Abstract
Background
The neural mechanisms of anorexia nervosa (AN), a severe and chronic psychiatric illness, are still poorly understood. Altered body state processing, or interoception, has been documented in AN, and disturbances in aversive interoception may contribute to distorted body perception, extreme dietary restriction, and anxiety. As prior data implicate a potential mismatch between interoceptive expectation and experience in AN, we examined whether AN is associated with altered brain activation before, during, and after an unpleasant interoceptive state change.
Methods
Adult women remitted from AN (RAN; n = 17) and healthy control women (CW; n = 25) underwent functional magnetic resonance imaging during an inspiratory breathing load paradigm.
Results
During stimulus anticipation, the RAN group, relative to CW, showed reduced activation in right mid-insula. In contrast, during the aversive breathing load, the RAN group showed increased activation compared with CW in striatum and cingulate and prefrontal cortices. The RAN group also showed increased activation in prefrontal cortices, bilateral insula, striatum, and amygdala after stimulus offset. Time course analyses indicated that RAN responses in interoceptive processing regions during breathing load increased more steeply than those of CW. Exploratory analyses revealed that hyperactivation during and after breathing load was associated with markers of past AN severity.
Conclusions
Anticipatory deactivation with a subsequent exaggerated brain response during and after an aversive body state may contribute to difficulty predicting and adapting to internal state fluctuation. Because eating changes our interoceptive state, restriction may be one method of avoiding aversive, unpredictable internal change in AN.
Introduction
Anorexia nervosa (AN) is a serious, often chronic eating disorder of unknown etiology. Aberrant interoception, or the perception and integration of signals relating to body homeostasis (e.g., hunger, heartbeat, respiration), has been postulated to contribute to AN symptoms including body image distortion, extreme restriction despite starvation, and alexithymia (Kerr et al. 2016; Nunn et al. 2011; Pollatos et al. 2008; Zucker et al. 2013). In support of this notion, reported sensory over-responsiveness (Brand-Gothelf et al. 2016) and altered neural responses to taste stimuli have been documented in individuals ill with and remitted from AN (Cowdrey et al. 2011; Oberndorfer et al. 2013b).
Signaling of both predicted and actual interoceptive experience may be particularly relevant for understanding AN. Learning depends on associations between anticipated and actual outcomes (Schultz et al. 1997), and differences between the expected and observed interoceptive state, or prediction errors, may promote avoidance learning and behavior (Paulus & Stein 2006). Women with AN have difficulty distinguishing actual from anticipated sensations (Khalsa et al. 2015), and women remitted from AN (RAN) show altered neural activation during anticipation and receipt of sucrose tastes (Frank et al. 2016; Oberndorfer et al. 2013b). Return to homeostasis after state changes may also be impaired in AN: Interoceptive accuracy in AN decreases after eating (Khalsa et al. 2015), and habituation to fullness is protracted (Zucker et al. 2013). Altered AN brain response before, during, and after interoceptive events could contribute to problematic learning from experience about body-related changes, thereby perpetuating maladaptive avoidance.
Many AN behaviors involve avoidance of unpleasant physical sensations (e.g., feelings of fullness; Courty et al. 2015), suggesting that brain responses before and during aversive interoceptive experiences may be closely associated with AN symptoms. In individuals with AN, unlike healthy individuals, eating and anticipating eating stimulate dysphoric mood (Steinglass et al. 2010) and aversive sensations (e.g., dyspnea; Khalsa et al. 2015). “Noisy” interoceptive signals may interfere with accurate prediction of the impact of future aversive stimuli, contributing to anxiety (Barrett & Simmons 2015; Paulus & Stein 2006; Paulus & Stein 2010). RAN women show altered insular and prefrontal activation in response to aversive tastes (Cowdrey et al. 2011) and before and during brief pain (Strigo et al. 2013). However, brief stimulation may not adequately capture the altered responsivity to prolonged aversive homeostatic state changes that could contribute to AN. Eating, for example, involves non-painful hunger-to-satiety state change. No study to date has examined anticipation of and adaptation to prolonged, non-painful, aversive state changes in AN.
Non-painful aversive interoception recruits circuitry including the insula, cingulate, lateral prefrontal cortices (PFC), striatum, and amygdala. Future events are predicted in the anterior insula (Craig 2002;2003), and direct sensation processing involves posterior insula. The difference between anticipated and experienced sensation is computed in the mid- to posterior insula, with afferents and efferents to anterior and posterior insula (Barrett & Simmons 2015). The amygdala and striatum are involved in evaluation of the rewarding or aversive aspects of the interoceptive experience (Craig 2002; Hayes & Northoff 2012; Khalsa et al. 2009; Simmons et al. 2013). Projections from anterior and mid-cingulate and orbitofrontal and lateral PFC to anterior insula may modulate cognitive reactions during the stimulus (Craig 2002; Critchley et al. 2004; Hayes & Northoff 2012; Khalsa et al. 2009; Simmons et al. 2013). Finally, return to homeostasis after aversive interoceptive experience is associated with activation in rostral anterior cingulate, lateral PFC, and anterior insula (Leknes et al. 2011; Peiffer 2009).
In this study, we used an inspiratory breathing load paradigm and fMRI to examine whether AN is associated with altered brain response before, during, and after aversive interoceptive state changes. We studied remitted participants to avoid confounding effects of malnutrition on neural function. The well-validated task has been shown to activate regions of the insula, PFC, striatum, and anterior cingulate (Berk et al. 2015; Galli et al. 2013; Paulus et al. 2012; Stewart et al. 2014). The majority of prior data from this paradigm suggest that less healthy or less resilient individuals tend to show reduced activation during anticipation and increased activation during breathing load (Berk et al. 2015; Haase et al. 2015; Haase et al. 2016; Haase et al. 2014; Paulus et al. 2012; see Table S1 for summary). Unlike brief pain, taste, or food picture tasks previously used to study RAN (Cowdrey et al. 2011; Oberndorfer et al. 2013a; Strigo et al. 2013), the breathing load paradigm uses prolonged, aversive stimulation to induce a negative interoceptive state while importantly avoiding the confounds of symptom-specific responses to food stimuli. We therefore preliminarily predicted that RAN, similar to less healthy or less resilient individuals in prior breathing load studies, would show increased activation in insular and prefrontal cortices during prolonged aversive stimulation and decreased activation in the anterior and mid-insula during anticipation relative to controls. We also predicted that the time course of the RAN response would differ from that of controls. Exploratory analyses examined associations between task-related activation and clinical symptoms.
Materials and Methods
Participants
Twenty RAN women and 26 healthy control women (CW) participated in this study. CW participants were recruited from San Diego, and RAN participants were recruited nationally. Masters-level or higher research staff administered standard structured interviews to confirm past eating disorder diagnosis and exclude current eating disorder diagnoses (see Supplement for further detail). AN remission was defined as in prior studies (Bailer et al. 2005; Bailer et al. 2004; Frank et al. 2005; Strigo et al. 2013; Wagner et al. 2008; Wagner et al. 2007; Wierenga et al. 2015). Women from both groups were excluded if they met diagnostic criteria for a current DSM-IV Axis I diagnosis, and CW participants with a history of any eating disorders were also excluded. Please see Supplementary Information for further eligibility criteria. Participants provided written, informed consent to participate in the study. The research was approved by the University of California, San Diego (UCSD) Human Research Protections Program.
Procedure
Participants completed fMRI scans during the first 10 days (the early follicular phase) of their menstrual cycles.
Aversive inspiratory breathing load paradigm
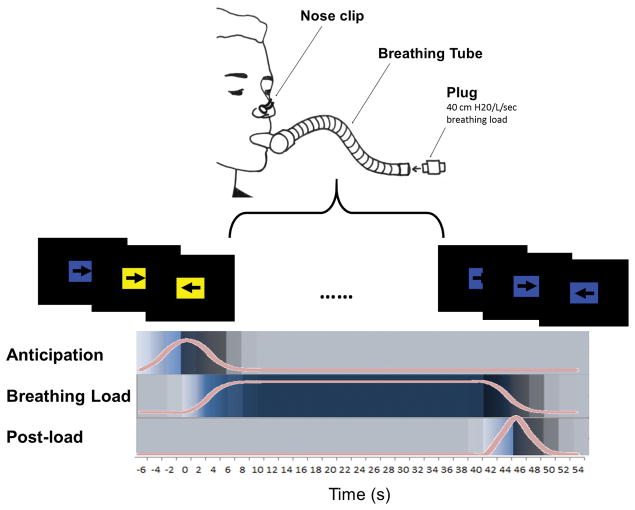
The inspiratory breathing load paradigm has been used to study aversive interoception in healthy and psychiatric populations (Berk et al. 2015; Galli et al. 2013; Haase et al. 2015; Haase et al. 2014; Paulus et al. 2012; Stewart et al. 2014). During fMRI scanning, participants wore a nose clip and were instructed to breathe through a hose that intermittently restricted breathing for 40-s periods via 40 cm H20/L/sec inspiratory loads (Figure 1). Colored rectangles signaled the likelihood of an upcoming breathing load period (yellow = 25% chance of subsequent breathing load, blue = 0% chance). Participants were made aware of these stimulus-outcome associations via oral instructions (“A blue box indicates that there will be no restriction placed on your breathing. However, a yellow box means there will be a 25% chance that your breathing will become restricted for 40 seconds. So, blue means no restriction and yellow means there is a 1-in-4 chance of breathing restriction.”) Overlaid on each rectangle, an arrow pointing left or right cued participants to press a left button or a right button, respectively. Accuracy and reaction time on this continuous performance task measured attention. During the task, cardiac and respiratory waveforms were recorded (see Supplementary Information for further detail).
Figure 1. Breathing Load Task Design.
Participants wore a nose clip and breathed through a hose that intermittently restricted their breathing via 40 cm H20/L/sec loads. During scanning, participants completed a continuous performance task. Participants were instructed to press a left or right button in response to left or right pointing arrows, respectively. During “anticipation” conditions, colored rectangles behind these arrows (mean duration 6 s) signaled the likelihood of an upcoming breathing load period (duration 40 s). A blue rectangle signaled no subsequent breathing load, and a yellow rectangle signaled a 25% chance of subsequent breathing load. A post-load period (mean duration 2 s) followed all breathing load conditions. The task was presented in an event-related design in two runs (total time =17 min and 4 s; TR = 2s). Across both runs, 34 baseline conditions and 32 anticipation conditions were jittered. Eight anticipation conditions were followed by breathing load. All conditions were jittered to optimize resolution of the hemodynamic response function.
Clinical symptoms and state measures
Participants completed the Eating Disorder Inventory-2 (Garner 1991), and the Beck Depression Inventory (BDI-II; Beck et al. 1996). Temperament and Character Inventory (TCI) Harm Avoidance subscale scores (TCI-HA; Cloninger et al. 1994), and Spielberger State-Trait Anxiety Inventory scores (STAI; Spielberger et al. 1970), which measure anxious temperament and trait anxiety, respectively, were available for a subset of participants and therefore were included only in secondary exploratory analyses (see Supplement). After experiencing breathing load for the first time pre-scan and again immediately after scanning, participants rated the pleasantness, unpleasantness, and intensity of the experience from “not at all” to “extremely” using 10 cm visual analogue scales (VAS). Additional post-scan VAS ratings of feelings of faintness, choking, abdominal distress, chest pain, and heart palpitations were also collected.
Image Acquisition
T2*-weighted echo-planar images were acquired on a 3T General Electric Discovery MR 750 (Milwaukee, WI). High-resolution T1-weighted anatomical images were acquired to permit activation localization and spatial normalization. EPI-based field maps corrected susceptibility-induced geometric distortions. See Supplementary Information additional image acquisition details.
Statistical Analysis
Behavioral task performance and VAS ratings
To examine whether behavioral performance on the continuous performance task differed between groups, which could indicate differential effects of the aversive breathing load, a Group (RAN, CW) x Condition (Anticipation, Breathing Load, Post-Breathing Load) repeated measures analysis of variance (ANOVA) was conducted on behavioral task reaction time (RT) and accuracy measures. Mean VAS intensity, pleasantness, and unpleasantness scores were submitted to a Group (RAN, CW) x Dimension (pleasantness, unpleasantness, and intensity) repeated measures ANOVA. Due to significant violations of the normality assumption, independent samples Mann-Whitney U tests compared groups on post-scan VAS ratings of other sensations.
MRI statistical analyses
Group-level statistical analyses were conducted using AFNI, FSL, and R statistical packages (see Supplement for additional detail).
Primary analyses examined whether groups differed in mean peak activation during breathing load anticipation, breathing load receipt, and after breathing load termination. We conducted between-group t tests (using AFNI’s 3dttest ++) within each condition. Effect sizes (Cohen’s d) were calculated for these within-condition, between-group contrasts. We also conducted an exploratory Group (CW, RAN) x Condition (anticipation, breathing load, post-breathing load) whole-brain linear mixed effects analysis (LME) analysis (see Supplement).
Secondary analyses examined potential group differences in the time course of BOLD responses across all stages of interoception (from anticipation to breathing load receipt to post-breathing load). Time course data were modeled using AFNI’s 3dDeconvolve TENT function across all three conditions (see Supplement). A Group (RAN, CW) × Time LME using AFNI’s 3dLME was used to compare time-course data from the RAN and CW groups in our a priori search regions of interest (ROIs).
To limit multiple comparisons, we restricted our fMRI analyses a priori to six bilateral regions involved in non-painful aversive interoception (Hayes & Northoff 2012) and implicated in aversive interoceptive prediction error (Barrett & Simmons 2015): 1) the insula in its entirety; 2) an anterior/mid-cingulate (ACC) region, comprised of subgenual, rostral, and dorsal components of the anterior cingulate gyrus, traditionally associated with affective and cognitive processing, respectively; 3) the posterior cingulate (PCC); 4) a lateral PFC region, comprised of ventrolateral and dorsolateral PFC; 5) a striatal region, including the caudate, putamen, and ventral striatum; and 6) the amygdalae (Figure S1). Region selection was also informed by control activation documented in the breathing load task (Berk et al. 2015; Galli et al. 2013; Haase et al. 2014; Paulus et al. 2012; Stewart et al. 2014) and locations of altered anticipatory and processing-related activation in women remitted from AN during aversive taste and pain tasks (Cowdrey et al. 2011; Strigo et al. 2013). Regions were derived from the Harvard-Oxford atlas (Desikan et al. 2006).
Small-volume correction was applied as follows: Intrinsic smoothness was estimated using the spatial autocorrelation function (ACF) option in AFNI’s 3dFWHMx. Results in each region were then corrected for multiple comparisons using Monte Carlo simulations via AFNI’s 3dClustSim, including ACF estimates. This robust approach employs non-Gaussian models and spatial autocorrelation functions (Eklund et al. 2016). First-nearest neighbor clustering and a per-voxel threshold of p < 0.01 (two-tailed) was set for each of the six search regions with a cluster-wise two-tailed alpha threshold of 0.05.
Exploratory associations with clinical variables and potential confounds
Mean percent signal change was extracted from significant clusters resulting from within-condition t tests. Huber robust regressions (Huber 1964), were conducted in R to examine associations of percent signal change with illness duration, lowest post-pubertal BMI, months since last AN symptoms, VAS ratings, and psychological measures. To control for family-wise error, these exploratory analyses were Bonferroni-corrected for the number of significant clusters within each breathing task condition and the number of clinical assessments tested (corrected alphas = 0.0033, 0.0002, and 0.00013 for anticipation, breathing load, and post-breathing load analyses, respectively). Additional exploratory analyses examined the potential confounding impact of past comorbidities and AN subtype.
Results
Participant Characteristics
One RAN and one CW did not complete the second run of the task, and two RAN participants were excluded because of excessive head movement, yielding a final sample of 17 RAN (11 restricting subtype, 6 binge-eating/purging subtype) and 25 CW who completed two full runs of the task and were included in analyses. The RAN and CW groups did not differ in age (RAN mean = 26.5; CW mean = 28.3; p = 0.449), body mass index (RAN mean = 21.6; CW mean = 22.0; p = 0.568), or years of education (RAN mean = 15.9; CW mean = 15.3; p = 0.294; Table S1). Consistent with prior findings (e.g., Wierenga et al. 2015), past mood and anxiety disorders were more common in the RAN group, and available data suggested that compared with controls, the RAN group reported higher, but non-clinically significant, levels of depressive symptoms, trait anxiety, and harm avoidance (Table S2).
Task Performance, VAS Ratings, and Physiology
There was no statistically significant Group x Condition interaction on accuracy or reaction time on the task, and there were no main effects of group on task performance (Table S2). RAN participants reported stronger post-scan choking sensations relative to CW, but groups did not differ on ratings of pleasantness, unpleasantness, or intensity of the breathing load experience, nor did they differ on reported feelings of faintness, heart palpitations, chest pain, or abdominal distress after the scan (Table S3).
Groups did not differ in average (RAN: 0.59 ± 0.39 mm; CW: 0.67 ± 0.44 mm; p = 0.501) or maximum (RAN: 1.80 ± 0.84 mm; CW: 1.74 ± 0.86 mm; p = 0.832) motion during the task. Because of hardware malfunction, physiological data could be analyzed for only a subset of participants; however, analyses of available data provide some indication that groups did not differ in heart rate, breathing rate, or breathing rate variability (see Supplement).
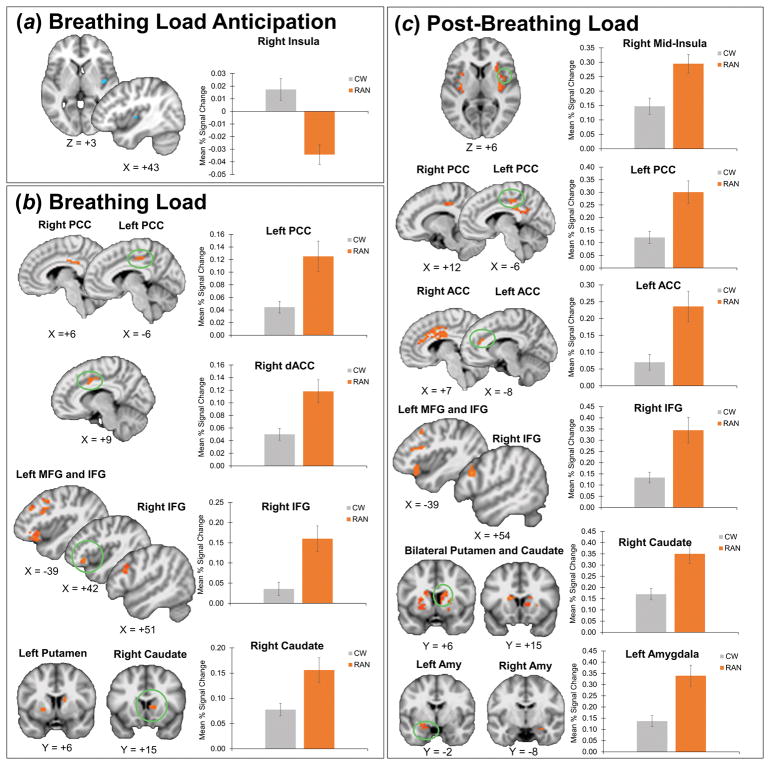
Group Differences in Peak Activation within Conditions
Results of between-group t-tests within each task condition are presented in Table 1 and Figure 2. Effect size maps of group effects are shown in Figure S2. Results of exploratory Group x Condition whole-brain voxelwise analyses are in Table S4.
Table 1.
Between-group Differences in Activation During Breathing Load Anticipation, Breathing Load, and Post-Breathing Load
| Center of Mass MNI Coordinates
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hem | Region | BA | voxels | CThr | x | y | z | t | Cluster Cohen’s d | % Overlap with Time Course Cluster |
| Anticipation: RAN < CW | ||||||||||
|
| ||||||||||
| R | Mid-Insula | 13 | 9 | 9 | 43 | −8 | 3 | −3.18 | 1.03 | |
|
| ||||||||||
| Breathing Load: RAN > CW | ||||||||||
|
| ||||||||||
| R | Cingulate Gyrus | 24 | 29 | 8 | 10 | 5 | 40 | 3.08 | 0.99 | 38% |
| L | Posterior Cingulate Gyrus | 31 | 20 | 12 | −5 | −26 | 44 | 3.03 | 0.98 | |
| R | Posterior Cingulate Gyrus | 23 | 13 | 12 | 6 | −28 | 30 | 3.03 | 0.97 | 77% |
| L | Inferior Frontal Gyrus | 47 | 106 | 13 | −41 | 28 | −11 | 3.20 | 1.03 | 30% |
| L | Middle Frontal Gyrus | 9 | 81 | 13 | −41 | 12 | 32 | 3.27 | 1.05 | 27% |
| L | Inferior Frontal Gyrus | 44 | 48 | 13 | −54 | 14 | 14 | 3.10 | 1.00 | |
| R | Inferior Frontal Gyrus | 9 | 36 | 13 | 53 | 19 | 25 | 3.09 | 0.99 | 50% |
| L | Middle Frontal Gyrus | 45 | 32 | 13 | −50 | 26 | 27 | 2.94 | 0.95 | 22% |
| L | Middle Frontal Gyrus | 8 | 22 | 13 | −42 | 12 | 46 | 3.10 | 1.00 | 36% |
| R | Inferior Frontal Gyrus | 47 | 21 | 13 | 43 | 29 | −8 | 3.07 | 0.99 | 14% |
| L | Middle Frontal Gyrus | 9 | 17 | 13 | −41 | 33 | 33 | 3.13 | 1.01 | 35% |
| R | Caudate | 16 | 11 | 12 | 16 | 4 | 2.94 | 0.95 | ||
| R | Caudate | 13 | 11 | 14 | 9 | 14 | 3.01 | 0.97 | ||
| L | Putamen | 11 | 11 | −22 | 6 | −2 | 2.85 | 0.92 | ||
|
| ||||||||||
| Post-Load: RAN > CW | ||||||||||
|
| ||||||||||
| R | Posterior Insula | 13 | 150 | 9 | 36 | −13 | 7 | 3.37 | 1.08 | 12% |
| L | Posterior Insula | 13 | 107 | 9 | −36 | −11 | 3 | 3.12 | 1.00 | 7% |
| R | Anterior Insula | 47 | 25 | 9 | 30 | 16 | 7 | 2.95 | 0.95 | |
| L | Mid/Anterior Insula | 13 | 20 | 9 | −30 | 5 | 11 | 3.32 | 1.07 | |
| L | Anterior Insula | 47 | 13 | 9 | −38 | 18 | −6 | 3.09 | 0.99 | |
| R | Mid Insula | 13 | 10 | 9 | 38 | 2 | 5 | 2.99 | 0.96 | |
| R | Mid Cingulate | 24 | 159 | 8 | 7 | 8 | 33 | 3.23 | 1.04 | 31% |
| L | Anterior Cingulate | 24 | 18 | 8 | −8 | 33 | 20 | 3.06 | 0.99 | |
| R | Posterior Cingulate Gyrus | 31 | 144 | 12 | 1 | −40 | 28 | 3.28 | 1.06 | 29% |
| R | Posterior Cingulate Gyrus | 31 | 36 | 12 | 11 | −23 | 40 | 3.20 | 1.03 | 19% |
| L | Posterior Cingulate Gyrus | 31 | 31 | 12 | −6 | −28 | 43 | 3.23 | 1.04 | |
| L | Middle Frontal Gyrus | 8 | 92 | 13 | −32 | 24 | 46 | 3.11 | 1.00 | 28% |
| L | Inferior Frontal Gyrus | 47 | 48 | 13 | −39 | 25 | −12 | 3.06 | 0.98 | 27% |
| L | Middle Frontal Gyrus | 46 | 41 | 13 | −50 | 24 | 29 | 3.03 | 0.98 | 51% |
| R | Middle Frontal Gyrus | 8 | 36 | 13 | 37 | 22 | 45 | 3.04 | 0.98 | 53% |
| R | Middle Frontal Gyrus | 6 | 32 | 13 | 32 | 3 | 51 | 3.14 | 1.01 | 50% |
| L | Inferior Frontal Gyrus | 13 | 24 | 13 | −38 | 24 | 20 | 2.99 | 0.96 | 25% |
| L | Inferior Frontal Gyrus | 44 | 23 | 13 | −48 | 10 | 12 | 3.13 | 1.01 | |
| R | Inferior Frontal Gyrus | 9 | 21 | 13 | 54 | 20 | 25 | 3.14 | 1.01 | 67% |
| L | Putamen | 121 | 11 | −26 | −1 | 3 | 3.10 | 1.00 | ||
| R | Caudate | 75 | 11 | 13 | 7 | 13 | 3.17 | 1.02 | ||
| L | Caudate | 55 | 11 | −15 | 8 | 17 | 3.31 | 1.07 | ||
| R | Putamen | 52 | 11 | 28 | −6 | 0 | 3.21 | 1.03 | ||
| R | Putamen | 34 | 11 | 24 | 6 | 6 | 2.99 | 0.96 | ||
| L | Amygdala | 34 | 28 | 4 | −22 | −2 | −17 | 3.27 | 1.05 | 4% |
| R | Amygdala | 34 | 5 | 4 | 27 | −8 | −18 | 3.49 | 1.12 | |
Coordinates are presented in LPI format. Clusters within each region of interest in which Group x Time tent function analysis results (see Table 2) overlap with between-group, within-condition t-tests are highlighted in grey, and the percentage overlap is noted in the last column. Hem, hemisphere; L, left; R, right; BA, Brodmann area; CThr, cluster threshold (p < 0.05, small-volume corrected (voxelwise p < 0.01) with Monte Carlo simulations (via Analysis of Functional NeuroImages 3dClustSim) to guard against false-positive results).
Figure 2. Within-condition Group Differences in Peak Activation.
A) During breathing load anticipation, the RAN group showed reduced activation in the right mid-insula compared with controls. RAN participants deactivated right mid-insula during aversive interoceptive stimulus anticipation, whereas healthy controls activated this region. B) During breathing load, the RAN group showed increased activation compared with controls in bilateral PCC, right dACC, bilateral IFG and left MFG, left putamen, and right caudate. C) After breathing load, the RAN group showed increased activation compared with controls in bilateral anterior insula, mid-insula, and posterior insula, (graph shown for right mid-insula), bilateral caudate and putamen (graph shown for right putamen), left amygdala, bilateral PCC, bilateral ACC, bilateral IFG and left MFG, bilateral putamen and caudate, and bilateral amygdala. Error bars represent standard error of the mean. When multiple clusters are shown, the graphs correspond to circled clusters. RAN, women remitted from anorexia nervosa; CW, healthy control women; PCC, posterior cingulate cortex; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; dACC, dorsal anterior cingulate cortex; amy, amygdala.
Breathing load anticipation
The RAN group showed reduced activation compared with CW in right mid-insula (Figure 2A).
Breathing load
During breathing load, the RAN group compared with CW showed increased activation in right dorsal ACC (dACC), bilateral PCC, bilateral inferior frontal and left middle frontal gyri (IFG and MFG), right caudate, and left putamen (Figure 2B).
Post-breathing load
After breathing load, the RAN group showed increased activation compared with CW in clusters within all ROIs: bilateral anterior, mid-, and posterior insula, bilateral dACC, right PCC, bilateral IFG and MFG, bilateral caudate and putamen, and bilateral amygdala (Figure 2C).
Group Differences in the Time Course of Activation
Statistically significant Group x Time interactions were detected in clusters in mid and posterior insula, subcallosal and dACC, PCC, MFG, IFG, and left amygdala, indicating that the time course of the BOLD response, from aversive stimulus anticipation to termination, differed in RAN and CW in all of these regions (Table 2; Figures 3 & S3).
Table 2.
Group x Time Interactions: Mixed Effects Time Series Analysis
| Hem | Region | BA | voxels | CThr | Center of Mass MNI Coordinates
|
t | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| R | Posterior Insula | 13 | 18 | 9 | 33 | −21 | 16 | 1.984 |
| L | Mid-insula | 13 | 16 | 9 | −38 | −3 | −6 | 1.958 |
| R | Cingulate Gyrus | 32 | 21 | 8 | 7 | 9 | 40 | 2.072 |
| R | Cingulate Gyrus | 24 | 11 | 8 | 10 | −11 | 34 | 1.920 |
| R | Cingulate Gyrus | 32 | 11 | 8 | 9 | 20 | 31 | 2.106 |
| R | Subcallosal Gyrus | 25 | 10 | 8 | 2 | 8 | −13 | 2.092 |
| L | Anterior Cingulate | 32 | 10 | 8 | −9 | 23 | −6 | 1.900 |
| R | Cingulate Gyrus | 24 | 10 | 8 | 5 | 1 | 25 | 1.974 |
| R | Cingulate Gyrus | 24 | 9 | 8 | 3 | 1 | 45 | 2.100 |
| R | Posterior Cingulate Gyrus | 31 | 64 | 12 | 5 | −32 | 31 | 2.194 |
| R | Middle Frontal Gyrus | 9 | 121 | 13 | 38 | 19 | 43 | 2.089 |
| L | Middle Frontal Gyrus | 46 | 51 | 13 | −41 | 32 | 29 | 1.979 |
| L | Middle Frontal Gyrus | 8 | 39 | 13 | −48 | 15 | 44 | 2.086 |
| R | Middle Frontal Gyrus | 6 | 39 | 13 | 30 | 3 | 55 | 2.211 |
| L | Middle Frontal Gyrus | 6 | 36 | 13 | −30 | 7 | 55 | 2.068 |
| R | Inferior Frontal Gyrus | 9 | 31 | 13 | 51 | 17 | 25 | 1.948 |
| L | Inferior Frontal Gyrus | 47 | 27 | 13 | −36 | 28 | −9 | 1.961 |
| L | Inferior Frontal Gyrus | 47 | 25 | 13 | −48 | 28 | −9 | 2.148 |
| L | Middle Frontal Gyrus | 46 | 18 | 13 | −40 | 15 | 26 | 1.962 |
| R | Inferior Frontal Gyrus | 45 | 15 | 13 | 47 | 28 | 1 | 2.006 |
| L | Amygdala | 34 | 5 | 4 | −28 | −5 | −21 | 2.111 |
Coordinates are presented in LPI format. Hem, hemisphere; L, left; R, right; BA, Brodmann area; CThr, cluster threshold (voxel-wise p < 0.01, cluster-wise p < 0.05, small-volume corrected with Monte Carlo simulations (via Analysis of Functional NeuroImages 3dClustSim) to guard against false-positive results).
Figure 3. Group Differences in Activation Time Course.
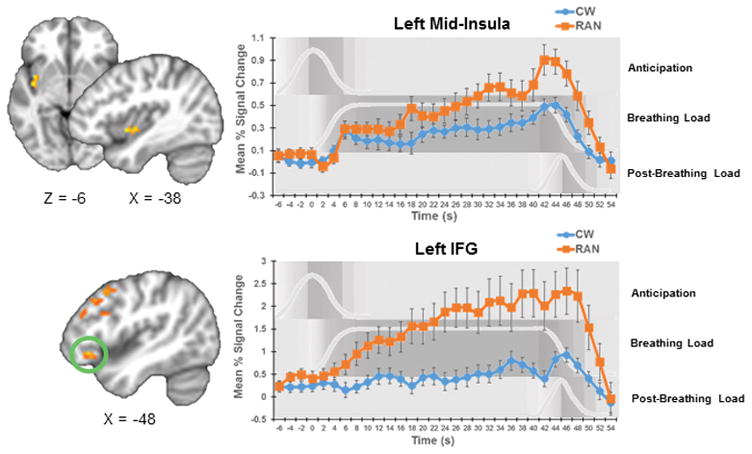
Group x Time interaction results suggested that the time course of activation in RAN participants statistically significantly differed from those of controls in left mid-insula and left IFG. Additional clusters in which time course differed between groups are shown in Figure S3. Time course graphs for anticipation, breathing load, and post-breathing load conditions are shown in in light gray and with gray shading the background. Error bars represent standard error of the mean. RAN, women remitted from anorexia nervosa; CW, healthy control women; IFG, inferior frontal gyrus.
In general, CW activation these areas plateaued or gradually increased over the course of the task. In contrast, RAN showed steadily increasing activation during the breathing load that peaked after the offset of the aversive stimulus (Figures 3 and S3).
Exploratory Associations with Clinical Variables
Exploratory Huber robust regression analyses revealed that RAN participants with the longest durations of illness showed the greatest hyperactivation of right MFG (p < 0.0001) and left caudate (p < 0.0001) after breathing load termination (Figure S4A). In addition, RAN participants with the greatest hyperactivation of right posterior insula after breathing load termination reported the most pronounced feelings of faintness after the scan (p < 0.0001; Figure S4B). RAN participants with the longest illness durations also tended to show the greatest hyperactivation of right IFG during breathing load (p = 0.0004), and RAN participants with the lowest lifetime post-pubertal BMIs trended toward more pronounced hyperactivation of right IFG after breathing load termination (p = 0.001), but these findings did not survive correction for multiple comparisons and should not be interpreted without replication. Results of additional regression analyses are reported in the Supplement.
AN subtype and history of major depression or an anxiety disorder did not have an appreciable effect on RAN activation (see Supplement).
Discussion
This investigation tested the hypothesis that AN is associated with altered processing of non-painful aversive interoceptive afferents. Several results support this hypothesis. First, although RAN and CW rated the experience similarly, RAN showed altered activation in aversive interoception circuitry before, during, and after breathing load. Results of within-condition analyses revealed reduced RAN anticipatory activation in right posterior mid-insula, a region implicated in the computation of the difference between anticipated and experienced stimuli. In contrast, RAN showed increased activation during and after aversive stimulus processing in frontal, striatal, and limbic regions involved in the evaluation of stimulus salience and modulation of cognitive reactions to stimuli. These between-group differences were consistently associated with large effect sizes. Second, RAN women showed elevated and steeply increasing activation during the receipt of an aversive stimulus, whereas in CW, activation remained fairly steady or more gradually increased during the sustained aversive experience. Taken together, these findings are consistent with altered anticipatory signaling and interoceptive network hyper-responsivity in RAN. Consequently, RAN may be more sensitive to and have more difficulty adjusting to changes in homeostatic state. Our exploratory results preliminarily indicate that altered processing of interoceptive state changes in RAN may contribute to or result from increased sensitivity to interoceptive sensations, prolonged periods of starvation, and ultimately more severe AN.
Anticipation Phase: Interoceptive Prediction Signaling
In contrast to prior findings of increased RAN activation in the insula during the anticipation of acute pain (Strigo et al. 2013) and food image exposure (Oberndorfer et al. 2013a), anticipation of breathing load was associated with decreased insular activation in RAN compared with CW. This was one of the largest between-group differences observed (d = 1.03). Whereas in prior pain and food tasks, interoceptive stimuli are rapidly presented (1–16 s), aversive breathing load is chronically applied for 40-s intervals. Thus, unlike anticipation periods in these rapid tasks, the breathing task’s anticipation period precedes a prolonged interoceptive state change. RAN deactivation of the mid-insula during anticipation of an aversive experience may therefore constitute a faulty prediction or preparatory signal for aversive state change. Unlike RAN, highly resilient elite athletes show increased mid-insula anticipatory response, and decreased receipt response to breathing load (Paulus et al. 2012). Thus, a decreased RAN response during anticipation may ultimately interfere with adaptive processing of the protracted aversive experience. Our results add to a growing literature suggesting anticipatory signals in RAN are altered.
Receipt Phase: Interoceptive Processing
The integration and processing of interoceptive stimuli also involves mid and posterior insula (Craig 2002;2003), and mid-insula may be essential to online monitoring of interoceptive state change (Klein et al. 2013). Again, in contrast to brief pain paradigm results, we did not find reduced insula activation in RAN during stimulus receipt. Breathing load tasks have not previously been used to study individuals with eating, anxiety, mood, or obsessive compulsive-related disorders, but findings in participants with substance use disorders and individuals high and low in resilience provide interesting comparisons to our RAN results. The time course of the mid-insula and posterior insula BOLD response in RAN increased more steeply during breathing load than that of CW. The pattern of reduced activation during anticipation and steeply increasing insular activation during breathing load is similar to prior findings of decreased anticipatory insular activation and increased activation during breathing load in adolescents with substance use disorders compared with controls (Berk et al. 2015). The response to the aversive stimulus observed in RAN is also consistent with that seen in individuals low in resilience (Paulus et al. 2012). Thus, the observed RAN pattern of escalating mid-insular activation during aversive stimulation may represent a transdiagnostic neural signature of difficulty adapting to interoceptive change.
The mid-insular location of altered RAN response time course and reduced activation during anticipation is very near clusters of aberrant activation previously observed in RAN women during stomach sensation processing (Kerr et al. 2016), food picture viewing (Oberndorfer et al. 2013a) and unexpected sucrose taste omission (Frank et al. 2016). Our findings are also consistent with insular anatomical abnormalities documented in both ill adult and adolescent AN and remitted adults (Frank 2015; Shott et al. 2015).
RAN women also showed aberrant neural responses during breathing load within the larger aversive interoceptive circuit, including the striatum, lateral PFC, and PCC. Similarly increased activation in PFC has been observed in RAN during receipt of pain (Strigo et al. 2013) and aversive taste (Cowdrey et al. 2011) and during breathing load in adolescents with active substance use disorders (Berk et al. 2015). Increased RAN activation in prefrontal top-down modulatory regions may represent an effort to downregulate “noisy interoceptive afferents” (Paulus & Stein 2010). Increased activation in RAN during breathing load in caudate, putamen and MFG and IFG is consistent with findings of increased RAN response to aversive taste in these visceral and sensory information processing and integration regions (Cowdrey et al. 2011), and further suggests a generalized AN hypersensitivity to aversive interoceptive events.
Increased and steeply rising prefrontal, insular, and cingulate activation during breathing load in RAN may also represent an error signal or an effort to adjust to a faulty prediction signal. The processing of unexpected compared with expected pain engages greater activation in similar regions to those hyperactivated or more steeply increasing in activation in RAN compared with CW during breathing load—bilateral PFC and insula (Seidel et al. 2015). Similarly, receipt of an unexpected sucrose taste is associated with increased activation in right cingulate and right medial frontal cortex in ill AN compared with CW (Frank et al. 2012). Overall, interoceptive circuit-wide RAN hyper-responsivity may suggest difficulty adapting to and regulating responses to aversive interoceptive change.
Return to Homeostasis Phase: Post-aversive Interoceptive Experience
RAN women also showed pronounced increases in activation and, in the case of right posterior insula, delayed decreases after breathing load termination. One hypothetical explanation is a delayed ability to return to baseline in RAN. Prolonged hyperactivation in RAN could add “noise” to aversive interoceptive processing signals and disrupt learning from experience. This could interfere with response to eating exposures during treatment and contribute to high relapse rates. Despite this potentially clinically-relevant and very preliminary hypothesis, increased post-load activation in RAN may instead represent carry-over of breathing-load-related (rather than post-load) hyperactivation. Further investigation is needed to understand neural response to interoceptive negative reinforcement in RAN.
Limitations and Directions for Future Study
Several study limitations are important to acknowledge. First, although group differences in carbon dioxide levels have not been documented in prior breathing load task studies, and analysis of available data suggests our groups did not differ in cardiac or respiratory rate, we did not collect carbon dioxide levels from participants. Future studies would benefit from systematically examining the relationship between breathing rate, expired carbon dioxide, and neural activation in AN. Second, differences in fitness levels between groups could explain our findings. Although RAN and CW groups did not differ on hours of exercise per week (p = 0.147), the relationship between aversive interoceptive state change processing and fitness should be examined in future studies of AN. Third, we were unable to use computational modeling to quantify prediction error as this approach requires a rapid, event-related design that would not have been appropriate for the breathing paradigm. Fourth, our sample size was modest, and physiological and self-report data were missing from some participants. Despite statistical investigation of the effects of past comorbidities in the RAN group, their potential impact cannot be ruled out completely. Future studies with larger sample sizes are necessary to thoroughly examine the potential influence of diagnostic subtype, anxiety symptoms, and past comorbidities on altered interoceptive processing associated with AN. Inclusion of clinical control groups (e.g., women with a history of panic disorder or panic attacks) may help to further characterize these influences. Further, since we included only remitted AN, our results should be compared to those from an ill sample. Additional research is needed to better understand why altered interoceptive network activation in RAN was not associated with subjective intensity or unpleasantness of the breathing load.
Clinical Implications
Our results have important implications for understanding AN pathogenesis and improving AN treatment. Neuropsychological task data suggest impairments or inefficiencies in cognitive flexibility in AN, some of which persist after recovery (Tchanturia et al. 2012), but no study to date has focused on the ability to adapt to a shift in interoceptive state. Our data suggest a brain-based difficulty predicting and adapting to internal milieu state shifts that may contribute to the severity and persistence of AN. Theories linking interoceptive prediction error to anxiety (Barrett & Simmons 2015; Paulus & Stein 2010), associations between perceived sensory sensitivity and emotion dysregulation in AN (Merwin et al. 2013), and our observed relationships among markers of AN severity and prefrontal and striatal hyperactivation after aversive interoception all support this notion. In addition, more pronounced insular hyperactivation after aversive interoception in RAN participants was associated with increased feelings of faintness after scanning. In the context of unreliable and unhelpful interoceptive signals and neural hypersensitivity to non-painful aversive interoceptive stimuli, patients may rely on external feedback to guide their behavior and pursue a state of interceptive “sameness.” Because eating changes our homeostatic state, starvation may be one method of avoiding aversive, unpredictable internal change in AN.
No empirically-supported psychotherapies exist for the treatment of adults with AN, and our results preliminarily suggest that this may be because existing interventions rely on new learning from experience. Integration of stimulus anticipation and receipt is critical for learning about and responding to salient stimuli (Schultz et al. 1997), and our findings add to a growing body of evidence that this integration is altered in AN (Frank et al. 2012). Determining next whether these alterations are plastic may help direct future treatment efforts for AN. Future studies should incorporate measures of cognitive flexibility, examine rate and magnitude of habituation across samples, and apply computational modeling to aversive interoceptive tasks to clarify how aversive learning may contribute to AN treatment resistance. Additionally, future research should further investigate the role of anticipation, processing, and recovery from interoceptive state changes in AN as they directly relate to eating behavior.
Supplementary Material
Acknowledgments
Funding:
This research and preparation of this manuscript were supported in part by grants from the National Institute of Mental Health to WHK (R01 MH042984), WHK and UB (R01 MH092793), and LAB (F32 MH108311), the Price Foundation, and the Hilda and Preston Davis Foundation. The authors declare no conflict of interest.
The authors thank Miki Ogasawara for assistance with fMRI data collection and pre-processing. We would also like to thank Daria Orlowska, Grace Rasmusson, Laura Greathouse, Raesana Williams, and Jocelyn Danielle Bulante for assistance with participant screening and data collection. Finally, we thank the women who participated in this study for their time.
References
- Bailer UF, Frank GK, Henry SE, Price JC, Meltzer CC, Weissfeld L, Mathis CA, Drevets WC, Wagner A, Hoge J, Ziolko SK, McConaha CW, Kaye WH. Altered brain serotonin 5-HT1A receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [carbonyl11C]WAY-100635. Archives of General Psychiatry. 2005;62:1032–1041. doi: 10.1001/archpsyc.62.9.1032. [DOI] [PubMed] [Google Scholar]
- Bailer UF, Price JC, Meltzer CC, Mathis CA, Frank GK, Weissfeld L, McConaha CW, Henry SE, Brooks-Achenbach S, Barbarich NC, Kaye WH. Altered 5-HT(2A) receptor binding after recovery from bulimia-type anorexia nervosa: relationships to harm avoidance and drive for thinness. Neuropsychopharmacology. 2004;29:1143–55. doi: 10.1038/sj.npp.1300430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nature Reviews Neuroscience. 2015;16:419–429. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Berk L, Stewart JL, May AC, Wiers RW, Davenport PW, Paulus MP, Tapert SF. Under pressure: adolescent substance users show exaggerated neural processing of aversive interoceptive stimuli. Addiction. 2015;110:2025–2036. doi: 10.1111/add.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Gothelf A, Parush S, Eitan Y, Admoni S, Gur E, Stein D. Sensory modulation disorder symptoms in anorexia nervosa and bulimia nervosa: A pilot study. International Journal of Eating Disorders. 2016;49:59–68. doi: 10.1002/eat.22460. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM. The Temperament and Character Inventory (TCI): A guide to its development and use. Center for Psychobiology of Personality, Washington University St; Louis, MO: 1994. [Google Scholar]
- Courty A, Godart N, Lalanne C, Berthoz S. Alexithymia, a compounding factor for eating and social avoidance symptoms in anorexia nervosa. Comprehensive Psychiatry. 2015;56:217–228. doi: 10.1016/j.comppsych.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Cowdrey FA, Park RJ, Harmer CJ, McCabe C. Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biological Psychiatry. 2011;70:736–43. doi: 10.1016/j.biopsych.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols T, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Science. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK, Bailer UF, Henry SE, Drevets W, Meltzer CC, Price JC, Mathis CA, Wagner A, Hoge J, Ziolko S, Barbarich-Marsteller N, Weissfeld L, Kaye WH. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biological Psychiatry. 2005;58:908–12. doi: 10.1016/j.biopsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Frank GK, Collier S, Shott ME, O’Reilly RC. Prediction error and somatosensory insula activation in women recovered from anorexia nervosa. Journal of Psychiatry and Neuroscience. 2016;41:150103. doi: 10.1503/jpn.150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GKW. Advances from neuroimaging studies in eating disorders. CNS Spectrums. 2015;20:391–400. doi: 10.1017/S1092852915000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GKW, Reynolds JR, Shott ME, Jappe L, Yang TT, Tregellas JR, O’Reilly RC. Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology. 2012;37:2031–2046. doi: 10.1038/npp.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G, Shukla A, Simmons AN, Davenport PW, Paulus MP. Sex Differences in the Neural Processing of Aversive Interoceptive Events: The Benefit of Relief. PLoS ONE. 2013;8:e84044. doi: 10.1371/journal.pone.0084044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner DM. Eating Disorder Inventory-2 Professional Manual. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- Haase L, May AC, Falahpour M, Isakovic S, Simmons AN, Hickman SD, Liu TT, Paulus MP. A pilot study investigating changes in neural processing after mindfulness training in elite athletes. Frontiers in Behavioral Neuroscience. 2015;9:229. doi: 10.3389/fnbeh.2015.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase L, Stewart JL, Youssef B, May AC, Isakovic S, Simmons AN, Johnson DC, Potterat EG, Paulus MP. When the brain does not adequately feel the body: Links between low resilience and interoception. Biological Psychology. 2016;113:37–45. doi: 10.1016/j.biopsycho.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase L, Thom NJ, Shukla A, Davenport PW, Simmons AN, Paulus MP, Johnson DC. Mindfulness-based training attenuates insula response to an aversive interoceptive challenge. Social Cognitive and Affective Neuroscience. 2014:nsu042. doi: 10.1093/scan/nsu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DJ, Northoff G. Common brain activations for painful and non-painful aversive stimuli. BMC Neuroscience. 2012;13:60. doi: 10.1186/1471-2202-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber P. Robust Estimation of Location Parameter. Annals of Mathematical Statistics. 1964:35. [Google Scholar]
- Kerr KL, Moseman SE, Avery JA, Bodurka J, Zucker NL, Simmons WK. Altered Insula Activity during Visceral Interoception in Weight-Restored Patients with Anorexia Nervosa. Neuropsychopharmacology. 2016;41:521–8. doi: 10.1038/npp.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Craske MG, Li W, Vangala S, Strober M, Feusner JD. Altered interoceptive awareness in anorexia nervosa: Effects of meal anticipation, consumption and bodily arousal. International Journal of Eating Disorders. 2015;48:889–897. doi: 10.1002/eat.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. The pathways of interoceptive awareness. Nature Neuroscience. 2009;12:1494–6. doi: 10.1038/nn.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TA, Ullsperger M, Danielmeier C. Error awareness and the insula: links to neurological and psychiatric diseases. Frontiers in Human Neuroscience. 2013:7. doi: 10.3389/fnhum.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Lee M, Berna C, Andersson J, Tracey I. Relief as a Reward: Hedonic and Neural Responses to Safety from Pain. PLoS ONE. 2011;6:e17870. doi: 10.1371/journal.pone.0017870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merwin RM, Moskovich AA, Wagner HR, Ritschel LA, Craighead LW, Zucker NL. Emotion regulation difficulties in anorexia nervosa: Relationship to self-perceived sensory sensitivity. Cognition and Emotion. 2013;27:441–52. doi: 10.1080/02699931.2012.719003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn K, Framptom I, Fuglset T, Torzsok-Sonnevend M, Lask B. Anorexia nervosa and the insula. Medical Hypotheses. 2011;76:353–357. doi: 10.1016/j.mehy.2010.10.038. [DOI] [PubMed] [Google Scholar]
- Oberndorfer T, Simmons A, McCurdy D, Strigo I, Matthews S, Yang T, Irvine Z, Kaye W. Greater anterior insula activation during anticipation of food images in women recovered from anorexia nervosa versus controls. Psychiatry Research: Neuroimaging. 2013a;214:132–141. doi: 10.1016/j.pscychresns.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberndorfer TA, Frank GK, Simmons AN, Wagner A, McCurdy D, Fudge JL, Yang TT, Paulus MP, Kaye WH. Altered insula response to sweet taste processing after recovery from anorexia and bulimia nervosa. The American Journal of Psychiatry. 2013b;170:1143–51. doi: 10.1176/appi.ajp.2013.11111745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Flagan T, Simmons AN, Gillis K, Kotturi S, Thom N, Johnson DC, Van Orden KF, Davenport PW, Swain JL. Subjecting elite athletes to inspiratory breathing load reveals behavioral and neural signatures of optimal performers in extreme environments. PLos ONE. 2012:7. doi: 10.1371/journal.pone.0029394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60:383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Structure and Function. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer C. Dyspnea relief: more than just the perception of a decrease in dyspnea. Respiratory Physiology & Neurobiology. 2009;167:61–71. doi: 10.1016/j.resp.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Kurz A-L, Albrecht J, Schreder T, Kleemann A, Schopf V, Kopietz R, Weismann M, Schandry R. Reduced perception of bodily signals in anorexia nervosa. Eating Behaviors. 2008;9:381–388. doi: 10.1016/j.eatbeh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Seidel E, Pfabigan DM, Hahn A, Sladky R, Grahl A, Paul K, Kraus C, Kublbock M, Kranz GS, Hummer A, Lanzenberger R, Windischberger C, Lamm C. Uncertainty during pain anticipation: the adaptive value of preparatory processes. Human Brain Mapping. 2015;36:744–755. doi: 10.1002/hbm.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shott ME, Pryor TL, Yang TT, Frank GKW. Greater insula white matter fiber connectivity in women recovered from anorexia nervosa. Neuropsychopharmacology. 2015;41:498–507. doi: 10.1038/npp.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Keeping the body in mind: Insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Human Brain Mapping. 2013;34:2944–2958. doi: 10.1002/hbm.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RD. State-Trait Anxiety Inventory Manual. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Steinglass JE, Sysko R, Mayer L, Berner LA, Schebendach J, Wang Y, Chen H, Albano AM, Simpson HB, Walsh BT. Pre-meal anxiety and food intake in anorexia nervosa. Appetite. 2010;55:214–218. doi: 10.1016/j.appet.2010.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, May AC, Poppa T, Davenport PW, Tapert SF, Paulus MP. You are the danger: Attenuated insula response in methamphetamine users during aversive interoceptive decision-making. Drug and Alcohol Dependence. 2014;142:110–119. doi: 10.1016/j.drugalcdep.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigo IA, Matthews SC, Simmons AN, Oberndorfer T, Klabunde M, Reinhardt LE, Kaye WH. Altered insula activation during pain anticipation in individuals recovered from anorexia nervosa: Evidence of interoceptive dysregulation. International Journal of Eating Disorders. 2013;46:23–33. doi: 10.1002/eat.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchanturia K, Davies H, Roberts M, Harrison A, Nakazato M, Schmidt U, Treasure J, Morris R. Poor Cognitive Flexibility in Eating Disorders: Examining the Evidence using the Wisconsin Card Sorting Task. PLoS ONE. 2012;7:e28331. doi: 10.1371/journal.pone.0028331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A, Aizenstein H, Mazurkewicz L, Fudge J, Frank GK, Putnam K, Bailer UF, Fischer L, Kaye WH. Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology. 2008;33:513–23. doi: 10.1038/sj.npp.1301443. [DOI] [PubMed] [Google Scholar]
- Wagner A, Aizenstein H, Venkatraman VK, Fudge J, May JC, Mazurkewicz L, Frank GK, Bailer UF, Fischer L, Nguyen V, Carter C, Putnam K, Kaye WH. Altered reward processing in women recovered from anorexia nervosa. American Journal of Psychiatry. 2007;164:1842–9. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Bischoff-Grethe A, Melrose AJ, Irvine Z, Torres L, Bailer UF, Simmons A, Fudge JL, McClure SM, Ely A. Hunger does not motivate reward in women remitted from anorexia nervosa. Biological Psychiatry. 2015;77:642–652. doi: 10.1016/j.biopsych.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker NL, Merwin RM, Bulik CM, Moskovich A, Wildes JE, Groh J. Subjective experience of sensation in anorexia nervosa. Behaviour Research and Therapy. 2013;51:256–265. doi: 10.1016/j.brat.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.