Abstract
Non-thermal irreversible electroporation (NTIRE) is a biophysical phenomenon in which certain electric fields delivered across the cell membrane in tissue, cause cell death, without affecting the extracellular matrix. “Minimally invasive regenerative surgery” is a new medical modality for treatment of end-stage organ or tissue failure in which exogenous cells are implanted in a decellularized niche in tissue, formed by the delivery of NTIRE electric fields across a targeted volume of tissue. We anticipate that the success of the procedure will depend on the time of implantation relative to the application of NTIRE. This study was performed to elucidate the histological and molecular events that occur within 24h after NTIRE, in the context of optimal criteria for the time of implantation. To this end, we examined the histology of NTIRE treated rat liver with H&E, Masson trichrome and TUNEL staining. Western blot was used to examine pro and cleaved caspase-3 (marker for apoptosis), pro and cleaved caspase-1 and gasdermin D (markers for pyroptosis), and RIP3 and MLKL (markers for necroptosis). The key findings are that, complete hepatocytes disintegration within an intact extracellular matrix is seen at 6h and, new hepatocytes are seen in the treated region at 24h, after NTIRE. There is no evidence of apoptotic cell death from NTIRE, contrary to commonly made claims in the NTIRE literature. However, molecular pathways of pyroptosis and necroptosis, programed necrosis associated with inflammation, are activated at 6h after NTIRE and are not evident at 24h after NTIRE. These are fundamental new findings of basic value to the field of NTIRE in all its applications. Taken together the results suggest the hypothesis that an optimal time for implantation is about 24h after NTIRE. Future studies in which exogenous cells are implanted at different times after NTIRE are required to examine this hypothesis.
Keywords: Non thermal irreversible electroporation, liver, cell death mechanism, minimally invasive regenerative surgery
Introduction
Recently [1], we have pioneered “minimally invasive regenerative surgery” (MIRS) as a new medical modality that could become an alternative to whole organ transplant for treatment of end-stage organ failures, such as the liver, or for treatment of impaired organ function, such as diabetes. MIRS employs non-thermal irreversible electroporation (NTIRE), to create an in vivo niche of a decellularized intact extracellular matrix for exogenous cell engraftment [1]. Electroporation, is the permeabilization of the cell membrane with electric fields, delivered across a cell [2]. Electroporation can be reversible [3], in which case the cell returns to its original state a certain time after the electric field has ceased, or irreversible [4], when the cell succumbs to the permeabilization of the cell membrane. Non thermal irreversible electroporation (NTIRE), has recently acquired importance in minimally invasive ablation surgery of otherwise unresectable tumors, e.g. [5], [6] on the strength of its ability to cause cell death by selectively affecting only the cell membrane, while sparing the extracellular matrix and important tissue features [7]. Notably, this has led to the idea that treating tissues with NTIRE could be used to form a de-cellularized tissue scaffold, that serves as an in vivo generated niche for exogenous cell engraftment [1].
The optimal time for exogenous cell engraftment after NTIRE, is a key parameter in MIRS. In the liver, the optimal time for implantation should satisfy the following criteria: 1) the hepatocytes have disintegrated, to avoid encroaching the newly transplanted cells; 2) the extracellular matrix maintains a comparatively normal architecture to support the implanted cells; 3) inflammatory response are mild, so as to minimize short term immune response damage to the implanted cells. However, currently, the majority of studies on NTIRE focus on the long term effects, because these are important to clinical applications in the treatment of cancer. There is very little research on the processes which occur at the early stages after the NTIRE treatment, which is the time of relevance to MIRS. The goal of the study reported in this paper was to generate fundamental information on the physiological and molecular events which occur within the first 24 hours after NTIRE, to provide support for future studies in which exogenous cells will be implanted in the NTIRE formed niche at the optimal time suggested by this study. We have used H&E and Masson’s trichrome stains to follow the physiological events that occur after NTIRE and to determine the optimal time for criteria 1) and 2) above. Concerning molecular events, the majority of publications on NTIRE, credit cell death to apoptosis, primarily on the basis of TUNEL stain based experiments e.g. [5], [8]. In regards to criteria 3) avoidance of inflammation, it is important to confirm that cell death is by apoptosis or not. Apoptosis generates a complex mixture of cell surface–associated molecules that are released by or displayed on apoptotic cells to help phagocytes find and engulf them without triggering inflammation [9]. In contrast cells undergoing necrotic death rupture release factors that stimulate inflammation. Pyroptosis and necroptosis are two inflammatory programed necrosis molecular pathways that involve membrane rupture and release of cytoplasmic contents and are distinct from apoptosis. Pyroptosis is driven by the inflammatory caspases: caspase 1, 4, 5 and 11. Once caspases 1, 11, 4 or 5 have been activated, they trigger pyroptosis by cleaving gasdermin D [10]. Activation of caspase 1 and failure to activate caspase 3 (which is associated with apoptosis) are considered a key marker of pyroptosis [11]. Necroptosis is also a programmed form of necrosis that is dependent on activation of receptor-interacting kinase (RIPK3)[12] and the mixed lineage kinase domain-like (MLKL)[13]. In this study, we examined molecular markers for apoptosis, pyroptosis and necroptosis, throughout the first 24 hours after NTIRE, in the context of 3) above.
Materials and Methods
Sprague–Dawley rats weighing 250–350 g were used in this study. All animals received humane care from properly trained professionals in compliance with both the Principals of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals, published by the National Institute of Health (NIH publication no. 85-23, revised 1985), and treated according to an animal protocol approved by the Animal Care and Use Committee of the University of California, Berkeley.
The surgical procedure followed a protocol described in detail in [14]. The animals were anesthetized, and the peritoneal cavity is entered via a midline incision of the abdomen. Three lobes were treated in each animal and each lobe was treated once. After partial mobilization of the liver from adjacent tissue, the treated liver lobe was gently clamped between the two 10 mm diameter electrodes, separated by 3 mm (Harvard Apparatus, Holliston, MA, USA), as described in [14]. A sequence of 10 square pulse with an electric field of 1000 V/cm, 100 μs pulse width, separated by 100 ms was applied between the electrodes, across the liver, using an electroporator (ECM 830, Harvard Apparatus, Holliston, MA, USA). (Previous studies with the experimental configuration in this study have shown that this electroporation protocol produces minimal thermal damage, and the tissue is affected by irreversible electroporation only [15].) At the end of the NTIRE experiment, the abdomen wall was closed and sutured. We examine the liver via liver harvest at the following times after the procedure: 1h, 3h, 6h, 24h.
For histological examination, tissue samples were cut normal to the liver lobe surface, distal to the treated region and through the center of the treated lesion, fixed in formalin, embedded in paraffin and sectioned 5-µm thick. They were stained with hematoxylin and eosin, Masson’s trichrome, and TUNEL assays by (Histo-Tec Laboratory, Hayward, CA, USA) and (Histowiz Inc, Brooklyn, NY, USA).
The liver tissues were lysed, and the lysates were subjected to Western blot assays. Anti-caspase-1 and anti-RPI3 were purchased from Abcam, anti-GSDMD was purchased from abbexa, anti-MLKL was purchased from Abclonal, anti-caspase-3 was purchased from Cell signaling technology; and visualized using the Western Bright ECL detection system (Biorad Hercules, CA) after incubation.
Results
We have performed four repeats for each experimental condition and the results shown here are typical to all the repeats. Continuous monitoring of the animals indicated that the animals did not experience any adverse effects due to the non-electrolytic NTIRE treatment procedure.
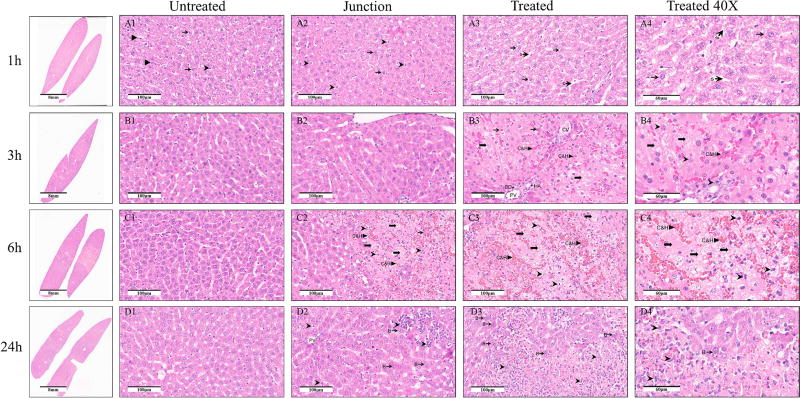
Figure 1 shows the histology of the H&E stained liver in different regions of the treated liver at different times after NTIRE treatment, as listed in the figure legend. Panel 1-A1 shows the normal liver and arrows point to a normal hepatocyte, an endothelial cell and a Kupffer or macrophage cell. Panel 1-A2 shows that hepatocytes in the treated and untreated part of the liver appear normal and there is no distinct interface between the treated and untreated areas. However, there seem to be more Kupffer cells or macrophages in the area corresponding to the treated tissues. Panels 1-A3 and 1-A4 show a relative large number of Kuppfer cells or macrophages, expanded sinusoids, and some ballooned but intact hepatocytes. Panels 1-B show micrographs taken 3h after the treatment. The H&E staining shows more ballooned hepatocyte than after 1h, vascular congestion and hemorrhagic change in ablation area. Cytoplasmic limits between the cells are barely distinguishable. Kupffer cells infiltration is seen. There are sites at which only the extracellular matrix is seen with no cell structure. It is interesting to notice in panel 1-B3 that the large blood vessels, (portal vein, hepatic artery and bile duct structure appear morphologically intact). Panels 1-C show the H&E stained tissue 6h after NTIRE. Panel 1-C2 shows a clear, cell scale resolution, line of demarcation between the non-electrolytic NTIRE treated zone (right hand side) and the normal liver (left hand side). The difference in appearance between the treated and untreated regions is striking. In the normal liver the sinusoids are patent and the hepatocytes look normal. In the treated region, which is separated from the normal liver by only several layers of cells, the liver architecture is completely lost and vesicle without nuclei and inflammatory cells are seen throughout. The treated region has experienced severe congestion and hemorrhage. Panels 1-D show the H&E stained region 24h after NTIRE. Panel 1-D2 shows that the distinction between the treated and untreated has become vague, strikingly different from the pronounced margin 6h after the treatment. In the untreated zone, the tissue structure is comparatively normal, with some nonspecific cell swelling and inflammatory cell infiltration. In the ablation zone, it is still possible to see inflammatory cells infiltration. Scattered congestion and hemorrhagic change are seen. However, normal hepatocytes are also seen throughout the treated region. In panels 1-D3 and 1-D4, numerous bi-nucleated hepatocytes are seen in the NTIRE treated area.
Figure 1. The histology of the H&E stained liver at different locations relative to the treatment zone and at different times after treatment.
The columns from left are: macroscopic cross section; untreated region; the interface between the untreated (left side) and treated (right side) regions; core of the treated region at (×20); core of the treated region (×40). The rows are, from top to bottom: 1h, 3h, 6h, and 24h after treatment. Scale bar given in the figures. Thin arrow, triangle arrow, arrowhead, arrow with flat tail, point to hepatocytes, endothelial cells, Kupffer cell and vacuolated hepatocyte without nuclei structure, respectively. Arrows marked with S point to wide sinusoids. Arrow marked with C&H point to areas of congestion and hemorrhagic change. Arrow marked with B point to bi-nucleate hepatocytes. CV means central vein. BD means bile duct.
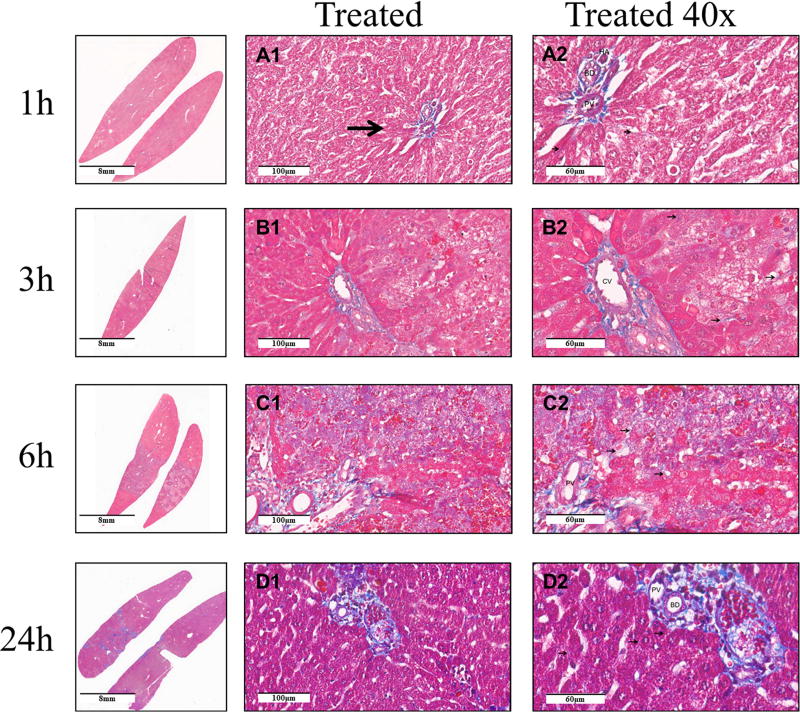
Masson’s trichrome stain was used to examine the effect of non-electrolytic NTIRE on the extracellular matrix. With this stain, the collagen fibers are stained blue, the cytoplasm is stained red, and the nucleus is stained dark brown. The results are shown in Figure 2. The micrographs taken 1h and 3h after the treatment show a normal thin layer of peri-hepatocyte bluish extracellular matrix. Vacuolated hepatocytes without nuclei retained normal peri-cell collagen at 3 hours (marked by red arrow). The micrographs taken 6h after the NTIRE treatment show vacuolated hepatocytes without nuclei retaining normal peri-cell collagen. Twenty-four hours after NTIRE photomicrographs show regenerated hepatocyte with a normal thin layer of peri-cell collagen around the cell. Patent portal veins, hepatic arteries and bile ducts are seen throughout the treated region at all times.
Figure 2. Histology with Mason’s trichrome stain for evaluation of collagen after NTIRE.
The columns from left are: macroscopic cross section; untreated region; the interface between the untreated (left side) and treated (right side) regions; core of the treated region at (×20); core of the treated region (×40). The rows are, from top to bottom: 1h, 3h, 6h, and 24h after treatment. Scale bar in the micrographs. Thin arrow points to collagen. PV means portal vein, HA – hepatic artery. BD means bile duct. CV means central vein.
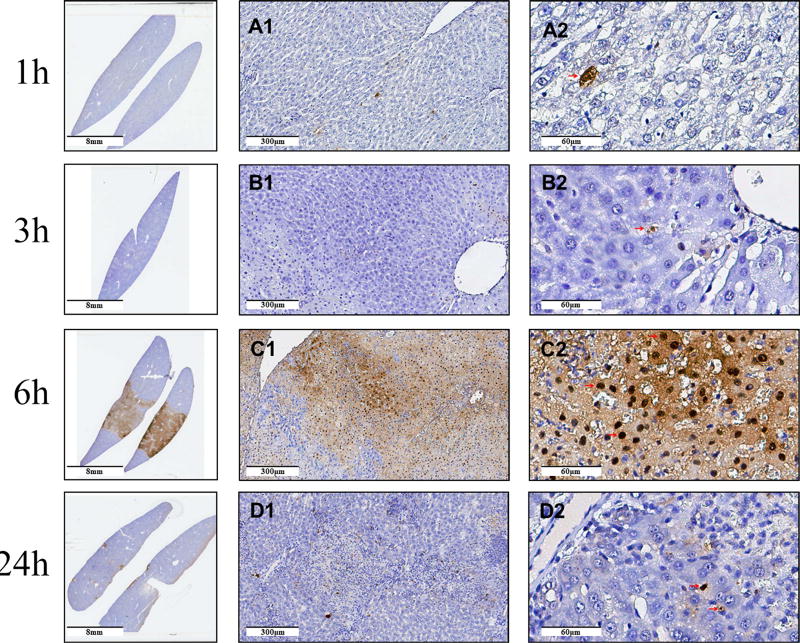
TUNEL stain results are shown in Figure 3. It is evident that very few cells are stained 1h and 3h after treatment. Six hours after treatment the TUNEL stain is spread throughout the treated tissue, and is not localized in the nucleus. The macroscopic cross section shows nicely that the treated region outside the nuclei is uniformly stained. Twenty-four hours after the non-electrolytic NTIRE treatment there is comparatively less stain than after 6h. Patent large blood vessels are seen throughout the treated region.
Figure 3. Histology with TUNEL stain.
The first column shows a macroscopic cross section through the treated lobe, the second column shows micrographs from the center of the treated lesion at ×10 and the third column shows the same site at × 40. The rows are for 1h, 3h, 6h, and 24h after the treatment. Scale bar in the micrographs.
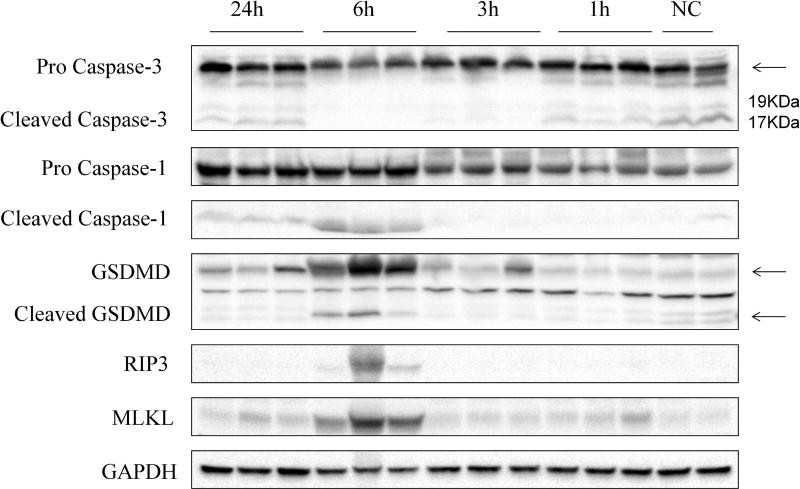
Figure 4 shows results for various molecular markers. Antibodies against pro Caspase 3, cleaved Caspase 3, pro Caspase 1, cleaved Caspase 1, GSDMD, cleaved GSDMD, RIP3 and MLKL were used to examine the activities of the apoptosis, pyroptosis and necroptosis signaling pathways. GAPDH was used as a loading control. An important measure of apoptosis is the cleaved caspase - 3. Interestingly, cleaved caspase 3 was present in the normal control and began disappearing after NTIRE. Notably, no cleaved caspase 3 was evident at measurements done 3h and 6h after NTIRE, but began reappearing 24 hours after NTIRE. To examine the mechanisms of cell death by pyroptosis and necroptosis, we examined the activation of caspase-1 and gasdermin D (GSDMD). Both caspase-1 and GSDMD were up-regulated at 6h and 24h. Importantly, both cleaved-caspase-1 and cleaved GSDMD increased at the same time. RIP3 and MLKL, are markers relevant to necroptosis. We found that both RIP3 and MLKL were also elevated after 6h.
Figure 4. Western blot.
Response to antibodies against pro Caspase 3, cleaved Caspase 3, pro Caspase 1, cleaved Caspase 1, GSDMD, cleaved GSDMD, RIP3 and MLKL are shown GAPDH was used as a loading control. The figure shows, from right to left, activities of not treated controls (NC) and NTIRE treated tissues, 1h, 3h, 6h and 24h after the treatment. The columns and rows are labeled.
Discussion
The goal of this study was to generate information on the physiological and molecular events that occur within 24h after NTIRE, to provide guidance for the optimal time of implantation of exogenous cells in MIRS, according to the criterial listed in the introduction.
The H&E stained micrographs (Figure 1) provide information on the pathological events within 24h after NTIRE. At 1h, there are no major histological changes in the treated tissue. This shows that the NTIRE electric fields do not cause an acute disintegration of the cells. An interesting observation is the increased number of Kupffer cells or macrophages, which apparently play an active role in the events after NTIRE. It should be noted, however, that Kupffer cells have a function in both apoptosis and necrosis. Three hours after NTIRE, while the sinusoids experienced vascular congestion, the large lumen structures, (portal vein, hepatic artery and bile duct) appear morphologically intact and patent. This is an important feature of NTIRE which suggests that the treated region is not completely occluded from the blood circulation. A sharp demarcation of the NTIRE treated region from the normal liver, is seen 6h after the treatment. No intact cells are seen in this region; with pyknotic and hyperchromatic nuclei and neutrophil infiltration throughout the treated volume. The process of cell death that has begun after the NTIRE treatment has reached completion 6h later. At 24h, normal hepatocytes are seen throughout the NTIRE treated zone, among them, numerous bi-nucleated hepatocytes. This is probably related to the observation of patent large blood vessels, which provide for the new hepatocytes. It shows that the conditions in the non-electrolytic NTIRE treated zone 24h after treatment allow the growth of new cells.
The Masson trichrome stains are used to examine changes in collagen, after the NTIRE. The micrographs (Figure 2) indicate that peri-cellular collagen (extracellular matrix) remains intact after the treatment. As shown in panel 2-C2, the peri-cellular collagen presented a comparatively intact structure after the hepatocytes disappeared. This is as expected in NTIRE, because in electroporation there is no mechanism to affect the molecules of the tissue. Electroporation affects only the permeability of the cell membrane and can affect the interior of the cell. Unlike thermal ablation, there is no mechanism for protein denaturation. The presence of an intact extracellular matrix, serving as a scaffold that facilitates the regrowth of the hepatocytes is the basis for the concept of exogenous cell implantation.
The histological study (Figs 1 and 2), provides relevant information on criteria 1) and 2) in the introduction. NTIRE causes hepatocyte disintegration within 6h after treatment and leaves behind an apparently intact extracellular matrix and patent large blood vessels, which are conducive to the survival of extraneous cell implants. At 24h, the conditions are conducive to growth of new cells within the treated region. Therefore, the optimal window of opportunity for exogenous cells implantation in the NTIRE generated niche seems to be between 6h and 24h after NTIRE.
Nowadays, apoptosis is widely recognized as a non-inflammatory reason of cell death after NTIRE [5], [8], on the basis of TUNEL stain experiments. To determine if the mechanism of cell death by NTIRE is from apoptosis we used TUNEL stain to examine the treated tissue. The results are in Figure 3. Very few cells are stained 1h and 3h after NTIRE. In contrast, the TUNEL stain 6h after NTIRE treatment is spread throughout the treated region showing a nice delineation of the treated region from the untreated region. The spread of the stain in the treated region at 6h is probably caused by the diffusion of fragmented DNA, across the breached cell membrane. The appearance is different from typical TUNEL stains of apoptotic cells, in which the nucleus is stained. It can be taken as evidence that the cell membrane is breached at 6h, and the DNA has been fragmented but not of apoptosis. The conclusion that NTIRE does not trigger apoptosis is also supported by a molecular study with caspase-3. In Figure 4, cleaved Caspase 3 was not upregulated at any time after NTIRE. However, cleaved caspase-3 was seen in controls and 1h, and 24h after NTIRE. The observation of cleaved caspase 3 in the controls is expected. The normal liver endures apoptosis at all times [16]. More relevant is that, cleaved caspase 3 disappeared 6h, reappeared at 24h after NTIRE. The disappearance of cleaved caspase-3 at 6h after the NTIRE treatment seems to indicate the time when hepatocytes have ceased to function as normal hepatocytes. The reappearance at 24h coincides with the histological evidence that new hepatocytes appear in the treated area. This confirms that the cleaved caspase-3 is a measure of normal functioning hepatocytes as well as hepatocytes regenerate in the treated area 24 hours after the NTIRE treatment. Taken together the TUNEL stain histology and the caspase-3 results indicated that apoptosis, a non-inflammatory mechanism of cell death, did not contribute to the cell death from NTIRE. We believe that this finding should be also adopted in the literature on the treatment of cancer with NTIRE, because cell death from NTIRE is almost universally attributed to apoptosis e.g. [5], [8].
The understanding of the mechanisms that lead to cell death have evolved well beyond the concepts of simple necrosis and apoptosis. A variety of other molecular mechanisms can lead to various modes of programed necrosis, such as pyroptosis and necroptosis. Pyroptosis is an inflammatory caspase-dependent form of programmed necrosis [17]. This form of cell death is driven by the inflammatory caspases: caspase 1, 4, 5 and 11. Activation of caspase 1 and failure to activate caspase 3 (which is associated with apoptosis) are considered a key marker of pyroptosis, which also eliminates apoptosis as a mechanism of cell death [11]. Once caspases 1, 11, 4 or 5 have been activated, they trigger pyroptosis by cleaving gasdermin D between Asp276 and Gly277[10]. Morphologically, pyroptotic cells display cell swelling and rapid plasma membrane lysis and release of cellular contents. This form of cell death is distinct from apoptosis. In this study, we find upregulated cleaved caspase-1, cleaved GSDMD and down-regulated cleaved caspase-3 6h after NTIRE, when histological evaluation shows the compete disruption of the cell membrane, release of intracellular content and uniformly distributed TUNEL stain. Necroptosis is also a programmed form of necrosis that is dependent on activation of receptor-interacting kinase (RIPK3)[12] and the mixed lineage kinase domain-like (MLKL)[13]. This form of cell death also involves membrane rupture and release of cytoplasmic contents and is also distinct from apoptosis. Here we find we found that both RIP3 and MLKL were also elevated 6 hours after NTIRE indicating that necroptosis pathway were also induced. Both markers for pyroptosis and necroptosis, are down regulated at 24h after NTIRE. Pyroptosis and necroptosis are modes of programed necrosis, which is an inflammatory mode of cell death. The markers are upregulated at 6h after NTIRE and are not evident at 24h. According to criterion 3) in the introduction, this suggests that an optimal time for implantation, that avoids an interaction with the local immunes system, may be at about 24h after NTIRE.
In summary, our study suggests that an optimal time for implantation of exogenous cells may be between 6 and 24 hours, probably closer to 24 hours. This is the window in time that we will study in our future studies on exogenous cell implantation. The implantation can be done in the core of the niche formed in the liver by non-electrolytic NTIRE. The pyroptosis and necroptosis, not apoptosis, contribute to the cell death after NTIRE treatment. We will follow this research with a study that will examine the hypothesis that 24h past NTIRE is the optimal time for implantation of exogenous cells in an NTIRE generated niche in the liver.
Supplementary Material
Highlights.
Hepatocytes completely disintegrate within 6h after NTIRE
The extracellular matrix in the treated volume is intact after NTIRE
Hepatocytes, normal and bi-nucleated, are seen in the treated volume, 24h after NTIRE
TUNEL stain is seen as a diffuse stain throughout the treated volume, 6h after NTIRE
Cleaved caspase-3 is actually downregulated after NTIRE.
Cleaved caspase-1, gasdermin D, RIP3 and MLKL are upregulated 6h, and downregulated 24h after NTIRE
Acknowledgments
The study was supported by NIH/NIBIB R21-EB02413 to T.T.C. and B.R. We thank professor Daniela Kaufer who generously provided the experimental devices for doing the Western Blot.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang TT, Zhou VX, Rubinsky B. Using non-thermal irreversible electroporation to create an in vivo niche for exogenous cell engraftment. Biotechniques. 2017;62:229–231. doi: 10.2144/000114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver JC, Chizmadzhev YA. Theory of electroporation: a review. Bioelectrochem. Bioenerg. 1996;41:135–160. [Google Scholar]
- 3.Neumann E, Schaeffer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lymphoma cells by electroporation in high electric fields. EMBO J. 1982;1:841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davalos RV, Mir LM, Rubinsky B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005;33:223–231. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 5.Vogel JA, van Veldhuisen E, Agnass P, Crezee J, Dijk F, Verheij J, van Gunk TM, Meijerink MR, Vroomen LG, van Lienden KP, Besselink MG. Time-Dependent Impact of Irreversible Electroporation on Pancreas, Liver, Blood Vessels and Nerves: A Systematic Review of Experimental Studies. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin RCG, Durham AN, Besselink MG, Iannitti D, Weiss MJ, Wolfgang CL, Huang KW. Irreversible electroporation in locally advanced pancreatic cancer: A call for standardization of energy delivery. J. Surg. Oncol. 2016;114:865–871. doi: 10.1002/jso.24404. [DOI] [PubMed] [Google Scholar]
- 7.Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality--clinical implications. Technol. Cancer Res. Treat. 2007;6:37–48. doi: 10.1177/153303460700600106. http://www.ncbi.nlm.nih.gov/pubmed/17241099. [DOI] [PubMed] [Google Scholar]
- 8.Lee EW, Loh CT, Kee ST. Imaging guided percutaneous irreversible electroporation: Ultrasound and immunohistological correlation. Technol. Cancer Res. Treat. 2007;6:287–293. doi: 10.1177/153303460700600404. [DOI] [PubMed] [Google Scholar]
- 9.VanHook AM. Avoiding Inflammation During Apoptosis. Sci. Signal. 2014;7:ec89–ec89. doi: 10.1126/scisignal.2005314. [DOI] [Google Scholar]
- 10.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 11.Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 12.Moriwaki K, Chan FKM. RIP3: A molecular switch for necrosis and inflammation. Genes Dev. 2013;27:1640–1649. doi: 10.1101/gad.223321.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 14.Phillips M, Rubinsky L, Meir A, Raju N, Rubinsky B. Combining electrolysis and electroporation for tissue ablation. Technol. Cancer Res. Treat. 2015;14:395–410. doi: 10.1177/1533034614560102. [DOI] [PubMed] [Google Scholar]
- 15.Phillips M, Maor E, Rubinsky B. Nonthermal irreversible electroporation for tissue decellularization. J. Biomech. Eng. 2010;132:91003. doi: 10.1115/1.4001882. [DOI] [PubMed] [Google Scholar]
- 16.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: A tale of two deaths? Hepatology. 2006;43 doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 17.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/S0966-842X(00)01936-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.