Highlights
-
•
fMRI was used to examine the brain mechanisms through which tactile stimuli disrupt processing of social cues in youth with ASD.
-
•
Tactile stimuli caused up-regulation of auditory language areas in TD youth but decreases in these areas in ASD youth.
-
•
Directing attention to social cues mitigated the effect of the sensory distracter so that activation was sustained in auditory-language areas.
-
•
Attentional direction to social cues was associated with increases in medial prefrontal cortex for ASD youth.
-
•
Severity of sensory over-responsivity modulated the effect of the distracter and attentional direction on brain processing of social cues.
Abbreviations: ASD, autism spectrum disorder; fMRI, functional magnetic resonance imaging; SOR, sensory over-responsivity; TD, typically-developing
Keywords: Autism, fMRI, Sensory over-responsivity, Social cognition
Abstract
Sensory over-responsivity (SOR) is a common condition in autism spectrum disorders (ASD) that is associated with greater social impairment. However, the mechanisms through which sensory stimuli may affect social functioning are not well understood. This study used fMRI to examine brain activity while interpreting communicative intent in 15 high-functioning youth with ASD and 16 age- and IQ-matched typically-developing (TD) controls. Participants completed the task with and without a tactile sensory distracter, and with and without instructions directing their attention to relevant social cues. When completing the task in the presence of the sensory distracter, TD youth showed increased activity in auditory language and frontal regions whereas ASD youth showed decreased activation in these areas. Instructions mitigated this effect such that ASD youth did not decrease activation during tactile stimulation; instead, the ASD group showed increased medial prefrontal activity. SOR severity modulated the effect of the tactile stimulus on social processing. Results demonstrate for the first time a neural mechanism through which sensory stimuli cause disruption of social cognition, and that attentional modulation can restore neural processing of social cues through prefrontal regulation. Findings have implications for novel, integrative interventions that incorporate attentional directives to target both sensory and social symptoms.
1. Introduction
Sensory over-responsivity (SOR) is an impairing condition manifested as extreme sensitivity to stimuli such as unexpected loud noises or being touched. SOR is particularly common (rates of 56–70%) in autism spectrum disorders (ASD) (Ben-Sasson et al., 2008) and, notably, it is associated with higher impairment including greater deficits in social and adaptive behavior (Ben-Sasson et al., 2008). Although SOR is strongly linked to impairment, the mechanisms through which it disrupts daily life, and particularly social functioning, are not well understood. In this study, we used functional magnetic resonance imaging (fMRI) to examine the effect of a tactile sensory distracter on the brain’s processing of social information in youth with and without ASD.
Recently, the association between SOR and social functioning has been gaining increased attention. Multiple parent-report studies have shown that SOR is associated with poorer social functioning in children with ASD including lower Vineland, Social Responsiveness Scale (SRS), and DSM-IV social scales (Glod et al., 2015). However, there is little experimental or observational research directly examining the effect of SOR on social functioning. One exception is a recent study finding that SOR was related to greater cortisol response to a peer interaction and increased stress response in multiple contexts (Corbett et al., 2016).
Recent neuroimaging work has begun to characterize the neurobiological basis of SOR, providing important clues about the mechanisms through which SOR is associated with social impairment. These studies show that, within ASD, SOR is related to over-reactive responses and decreased habituation to mildly aversive sensory stimuli in primary sensory processing brain regions, as well as in brain regions related to affective valence, salience, and attention such as the amygdala and insula (Green et al., 2013, Green et al., 2015). These findings suggest that SOR is related to over-attribution of salience to extraneous sensory information. Consistent with this idea, ASD has been linked to altered resting-state connectivity in the salience network, which is thought to influence attention and behavioral response to stimuli, particularly those that are emotionally or socially salient (Uddin et al., 2013). Further, SOR has been linked to greater functional connectivity between the anterior insula – the hub of the salience network – and both amygdala and sensory processing regions, but less connectivity between the salience network and areas associated with social cognition (e.g., fusiform gyrus and precuneus) (Green et al., 2016).
Taken together, these studies suggest that SOR may be related to increased attribution of salience to sensory information as compared to social cues, leading to over-attention to extraneous sensory information at the cost of attention to social information. Yet, the effect of sensory distracters on the brain’s ability to process social information has not been tested directly. Multiple studies have shown that individuals with ASD show reduced activity in social cognition regions of the brain in response to basic social cues. For example, compared to neurotypical controls, individuals with ASD show reduced fusiform face area activation in response to faces and facial emotions (Dalton et al., 2005, Pierce et al., 2001, Hubl et al., 2003) and reduced activity in superior temporal sulcus (STS) in response to voices (Gervais et al., 2004). However, real-world processing of social cues, which requires integration of multiple, often ambiguous types of information, is even more likely to be disrupted by extraneous sensory stimuli.
In this study, we examined this effect using a “sarcasm” task that requires participants to interpret communicative intent in non-literal language, an area of difficulty for individuals with ASD (Pexman et al., 2010, Kaland et al., 2002). Interestingly, even when high-functioning individuals with ASD can detect non-literal language such as sarcasm, they have difficulty explaining their responses and are often unable to appropriately use such language in their everyday lives (Leekam and Prior, 1994). Neuroimaging research supports these findings: although ASD and TD children and adolescents perform equally well on this sarcasm task, children with ASD lack the neural modulation seen in TD children when interpreting ambiguous versus unambiguous social information. Specifically, in a study by Wang et al. (2007) TD but not ASD children showed significant activity in medial prefrontal cortex (mPFC) and temporal regions when interpreting the meaning of potentially sarcastic comments. However, when participants were explicitly instructed to pay attention to the speaker’s facial expression or tone of voice, the ASD (but not TD) group increased activation in the mPFC, though the TD group continued to have greater superior temporal activation. Regardless of instructions, greater activity in the mPFC and temporal gyri were associated with lower levels of social impairment. Taken together, the results of this study indicate that children with ASD had more difficulty integrating facial, prosodic, and contextual cues to infer a speaker’s ambiguous intent, but that explicit instructions to attend to vocal and facial affective cues elicited greater activity in areas that allow integrative processing, such as mPFC, which are normally automatically activated in TD individuals. Findings from other studies support the idea that explicitly directing attention to social stimuli can increase activation in brain areas that are usually activated in neurotypical individuals: for example, Hadjikhani et al. (2004) found that levels of fusiform activity in response to faces in individuals with ASD increased to typical levels when participants were directed to look at the eyes. However, in a natural setting, many stimuli simultaneously compete for attention, and this is likely to be particularly true for ASD individuals with SOR. Thus, it is unclear whether, for individuals with ASD, explicit instructions would continue to increase neural recruitment of social cognition regions even in a real-world setting with multiple sensory distracters, or if the brain – “flooded” with the sensory information – would be unable to redirect attention. This question has important implications for intervention, especially given that mPFC recruitment during social inference is related to greater social competence in children with ASD (Wang et al., 2007).
In this study, we tested the effect of a sensory distracter on brain responses to the same sarcasm social cognition task used in Wang et al. (2007) with and without explicit instructions. We chose a tactile sensory stimulus because, along with auditory stimuli, responsivity to these stimuli has been found to best distinguish individuals with and without SOR (Kern, 2006, Leekam et al., 2006, Tomchek and Dunn, 2007); we did not use auditory stimuli as we wanted to differentiate between SOR and auditory filtering problems. We hypothesized that the addition of a tactile distracter would differentially affect brain activity during the sarcasm task in youth with and without ASD. Specifically, we expected that TD youth would up-regulate auditory language and prefrontal areas, as activity in these regions has been found to increase with task complexity and with attentional demand (Culham et al., 2001, Giraud et al., 2004, Colich et al., 2012). Conversely, we expected that ASD youth would show decreases in these areas in the presence of the tactile stimulus, reflecting greater difficulty maintaining integrative processing of auditory and social cues. However, we hypothesized that with explicit redirection of attention to relevant social cues, the tactile stimulus would cause less decreased attention in social cognition regions, coupled with greater increases in medial prefrontal cortex in the ASD group. We also hypothesized that within the ASD group, SOR would be correlated with reduced activity in social cognition regions during tactile stimulation, and fewer increases in medial prefrontal cortex with attentional instructions.
2. Methods
2.1. Participants
Participants were 15 youth with ASD and 16 TD matched controls aged 9–17.6 years (M = 13.66; SD = 2.11); the groups did not differ significantly in age, IQ, or motion during fMRI (see Table 1). All participants had full-scale, verbal, and performance IQs of 75 or greater based on the Weschler Abbreviated Scales of Intelligence (WASI (Wechsler, 1999)), the Weschler Intelligence Scale for Children–4th Edition (WISC-IV (Wechsler, 2003)), or the Differential Abilities Scales (DAS) (Elliott, 1990). ASD participants had a prior diagnosis of Autism Spectrum Disorder, confirmed using the Autism Diagnostic Interview – Revised (ADI-R (Lord et al., 1994)) and the Autism Diagnostic Observation Schedule – 2nd Edition (ADOS-2 (Lord et al., 2012)). Data were originally acquired for 18 ASD and 17 TD subjects; 3 ASD participants and 1 TD participant were excluded due to excessive motion. No participants reported loss of consciousness for longer than 5 min or any neurological (e.g., epilepsy), genetic (e.g., Fragile X), or severe psychiatric disorder (e.g., schizophrenia) other than autism. Additionally, no TD participants had comorbid psychiatric disorders (e.g., ADHD, mood disorders, anxiety). Participants were recruited from the UCLA Child and Adult Neurodevelopmental Clinic, from flyers poster around UCLA and in the community, and through local autism organizations. Recruitment was conducted in part under the auspices of The Help Group-UCLA Autism Research Alliance. All parents provided written informed consent and children gave written assent. Study procedures were approved by the UCLA Institutional Review Board.
Table 1.
Descriptive statistics.
| ASD | TD | t or χ2 | |
|---|---|---|---|
| Age | 14.09 (2.70) | 14.97 (2.44) | 0.95 |
| Gender (% male) | 73% (n = 11) | 75% (n = 12) | 0.01 |
| Handedness (% right-handed) | 100% (n = 15) | 94% (n = 15) | 0.97 |
| FSIQ | 105.00 (16.84) | 109.44 (10.26) | 0.89 |
| VIQ | 103.33 (17.22) | 108.94 (11.32) | 1.06 |
| PIQ | 107.87 (18.80) | 105.06(8.27) | −0.53 |
| Mean Absolute Motion | 0.64 (0.39) | 0.45 (0.30) | −1.5 |
| Max Absolute Motion | 1.82 (1.85) | 1.62 (1.57) | −0.33 |
| Mean Relative motion | 0.14 (0.14) | 0.11 (0.06) | −0.68 |
| Max Relative Motion | 1.46 (1.87) | 1.13 (0.94) | −0.63 |
| Mean Volumes Censored | 13.47 (7.27) | 11.63 (5.29) | −0.81 |
| SensOR tactile count | 10.60 (8.90) | 1.63 (1.78) | 3.84** |
| SSP tactile sensitivity | 28.73 (4.80) | 34.00 (2.07) | 3.92** |
| Tactile SOR composite | 0.61 (0.93\4) | −0.57 (0.28) | −4.63*** |
| NINT accuracy | 89.47 (21.21) | 100.00 (0) | −1.92+ |
| NIT accuracy | 83.33 (32.27) | 100.00 (0) | −2.00+ |
| INT accuracy | 88.33 (16.0) | 98.33 (6.46) | −2.25* |
| IT accuracy | 89.47 (28.14) | 93.33 (11.44) | −0.51 |
Note: Lower SSP scores indicate higher symptom severity. N = 15 ASD, 16 TD.
p < 0.10.
p < 0.05.
p < 0.01.
p < 0.001.
2.2. MRI data acquisition
MRI data were acquired on a Siemens Trio 3 T scanner. A high-resolution structural T2-weighted echo-planar imaging volume (spin-echo, TR = 5000 ms, TE = 33 ms, 128 × 128 matrix, 20 cm FOV, 36 slices, 1.56 mm in-plane resolution, 3 mm thick) was acquired coplanar to the functional scans in order to ensure identical distortion characteristics. Each functional run involved the acquisition of 176 EPI volumes (gradient-echo, TR = 2500 ms, TE = 30 ms, flip angle = 90, 64 × 64 matrix, 20 cm FOV, 33 slices, 3.125 mm in-plane resolution, 3 mm thick). Auditory stimuli were presented to the participant using magnet-compatible headphones under computer control (Resonance Technologies, Inc.). Participants wore earplugs and headphones to reduce interference of the auditory stimuli from the scanner noise.
2.3. FMRI sensory paradigm
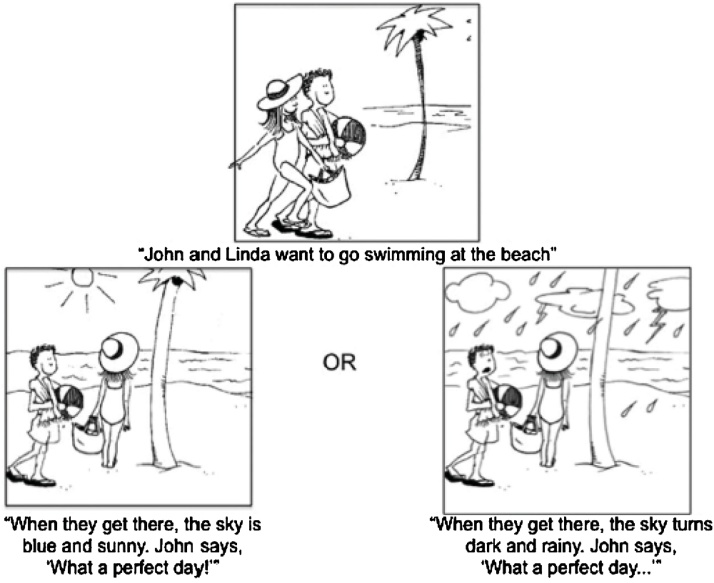
The sarcasm task has been previously reported on by our group (Wang et al., 2007, Wang et al., 2006a, Wang et al., 2006b, Colich et al., 2012) (see Wang et al., 2006a, Wang et al., 2006b, Wang et al., 2007 for details on the task design). The task consisted of 16 different scenarios in which participants first saw a picture with auditory narration setting up the story, followed by a second picture depicting an outcome accompanied by either a sincere or sarcastic remark (Fig. 1). Participants were then asked, “Did [name] mean what he (or she) said?” and responded yes or no using a button box. Accuracy was recorded as percentage of total responded trials for which participants correctly respond “yes” to sincere trials and “no” to sarcastic trials, and did not include trials with no response (4 in the ASD group, 1 in the TD group). Due to a computer malfunction, accuracy data was not available for one TD participant; thus, accuracy analyses were conducted with 15 ASD and 15 TD participants. The scenarios were presented in four 60 s blocks which included four scenarios each. Each block contained two scenarios with a sincere ending and two with a sarcastic ending; the ending given to a particular scenario as well as the order of the scenarios was counterbalanced across participants within each group. All analyses were conducted combining sarcastic and sincere scenarios within a given block, as our focus was on the brain processing underlying the interpretation of a speaker’s communicative intent rather than differential brain responses to the prosody of the speech (see Colich et al., 2012). The task had a 2 × 2 design, with tactile condition (tactile stimulus present or not present) and instructions (general or specific attentional directives) as the two independent variables. During the first two “No Instruction” blocks, the participants were simply given the instruction to “pay attention” (No Instruction No Tactile, NINT; and No Instruction Tactile, NIT). During the second two “Instruction” blocks, the participants were primed with the instructions, “Pay attention to the tone of voice and the look on the face” (Instruction No Tactile, INT; and Instruction Tactile, IT). One block of No Instructions and one block of Instructions was presented with simultaneous tactile stimulation on the inner left arm from elbow to wrist at the rate of 1 stroke/s. The tactile stimulus consisted of a scratchy wool fabric attached to a handle so that only the fabric and not the experimenter touched the participant. The order of the tactile and no tactile blocks was counterbalanced among participants. Alternating between scenario blocks were blocks of 15 s tactile stimulation only (not analyzed for this study). Between each block of the social cognition task and tactile stimulation only was 15 s of fixation, with an additional 15 s of initial and final fixation. Total scan length was 7 min 20 s.
Fig. 1.
Sarcasm task example. The setup (top) is shared by sincere and sarcastic versions. Participants see either a sincere (bottom left) or sarcastic (bottom right) ending. The text represents the auditory stimuli accompanying the drawing. After viewing the outcome, participants answer the question, “Did John mean what he said?.
2.4. Behavioral measures
Diagnostic and cognitive measures were administered at a clinical assessment visit; child sensory questionnaires were completed by parents (see Table 1).
2.4.1. Short sensory profile (SSP (Dunn, 1999))
The SSP is a widely used, parent report measure of sensory dysregulation across modalities. We used the Auditory/Visual, and Tactile Sensitivity subscales. Higher scores on the SSP indicate lower impairment. This measure has strong reliability and validity (McIntosh and Miller, 1999).
2.4.2. Sensory over-responsivity (SensOR) inventory (Schoen et al., 2008)
The SensOR Inventory is a parent checklist of sensory sensations that bother their child. For this study, we used the tactile subscale. The number of items parents rate as bothering their child has been shown to discriminate between children with and without SOR (Schoen et al., 2008).
2.4.3. SOR composite
An SOR composite score was created by standardizing (creating Z-scores) and averaging tactile-related subscales of the SOR measures (SSP reverse-scored tactile sensitivity scale and SensOR tactile scores) across all participants, as in our previous work (Green et al., 2013, Green et al., 2015).
2.5. fMRI data analysis
Analyses were performed using FSL Version 5.0.8 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Preprocessing included motion correction to the mean image, spatial smoothing (Gaussian Kernel FWHM = 5 mm), and high-pass temporal filtering (t > 0.01 Hz). Functional data were linearly registered to a common stereotaxic space by first registering to the in-plane T2 image (6 ° of freedom) then to the MNI152 T1 2 mm brain (12 ° of freedom). FSL’s fMRI Expert Analysis Tool (FEAT), Version 6.00 was used for statistical analyses. Fixed-effects models were run separately for each subject, then combined in a higher-level mixed-effects model to investigate within and between-group differences. Volumes identified as containing excessive motion using FSL’s motion outliers tool (default setting: root mean squared (RMS) intensity difference outliers compared to the center volume) were excluded using censoring. For two ASD subjects, excluding RMS outliers was not sufficient to reduce max motion below 1.5 mm framewise displacement, so additional volumes were censored (7 additional for one, 6 additional for the other). Single-subject models included six motion parameters and the motion censor as covariates. Each experimental condition (No Instructions No Tactile, NINT; No Instructions Tactile, NIT; Instructions No Tactile, INT; and Instructions Tactile, IT) was modeled with respect to the fixation condition during rest. Tactile conditions within each instruction condition were then compared to the no tactile conditions (NIT > NINT and IT > INT). Higher-level group analyses were carried out using FSL’s FLAME (FMRIB’s Local Analysis of Mixed Effects State) stage 1 (Beckmann et al., 2003, Woolrich, 2008, Woolrich et al., 2004).
Within-group activation maps for each condition (vs. fixation) were thresholded at Z > 2.3 (p<0.01) and whole-brain cluster-corrected at p < 0.05 using FSL (http://www.fmrib.ox.ac.uk/fsl). Between-group comparisons were thresholded at Z > 1.7 (p< 0.05), whole-brain cluster-corrected at p<0.05; only clusters with peaks of Z > 2.3 are reported as significant.
2.6. Correlation with SOR scores
To determine whether the effect of the tactile distracter on neural response to the sarcasm task varied as a function of SOR, regression analyses were performed with the SOR composite (centered within-group) as the independent variable predicting change in BOLD response with the addition of the tactile stimulus (i.e., No Instruction Tactile > No Instruction No Tactile and Instruction Tactile > Instruction No Tactile). These analyses were performed only within the ASD group, as there was very little variability in SOR in the TD group. Parameter estimates from significant clusters were extracted and plotted to ensure that the correlations were not driven by outliers. These plots are displayed in Supplementary Figs. 1 and 2; TD parameter estimates for the same regions are plotted as well for purposes of comparison, although there were no significant associations with SOR severity.
3. Results
3.1. Behavioral results
3.1.1. SOR
Independent-sample t-tests showed that, as expected, the ASD group was rated as having significantly more severe tactile SOR symptoms than the TD group on both sensory measures (Table 1), as well as higher scores on the SRS. The correlation between SRS total and the SOR tactile composite was significant in the ASD group only (TD: r= 0.01, p= 0.48; ASD: r= 0.59, p= 0.01).
3.1.2. Accuracy
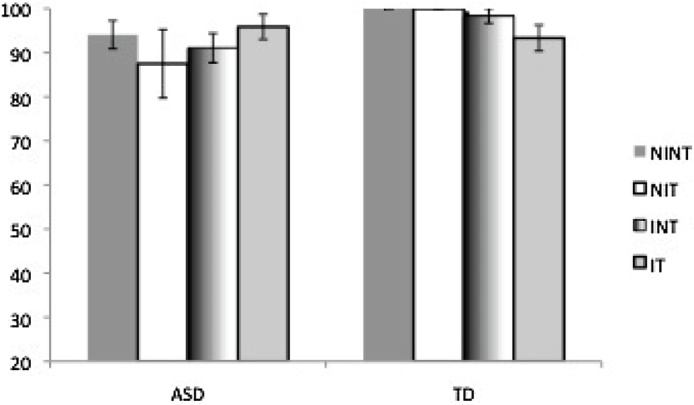
The ASD group had marginally lower accuracy for the No Instruction conditions and significantly lower accuracy for the Instruction No Tactile (INT) condition compared to the TD group (see Table 1). We tested for GroupXCondition differences in accuracy using a 2 × 2 repeated-measures ANOVA with Instruction (No Instruction and Instruction) and Tactile (No Tactile and Tactile) as within-group variables, Group (TD or ASD) as a between-group variable, and age as a covariate. This analysis revealed a significant InstructionXTactileXAge interaction (F(27) = 7.17, p= 0.01). Follow-up analysis revealed that this interaction was accounted for by one young ASD participant with very low accuracy (25% average accuracy). The ANOVA was run again excluding this participant and showed a significant InstructionXTactileXGroup interaction (see Fig. 2) indicating that, in the ASD group, the tactile stimulus caused accuracy to go down in the No Instruction condition, but to go up in the Instruction condition. In the TD group, accuracy was consistent across Tactile and No Tactile for the No Instruction conditions, but went down for Tactile only in the Instruction condition. There were no other main effects or interactions. With the accuracy outlier removed, there were no significant group differences in accuracy, although differences approached significance for the NINT condition (ASD mean = 94, SD = 11.92; TD mean = 100, SD = 0, t(13) = −1.86, p= 0.09) and the INT condition (ASD mean = 91.07, SD = 12.43, TD mean = 98.33, SD = 6.46, t(19) = −1.95, p= 0.07). All subsequent fMRI analyses were conducted with and without the participant with low accuracy, and none of the results differed, so that participant was included in final analyses.
Fig. 2.
Accuracy data (percent correct responses out of total responded trials) for each group with no explicit instructions, with and without the tactile stimulus (NINT and NIT) and with explicit instructions, with and without the tactile stimulus (INT and IT). Figure shows 15 ASD (autism spectrum disorder) and 15 TD (typically developing) participants, excluding one ASD outlier with extremely low accuracy. Group pairwise comparisons were not significantly different, but a GroupXCondition ANOVA showed an interaction effect by which the ASD group increased in accuracy but the TD group decreased in accuracy when instructions were added to the tactile condition.
4. fMRI results
4.1. No instruction conditions
4.1.1. No instruction, no tactile condition (Table 2, Fig. 3)
Table 2.
MNI coordinates for the No Instructions No Tactile condition as compared to baseline.
| TD |
ASD |
ASD > TD |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI Peak (mm) |
Max | MNI Peak (mm) |
Max | MNI Peak (mm) |
Max | ||||||||||
| Voxels | x | y | z | Z | Voxels | x | y | z | Z | Voxels | x | y | z | Z | |
| Occipital Pole (V1) | 24238 | 12 | −94 | 18 | 6.17 | 41715 | −8 | −100 | 10 | 6.20 | |||||
| Right Lateral Occipital Cortex | 32 | −86 | −8 | 5.99 | |||||||||||
| Left Superior/Middle Temporal Gyrus | −62 | −42 | 8 | 5.99 | |||||||||||
| Left Heschl's Gyrus | −48 | −20 | 4 | 6.12 | |||||||||||
| Left Orbitofrontal Cortex/Inferior Frontal Gyrus | −50 | 32 | −10 | 4.17 | |||||||||||
| Left Inferior Frontal Gyrus | −44 | 10 | −22 | 4.32 | 1468 | −44 | 12 | 22 | 3.02 | ||||||
| Left Middle Frontal Gyrus | −36 | 2 | 48 | 4.07 | −38 | 4 | 58 | 2.93 | |||||||
| Left Occipital Fusiform | −18 | −82 | −10 | 5.48 | 1783 | −26 | −80 | −6 | 3.88 | ||||||
| Left Temporal Occipital Fusiform | −44 | −56 | −14 | 3.56 | |||||||||||
| Left Lateral Occipital Cortex | −34 | −82 | 6 | 5.85 | −50 | −64 | 14 | 3.46 | |||||||
| Left Inferior Temporal Gyrus | −48 | −62 | −16 | 3.25 | |||||||||||
| Left Superior Frontal Gyrus | 1926 | −8 | 48 | 46 | 4.54 | 10 | 52 | 34 | 3.92 | ||||||
| Right Orbitofrontal Cortex | 42 | 32 | −12 | 3.56 | 2329 | 48 | 46 | −16 | 3.51 | ||||||
| Right Orbitofrontall/Inferior Frontal Gyrus | 54 | 28 | 6 | 4.22 | 44 | 44 | −20 | 3.39 | |||||||
| Paracingulate Gyrus/Supp Motor Cortex | 2306 | −2 | 6 | 54 | 4.86 | ||||||||||
| Right Superior/Middle Temporal Gyrus | 3191 | 60 | −26 | 8 | 5.80 | 68 | −20 | 0 | 5.92 | 2513 | 66 | −52 | 6 | 3.62 | |
| Right Planum Temporale | 60 | −18 | 4 | 4.90 | |||||||||||
| Left Precentral Gyrus | 1658 | −38 | −20 | 68 | 4.37 | ||||||||||
| Left Postcentral Gyrus | −46 | −18 | 58 | 4.18 | |||||||||||
Note: x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively; Z refers to the Z-score at those coordinates (local maxima in bold, submaxima underneath). Voxels refers to number of voxels in the cluster; labels without voxels indicate submaxima within the same cluster as label above with voxel size. Within-group analyses are cluster corrected for multiple comparisons, Z > 2.3, p < 0.05; between-group analyses are cluster corrected at Z > 1.7, p < 0.05. Between-group analyses are masked by regions of significant activation in either within-group analysis at Z > 1.7, corrected.
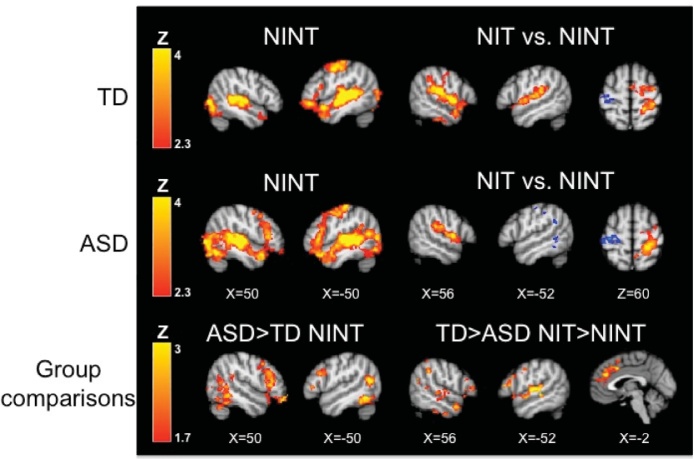
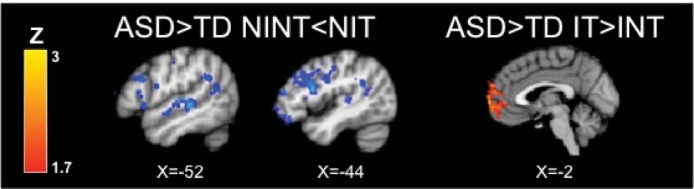
Fig. 3.
Within- and between-group results for the sarcasm task without explicit instructions. In the No Instruction No Tactile (NINT) condition, both ASD and TD youth show activation of brain regions including frontal cortex and primary auditory and visual areas (Table 2); compared to TD youth, ASD participants show significantly greater activation in left inferior frontal gyrus, temporal gyrus, and occipital cortex. When the tactile stimulus was added (No Instructions Tactile, NIT compared to No Instruction No Tactile, NINT), both TD and ASD youth demonstrate increased neural activity in primary somatosensory and association areas (red; Table 6). TD youth show greater increases in brain regions implicated in social cognition (e.g., prefrontal cortex), and language processing (e.g., inferior frontal gyrus) compared to ASD youth (Table 6). Within-group contrasts thresholded at Z > 2.3, corrected (p< 0.05). Between-group contrasts thresholded at Z > 1.7, corrected.
During the No Instructions, No Tactile (NINT) condition compared to rest, both groups showed significant activation in bilateral primary auditory and visual cortices and association areas, dorsomedial prefrontal cortex, left inferior frontal gyrus (IFG), and left precentral gyrus (button press was with the right hand). The ASD group also had significant activation in the right IFG. Between-group comparisons showed that the ASD group had greater activation in right temporal gyrus, left lateral occipital cortex, and bilateral IFG. There were no significant areas where the TD showed great activity than the ASD group.
4.1.2. No instructions tactile condition (Tables 3, 6, & 7, and Figs. 1 & 5)
Table 3.
MNI coordinates for the No Instructions Tactile condition as compared to baseline.
| TD |
ASD |
ASD > TD |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI Peak (mm) |
Max | MNI Peak (mm) |
Max | MNI Peak (mm) |
Max | ||||||||||
| Voxels | x | y | z | Z | Voxels | x | y | z | Z | Voxels | x | y | z | Z | |
| Right Superior Temporal/Heschl's Gyrus | 25825 | 54 | −12 | 6 | 6.17 | 35963 | 58 | −4 | −6 | 5.96 | |||||
| Right Planum Temporale | 62 | −22 | 10 | 5.87 | |||||||||||
| Occipital Pole (V1) | −8 | −94 | −2 | 5.86 | 18 | −96 | 16 | 5.90 | |||||||
| Left Occipital Fusiform | −24 | −76 | −14 | 5.86 | |||||||||||
| Right Occipital Fusiform | 44 | −64 | −18 | 5.74 | |||||||||||
| Left Superior Temporal/Heschl's Gyrus | 8435 | −46 | −24 | 10 | 6.46 | ||||||||||
| Left Planum Temporale/STG | −48 | −34 | 12 | 6.22 | 56 | −16 | −2 | 5.38 | |||||||
| Right Postcentral/Precentral Gyrus | 3897 | 24 | −16 | 70 | 5.07 | 28 | −36 | 74 | 4.84 | ||||||
| Paracingulate/Superior Frontal Gyrus | −4 | 54 | 28 | 4.21 | 417 | −8 | 14 | 64 | 3.90 | ||||||
| Left Precentral Gyrus | 937 | −52 | 0 | 42 | 4.29 | ||||||||||
| Left Postcentral Gyrus | −54 | −14 | 48 | 4.17 | |||||||||||
| Left Superior Frontal Gyrus | 725 | −10 | 50 | 42 | 3.90 | ||||||||||
| Cerebellum | 1137 | 50 | −62 | −30 | 3.10 | ||||||||||
| Right Inferior/Middle Temporal Gyrus | 50 | −48 | −24 | 3.09 | |||||||||||
| Right Lateral Occipital Cortex | 56 | −66 | −6 | 2.94 | |||||||||||
Note: x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively; Z refers to the Z-score at those coordinates (local maxima in bold, submaxima underneath). Voxels refers to number of voxels in the cluster; labels without voxels indicate submaxima within the same cluster as label above with voxel size. Within-group analyses are cluster corrected for multiple comparisons, Z > 2.3, p < 0.05; between-group analyses are cluster corrected at Z > 1.7, p < 0.05. Between-group analyses are masked by regions of significant activation in either within-group analysis at Z > 1.7, corrected.
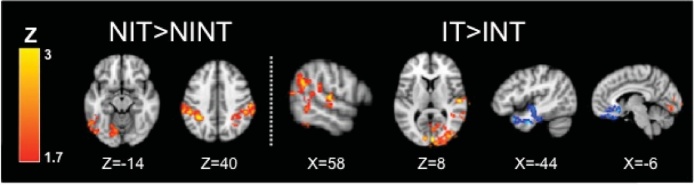
Next, we examined change in brain activation when the tactile stimulus was added to the No Instruction condition (NIT > NINT). As expected given that tactile stimulation was to the right arm, both groups showed increases in the right postcentral gyrus (somatosensory cortex) and right insula and operculum, and decreases in left pre- and postcentral gyri. The TD group also showed increases in left auditory language areas and anterior cingulate gyrus. The ASD group also showed decreases in left auditory language areas (angular gyrus) and occipital cortex. Direct between-group comparisons showed an interaction effect (TD > ASD, NIT > NINT and ASD > TD, NINT > NIT), such that tactile stimulation was associated with increases in the TD group but decreases in the ASD group in several areas associated with social cognition and language processing including auditory language regions, left IFG, orbital frontal cortex (OFC), and left dorsolateral prefrontal cortex. The TD group also showed significantly greater increases in medial prefrontal cortex and anterior cingulate gyrus/paracingulate.
4.2. Instruction conditions
4.2.1. Instructions-No Tactile condition (Table 4 and Fig. 4)
Table 4.
MNI coordinates for the Instructions No Tactile condition as compared to baseline.
| TD |
ASD |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MNI Peak (mm) |
Max | MNI Peak (mm) |
Max | |||||||
| Voxels | x | y | z | Z | Voxels | x | y | z | Z | |
| Occipital Pole | 21305 | 18 | −98 | −2 | 6.09 | 24493 | −18 | −98 | 6 | 6.18 |
| Right Occipital Fusiform | −28 | −84 | −12 | 5.77 | ||||||
| Left Superior Temporal/Heschl's Gyrus | −64 | −38 | 6 | 5.72 | −52 | −18 | 2 | 6.05 | ||
| Right Superior/Middle Temporal Gyrus | 3787 | 52 | −20 | −8 | 5.65 | 56 | −16 | −2 | 5.82 | |
| Left Postcentral/Precentral Gyrus | 755 | −50 | −14 | 52 | 3.71 | 1257 | −40 | −22 | 66 | 4.10 |
| Left Middle Frontal Gyrus | −44 | 4 | 50 | 4.78 | ||||||
| Superior Frontal Gyrus/mPFC | 1652 | −6 | 52 | 28 | 4.06 | 834 | −12 | 52 | 40 | 4.02 |
| Supplementary Motor Cortex | −2 | 2 | 60 | 3.96 | ||||||
| Left Hippocampus | 585 | −24 | −32 | −4 | 4.64 | |||||
| Left Amygdala | −20 | −6 | −18 | 3.21 | ||||||
Note: x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively; Z refers to the Z-score at those coordinates (local maxima in bold, submaxima underneath). Voxels refers to number of voxels in the cluster; labels without voxels indicate submaxima within the same cluster as label above with voxel size. Within-group analyses are cluster corrected for multiple comparisons, Z > 2.3, p < 0.05; between-group analyses are cluster corrected at Z > 1.7, p < 0.05.
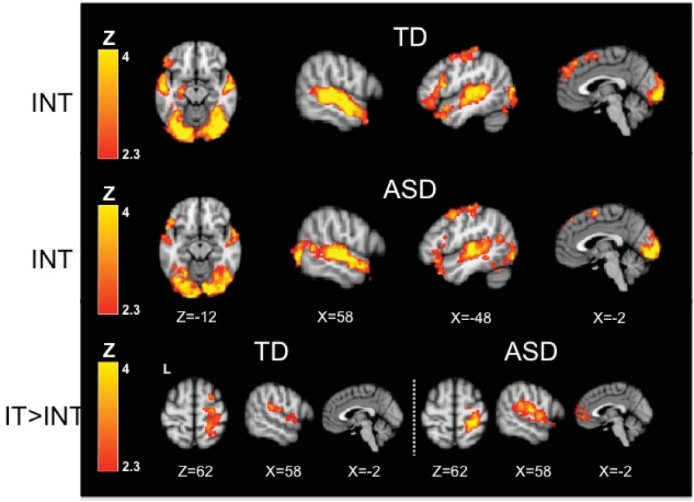
Fig. 4.
Within-group results for the sarcasm task with explicit attentional direction to the facial expression and tone of voice. The Instructions No Tactile (INT) condition elicited activation of auditory and visual cortex, motor cortex, and inferior frontal gyrus in both TD and ASD participants (Table 4). There were no significant between-group differences in the INT condition. With the addition of the tactile stimulus (Instructions with Tactile, IT compared to Instructions No Tactile, INT), both groups show increased activity in auditory and somatosensory cortices; ASD youth show additional up-regulation of medial prefrontal cortex (Table 8). Within-group contrasts thresholded at Z > 2.3, corrected (p< 0.05).
With explicit instructions to attend to the speaker’s face and tone of voice (but no tactile; INT), both groups showed a similar pattern of activation as in the NINT condition. Both groups showed activation in bilateral occipital and temporal lobes, left pre- and postcentral gyri, and left IFG. The TD group also had activation in left amygdala and hippocampus. There were no significant between-group differences, or significant differences comparing the no tactile conditions with and without instructions in either group.
4.2.2. Instructions-tactile condition (Tables 5 & 8, Figs. 4 & 5)
Table 5.
MNI coordinates for the Instructions Tactile condition as compared to baseline.
| TD |
ASD |
ASD > TD |
TD > ASD |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI Peak (mm) |
Max | MNI Peak (mm) |
Max | MNI Peak (mm) |
Max | MNI Peak (mm) |
Max | |||||||||||||
| Voxels | x | y | z | Z | Voxels | x | y | z | Z | Voxels | x | y | z | Z | Voxels | x | y | z | Z | |
| Right Superior Temporal/Heschl's Gyrus | 34880 | 52 | −40 | 6 | 6.11 | 42161 | 46 | −20 | 8 | 6.52 | 3340 | 68 | −6 | 8 | 3.35 | |||||
| Occipital Pole | −28 | −92 | 10 | 6.17 | −10 | −104 | −2 | 6.01 | ||||||||||||
| Left Occipital Fusiform | −24 | −76 | −12 | 6.16 | ||||||||||||||||
| Right Occipital Fusiform | 24 | −86 | −8 | 5.75 | ||||||||||||||||
| Right Lateral Occipital Cortex | 60 | −64 | 4 | 3.29 | ||||||||||||||||
| Right Middle Frontal Gyrus | 54 | 14 | 44 | 3.29 | ||||||||||||||||
| Left Superior Temporal/Heschl's Gyrus | 10215 | −60 | −36 | 10 | 5.41 | −58 | −24 | −2 | 5.93 | 909 | −62 | −38 | 4 | 2.88 | ||||||
| Left Middle Temporal Gyrus | −50 | −16 | −16 | 3.09 | ||||||||||||||||
| Left Supplementary Motor Cortex | 4415 | −4 | 6 | 58 | 4.29 | |||||||||||||||
| Superior Frontal Gyrus/mPFC | 0 | 48 | 34 | 4.16 | −6 | 40 | 54 | 4.25 | ||||||||||||
| Right Precentral Gyrus | 383 | 50 | 6 | 46 | 3.91 | |||||||||||||||
| Right Postcentral Gyrus | 58 | −8 | 42 | 3.71 | 869 | 34 | −32 | 62 | 4.26 | |||||||||||
| Left Thalamus (pulvinar) | 1203 | −14 | −32 | −4 | 4.96 | 50 | −20 | 38 | 2.94 | |||||||||||
| Left Hippocampus | −24 | −20 | −12 | 4.18 | ||||||||||||||||
| Left Amygdala | −22 | −10 | −10 | 3.30 | ||||||||||||||||
| Right Thalamus (pulvinar) | 700 | 16 | −30 | −6 | 4.47 | |||||||||||||||
| Right Thalamus (medial dorsal nucleus) | 8 | −30 | 4 | 2.92 | ||||||||||||||||
| Cerebellum | 1034 | −28 | −96 | −24 | 3.53 | |||||||||||||||
Note: x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively; Z refers to the Z-score at those coordinates (local maxima in bold, submaxima underneath). Voxels refers to number of voxels in the cluster; labels without voxels indicate submaxima within the same cluster as label above with voxel size. Within-group analyses are cluster corrected for multiple comparisons, Z > 2.3, p < 0.05; between-group analyses are cluster corrected at Z > 1.7, p < 0.05. Between-group analyses are masked by regions of significant activation in either within-group analysis at Z > 1.7, corrected.
With the addition of tactile stimulation (IT > INT), both groups showed increases in right pre- and postcentral gyri, right auditory regions, and insular/opercular regions. The ASD group also showed increases in left opercular cortex and medial prefrontal cortex. A direct group comparison showed a significant interaction (ASD > TD, IT > INT) whereby the ASD group showed greater increases in medial prefrontal cortex (mPFC) compared to the TD group with the addition of the tactile stimulus.
Table 7.
MNI coordinates for brain regions with greater activation in the No Instructions No Tactile condition as compared to the No Instructions Tactile condition.
| TD |
ASD |
ASD > TD |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI Peak (mm) |
Max | MNI Peak (mm) |
Max | MNI Peak (mm) |
Max | ||||||||||
| Voxels | x | y | z | Z | Voxels | x | y | z | Z | Voxels | x | y | z | Z | |
| Left Middle Frontal Gyrus | 852 | −48 | 18 | 28 | 3.16 | ||||||||||
| Left Frontal Pole | 316 | −40 | 48 | 16 | 3.00 | ||||||||||
| Left Orbitofrontal Cortex | 223 | −32 | 24 | −14 | 2.71 | ||||||||||
| Medial Orbitofrontal cortex | 184 | −14 | 56 | −16 | 3.62 | ||||||||||
| Left Inferior Frontal Gyrus | 59 | −56 | 20 | 10 | 2.98 | ||||||||||
| Left Precentral Gyrus | 449 | −26 | −22 | 78 | 3.89 | 803 | −38 | −18 | 66 | 3.72 | 322 | −34 | −2 | 48 | 2.67 |
| Left Postcentral Gyrus | −36 | −24 | 64 | 3.30 | −38 | −24 | 62 | 3.68 | |||||||
| Left Planum Temporale | 569 | −62 | −26 | 8 | 3.73 | ||||||||||
| Left Superior Temporal Gyrus | −50 | −32 | 0 | 3.48 | |||||||||||
| Left Angular Gyrus | 749 | −42 | −56 | 48 | 3.27 | 255 | −50 | −58 | 18 | 2.42 | |||||
| Left Lateral Occipital Cortex | 449 | −36 | −66 | 0 | 3.57 | ||||||||||
| Left Occipital Fusiform Gyrus | −30 | −64 | −2 | 3.43 | |||||||||||
| Left Middle Temporal Gyrus | −46 | −52 | 4 | 3.33 | |||||||||||
Note: x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively; Z refers to the Z-score at those coordinates (local maxima in bold, submaxima underneath). Voxels refers to number of voxels in the cluster; coordinates without voxels indicate submaxima within the same cluster as coordinate above with voxel size. Within-group analyses are cluster corrected for multiple comparisons, Z > 2.3, p < 0.05; between-group analyses are cluster corrected at Z > 1.7, p < 0.05. Between-group analyses are masked by regions of significant activation in either within-group analysis at Z > 1.7, corrected.
Fig. 5.
Between-group results for the ASD group compared to the TD group. Compared to TD youth, ASD participants show greater decreases (blue) with the addition of the tactile stimulus in the no instruction condition (i.e., No Instruction No Tactile, NINT > No Instructions Tactile, NIT) in brain regions implicated in social and language processing (inferior frontal gyrus, orbital frontal cortex, auditory cortex; Table 7). In conditions in which instructions to attend were given, addition of the tactile stimulus (i.e., Instructions Tactile, IT > Instructions No Tactile, INT) was associated with greater activity in ASD youth compared to TD youth in the medial prefrontal cortex (red; Table 8). There were no significant findings for the opposite comparisons (i.e. ASD > TD NIT > NINT and ASD > TD INT > IT).
4.3. Correlations with SOR severity in ASD
In the No Instruction conditions, higher SOR was correlated with greater signal increases in lateral occipital cortex and bilateral supramarginal gyrus with the addition of the tactile stimulus (NIT > NINT; Table 6, Fig. 6, and Supp Fig. 1). In the Instruction conditions, higher SOR was correlated with greater signal increases in occipital cortex and right temporal regions (including primary auditory, inferior and middle temporal gyrus, and planum temporale) with the addition of the tactile stimulus (IT > INT; Table 8, Fig. 6, and Supp Fig. 2). Lower SOR was correlated with greater signal increases in left temporal pole/temporal fusiform gyrus and medial prefrontal cortex with the addition of the tactile stimulus (IT > INT). To determine whether these correlations were truly driven by SOR and not social functioning (given the high correlation between SOR and the SRS), we extracted parameter estimates from each cluster where activity was significantly correlated with SOR and correlated these parameter estimates with SRS total scores. Only the occipital and right temporal difference scores (IT > INT) were significantly correlated with SRS, and the correlation with SOR remained significant even after regressing out the effect of SRS score.
Table 6.
MNI coordinates for brain regions with greater activation in the No Instructions Tactile condition as compared to the No Instructions No Tactile condition.
| TD |
ASD |
TD > ASD |
SOR correlation |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI Peak (mm) |
Max | MNI Peak (mm) |
Max | MNI Peak (mm) |
Max | MNI Peak (mm) |
Max | |||||||||||||
| Voxels | x | y | z | Z | Voxels | x | y | z | Z | Voxels | x | y | z | Z | Voxels | x | y | z | Z | |
| Right Supramarginal Gyrus | 3022 | 60 | −24 | 40 | 3.38 | |||||||||||||||
| Right Operculum | 10823 | 42 | −20 | 18 | 5.66 | 2430 | 50 | −26 | 20 | 4.35 | 52 | −36 | 26 | 3.37 | ||||||
| Right Planum Temporale | 62 | −34 | 18 | 4.92 | ||||||||||||||||
| Right Insula | 40 | −2 | −12 | 4.75 | 34 | −16 | 20 | 4.12 | ||||||||||||
| Right Superior/Middle Temporal Gyrus | 2618 | 66 | −38 | 6 | 3.32 | |||||||||||||||
| Right Postcentral Gyrus | 3877 | 30 | −30 | 74 | 4.68 | 2648 | 28 | −36 | 72 | 5.74 | ||||||||||
| Right Precentral Gyrus | 24 | −16 | 72 | 4.90 | 22 | −12 | 72 | 3.96 | ||||||||||||
| Anterior Cingulate/Paracingulate | 10 | 18 | 34 | 3.49 | 1765 | −2 | 36 | 28 | 3.31 | |||||||||||
| Left Frontal Pole | −40 | 48 | 16 | 3.00 | ||||||||||||||||
| Left Supramarginal Gyrus | 2401 | −44 | −44 | 60 | 3.45 | |||||||||||||||
| Left Operculum | 2869 | −46 | −32 | 18 | 4.96 | 1027 | −50 | −30 | 14 | 3.13 | ||||||||||
| Left Planum Temporale | −62 | −22 | 12 | 4.39 | −62 | −26 | 8 | 3.73 | ||||||||||||
| Left Superior Temporal Gyrus | −50 | −32 | 0 | 3.48 | ||||||||||||||||
| Left Precentral Gyrus | −58 | 4 | 4 | 4.25 | 53 | −56 | −4 | 38 | 2.84 | |||||||||||
| Right Frontal Pole/Superior Frontal Gyrus | 390 | 30 | 54 | 32 | 3.58 | 518 | 36 | 48 | 28 | 3.31 | ||||||||||
| Left Frontal Pole/vmPFC | 423 | −14 | 56 | −16 | 3.62 | |||||||||||||||
| Left Caudate | −12 | 18 | 2 | 2.40 | ||||||||||||||||
| Medial Orbitofrontal Cortex | −14 | 18 | −16 | 2.36 | ||||||||||||||||
| Left Middle Frontal Gyrus | 153 | −48 | 18 | 28 | 3.16 | |||||||||||||||
| Left Inferior Frontal Gyrus | −44 | 10 | 24 | 2.99 | ||||||||||||||||
| Left Lateral Occipital Cortex | 4030 | −48 | −76 | −12 | 2.67 | |||||||||||||||
| Cerebellum | 273 | 32 | −62 | −32 | 2.76 | 4030 | −18 | −78 | −50 | 3.32 | ||||||||||
Note: x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively; Z refers to the Z-score at those coordinates (local maxima in bold, submaxima underneath). Voxels refers to number of voxels in the cluster; coordinates without voxels indicate submaxima within the same cluster as coordinate above with voxel size. Within-group analyses are cluster corrected for multiple comparisons, Z > 2.3, p < 0.05; between-group and correlation analyses are cluster corrected at Z > 1.7, p < 0.05. Between-group analyses are masked by regions of significant activation in either within-group analysis at Z > 1.7, corrected.
Fig. 6.
Areas of change with the tactile stimulus that were correlated with tactile sensory over-responsivity (SOR) scores in ASD youth. When assessing the effect of the tactile stimulus in the no instruction condition (i.e., No Instructions Tactile, NIT > No Instructions No Tactile, NINT), higher SOR is associated with greater increases in supramarginal gyrus and occipital cortex (red; Table 6). When assessing the effect of the tactile stimulus with instructions to attend (i.e., Instructions Tactile, IT > Instructions No Tactile, INT), higher SOR is correlated with greater increases in occipital and temporal gyrus (red); lower SOR is associated with greater increases in left temporal pole and medial prefrontal gyrus (blue; Table 8).
Table 8.
MNI coordinates for brain regions with greater activation in the Instructions Tactile condition as compared to the Instructions No Tactile condition.
| TD |
ASD |
ASD > TD |
SOR correlation |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI Peak (mm) |
Max | MNI Peak (mm) |
Max | MNI Peak (mm) |
Max | MNI Peak (mm) |
Max | Corr. | |||||||||||||
| Voxels | x | y | z | Z | Voxels | x | y | z | Z | Voxels | x | y | z | Z | Voxels | x | y | z | Z | Dir. | |
| Occipital Pole (V1) | 7803 | 2 | −90 | −2 | 3.89 | POS | |||||||||||||||
| Occipital Fusiform Gyrus | 20 | −76 | −14 | 3.75 | POS | ||||||||||||||||
| Right Lateral Occipital Cortex | 46 | −84 | 6 | 3.57 | POS | ||||||||||||||||
| Right Angular Gyrus | 60 | −52 | 24 | 2.95 | POS | ||||||||||||||||
| Cerebellum | 34 | −88 | −30 | 3.52 | POS | ||||||||||||||||
| Right Middle Frontal Gyrus | 2901 | 28 | 22 | 38 | 3.76 | ||||||||||||||||
| Right Paracingulate/vmPFC | 1033 | 0 | 52 | −4 | 3.65 | 1597 | −10 | 70 | 2 | 3.39 | |||||||||||
| Right Superior Frontal Gyrus | 20 | 38 | 38 | 3.56 | |||||||||||||||||
| Right Orbitofrontal Cortex | 40 | 32 | −8 | 3.48 | 28 | 58 | −10 | 3.49 | 26 | 60 | −12 | 3.04 | |||||||||
| Right Insula | |||||||||||||||||||||
| Right Postcentral Gyrus | 1999 | 36 | −32 | 52 | 4.16 | 907 | 24 | −36 | 64 | 4.66 | |||||||||||
| Right Precentral Gyrus | 24 | −18 | 72 | 4.28 | |||||||||||||||||
| Right Operculum/Superior Temporal Gyrus | 1246 | 40 | −20 | 22 | 3.77 | 3457 | 54 | −14 | 12 | 4.44 | 42 | −22 | 20 | 2.68 | POS | ||||||
| Right Insula | 42 | −4 | 4 | 3.15 | 36 | −18 | 12 | 4.12 | |||||||||||||
| Right Temporal Fusiform Gyrus | 40 | −20 | −30 | 3.05 | |||||||||||||||||
| Right Superior Temporal Gyrus | 42 | −22 | 14 | 3.95 | 66 | −40 | −16 | 3.04 | POS | ||||||||||||
| Right Heschl's Gyrus | 54 | −18 | 10 | 3.29 | POS | ||||||||||||||||
| Right Planum Temporale | 64 | −16 | 6 | 2.46 | POS | ||||||||||||||||
| Left Superior Temporal Gyrus | 1654 | −58 | −28 | 22 | 4.13 | ||||||||||||||||
| Left Operculum | −62 | −12 | 10 | 3.50 | |||||||||||||||||
| Left Inferior Temporal Gyrus/Temporal Pole | 61773 | −44 | −6 | −32 | 3.37 | NEG | |||||||||||||||
| Left Temporal Fusiform Gyrus | −38 | −12 | −34 | 3.33 | NEG | ||||||||||||||||
| Medial Orbitofrontal Cortex | 10 | 26 | −16 | 3.26 | NEG | ||||||||||||||||
Note: x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively; Z refers to the Z-score at those coordinates (local maxima in bold, submaxima underneath). Voxels refers to number of voxels in the cluster; coordinates without voxels indicate submaxima within the same cluster as coordinate above with voxel size. Corr. Dir. indicates whether correlations with sensory over-responsivity (SOR) composite are positive or negative. Within-group analyses are cluster corrected for multiple comparisons, Z > 2.3, p < 0.05; between-group and correlation analyses are cluster corrected at Z > 1.7, p < 0.05. Between-group analyses are masked by regions of significant activation in either within-group analysis at Z > 1.7, corrected.
5. Discussion
This study investigated the effect of a mildly aversive tactile sensory distracter on brain activation during a social cognition task requiring integration of visual and auditory social cues to interpret a speaker’s communicative intent (sincere or sarcastic). We additionally explored whether explicit instructions to attend to important social cues – such as those conveyed by the speaker’s facial expression and tone of voice – could mitigate the effect of the sensory distracter. Finally, we examined how SOR might be related to changes in brain responses during this task in the presence (vs. absence) of distracting sensory stimulation.
First, our results are consistent with previous studies using the same task, as we found that ASD participants were able to understand and complete the sarcasm task at high levels of accuracy (Wang et al., 2007, Colich et al., 2012, Wang et al., 2006a). Furthermore, brain activation patterns in response to the task with no explicit instructions and no sensory distracter were generally consistent with previous studies in that the ASD group showed greater activation in auditory cortex and inferior frontal gyrus (Wang et al., 2006a). As suggested in Wang et al. (2006a), this pattern of greater activation may reflect more effortful processing in the ASD group to interpret a speaker’s communicative intent, particularly in the case of sarcasm.
With the addition of the sensory distracter, the TD participants showed increases in multiple brain regions including fronto-temporal language processing regions, as well as dorsolateral and dorsomedial prefrontal cortex. These increases may reflect the up-regulation of relevant language and social processing areas as the TD participants increase effort to process relevant task information in the presence of distracting tactile stimulation (Culham et al., 2001, Wild et al., 2012). Conversely, the ASD participants showed decreases in activation in these regions, possibly because they shifted attention away from the task and towards the sensory stimulus. Thus, findings suggest that they were unable to sustain effortful processing of social information during simultaneous sensory stimulation. The behavioral accuracy data were consistent with these findings in that the ASD group, but not the TD group, had slight decreases in accuracy (though they retained over 80% accuracy) when the tactile stimulus was added. Interestingly, none of the areas showing decreased activity in the presence of the sensory distracter were correlated with SOR; this may reflect the relatively small sample size, but it might suggest that the decreases in social processing regions are more related to social deficits in the ASD group as a whole (e.g., difficulty maintaining attention to complex social cues in the face of distraction) as opposed to within-group differences in sensory over-responsivity. This is consistent with our finding that TD and ASD youth did not show significant differences in brain areas related to processing the sensory stimulus such as somatosensory cortex, insula, or amygdala. However, in the ASD group, tactile SOR was positively correlated with widespread increases in bilateral supramarginal gyrus in response to the addition of the tactile stimulus. The supramarginal gyrus is considered a somatosensory association area, receiving inputs from primary somatosensory cortex (Iwamura, 2003, Maldjian et al., 1999), and is involved in interpretation of tactile stimuli such as tactile object recognition (Reed et al., 2004). Thus, it is likely that increases in this area reflect that ASD individuals with higher SOR are devoting more brain resources to processing the tactile stimulus. Contrary to our expectations, SOR was also positively correlated with increases in occipital cortex in the presence of tactile stimulation. It is possible that the tactile stimulus made it particularly difficult for youth with high SOR to interpret the ambiguous verbal statement, so they relied more heavily on visual clues to decide whether the narrator meant what he/she said.
In the second half of the task, participants were explicitly directed to pay attention to the facial expression and tone of voice of the speaker. With these instructions, the addition of tactile stimulation no longer caused decreases in activation in the ASD group. Thus, explicit instructions to attend to relevant social cues appear to mitigate the effect of the sensory distracter in that the ASD group now showed a level of activation in auditory language regions similar to what was observed in the absence of the tactile distracter. Additionally, when the tactile stimulation was accompanied by attentional instructions, the ASD (but not TD) group showed increased activity in medial prefrontal cortex (mPFC). This is consistent with our previous work demonstrating that explicit instructions increases medial prefrontal activation during this sarcasm task (Wang et al., 2007); here we further extend these findings to show that greater mPFC activation in response to attentional modulation may play an important role in helping the ASD brain regulate responses to sensory distraction in order to sustain adequate processing of social cues.
Finally, we found that, when attention was explicitly directed to the speaker’s face and tone of voice during simultaneous tactile stimulation, higher SOR was associated with greater activity in primary auditory and visual cortex as well as higher-level language and face processing regions (e.g., angular gyrus, planum temporale, and fusiform gyrus). Conversely, lower SOR was associated with greater activity in left temporal pole and mPFC, regions associated with social cognition including inference, theory of mind, and mentalising (Wang et al., 2007, Gallagher et al., 2000, Dumontheil et al., 2010, Blakemore et al., 2007). These findings suggest that, similar to the condition without implicit instructions, the ASD youth with higher SOR may continue to use visual strategies to complete the task, and through the attentional modulation of the instructions, they are additionally able to recruit some language regions. This suggests that youth with high-SOR may be independently evaluating the visual and auditory social cues, a likely less efficient strategy than the one seemingly at play in youth with lower SOR, for whom the attentional instructions lead to greater recruitment of regions involved in integrating and interpreting multiple social cues. These results are also consistent with our previous findings that ASD youth with low SOR show more prefrontal inhibition of amygdala response to aversive tactile and auditory stimuli (Green et al., 2015). Of course, given the relatively small sample size, these correlations with SOR in our ASD group should be considered preliminary and future studies using larger samples are needed to better characterize the interplay between sensory and social symptoms and their relationship to brain activity during social tasks. Further, it should be noted that some studies have found differences in brain responses to affective versus non-affective touch. Compared to the tactile stimulation in this study, which was designed to be aversive, affective touch is considered to be slow, gentle, often pleasant, touch that selectively activates a distinct class of unmyelinated afferents known as C-touch (CT) fibers (Olausson et al., 2002). CT afferents are activated with stroking rates of about 1–10 cm/s on the face or hairy skin (Vallbo et al., 1999, Löken et al., 2009), as opposed to the rough touch used for this study at a rate of about 15–20 cm/s (depending on the size of the child’s arm) on the smooth underside of the wrist/forearm. Affective touch has been associated with hypoactive brain response in both adults with ASD46 and neurotypical adults with autistic traits (Voos et al., 2013). Importantly, a given individual with ASD may be hypoactive to affective touch but hyperactive to rough or unpleasant touch (Cascio et al., 2012). Therefore, future research should seek to differentiate the effect of affective versus non-affective on social functioning and related brain processing.
Taken together, these findings have clear implications for intervention. First, our results show that although high-functioning youth with ASD are able to increase effortful processing to interpret ambiguous social cues, the addition of a sensory distracter disrupted that process. This effect is likely to be even stronger in the real world, where contextual clues are not always available and where distracting sensory stimulation is often unexpected, frequent, and multi-modal. This would suggest that interventions could focus on either (a) reducing environmental distractions as much as possible during social interactions, or (b) reducing the effort needed to maintain attention towards relevant social cues. Explicit instructions to direct attention to key social cues may be one way to reduce effort, perhaps by allowing individuals with ASD to ‘zoom in’ on the most relevant information rather than attempting to process and sort through all incoming information. The present findings also suggest that while explicitly directing attention is beneficial for ASD youth with both high and low SOR, the attentional modulation may work through different mechanisms depending on the level of sensory over-responsiveness. For example, ASD youth with high SOR may benefit from explicit attentional direction to each relevant social cue, whereas high-functioning youth with lower levels of SOR may be more able to benefit from practice integrating the cues. However, if youth with high SOR are using strategies that are less efficient, they may be less likely to benefit from attentional direction under conditions of stress (e.g., fatigue, hunger, unexpected sensory input), or when social cues are more subtle. More research is needed to understand the most useful types of attentional direction for youth with high SOR and the parameters under which do or do not benefit from such direction.
In summary, here we show that even mildly aversive sensory stimuli can disrupt the neural networks supporting effortful processing of social information in youth with ASD, and, importantly, that explicitly directing their attention to relevant social cues can mitigate this effect through recruitment of regions implicated in social cognition and mentalizing. To our knowledge, this is the first fMRI study to directly examine the interaction between social and sensory processing in ASD, thus beginning to inform our understanding of the mechanisms through which altered sensory responsivity may interfere with social functioning. These findings not only have important clinical implications but they also highlight the need for future studies to examine how social and sensory symptoms interact in ASD early in development. Such integrative studies promise to shed new light on the etiology of social communication deficits in ASD and lead to the development of more effective interventions to address social deficits in the context of a better understanding of their interplay with sensory sensitivities.
Conflict of interest
None.
Acknowledgments and funding
This work was supported by grants from the National Institute of Child Health and Human Development (grant number P50 HD055786), the National Institute of Mental Health (grant number 1 R01 HD065280-01), the Simons Foundation Autism Research Initiative (grant number 345389) as well as a National Institute of Health National Research Service Award postdoctoral fellowship to S.G. (grant number F32MH105167-01).
For generous support, the authors also wish to thank the Brain Mapping Medical Research Organization, the Brain Mapping Support Foundation, the Pierson-Lovelace Foundation, The Ahmanson Foundation, the Staglin IMHRO Center for Cognitive Neuroscience, the William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, the Tamkin Foundation, the Jennifer Jones-Simon Foundation, the Capital Group Companies Charitable Foundation, the Robson Family, and the North Star Fund. The project described was supported by grant numbers RR12169, RR13642, and RR00865 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCR or NIH.
The funding sources and organizations listed above had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2017.02.005.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Beckmann C.F., Jenkinson M., Smith S.M. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A., Hen L., Fluss R., Cermak S.A., Engel-Yeger B., Gal E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. J. Autism Dev. Disord. 2008;39(1):1–11. doi: 10.1007/s10803-008-0593-3. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Ouden den H., Choudhury S., Frith C. Adolescent development of the neural circuitry for thinking about intentions. Soc. Cogn. Affect Neurosci. 2007;2(2):130–139. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio C.J., Moana-Filho E.J., Guest S. Perceptual and neural response to affective tactile texture stimulation in adults with autism spectrum disorders. Autism Res. 2012;5(4):231–244. doi: 10.1002/aur.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich N.L., Wang A.-T., Rudie J.D., Hernandez L.M., Bookheimer S.Y., Dapretto M. Atypical neural processing of ironic and sincere remarks in children and adolescents with autism spectrum disorders. Metaphor. Symb. 2012;27(1):70–92. doi: 10.1080/10926488.2012.638856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett B.A., Muscatello R.A., Blain S.D. Impact of sensory sensitivity on physiological stress response and novel peer interaction in children with and without autism spectrum disorder. Child Adolesc. Psychiatry. 2016;27:8. doi: 10.3389/fnins.2016.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham J.C., Cavanagh P., Kanwisher N.G. Attention response functions: characterizing brain areas using fMRI activation during parametric variations of attentional load. Neuron. 2001;32(4):737–745. doi: 10.1016/s0896-6273(01)00499-8. [DOI] [PubMed] [Google Scholar]
- Dalton K.M., Nacewicz B.M., Johnstone T. Gaze fixation and the neural circuitry of face processing in autism. Nat. Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I., Küster O., Apperly I.A., Blakemore S.-J. Taking perspective into account in a communicative task. NeuroImage. 2010;52(4):1574–1583. doi: 10.1016/j.neuroimage.2010.05.056. [DOI] [PubMed] [Google Scholar]
- Dunn W. Psychological Corporation; San Antonio, TX: 1999. The Sensory Profile: User’s Manual. [Google Scholar]
- Elliott C.D. Psychological Corporation; San Antonio, TX: 1990. Differential Abilities Scale (DAS) [Google Scholar]
- Gallagher H.L., Happé F., Brunswick N., Fletcher P.C., Frith U., Frith C.D. Reading the mind in cartoons and stories: an fMRI study of theory of mind in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gervais H., Belin P., Boddaert N. Abnormal cortical voice processing in autism. Nat. Neurosci. 2004;7(8):801–802. doi: 10.1038/nn1291. [DOI] [PubMed] [Google Scholar]
- Giraud A.L., Kell C., Thierfelder C. Contributions of sensory input, auditory search and verbal comprehension to cortical activity during speech processing. Cereb. Cortex. 2004;14(3):247–255. doi: 10.1093/cercor/bhg124. [DOI] [PubMed] [Google Scholar]
- Glod M., Riby D.M., Honey E., Rodgers J. Psychological correlates of sensory processing patterns in individuals with autism spectrum disorder: a systematic review. Rev. J. Autism Dev. Disord. 2015;2(2):199–221. [Google Scholar]
- Green S.A., Rudie J.D., Colich N.L. Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52(11):1158–1172. doi: 10.1016/j.jaac.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S.A., Hernandez L., Tottenham N., Krasileva K., Bookheimer S.Y., Dapretto M. Neurobiology of sensory overresponsivity in youth with autism spectrum disorders. JAMA Psychiatry. 2015;72(8):778–786. doi: 10.1001/jamapsychiatry.2015.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S.A., Hernandez L., Bookheimer S.Y., Dapretto M. Salience network connectivity in autism is related to brain and behavioral markers of sensory overresponsivity. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55(7):618–626. doi: 10.1016/j.jaac.2016.04.013. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N., Joseph R.M., Snyder J. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. NeuroImage. 2004;22(3):1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Hubl D., Bölte S., Feineis–Matthews S. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61(9):1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- Iwamura Y. Somatosensory association cortices. Int. Congr. Ser. 2003;1250:3–14. [Google Scholar]
- Kaland N., Møller-Nielsen A., Callesen K., Mortensen E.L., Gottlieb D., Smith L. A new ‘advanced’ test of theory of mind: evidence from children and adolescents with Asperger syndrome. J. Child Psychol. Psychiatry. 2002;43(4):517–528. doi: 10.1111/1469-7610.00042. [DOI] [PubMed] [Google Scholar]
- Kern J.K. The pattern of sensory processing abnormalities in autism. Autism. 2006;10(5):480–494. doi: 10.1177/1362361306066564. [DOI] [PubMed] [Google Scholar]
- Löken L.S., Wessberg J., Morrison I., McGlone F., Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009;12(5):547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Leekam S.R., Prior M. Can autistic children distinguish lies from jokes? A second look at second-order belief attribution. J. Child Psychol. Psychiatry. 1994;35(5):901–915. doi: 10.1111/j.1469-7610.1994.tb02301.x. [DOI] [PubMed] [Google Scholar]
- Leekam S.R., Nieto C., Libby S.J., Wing L., Gould J. Describing the sensory abnormalities of children and adults with autism. J. Autism Dev. Disord. 2006;37(5):894–910. doi: 10.1007/s10803-006-0218-7. [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P., Risi S., Gotham K., Bishop S. Western Psychological Services; 2012. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) [Google Scholar]
- Maldjian J.A., Gottschalk A., Patel R.S., Pincus D., Detre J.A., Alsop D.C. Mapping of secondary somatosensory cortex activation induced by vibrational stimulation: an fMRI study. Brain Res. 1999;824(2):291–295. doi: 10.1016/s0006-8993(99)01126-9. [DOI] [PubMed] [Google Scholar]
- McIntosh D.N., Miller L.J. Evaluation of sensory processing. In: Dunn W., editor. The Sensory Profile: Examiner’s Manual. The Psychological Corporation; San Antonio, TX: 1999. pp. 59–73. [Google Scholar]
- Olausson H., Lamarre Y., Backlund H. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 2002;5(9):900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Pexman P.M., Rostad K.R., McMorris C.A., Climie E.A., Stowkowy J., Glenwright M.R. Processing of ironic language in children with high-functioning autism spectrum disorder. J. Autism Dev. Disord. 2010;41(8):1097–1112. doi: 10.1007/s10803-010-1131-7. [DOI] [PubMed] [Google Scholar]
- Pierce K., Müller R.-A., Ambrose J., Allen G., Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124(10):2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Reed C.L., Shoham S., Halgren E. Neural substrates of tactile object recognition: an fMRI study. Hum. Brain Mapp. 2004;21(4):236–246. doi: 10.1002/hbm.10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen S.A., Miller L.J., Green K.E. Pilot study of the sensory over-responsivity scales: assessment and inventory. Am. J. Occup. Ther. 2008;62:393–406. doi: 10.5014/ajot.62.4.393. [DOI] [PubMed] [Google Scholar]
- Tomchek S.D., Dunn W. Sensory processing in children with and without autism: a comparative study using the short sensory profile. Am. J. Occup. Ther. 2007;61(2):190–200. doi: 10.5014/ajot.61.2.190. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Lynch C.J. SAlience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 2013;70(8):869–879. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo Å.B., Olausson H., Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J. Neurophysiol. 1999;81(6):2753–2763. doi: 10.1152/jn.1999.81.6.2753. [DOI] [PubMed] [Google Scholar]
- Voos A.C., Pelphrey K.A., Kaiser M.D. Autistic traits are associated with diminished neural response to affective touch. Soc. Cogn. Affect. Neurosci. 2013;8(4):378–386. doi: 10.1093/scan/nss009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.T., Lee S.S., Sigman M., Dapretto M. Neural basis of irony comprehension in children with autism: the role of prosody and context. Brain. 2006;129(4):932–943. doi: 10.1093/brain/awl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.T., Lee S.S., Sigman M., Dapretto M. Developmental changes in the neural basis of interpreting communicative intent. Soc. Cogn. Affect. Neurosci. 2006;1(2):107–121. doi: 10.1093/scan/nsl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Lee S.S., Sigman M., Dapretto M. Reading affect in the face and voice: neural correlates of interpreting communicative intent in children and adolescents with autism spectrum disorders. Arch. Gen. Psychiatry. 2007;64(6):698–708. doi: 10.1001/archpsyc.64.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation: Harbourt Brace & Company; New York, NY: 1999. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- Wechsler D. 4th ed. Psychological Corporation; San Antonio, TX: 2003. Wechsler Intelligence Scale for Children. [Google Scholar]
- Wild C.J., Yusuf A., Wilson D.E., Peelle J.E., Davis M.H., Johnsrude I.S. Effortful listening: the processing of degraded speech depends critically on attention. J. Neurosci. 2012;32(40):14010–14021. doi: 10.1523/JNEUROSCI.1528-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M.W., Behrens T.E.J., Beckmann C.F., Jenkinson M., Smith S.M. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. Neuroimage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.