google Scholar]
- 27.Muller T, Woitalla D, Saft C, Kuhn W. Levodopa in plasma correlates with body weight of parkinsonian patients. Parkinsonism Relat Disord. 2000;6(3):171–173. doi: 10.1016/s1353-8020(00)00005-5. [DOI] [PubMed] [Google Scholar]
- 28.Strong JA, Dalvi A, Revilla FJ, et al. Genotype and smoking history affect risk of levodopa-induced dyskinesias in Parkinson's disease. Mov Disord. 2006;21(5):654–659. doi: 10.1002/mds.20785. [DOI] [PubMed] [Google Scholar]
- 29.Lee J-Y, Cho J, Lee E-K, Park S-S, Jeon BS. Differential genetic susceptibility in diphasic and peak-dose dyskinesias in Parkinson's disease. Mov Disord. 2011;26(1):73–79. doi: 10.1002/mds.23400. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan N, Vituri A, Korczyn AD, et al. Sequence variants in SLC6A3, DRD2, and BDNF genes and time to levodopa-induced dyskinesias in Parkinson's disease. J Mol Neurosci. 2014;53(2):183–188. doi: 10.1007/s12031-014-0276-9. [DOI] [PubMed] [Google Scholar]
- 31.Lee JY, Beom BJ. Risk Factors for Levodopa-induced Dyskinesia. In: Fox SH, Brotchie JM, editors. Levodopa-Induced Dyskinesia in Parkinson's Disease. London: Springer; 2014. p. 51. [Google Scholar]
- 32.Dekker MCJ, Bonifati V, van Duijn CM. Parkinson's disease: piecing together a genetic jigsaw. Brain. 2003;126(Pt 8):1722–1733. doi: 10.1093/brain/awg172. [DOI] [PubMed] [Google Scholar]
- 33.Oliveri RL, Annesi G, Zappia M, et al. Dopamine D2 receptor gene polymorphism and the risk of levodopa-induced dyskinesias in PD. Neurology. 1999;53(7):1425–1430. doi: 10.1212/wnl.53.7.1425. [DOI] [PubMed] [Google Scholar]
- 34.Cheshire P, Bertram K, Ling H, et al. Influence of single nucleotide polymorphisms in COMT, MAO-A and BDNF genes on dyskinesias and levodopa use in Parkinson's disease. Neurodegener Dis. 2014;13(1):24–28. doi: 10.1159/000351097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM. The pharmacology of L-DOPA-induced dyskinesia in Parkinson's disease. Pharmacol Rev. 2013;65(1):171–222. doi: 10.1124/pr.111.005678. [DOI] [PubMed] [Google Scholar]
- 36.Bateman DE, Levett K, Marsden CD. Sleep benefit in Parkinson's disease. J Neurol Neurosurg Psychiatr. 1999;67(3):384–385. doi: 10.1136/jnnp.67.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Gilst MM, Bloem BR, Overeem S. “Sleep benefit” in Parkinson's disease: a systematic review. Parkinsonism Relat Disord. 2013;19(7):654–659. doi: 10.1016/j.parkreldis.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Hutton JT, Morris JL. Long-term evaluation of Sinemet CR in parkinsonian patients with motor fluctuations. Can J Neurol Sci. 1991;18(4):467–471. doi: 10.1017/s0317167100032170. [DOI] [PubMed] [Google Scholar]
- 39.Yeh KC, August TF, Bush DF, Titus DC. Pharmacokinetics and bioavailability of Sinemet CR: a summary of human studies. Neurology. 1989;39(11 Suppl 2):25–38. [PubMed] [Google Scholar]
- 40.DeMaagd G, Philip A. Parkinson's Disease and Its Management: Part 4: Treatment of Motor Complications. P T. 2015;40(11):747–773. doi: 10.1016/j.ejphar.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gottwald MD, Aminoff MJ. Therapies for dopaminergic-induced dyskinesias in Parkinson disease. Ann Neurol. 2011;69(6):919–927. doi: 10.1002/ana.22423. [DOI] [PubMed] [Google Scholar]
- 42.Okuma Y, Yanagisawa N. The clinical spectrum of freezing of gait in Parkinson's disease. Mov Disord. 2008;23(Suppl 2(S2)):S426–S430. doi: 10.1002/mds.21934. [DOI] [PubMed] [Google Scholar]
- 43.Espay AJ, Fasano A, van Nuenen BFL, Payne MM, Snijders AH, Bloem BR. “On” state freezing of gait in Parkinson disease: a paradoxical levodopa-induced complication. Neurology. 2012;78(7):454–457. doi: 10.1212/WNL.0b013e3182477ec0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gazibara T, Pekmezovic T, Kisic-Tepavcevic D, et al. Incidence and prediction of falls in Parkinson's disease: a prospective cohort study. Eur J Epidemiol. 2015;30(4):349–352. doi: 10.1007/s10654-015-0019-4. [DOI] [PubMed] [Google Scholar]
- 45.Malkani R, Zadikoff C, Melen O, Videnovic A, Borushko E, Simuni T. Amantadine for freezing of gait in patients with Parkinson disease. Clinical Neuropharmacology. 2012;35(6):266–268. doi: 10.1097/WNF.0b013e31826e3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim YE, Yun JY, Yang HJ, et al. Intravenous amantadine for freezing of gait resistant to dopaminergic therapy: a randomized, double-blind, placebo-controlled, cross-over clinical trial. PLoS ONE. 2012;7(11):e48890. doi: 10.1371/journal.pone.0048890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J-Y, Oh S, Kim J-M, et al. Intravenous amantadine on freezing of gait in Parkinson's disease: a randomized controlled trial. J Neurol. 2013;260(12):3030–3038. doi: 10.1007/s00415-013-7108-7. [DOI] [PubMed] [Google Scholar]
- 48.Parkinson Study Group. A randomized placebo-controlled trial of rasagiline in levodopa-treated patients with Parkinson disease and motor fluctuations: the PRESTO study. Arch Neurol. 2005;62(2):241–248. doi: 10.1001/archneur.62.2.241. [DOI] [PubMed] [Google Scholar]
- 49.Rascol O, Brooks DJ, Melamed E, et al. Rasagiline as an adjunct to levodopa in patients with Parkinson's disease and motor fluctuations (LARGO, Lasting effect in Adjunct therapy with Rasagiline Given Once daily, study): a randomised, double-blind, parallel-group trial. Lancet. 2005;365(9463):947–954. doi: 10.1016/S0140-6736(05)71083-7. [DOI] [PubMed] [Google Scholar]
- 50.Rahimi F, Roberts AC, Jog M. Patterns and predictors of freezing of gait improvement following rasagiline therapy: A pilot study. Clin Neurol Neurosurg. 2016;150:117–124. doi: 10.1016/j.clineuro.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 51.Espay AJ, Dwivedi AK, Payne M, et al. Methylphenidate for gait impairment in Parkinson disease: a randomized clinical trial. Neurology. 2011;76(14):1256–1262. doi: 10.1212/WNL.0b013e3182143537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devos D, Moreau C, Delval A, Dujardin K, Defebvre L, Bordet R. Methylphenidate : a treatment for Parkinson's disease? CNS Drugs. 2013;27(1):1–14. doi: 10.1007/s40263-012-0017-y. [DOI] [PubMed] [Google Scholar]
- 53.Moreau C, Delval A, Defebvre L, et al. Methylphenidate for gait hypokinesia and freezing in patients with Parkinson's disease undergoing subthalamic stimulation: a multicentre, parallel, randomised, placebo-controlled trial. Lancet Neurol. 2012;11(7):589–596. doi: 10.1016/S1474-4422(12)70106-0. [DOI] [PubMed] [Google Scholar]
- 54.Bastide MF, Meissner WG, Picconi B, et al. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson's disease. Progress in Neurobiology. 2015;132:96–168. doi: 10.1016/j.pneurobio.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Guridi J, González-Redondo R, Obeso JA. Clinical features, pathophysiology, and treatment of levodopa-induced dyskinesias in Parkinson's disease. Parkinsons Dis. 2012;2012:943159. doi: 10.1155/2012/943159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poewe WH, Lees AJ, Stern GM. Dystonia in Parkinson's disease: clinical and pharmacological features. Ann Neurol. 1988;23(1):73–78. doi: 10.1002/ana.410230112. [DOI] [PubMed] [Google Scholar]
- 57.Tolosa E, Compta Y. Dystonia in Parkinson's disease. J Neurol. 2006;253(Suppl 7):VII7–VII13. doi: 10.1007/s00415-006-7003-6. [DOI] [PubMed] [Google Scholar]
- 58.Luquin MR, Scipioni O, Vaamonde J, Gershanik O, Obeso JA. Levodopa-induced dyskinesias in Parkinson's disease: clinical and pharmacological classification. Mov Disord. 1992;7(2):117–124. doi: 10.1002/mds.870070204. [DOI] [PubMed] [Google Scholar]
- 59.Chen R, Ashby P, Lang AE. Stimulus-sensitive myoclonus in akinetic-rigid syndromes. Brain. 1992;115(Pt 6):1875–1888. doi: 10.1093/brain/115.6.1875. [DOI] [PubMed] [Google Scholar]
- 60.Grötzsch H, Sztajzel R, Burkhard PR. Levodopa-induced ocular dyskinesia in Parkinson's disease. Eur J Neurol. 2007;14(10):1124–1128. doi: 10.1111/j.1468-1331.2007.01919.x. [DOI] [PubMed] [Google Scholar]
- 61.LeWitt PA. Conjugate eye deviations as dyskinesias induced by levodopa in Parkinson's disease. Mov Disord. 1998;13(4):731–734. doi: 10.1002/mds.870130421. [DOI] [PubMed] [Google Scholar]
- 62.Linazasoro G, Van Blercom N, Lasa A, Indakoetxea B, Ruiz J. Levodopa-induced ocular dyskinesias in Parkinson's disease. Mov Disord. 2002;17(1):186–187. doi: 10.1002/mds.10017. [DOI] [PubMed] [Google Scholar]
- 63.Rich MW, Radwany SM. Respiratory dyskinesia. An underrecognized phenomenon. Chest. 1994;105(6):1826–1832. doi: 10.1378/chest.105.6.1826. [DOI] [PubMed] [Google Scholar]
- 64.Rice JE, Antic R, Thompson PD. Disordered respiration as a levodopa-induced dyskinesia in Parkinson's disease. Mov Disord. 2002;17(3):524–527. doi: 10.1002/mds.10072. [DOI] [PubMed] [Google Scholar]
- 65.Pietracupa S, Fasano A, Fabbrini G, et al. Poor self-awareness of levodopa-induced dyskinesias in Parkinson's disease: clinical features and mechanisms. Parkinsonism Relat Disord. 2013;19(11):1004–1008. doi: 10.1016/j.parkreldis.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 66.Amanzio M, Palermo S, Zibetti M, et al. Self-unawareness of levodopa induced dyskinesias in patients with Parkinson's disease. Brain Cogn. 2014;90:135–141. doi: 10.1016/j.bandc.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 67.Marconi R, Lefebvre-Caparros D, Bonnet AM, Vidailhet M, Dubois B, Agid Y. Levodopa-induced dyskinesias in Parkinson's disease phenomenology and pathophysiology. Mov Disord. 1994;9(1):2–12. doi: 10.1002/mds.870090103. [DOI] [PubMed] [Google Scholar]
- 68.Wirdefeldt K, Odin P, Nyholm D. Levodopa-Carbidopa Intestinal Gel in Patients with Parkinson's Disease: A Systematic Review. CNS Drugs. 2016 Apr;:1–24. doi: 10.1007/s40263-016-0336-5. [DOI] [PubMed] [Google Scholar]
- 69.Krack P, Pollak P, Limousin P, Benazzouz A, Deuschl G, Benabid AL. From off-period dystonia to peak-dose chorea. The clinical spectrum of varying subthalamic nucleus activity. Brain. 1999;122(Pt 6):1133–1146. doi: 10.1093/brain/122.6.1133. [DOI] [PubMed] [Google Scholar]
- 70.Jankovic J. Motor fluctuations and dyskinesias in Parkinson's disease: clinical manifestations. Mov Disord. 2005;20(Suppl 11):S11–S16. doi: 10.1002/mds.20458. [DOI] [PubMed] [Google Scholar]
- 71.Vijayakumar D, Jankovic J. Drug-Induced Dyskinesia, Part 1: Treatment of Levodopa-Induced Dyskinesia. Drugs. 2016;76(7):759–777. doi: 10.1007/s40265-016-0566-3. [DOI] [PubMed] [Google Scholar]
- 72.Stowe R, Ives N, Clarke CE, et al. Evaluation of the efficacy and safety of adjuvant treatment to levodopa therapy in Parkinson s disease patients with motor complications. Cochrane Database Syst Rev. 2010;(7):CD007166. doi: 10.1002/14651858.CD007166.pub2. [DOI] [PubMed] [Google Scholar]
- 73.Morgan JC, Fox SH. Treating the Motor Symptoms of Parkinson Disease. Continuum (Minneap Minn) 2016;22:1064–1085. doi: 10.1212/CON.0000000000000355. 4 Movement Disorders. [DOI] [PubMed] [Google Scholar]
- 74.Mokhles MM, Trifirò G, Dieleman JP, et al. The risk of new onset heart failure associated with dopamine agonist use in Parkinson's disease. Pharmacol Res. 2012;65(3):358–364. doi: 10.1016/j.phrs.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 75.Perez-Lloret S, Rey MV, Crispo J, et al. Risk of heart failure following treatment with dopamine agonists in Parkinson's disease patients. Expert Opin Drug Saf. 2014;13(3):351–360. doi: 10.1517/14740338.2014.888057. [DOI] [PubMed] [Google Scholar]
- 76.Cannas A, Solla P, Floris G, et al. Reversible Pisa syndrome in patients with Parkinson's disease on dopaminergic therapy. J Neurol. 2009;256(3):390–395. doi: 10.1007/s00415-009-0072-6. [DOI] [PubMed] [Google Scholar]
- 77.Katzenschlager R, Head J, Schrag A, et al. Fourteen-year final report of the randomized PDRG-UK trial comparing three initial treatments in PD. Neurology. 2008;71(7):474–480. doi: 10.1212/01.wnl.0000310812.43352.66. [DOI] [PubMed] [Google Scholar]
- 78.Dewey RB, Hutton JT, LeWitt PA, Factor SA. A randomized, double-blind, placebo-controlled trial of subcutaneously injected apomorphine for parkinsonian off-state events. Arch Neurol. 2001;58(9):1385–1392. doi: 10.1001/archneur.58.9.1385. [DOI] [PubMed] [Google Scholar]
- 79.Trosch RM, Silver D, Bottini PB. Intermittent subcutaneous apomorphine therapy for 'off‘ episodes in Parkinson’s disease: a 6-month open-label study. CNS Drugs. 2008;22(6):519–527. doi: 10.2165/00023210-200822060-00005. [DOI] [PubMed] [Google Scholar]
- 80.Haasio K. Toxicology and safety of COMT inhibitors. Int Rev Neurobiol. 2010;95:163–189. doi: 10.1016/B978-0-12-381326-8.00007-7. [DOI] [PubMed] [Google Scholar]
- 81.Fox SH, Katzenschlager R, Lim S-Y, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the motor symptoms of Parkinson's disease. Mov Disord. 2011;26(Suppl 3):S2–S41. doi: 10.1002/mds.23829. [DOI] [PubMed] [Google Scholar]
- 82.Smith KM, Eyal E, Weintraub D. Combined Rasagiline and Antidepressant Use in Parkinson Disease in the ADAGIO Study. JAMA Neurol. 2015;72(1):88–88. doi: 10.1001/jamaneurol.2014.2472. [DOI] [PubMed] [Google Scholar]
- 83.Hauser RA, Hsu A, Kell S, et al. Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson's disease and motor fluctuations: a phase 3 randomised, double-blind trial. Lancet Neurol. 2013;12(4):346–356. doi: 10.1016/S1474-4422(13)70025-5. [DOI] [PubMed] [Google Scholar]
- 84.Stocchi F, Hsu A, Khanna S, et al. Comparison of IPX066 with carbidopa-levodopa plus entacapone in advanced PD patients. Parkinsonism Relat Disord. 2014;20(12):1335–1340. doi: 10.1016/j.parkreldis.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 85.Pahwa R, Lyons KE, Hauser RA, et al. Randomized trial of IPX066, carbidopa/levodopa extended release, in early Parkinson's disease. Parkinsonism Relat Disord. 2014;20(2):142–148. doi: 10.1016/j.parkreldis.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 86.Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13(2):141–149. doi: 10.1016/S1474-4422(13)70293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seeberger LC, Hauser RA. Carbidopa levodopa enteral suspension. Expert Opin Pharmacother. 2015;16(18):2807–2817. doi: 10.1517/14656566.2015.1111336. [DOI] [PubMed] [Google Scholar]
- 88.Freitas ME, Ruiz-Lopez M, Fox SH. Novel Levodopa Formulations for Parkinson's Disease. CNS Drugs. 2016 Oct; doi: 10.1007/s40263-016-0386-8. [Epubaheadofprint] [DOI] [PubMed] [Google Scholar]
- 89.Lewitt PA, Hauser RA, Grosset DG, et al. A randomized trial of inhaled levodopa (CVT-301) for motor fluctuations in Parkinson's disease. Mov Disord. 2016;31(9):1356–1365. doi: 10.1002/mds.26611. [DOI] [PubMed] [Google Scholar]
- 90.Hauser RA, Olanow CW, Dzyngel B, et al. Sublingual apomorphine (APL-130277) for the acute conversion of OFF to ON in Parkinson's disease. Mov Disord. 2016;31(9):1366–1372. doi: 10.1002/mds.26697. [DOI] [PubMed] [Google Scholar]
- 91.Freitas ME, Fox SH. Nondopaminergic treatments for Parkinson's disease: current and future prospects. Neurodegener Dis Manag. 2016;6(3):249–268. doi: 10.2217/nmt-2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vorovenci RJ, Antonini A. The efficacy of oral adenosine A(2A) antagonist istradefylline for the treatment of moderate to severe Parkinson's disease. Expert Rev Neurotherapeutics. 2015;15(12):1383–1390. doi: 10.1586/14737175.2015.1113131. [DOI] [PubMed] [Google Scholar]
- 93.Mizuno Y, Kondo T Japanese Istradefylline Study Group. Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson's disease. Mov Disord. 2013;28(8):1138–1141. doi: 10.1002/mds.25418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hauser RA, Olanow CW, Kieburtz KD, et al. Tozadenant (SYN115) in patients with Parkinson's disease who have motor fluctuations on levodopa: a phase 2b, double-blind, randomised trial. Lancet Neurol. 2014;13(8):767–776. doi: 10.1016/S1474-4422(14)70148-6. [DOI] [PubMed] [Google Scholar]
- 95.Borgohain R, Szasz J, Stanzione P, et al. Randomized trial of safinamide add-on to levodopa in Parkinson's disease with motor fluctuations. Mov Disord. 2014;29(2):229–237. doi: 10.1002/mds.25751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borgohain R, Szasz J, Stanzione P, et al. Two-year, randomized, controlled study of safinamide as add-on to levodopa in mid to late Parkinson's disease. Mov Disord. 2014;29(10):1273–1280. doi: 10.1002/mds.25961. [DOI] [PubMed] [Google Scholar]
- 97.Cattaneo C, Ferla RL, Bonizzoni E, Sardina M. Long-Term Effects of Safinamide on Dyskinesia in Mid- to Late-Stage Parkinson's Disease: A Post-Hoc Analysis. J Parkinsons Dis. 2015;5(3):475–481. doi: 10.3233/JPD-150569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pahwa R, Tanner CM, Hauser RA, et al. Amantadine extended release for levodopa-induced dyskinesia in Parkinson's disease (EASED Study) Mov Disord. 2015;30(6):788–795. doi: 10.1002/mds.26159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eskow KL, Gupta V, Alam S, Park JY, Bishop C. The partial 5-HT(1A) agonist buspirone reduces the expression and development of l-DOPA-induced dyskinesia in rats and improves l-DOPA efficacy. Pharmacol Biochem Behav. 2007;87(3):306–314. doi: 10.1016/j.pbb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 100.Svenningsson P, Rosenblad C, Af Edholm Arvidsson K, et al. Eltoprazine counteracts l-DOPA-induced dyskinesias in Parkinson's disease: a dose-finding study. Brain. 2015;138(Pt 4):963–973. doi: 10.1093/brain/awu409. [DOI] [PMC free article] [PubMed] [Google Scholar]