Abstract
Purpose
To compare the characteristics of the retinal and choroidal lesions including choroidal nevus, choroidal melanoma and congenital hypertrophy of the retina pigment epithelium using conventional color fundus photography (CFP) and multicolor imaging (MCI).
Methods
The paired images of patients with retinal or choroidal lesions were assessed for the visibility of lesion’s border, halo and drusen using a grading scale (0–2). The area of the lesion was measured on both imaging modalities. The same grading was also done on the individual color channels of MCI for a further evaluation.
Results
Thirty-three eyes of 33 patients were included. There were no significant differences in the mean border, drusen and halo visibility scores between the two imaging modalities (p=0.12, p=0.70, p=0.35). However, the mean area of the lesion was significantly smaller on MCI than that on CFP (14.9±3.3 versus 18.7±3.4 mm2, p=0.01).
Conclusion
The appearance of choroidal and/ or retinal lesions on MCI may be different than that on CFP. Though MCI can provide similar information with CFP for the features of retinal and/ or choroidal lesions including border, halo and drusen; the infrared light reflection on MCI underestimates the extent of the choroidal lesion by 33%.
Keywords: multicolor imaging, fundus photography, choroidal nevus, choroidal melanoma, congenital hypertrophy of the retina pigment epithelium, pseudocolor
INTRODUCTION
Choroidal nevus is the most common intraocular tumor with an estimated prevalence of 7% among the white population [1]. The nevus represents as a brown or tan mass with a round or oblong configuration, deep to the retina and retinal pigment epithelium (RPE). Overlying RPE alterations and drusen appears to common features of nevus. However, orange pigment, retinal edema or RPE detachment can rarely be seen in patients with choroidal nevus [2]. Despite the benign histological findings in choroidal nevus, it can lead to vision loss and can rarely evolve into life-threatening malignant melanoma [3]. Thus, proper documentation of choroidal nevi is important.
Uveal melanoma is the most common non-cutaneous melanoma with an incidence of 5–6 cases per million per year [3]. The current diagnosis of choroidal melanoma is based on both the clinical experience of the specialist and modern diagnostic tools including ultrasonography, fundus fluorescein angiography (FA), optical coherence tomography (OCT), indocyanine green angiography (ICG) and autofluorescence imaging [2–6].
Color fundus photography (CFP) has been available for decades in ophthalmology practice to document retinal disorders as seen on fundus examination with the advantage of giving the most identical appearance to clinical examination. A variety of imaging modalities can be used to improve the information obtained by CFP. Heidelberg engineering has recently developed MultiColor imaging (MCI) system (Spectralis HRA-OCT; Heidelberg Engineering, Heidelberg, Germany) which enables combined pseudo-color image using three different wavelengths including blue (486 nm), green reflectance (518 nm) and infrared (IR) reflectance (815 nm) with simultaneously or near simultaneously optical coherence tomography (OCT) and FA. Recently, this imaging technique, which indeed provides pseudocolor image of the retina, has gained great interest among the retinal physicians, as it offers multicolor fundus images with FA and co-located spectral-domain OCT in a timely manner [7,8,9].
In our routine practice, all patients with retina pathologies undergo both CFP and MCI imaging with OCT, when available, for documentation purposes. At the time of acquiring images of some patients with retinal and/ or choroidal lesions such as choroidal nevus, choroidal melanoma and congenital hypertrophy of retina pigment epithelium (CHRPE), we noticed some differences in the appearance of lesions. Moreover, since these instruments are designed based on different image acquiring algorithms, the interpretation of the images may be somewhat different on MCI than that on CFP. Therefore, we conducted this study to show whether MCI can provide same information for the characteristics of these lesions as CFP does. Also, we wished to highlight the difference between the appearance of such these lesions where lesion color and borders have great impact on the diagnosis. We are aware that multicolor imaging is not a standard way to document and/or diagnose these lesions. However, the advantage of acquiring simultaneously or near simultaneously FA and OCT images without using a bright light may lead this imaging technique to replace standard color photography in routine retina practice.
METHODS
Patients Population
This was a retrospective study including patients with choroidal melanoma, choroidal nevus, choroidal hemangioma or CHRPE who were referred to the Department of Ophthalmology, University of California San Diego (UCSD), Shiley Eye Institute between January 2016 and December 2016. Institutional Review Board approval from UCSD was obtained for the review of patients’ charts and images. The study adhered to the tenets of the Declaration of Helsinki for research involving human subjects and complied with Health Insurance Portability and Accountability Act (HIPAA) of regulations.
Patients who were imaged by both CFP and MCI at the same day and had good quality images were included. Color fundus photography was taken with the Topcon TRC50X digital fundus camera (Topcon, Tokyo, Japan) and multicolor images were acquired using Spectralis (HRA-OCT Spectralis Heidelberg, Germany, acquisition module version 6.3.2.0) after optimal pupil dilation with tropicamide and phenylephrine. Multicolor images were obtained using either 30° or 55° based on the lesion location, whereas all CFP images had a 55° of field of view. Additionally, as a routine imaging protocol in our institute, each patient underwent horizontal and vertical B-scans cutting through fovea as well as a 6-mmx6-mm macular cube comprising at least 37 horizontal B-scans using Spectralis device. When available, B-scan cutting through the lesion was also acquired.
Patients with poor quality images that did not allow proper documentation of the lesions and eyes that had far peripheral lesions that were only partially visible in any imaging modalities were excluded.
Analysis of Images
-
-
As a first step, names of the patients with given retinal or choroidal lesion were identified through a search of local database.
-
-
A masked observer (D.U.B.) removed the patients’ identification such as names and imaging date from the images (CF and MCI) and saved them as maximum quality TIFF image files without any modifications.
-
-
Then, 2 experienced retina specialists (M.G., W.R.F.) who have had at least 30-year experiences in the field reviewed and graded each image at different time points at least 24-hour apart in the same room under optimal room conditions using a computer unit with 2 monitors that had identical specifications and user settings (1,280 × 1,024 pixels, 17-inch liquid crystal display monitors, NEC Accusync LCD 92V) to avoid monitor-induced differences.
-
-
The visualization of the lesion border was scored using a grading scale between 0 and 2. “Grade 0” was given when the lesion margin was not visible on the imaging modality, “Grade 1” referred that the margin of the lesion was barely visible, “Grade 2” indicated that the margin was clearly visible. The same grading was also done for the assessment of halo and drusen. Halo was considered as yellow circumferential yellow ring, and drusen was regarded as tiny white or yellow deposits underneath the retina.9 When there was a disagreement in the grading score, a third observer’s impression was asked to achieve an acceptable result.
-
-
Masked single-wavelength images that were initially combined to obtain MCI images (i.e., near-infrared reflectance, blue reflectance, and green reflectance) were graded using the same grading scale as defined above to determine which reflectance image allowed better visualization of the lesion.
-
-
The area of the lesion was measured using the built-in measurement tool of the Spectralis (Heyex software viewing module version 6.3.4.0). The fundus image was loaded into the Spectralis software using the built-in import function. The Heyex software outline tool allows to draw a line around retinal features and measure the area encompassed by the line. The scanning laser ophthalmoscopy (SLO) images measurements are in square millimeter, while the fundus camera image measurements are in square pixel. In order to avoid any errors that may be related to difference in the magnification of the two devices, the area of the lesion was measured as follows: A retinal feature that was visible in both images was randomly selected. Typically, this feature was the distance between two vessel branching points on the superior and inferior branch vessels. The distance was measured in both images and the results were in micrometer for the SLO and pixel for the fundus camera. Using this scale, we converted the fundus camera image measurements from square pixel to square millimeter. By importing the fundus camera images into Heyex software and directly comparing retinal landmarks we eliminate any magnification differences between the two instruments. We measured the magnification linearity of the fundus camera across the image using a millimeter grid and found no distortions across the image sensor.
Statistical Analysis
The agreement between the two graders was evaluated by using Kappa (K) coefficient and the agreements among the graders for the visibility of border, halo and drusen were found to be high; K=0.870, K=0.930, and K=0.92, respectively. The agreement between the two graders for the measurement of lesion area was found perfect (intracorrelation coefficient=0.96).
Continuous variables were presented as mean and ± standard deviation and categorical variables were presented as percentage (%). The average visibility score on MCI and CFP was compared using Mann-Whitney-U test. Chi-square or Fisher-exact test was used to compare the distribution of visibility of halo, border, drusen using MCI and CFP. Paired t-test was used to compare area of the lesions on the two imaging modalities.
All statistics analysis was performed with SPSS statistical software version 23 (SPSS Inc, Chicago, IL, US). A two-sided p-value less than 0.05 was considered to be statistically significant.
RESULTS
Thirty-three eyes of 33 patients with clinical based diagnosis of choroidal nevus, choroidal melanoma, choroidal hemangioma or CHRPE that underwent same-day CFP and MCI were included in the study. The baseline characteristics of the patients are shown in Table 1. Most of the patients had choroidal nevus.
Table 1.
Baseline characteristics of the patients
| Age, mean ± SD (range) | 64.67 ± 13.47 (86–19) | |
|
| ||
| Gender (female/male) | 19/14 | |
|
| ||
| Diagnosis | Choroidal Nevus | 29 |
| Pigmented | 26 | |
| Amelonotic | 2 | |
| Mixed | 1 | |
|
| ||
| Choroidal Melanoma | 2 | |
|
| ||
| CHRPE | 1 | |
|
| ||
| Choroidal Hemangioma | 1 | |
SD=standard deviation, CHRPE=Congenital hypertrophy of retinal pigment epithelium
In 97% of eyes, MCI was able to delineate the border of lesions in all patients, specifically; in 24.2% of eyes, borders were graded as barely visible; in 72.7% of eyes, border was clearly visible (table 2). Though the percentage of eyes that had a clearly visible border was higher in CFP (90.9% versus 72.7%); overall, there was no significant difference in the mean border visibility score between the two imaging modalities (1.69±0.52 in MCI, 1.8±0.4 in CFP, p=0.12).
Table 2.
The distribution of visibility of lesion characteristics using multicolor imaging and color fundus photography
| Color Fundus Photography | Total | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Non Visible |
Barely Visible |
Clearly Visible |
|||||
|
|
|||||||
| Detection of Lesion Border | MCI | Non | n | 0 | 0 | 1 | 1 |
| Visible | % | 0.0% | 0.0% | 3.0% | 3.0% | ||
|
| |||||||
| Barely | n | 0 | 2 | 6 | 8 | ||
| Visible | % | 0.0% | 6.1% | 18.2% | 24.2% | ||
|
| |||||||
| Clearly | n | 1 | 0 | 23 | 24 | ||
| Visible | % | 3.0% | 0.0% | 69.7% | 72.7% | ||
|
|
|||||||
| Total | n | 1 | 2 | 30 | 33 | ||
| % | 3.0% | 6.1% | 90.9% | 100% | |||
|
|
|||||||
| Detection of Halo | MCI | Non | n | 25 | 0 | 0 | 25 |
| Visible | % | 75.8% | 0.0% | 0.0% | 75.8% | ||
|
| |||||||
| Barely | n | 0 | 1 | 0 | 1 | ||
| Visible | % | 0.0% | 3.0% | 0.0% | 3.0% | ||
|
| |||||||
| Clearly | n | 2 | 1 | 4 | 7 | ||
| Visible | % | 6.1% | 3.0% | 12.1% | 21.2% | ||
|
|
|||||||
| Detection of Drusen | Total | n | 27 | 2 | 4 | 33 | |
| % | 81.8% | 6.1% | 12.1% | 100% | |||
|
| |||||||
| MCI | Non | n | 16 | 1 | 0 | 17 | |
| Visible | % | 48.5% | 3.0% | 0.0% | 51.5% | ||
|
| |||||||
| Barely | n | 0 | 0 | 2 | 2 | ||
| Visible | % | 0.0% | 0.0% | 6.1% | 6.1% | ||
|
| |||||||
| Clearly | n | 1 | 0 | 13 | 14 | ||
| Visible | % | 3.0% | 0.0% | 39.4% | 42.4% | ||
|
| |||||||
| Total | n | 17 | 1 | 15 | 33 | ||
| % | 51.5% | 3.0% | 45.5% | 100% | |||
The average score for the visibility of drusen was similar between MCI and CFP (0.93±0.97 in MCI, 0.90±0.99 in CFP, p=0.70). There was no significant difference in the mean visibility score for the drusen and halo between the two imaging modalities (p=0.70, p=0.35). Specifically, the percentage of eyes with clearly visible drusen was similar on MCI (42.4%) and CFP (45.5%).
The mean area of the lesions was 18.7±3.4 (ranging, 0–86.26) mm2 on CFP, and 14.9±3.3 mm2 (ranging, 0.59–76.81) on MCI; there was a significant difference in the mean area of lesions obtained using the two imaging modalities (p=0.01).
When looking at the individual color channels (figure 1), IR-reflectance provided more information regarding the visibility of border, halo and drusen compared to blue-reflectance and green-reflectance (table 3). In addition, the area of the choroidal lesions by IR-reflectance was comparable to MCI and CFP; the extent of the choroidal lesion (area) was found significantly smaller in IR-reflectance images (14.9±3.3 mm2) compared to that on CFP (18.7±3.4 mm2) (p=0.01) (figure 2). There was also a significant difference in the mean size of the lesions between IR reflectance and CFP (p=0.009). In 78.7% of eyes (26 eyes), blue-reflectance could not detect the lesion border, and in 75.7% of eyes (25 eyes), the borders of the lesions were not visible on green-reflectance.
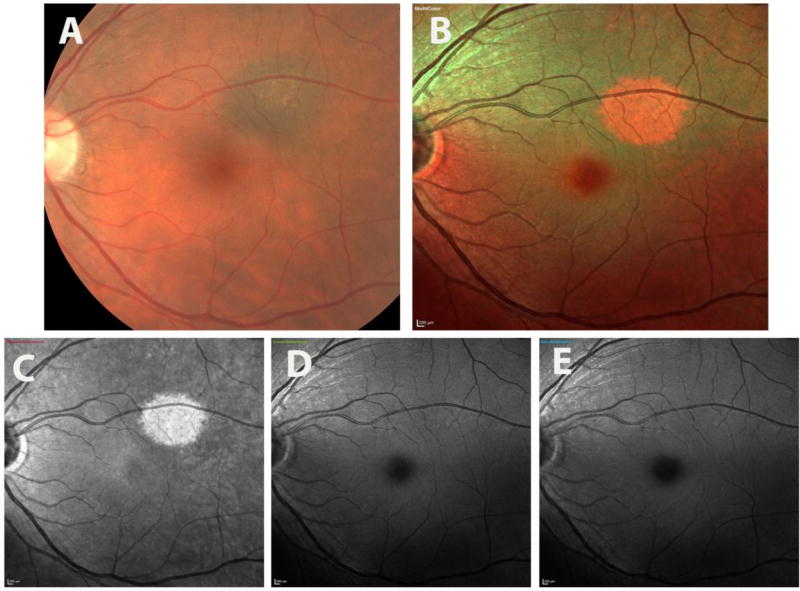
Figure 1.
illustrates the multicolor images and color fundus photography images of a patient with pigmented choroidal nevus in left eye. The nevus is visible on CFP (a), MCI (b), and on IR-reflectance (c) images, but not on green-reflectance (d) or blue reflectance (e). Note the difference in color rendition of nevus, which appears as dark brown on CFP (a), red and smaller in MCI (b).
Table 3.
The average visibility score of the lesion characteristics in individual color channels on multicolor imaging
| Drusen | Halo | Border | Size, mm2 | |
|---|---|---|---|---|
| IR-reflectance | 0.78±0.16 | 0.42±0.14 | 1.6±0.09 | 14.9±.3 |
| Blue-reflectance | 0.48±0.15 | 0.03±0.03 | 0.27±0.09 | 2.2±1.5 |
| Green-reflectance | 0.54±0.15 | 0.03±0.03 | 0.03±0.1 | 3.2±1.7 |
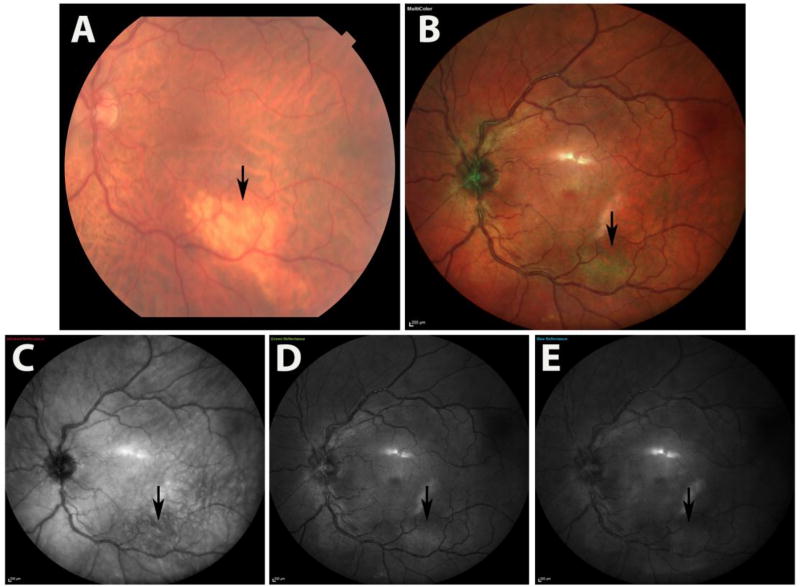
Figure 2.
illustrates the multicolor images and color fundus photography images of a patient with amelanotic choroidal nevus in left eye. The nevus is visible on CFP (a) and MCI (b), hypo reflective on IR-reflectance (c) images, but hyper reflective on green-reflectance (d) normal reflectivity on blue reflectance (e).
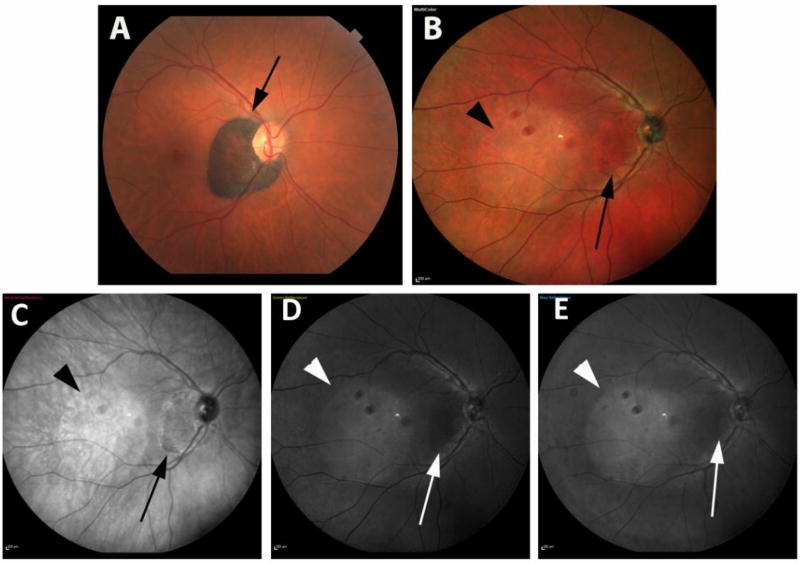
Figure 3 shows the paired images of a patient with CHRPE. Figure 4 shows a patient with choroidal melanoma.
Figure 3.
illustrates the multicolor images and color fundus photography images of a patient with congenital hypertrophy of RPE in right eye. CHRPE (Congenital hypertrophy of retinal pigment epithelium) appears dark brown on CFP (a) and red in MCI (b). Lesion has a hyperreflective border on infrared image (c) and is seen in black color without a prominent hyperreflective border (d, e). Note the artifacts associated with wide-field lens and tear film instability.
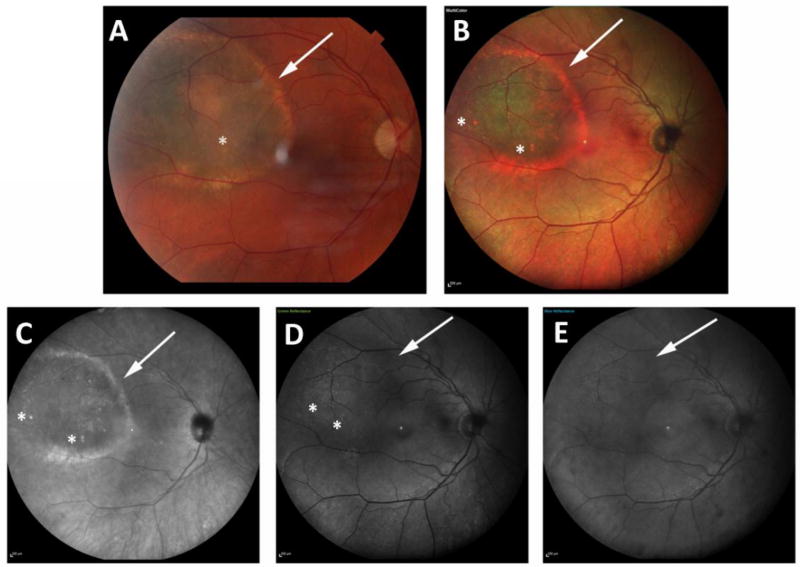
Figure 4.
Panel shows the color and multicolor images of a 67-year-old female patient with a choroidal melanoma. In color photography (a) and combined pseudocolor image (b), and infrared-reflectance image (c), visibility of lesion’s border, drusen and halo are all graded as clearly visible (score=2 for each feature). However, in green and blue-reflectance images (c, d), border and halo of the lesion are barely visible (graded as 1). Though green-reflectance can visualize drusen as clearly visible (graded as 2), in blue-reflectance image (e), the visibility of drusen is scored as 0 (non- visible).
DISCUSSION
Standard fundus photography remains certainly the most common imaging modality to document what can be seen by the ophthalmologist clinically. However, it may be time-consuming when the patient needs to undergo multiple images such as FA and OCT. Multicolor imaging is a new imaging method of the retina which can be performed nearly simultaneously with SD-OCT and/or FA using the Spectralis device.
In this the study, we compared the visualization of retinal and/ or choroidal lesions using the two different imaging modalities. We found that MCI is not inferior than CFP for the detection of lesion’s border, drusen and halo. However, the area of the choroidal or pigmented or non-pigmented retinal lesion on MCI is consistently smaller than CFP. We speculate that as the lesion gets thinner towards the periphery, the ability of SLO to detect the thin edge of masses decreases. Despite the ability of MCI on providing similar information on some features of the lesions, tendency to delineate the lesion borders smaller may not allow this imaging modality to be used as a first option to document the pigmentary lesions, particularly choroidal nevus in which the growth rate is important for the malign transformation.
Based on our observations, amelanotic lesions appear as tan/white on CFP, in contrary to a green appearance on MCI. In multicolor imaging, the green channel shows high reflectance, the blue channel shows normal reflectance while the infrared channel shows very low reflectance caused by the absence of melanin pigment. By combining the three channels with the assigned colors, the pseudo color image of the lesion was green due to the low IR channel reflectivity that emphasized green, the inverse color of red. This observation is similar to MC images of normal retina where choroidal vessels appear green. Again, the IR channel shows choroidal vessels as dark due to absorption of IR light by the blood vessels. The green and blue channel does not penetrate beyond the RPE and shows a bright reflectance from the RPE. Thus, the lack of red channel information from the IR image will make the choroidal blood vessels appear green. The discrepancies in the appearance of images can be explained by the above-mentioned hypothesis.
In pigmented lesions the CFP shows the lesions dark brown, in MCI the green and blue channels show low reflectivity while infrared channel shows high reflectivity due to the higher reflectivity of melanin in IR light [10]. By combining the three channels the pseudo color image shows the pigmented lesion as red because the IR channel is assigned the red color.
There have been limited reports on the utility of scanning laser ophthalmoscopy in patients with various retinal pathologies [11–14]. Previously, our group reported that MCI provided better delineation of epiretinal membranes compared to color fundus photography, particularly due to having green individual channel.11 To the best of our knowledge, our study is the first to discuss the optical principles of MCI that cause discrepancies on the features of pigmented retinal and choroidal lesions. In a study by Zapta et al. [14], exploring the utility of fundus autofluorescence and red-reflectance imaging using ultra-wide-field scanning laser ophthalmoscopy in choroidal nevi, red reflectance by Optos (Optos, Dunfermline, Scotland, UK) was found more sensitive than CFP for follow-up of choroidal nevi. In a series by Reznicek et al. [13], the standard autofluorescence technique provided subtle information on choroidal masses due to producing faint or little detectable autofluorescence, whereas RPE alterations produced more autofluorescence. Different from these studies, we evaluated whether MCI can be used to provide same information as conventional color photography did.
There are some limitations of the study that should be addressed. First, images taken using conventional color photography look very different from MCI images. Despite in an attempt to increase the objectivity of the grading method used, it was not possible to mask the observers to which modality they were grading. On the other hand, since the size of the choroidal lesion is the most important clinical factor related to prognosis and is a risk factor for the transformation of a choroidal nevus in to a choroidal melanoma, it is not clear if the MCI can be used to monitor the changes in the size of the choroidal nevus in the long-term follow-up. Thus, longitudinal studies with a higher number of patients would warranted to show whether MCI can be used as an imaging technique to follow the patients with choroidal melanocytic lesions. Also, since we have a heterogonous study population with varying degree of retina and choroid thicknesses, the appearance of lesions may vary depending on the lesion thickness. On the other hand, though ultrasonography and OCT images can be used to assess the features of the choroidal mass, we do not provide any information on this due to goal of comparing only MCI and CFP. Lastly, despite the ability of MCI to visualize choroidal and retinal lesions, the information obtained by MCI may be limited for the peripheral retina lesions, due to limited field of view.
In conclusion, MCI was found a useful tool to detect the characteristics of the choroidal and retinal pigmentary lesions including border, halo and drusen. However, this modality underestimated the extent of the choroidal lesion by 33%. When looking at the individual cSLO images obtained using different wavelengths, infrared-reflectance with an 815 nm wavelength provided more information than blue or green reflectance due to ability to penetrate deeper retinal layers. Also, clinicians should be aware of some differences on the appearance of lesions by MCI that requires some experience to interpret the lesion characteristics.
Acknowledgments
Funding: This work was supported in part by an unrestricted grant from Supported in part by NIH grant R01 EY016323-09A1 (D.U.B.) and a core grant from the National Eye Institute P30 EY022589, an unrestricted grant from Research to Prevent Blindness, NY (WRF). The funding organizations had no role in the design or conduct of this research.
Footnotes
Conflict of Interest: All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Sumich P, Mitchell P, Wang JJ. Choroidal nevi in a white population: The Blue Mountains Eye Study. Arch Ophthalmol. 1998;116:645–650. doi: 10.1001/archopht.116.5.645. [DOI] [PubMed] [Google Scholar]
- 2.Shields CL, Furuta M, Mashayekhi A, et al. Clinical spectrum of choroidal nevi based on age at presentation in 3422 consecutive eyes. Ophthalmology. 2008;115:546–552. doi: 10.1016/j.ophtha.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Singh AD, Kalyani P, Topham A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology. 2005;112:1784–1789. doi: 10.1016/j.ophtha.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Shields CL, Kaliki S, Rojanaporn D, et al. Enhanced depth imaging OCT of small choroidal melanoma: comparison with choroidal nevus. Arch Ophthalmol. 2012;130:850–856. doi: 10.1001/archophthalmol.2012.1135. [DOI] [PubMed] [Google Scholar]
- 5.Almeida A, Kaliki S, Shields CL. Autofluorescence of intraocular tumours. Curr Opin Ophthalmology. 2013;24:222–232. doi: 10.1097/ICU.0b013e32835f8ba1. [DOI] [PubMed] [Google Scholar]
- 6.Shields CL, Bianciotto C, Pirondini C, et al. Autofluorescence of choroidal melanoma in 51 cases. Br J Ophthalmol. 2008;92:617–622. doi: 10.1136/bjo.2007.130286. [DOI] [PubMed] [Google Scholar]
- 7.Ben Moussa N, Georges A, Capuano V, et al. MultiColor imaging in the evaluation of geographic atrophy due to age-related macular degeneration. Br J Ophthalmol. 2015;99:842–847. doi: 10.1136/bjophthalmol-2014-305643. [DOI] [PubMed] [Google Scholar]
- 8.Yu S, Bellone D, Lee SE, Yannuzzi LA. Multimodal imaging in foveal red spot syndrome. Retin Cases Brief Rep. 2015;9:97–101. doi: 10.1097/ICB.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 9.Shields CL, Maktabi AM, Jahnle E, Mashayekhi A, Lally SE, Shields JA. Halo nevus of the choroid in 150 cases: the 2010 Henry van Dyke Lecture. Arch Ophthalmol. 2010;128:859–864. doi: 10.1001/archophthalmol.2010.132. [DOI] [PubMed] [Google Scholar]
- 10.Van de Kraats J, Berendschot TT, Valen S, van Norren D. Fast assessment of the central macular pigment density with natural pupil using the macular pigment reflectometer. J Biomed Opt. 2006;11:064031. doi: 10.1117/1.2398925. [DOI] [PubMed] [Google Scholar]
- 11.Kilic Muftuoglu I, Bartsch DU, Barteselli G, et al. Visualization of macular pucker by multicolor scanning laser imaging. Retina. 2017 Feb 1; doi: 10.1097/IAE.0000000000001525. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan AC, Fleckenstein M, Schmitz-Valckenberg S, Holz FG. Clinical Application of Multicolor Imaging Technology. Ophthalmologica. 2016;236:8–18. doi: 10.1159/000446857. [DOI] [PubMed] [Google Scholar]
- 13.Reznicek L, Stumpf C, Seidensticker F, et al. Role of wide-field autofluorescence imaging and scanning laser ophthalmoscopy in differentiation of choroidal pigmented lesions. Int J Ophthalmol. 2014;7:697–703. doi: 10.3980/j.issn.2222-3959.2014.04.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zapta MA, Leila M, Teixidor T, Garcia-Arumi J. Compartive study between fundus autofluorescence and red reflectance imaging of choroidal naevi using ultra wide field scanning laser ophthalmoscopy. Retina. 2015;35:1202–1204. doi: 10.1097/IAE.0000000000000463. [DOI] [PubMed] [Google Scholar]