Abstract
STUDY QUESTION
Do environmental exposures, diet and lifestyle factors impact reproductive and pregnancy outcomes among subfertile couples attending a fertility clinic?
SUMMARY ANSWER
Environmental chemicals exposure in men and women were associated with reduced fertility and a higher risk of adverse outcomes, whereas some dietary factors improved the probability of successful reproductive outcomes.
WHAT IS KNOWN ALREADY
Accumulating epidemiologic evidence has shown associations of environmental chemicals and nutritional factors with reproductive and pregnancy outcomes. However, few studies have been designed to assess these factors simultaneously, and even fewer have collected such data among both men and women in the preconception period. Furthermore, early and sensitive reproductive endpoints (e.g. fertilization, implantation, biochemical pregnancy loss) are largely unobservable in population-based designs.
STUDY DESIGN SIZE, DURATION
The Environment and Reproductive Health (EARTH) Study is an ongoing prospective preconception cohort designed to investigate the impact of environmental, nutritional and lifestyle factors in both women and men on fertility and pregnancy outcomes. The study has been ongoing since 2004 and has recruited 799 women and 487 men (447 couples; 40 men joined without female partners) as of June 2017.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The study recruits women aged 18–45 years and men aged 18–55 years seeking fertility evaluation and treatment at a large academic hospital fertility center. Women and men are eligible to join either independently or as a couple. Participants are followed from study entry throughout each fertility treatment cycle, once per trimester of pregnancy (for those achieving pregnancy), and up to labor and delivery, or until they discontinue treatment or withdraw from the study. The study prospectively collects a combination of biological samples (e.g. blood, urine, semen), self-reported questionnaire data (including a validated food frequency questionnaire) and medical information abstracted from fertility clinic and hospital records.
MAIN RESULTS AND THE ROLE OF CHANCE
Among women in this cohort, higher urinary concentrations of some phthalate metabolites were associated with reduced oocyte yields, lower likelihood of clinical pregnancy, increased risk of pregnancy loss and lower likelihood of live birth following infertility treatment. Certain urinary phthalate metabolite concentrations among men was also associated with decreased odds of implantation and live birth. Maternal soy and folate intake significantly modified the association between bisphenol A (BPA) and IVF outcomes in women. While the EARTH Study has tested many a priori hypotheses, multiple comparisons were undertaken, and we cannot rule out the possibility that some of findings may be spurious or due to chance.
LIMITATIONS REASONS FOR CAUTION
While the fertility clinic setting provides the opportunity to measure environmental exposures, diet and lifestyle factors across different windows of vulnerability and to evaluate their potential effect on critical early fertility, pregnancy and delivery outcomes, the findings may be less generalizable to naturally conceived pregnancies.
WIDER IMPLICATIONS OF THE FINDINGS
The EARTH Study is one of the few cohorts designed to examine multiple windows of vulnerability, including the paternal and maternal preconception windows and the periconception and prenatal windows, in pregnancy. It is also one of the few human studies that has assessed potential interactions between environmental exposures and dietary factors.
STUDY FUNDING/COMPETING INTEREST(S)
The EARTH Study has been funded by the National Institute of Environmental Health Sciences since its inception in 2004. The authors declare no competing interests.
TRIAL REGISTRATION NUMBER
n/a.
Keywords: prospective, preconception, cohort, infertility, environmental exposures, diet, pregnancy, male and female reproduction
Introduction
Accumulating epidemiologic evidence over the last several decades has shown associations of environmental chemicals with adverse reproductive health outcomes, including male and female infertility, poor pregnancy outcomes and increased risk of diseases in childhood and beyond (Woodruff et al., 2008; Bergman et al., 2012). Nutritional factors also impact reproductive health both directly and by modifying the potential effects of some environmental chemicals on these same endpoints (Sharpe and Franks, 2002; Homan et al., 2007). Most studies to date have been designed to examine environmental or nutritional factors during pregnancy on fetal and infant health but few studies have simultaneously assessed environmental and nutritional exposures, and even fewer have included assessments during the preconception period. Experimental animal studies and limited human studies have shown that the sensitive window of exposure for fetal and infant health includes the preconception period in both women and men (Chapin et al., 2004; Braun et al., 2017; Louis et al., 2008). Investigating the maternal and paternal preconception period is challenging in most observational studies and requires a design that identifies and recruits women and men attempting pregnancy to be followed until conception and onward (Buck Louis et al., 2011). Furthermore, early and sensitive reproductive endpoints of interest (e.g. ovarian follicle growth, fertilization, implantation, biochemical pregnancy loss) in relation to diet and environmental chemical exposures are largely unobservable in population-based designs.
What does this mean for patients?
This article reports on a cohort known as the Environment and Reproductive Health (EARTH) Study, which looks at the impact of environment, diet and lifestyle factors on human reproduction. Previous research has found links between environmental chemicals and fertility and miscarriage, but this study was wider as it looked at the effects of exposures in both men and women in the preconception period, as well diet and lifestyle and followed the participants through each stage of their fertility journey.
The researchers looked at couples that had come to a large academic fertility center, and used questionnaires and samples such as blood, urine and semen to analyse what impact different exposures had on the chances of a successful pregnancy.
The study found that couples with certain environmental chemicals in their samples had a lower chance of a successful outcome from treatment, but a healthy diet had a positive impact on the chances of success for both men and women. Caffeine did not make a difference for women, but it did for men. Women doing jobs that involved heavy lifting were found to have a lower chance of success. Eating fruit and vegetables with high pesticide residue was linked to lower fertility.
The researchers suggest that since these couples were experiencing fertility problems they may be more sensitive to the factors examined in this study, but findings may still be of interest to others having difficulty conceiving.
In an effort to address these challenges, we established the Environment and Reproductive Health (EARTH) Study, an ongoing prospective preconception cohort of couples seeking care at the Massachusetts General Hospital (MGH) Fertility Center, Boston, USA, to investigate environmental, nutritional and lifestyle factors among both women and men in relation to fertility and pregnancy outcomes. The EARTH Study was designed to examine multiple potentially relevant periods of vulnerability, including the paternal and maternal preconception windows as well as the periconception and prenatal windows in pregnancy. The study has been funded by the National Institute of Environmental Health Sciences since its inception in 2004. A comprehensive assessment of diet was added in 2007. Future goals include following the children of the couples, as well as the mothers and fathers who enrolled in the EARTH Study.
Materials and Methods
Participant eligibility and recruitment
The EARTH Study recruits women and men seeking fertility evaluation and medically assisted reproductive treatment at the Massachusetts General Hospital (MGH) Fertility Center. Women aged 18–45 years, and men 18–55 years who have not had a vasectomy and who are not taking hormones at the time of enrollment, are eligible to join either independently or as a couple. The study has strong support and collaboration from physicians and other medical personnel from the MGH Fertility Center who identify potentially eligible patients in their practice and briefly inform them of the study at any point during their care, including at the start of their fertility investigation or after initiating treatment. A study staff member then approaches potential participants and further determines their eligibility and interest. The study staff provides each potential participant with complete information about the requirements and expectations of enrolling in the EARTH Study and answers questions. All participants agreeing to join in the study provide written informed consent. The study was approved by the Institutional Review Boards of MGH (Partners), Harvard T.H. Chan School of Public Health and the Centers for Disease Control and Prevention (CDC).
Design and follow-up
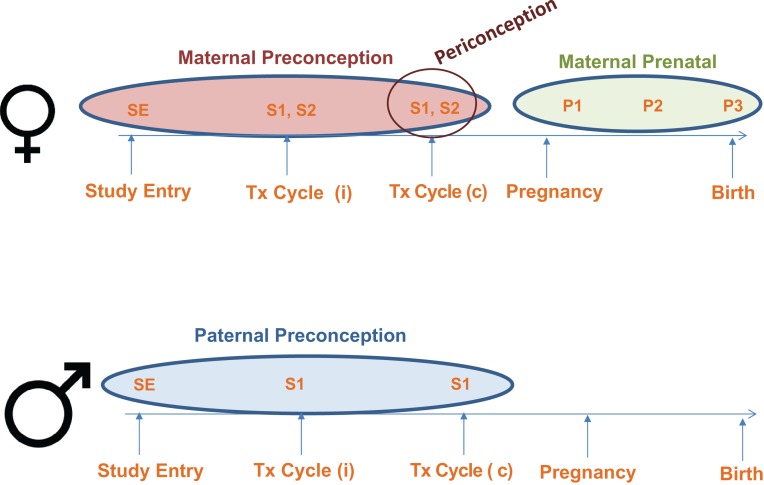
All participants enrolling in the EARTH Study are scheduled for a detailed entry visit with a study staff member. During this first visit, female and male participants complete a series of baseline questionnaires, undergo anthropometric measurements, and provide a spot urine and blood sample. They are also given a comprehensive self-reported questionnaire (take-home or online) (Fig. 1). Couples trying to conceive using medically assisted reproduction undergo different types of treatment, including IVF-based technologies (i.e. fresh or frozen IVF protocols, including ICSI) and non-IVF based treatments (i.e. IUI, ovulation induction and ovarian stimulation). Both IVF and non-IVF based treatments require careful and detailed cycle follow-up at the clinic. During the monitoring phase of the treatment cycle (approximate follicular Days 3–9), women provide a single spot urine sample and non-fasting blood sample, and at the same time complete a questionnaire regarding personal care product (PCP) use in the past 24 h. Following the monitoring phase, on the clinic visit day of the scheduled fertility procedure [i.e. on day of oocyte-retrieval (for fresh IVF protocols) or embryo transfer (for frozen IVF protocols) or on day of IUI procedure (for non-IVF based cycles)], women complete another PCP use questionnaire and provide an additional spot urine sample (Fig. 1). For women undergoing oocyte retrieval, a follicular fluid sample is also collected. All women are followed to determine pregnancy status after each individual treatment cycle, which includes a routine β-hCG blood test on Days 12–17 following the IVF or IUI procedure day. Women achieving a positive pregnancy test undergo an ultrasound scan at approximately gestational week 6 for clinical confirmation of an intrauterine pregnancy and are followed throughout the prenatal period. Pregnant participants provide a spot urine and non-fasting blood sample and complete a PCP use questionnaire once per trimester at ~6, 24 and 33 weeks gestation (Fig. 1).
Figure 1.
Maternal and Paternal Assessment in the Environment and Reproductive Health (EARTH) Study. Female participants: Study entry (SE) assessment includes: baseline urine and blood samples, and completion of the baseline and full questionnaires (includes the Food Frequency Questionnaire). Treatment (Tx) cycle (i) denotes any number of followed cycles including those treated with IVF-based technologies or non-IVF based procedures. Assessment at two points in time during each Tx cycle: S1—includes the first spot urine sample and blood sample collected during the follicular phase of the cycle (Days 3–9) and the completion of the Product Use Questionnaire at the same point in time. S2—includes the second spot urine sample collected at the time of scheduled treatment procedure (oocyte retrieval, embryo transfer or IUI) and a follicular fluid sample collected during oocyte retrievals. All SE, S1 and S2 samples represent exposure in the maternal preconception period. Tx (c) denotes the index cycle of conception. Clinical information about the mode of conception (IVF-based, non-IVF based or non-medically assisted) is abstracted from electronic medical records by trained study staff. S1 and S2 samples collected in the index conception represent exposure in the maternal periconception period. P1/P2/P3—includes a single urine sample and blood sample and Produce Use Questionnaires collected in the first, second and third trimester of pregnancy, respectively. P1, P2 and P3 samples collected following the index conception represent the maternal prenatal exposure period. Male participants: SE assessment includes: baseline urine and blood samples, and completion of the Baseline and Full Questionnaires (includes the Food Frequency Questionnaire). Men also provide a semen sample and an abstinence time questionnaire at baseline if their study entry visit coincides with a routine semen sample collection. Assessment at Tx cycle: S1 includes a spot urine sample, blood sample and semen sample along with the abstinence time questionnaire on the day their female partner undergoes their scheduled fertility treatment procedure. SE and S1 samples collected up to the index conception represent the paternal preconception exposure period.
In addition to baseline questionnaires, anthropometric measurements, and blood and urine specimens, men provide a semen sample and complete an abstinence time questionnaire at enrollment if their study entry visit coincides with a routine semen sample collection. On the day their female partner undergoes their scheduled fertility treatment procedure, male participants provide another spot urine sample, non-fasting blood sample and semen sample along with the abstinence time questionnaire (Fig. 1). For men participating without their female partner, we obtain consent to release the birth and newborn nursery records from the delivering hospital.
Data and biospecimen collection
The EARTH Study prospectively collects a combination of biological samples, self-reported questionnaire data and medical information abstracted from the fertility clinic and delivery records (Table I).
Table I.
Summary of measurements collected in women (X) and men (Y) in the Environment and Reproductive Health (EARTH) Study.
| Measurement category | Measurement or sample | Study visits | |||||
|---|---|---|---|---|---|---|---|
| Entry | Per cycle | Per pregnancy | |||||
| Visit | Visit 1 | Visit 2 | Visit 1 | Visit 2 | Visit 3 | ||
| Biological Samples | |||||||
| Urine | X, Y | X | X, Y | X | X | X | |
| Blood | X, Y | X, Y | X | X | X | ||
| Serum | X, Y | X, Y | X | X | X | ||
| Blood clot | X, Y | X, Y | X | X | X | ||
| Whole blood | X, Y | X, Y | X | X | X | ||
| Follicular fluid | X | ||||||
| Supernatant | |||||||
| Cell pellet | |||||||
| Semen | Y | Y | |||||
| Hair | X | ||||||
| Children’s teeth | |||||||
| Questionnaires | |||||||
| Demographics | X, Y | ||||||
| Medical history | X, Y | ||||||
| Reproductive history | X, Y | ||||||
| Occupation history | X, Y | ||||||
| Lifestyle | X, Y | ||||||
| Diet/food frequency | X, Y | ||||||
| Personal care product use | X, Y | X | X | ||||
| Male abstinence | Y | Y | |||||
| Data Abstraction | |||||||
| Fertility clinic records (infertility diagnosis) | X, Y | ||||||
| Fertility records (ART medications) | X | ||||||
| Fertility clinic (ART/IUI outcomes) | X | ||||||
| Pregnancy records (prenatal follow-up data) | X | X | X | ||||
| Labor/delivery records (maternal and infant delivery outcomes) | X | ||||||
| Anthropometry | |||||||
| Weight | X, Y | X | |||||
| Height | X, Y | ||||||
| Environmental Samples | |||||||
| Dust | X, Y | ||||||
Biological samples
The EARTH Study was designed to examine exposures across several windows: paternal and maternal preconception windows, and maternal periconception and prenatal windows. We obtain prospective repeated urine and blood samples at several times during these periods (Fig. 1). There is also an optional voluntary hair sample collection. All samples were collected using methods to minimize exogenous contamination by known environmental chemicals (Calafat et al., 2015). To date, we have collected 32 792 and 8967 urine aliquots, and 8156 and 3875 blood aliquots from women and men, respectively. These have been archived and stored at the Harvard T.H. Chan School of Public Health, Boston, MA, USA. The CDC has quantified urinary biomarkers of > 40 chemicals, including phthalates and di-isononyl cyclohexane-1,2-dicarboxylate metabolites, phenols (e.g. bisphenol A, triclosan, parabens), and pesticides (metabolites of organophosphates, pyrethroids, 2,4-dichlorophenoxyacetic acid and N,N-diethyl-m-toluamide). Organophosphate flame-retardants and polybrominated diphenyl ethers were measured at Duke University, Durham, NC, USA.
In whole blood, we have quantified heavy metals and metalloids (e.g. lead, cadmium, manganese) at the Mount Sinai School of Medicine, New York, NY, USA, in a subgroup of 150 women. We have measured serum folate, vitamin B12, fatty acids and vitamin D concentrations among 100 women. Among 558 women, we have also analyzed serum for thyroid hormones (thyroid-stimulating hormone, free thyroxine 4 (T4), T4, free tri-iodothyronine (T3), T3, thyroglobulin, and thyroperoxidase antibodies). To date, we have quantified mercury in more than 1200 hair samples. We have also analyzed more than 1200 semen samples for standard semen quality parameters. From participants undergoing oocyte retrieval, we have stored 6041 follicular fluid aliquots and we have to date analyzed 147 of them from 143 women for phthalate metabolites and phenols. In small pilot studies, we have measured non-coding micro-RNAs in semen, and obtained and archived amniotic fluid samples.
Self-reported questionnaires
Both female and male participants complete the Baseline Questionnaire (BQ), which includes demographic, medical history and lifestyle questions (Table I). They also complete the self-reported Full Questionnaire (FQ) with information on family, medical and reproductive history, occupational history and lifestyle (e.g. physical-activity, frequency of tobacco, alcohol and illicit substance use) and the Food Frequency Questionnaire (FFQ). Overall, 95% of women (n = 759/799) and 99% of men (n = 484/487) completed the BQ; 91% of women (n = 729/799) and 77% of men (n = 376/487) completed the FQ. The Product Use Questionnaire is administered at baseline and once per treatment cycle to identify recent exposure to, and time since last use of, common products including lotions, soaps, cleaning products, plastics, pesticides, smoking and secondhand tobacco smoke exposure, specific foods, weight loss/weight gain products, and over-the-counter and prescription pharmaceuticals.
Diet assessment
Diet is assessed using a previously validated self-administered FFQ (Rimm et al., 1992; Yuan et al., 2017). Participants are asked to report how often, on average, they consumed specified amounts of the 131 foods, beverages and supplements listed in the questionnaire over the past year with nine possible response categories ranging from never/almost never to ≥6 times per day. Open-ended questions are used for usual brand and type of margarine, cooking oil, cold breakfast cereal and multivitamins. Intakes for over 100 nutrients and non-nutritive food constituents are estimated by linking participant responses to a custom nutrient composition database maintained and updated by the Department of Nutrition, Harvard T. H. Chan School of Public Health.
Other environmental and biological samples
We have collected 240 home dust samples and 120 primary teeth from children of EARTH Study participants. For a small subset of volunteers (118 women and 52 men) we also measured personal electromagnetic fields using a portable magnetic field monitor. Recently, using couples’ self-reported residential addresses at study entry, we estimated distance to a major roadway, near-residence traffic density and particulate matter <2.5 µm, Black Carbon, NO2, CO and SO2 concentrations during each fertility treatment cycle.
Electronic medical record abstraction—cycle, pregnancy and delivery data
We have an extensive clinical abstraction process to obtain prospective data during each individual fertility treatment cycle and throughout follow-up (up to the birth of an infant for those achieving pregnancy). Trained study staff abstract pertinent clinical information from the electronic medical records at the MGH to ascertain the outcome of each cycle, including mode of conception, cycle cancellation, oocyte parameters, early embryo development, implantation, biochemical pregnancy (with β-hCG measurements), clinical pregnancy (with ultrasound assessment), physician-assigned infertility diagnosis, polycystic ovary syndrome, terminations, pregnancy complications and pathology, glucose tolerance tests during pregnancy and delivery outcomes (e.g. livebirths, stillbirths, birthweight, gestational age, infant sex, complications and pathologies).
Anthropometry
At study entry, trained study staff measure and record each participant’s height, weight and waist circumference. Additional weight measurements taken during routine prenatal visits are abstracted from electronic medical records.
Child follow-up
Two pilot studies have been conducted on small subsets of children born to EARTH Study participants. In one, we measured anogenital distance in male and female infants at 3–18 months of age. In the second, we assessed behavior in 166 children via parent-completed mailed questionnaires adapted from the Behavior Assessment System for Children (BASC, second edition), Social Responsiveness Scale and Preschool Activity Inventory (Golombok and Rust, 1993; Constantino and Gruber, 2012; Reynolds and Kamphaus, 1998).
Results
Study population
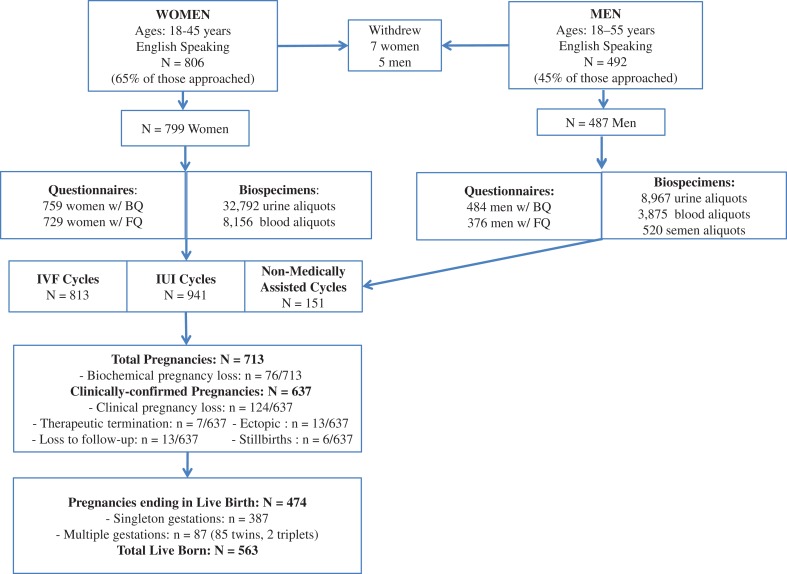
Among patients initially approached by the EARTH Study staff as of June 2017, ~65% (N = 806) of women and 45% of men (n = 492) were eligible and agreed to enroll (Fig. 2). Participants are followed from study entry throughout their fertility care, pregnancy and birth (for those achieving pregnancy), or until they discontinue treatment or withdraw from the study. During the course of follow-up, seven women and five men discontinued treatment or withdrew. As of June 2017, the cohort included 799 women and 487 men (447 couples; 40 men joined without female partners) (Fig. 2). Women in the EARTH Study were on average 34.7 years old with a BMI of 24.6 kg/m2 at time of enrollment (Table II). They are predominately Caucasian (81%), highly educated (49% have a graduate degree), never-smokers (73%) and nulliparous (87%). Approximately one-third of women (36%) have a female factor of infertility as their primary diagnosis. Men were on average 36.6 years old with a BMI of 27.5 kg/m2 at time of enrollment. Most men are Caucasian (86%), highly educated (41% have a graduate degree) never-smokers (67%), and 30% have a male factor as their primary infertility diagnosis (Table II).
Figure 2.
Participant Flow Chart for the EARTH Study. BQ, Baseline Questionnaire; FQ, Full Questionnaire (includes the Food Frequency Questionnaire). Biochemical pregnancy loss is defined as the demise of a β-hCG-confirmed pregnancy that was never visualized on ultrasound. Clinical pregnancy loss is defined as the demise of an ultrasound confirmed intrauterine pregnancy up to 20 weeks gestation. IVF cycles include fresh and frozen IVF-based protocols. IUI cycles include all non-IVF based procedures such as IUI, ovulation induction and ovarian stimulation. Non-medically assisted cycles are those that were conceived naturally without treatment.
Table II.
Characteristics of 799 women and 487 men (447 couples) participating in the EARTH Study from 2004 to 2017.
| Characteristic | Women N = 799 | Men N = 487 |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 34.7 (4.5) | 36.6 (5.4) |
| Age > 35, n (%) | 345 (43) | 273 (56) |
| Race, n (%) | ||
| White | 651 (81) | 419 (86) |
| Black | 39 (5) | 15 (3) |
| Asian | 71 (9) | 34 (7) |
| Other | 38 (5) | 19 (4) |
| BMI (kg/m2) | ||
| Mean (SD) | 24.6 (4.9) | 27.5 (4.5) |
| BMI > 25, n (%) | 283 (35) | 346 (71) |
| Education, n (%) | ||
| <College | 60 (8) | 55 (11) |
| College graduate | 231 (29) | 136 (28) |
| Graduate degree | 392 (49) | 198 (41) |
| Missing | 116 (14) | 98 (20) |
| Smoking status, n (%) | ||
| Never | 583 (73) | 327 (67) |
| Former | 192 (24) | 131 (27) |
| Current | 24 (3) | 29 (6) |
| Primary infertility diagnosis, n (%) | ||
| Male factor | 196 (24) | 146 (30) |
| Female factor | 285 (36) | 166 (34) |
| Diminished ovarian reserve | 90/285 | |
| Ovulation disorders | 106/285 | |
| Endometriosis | 36/285 | |
| Uterine disorders | 11/285 | |
| Tubal factor | 42/285 | |
| Unexplained | 318 (40) | 175 (36) |
| Nulliparous at study entry, n (%) | ||
| 698 (87) | – | |
| Live births, n (%) | ||
| Singletons, n (%) | 387/563 (69) | |
| Multiples, n (%) | 176/563 (31) | |
Cycle endpoints
Participants have been followed for a total of 813 IVF-based treatment cycles, 941 non-IVF based treatment cycles and 151 non-medically assisted/naturally conceived cycles during follow-up in the EARTH Study. These 1905 initiated cycles resulted in 713 pregnancies of which 11% (n = 76/713) were only chemically detected by a β-hCG blood test and not clinically visualized on ultrasound (biochemical losses). Among the remaining 637 ultrasound-confirmed pregnancies, 19% ended in a spontaneous loss before 20 weeks gestation, 1% ended in a therapeutic abortion, 2% in ectopic loss, 1% ended in stillbirth (loss on or after 20 weeks) or were lost to follow-up during pregnancy (2%) (Fig. 2). There have been 474 successful pregnancies resulting in 563 live births: 387 singletons and 176 multiples (85 pairs of twins, two sets of triplets). Among these births, 47 females and 17 males were recurrent participants who returned for further treatment and delivered (or their female partner delivered) one singleton and 46 twins. The overall live birth rate per initiated cycle is 26% (n = 487/1905) and the live birth rate among cycles achieving pregnancy is 68% (n = 487/713). Among IVF only cycles, the live birth rate per initiated cycle is 37% (n = 299/813) and the live birth rate among cycles achieving pregnancy is 80% (n = 299/375).
Key findings
A summary of key environmental chemical, dietary and lifestyle factor findings can be found in Table III.
Table III.
Key findings in the EARTH Study.
| Studies on endocrine disrupting chemicals | |||
|---|---|---|---|
| Study participant | EDC | Key finding | Reference |
| Women undergoing ART | DEHP | Decreased oocyte yield | Hauser et al. (2016) |
| Women undergoing ART | DEHP | Decreased probability of clinical pregnancy | Hauser et al. (2016) |
| Women undergoing ART | DEHP | Decreased probability of live birth | Hauser et al. (2016) |
| Women conceiving with ART or non-ART | DEHP | Increased pregnancy loss | Messerlian et al. (2016b) |
| Men with female partner undergoing ART | DOP and DiNP | Decreased odds of implantation | Dodge et al. (2015) |
| Men with female partner undergoing ART | DOP and DiNP | Decreased odds of live birth | Dodge et al. (2015) |
| Women undergoing ART | BPA (modification by soy) | Among women not consuming soy, BPA associated with decreased probability of implantation, clinical pregnancy and live birth | Minguez-Alarcon et al. (2016) |
| Women undergoing ART | BPA (modification by folate) | Among women consuming <400 μg food folate/day, BPA associated with decreased probability of implantation, clinical pregnancy and live birth | Chavarro et al. (2016) |
| Female EARTH Study participants | DEHP | Decreased number of antral follicles measured on Day 3 of an unstimulated cycle | Messerlian et al. (2016a) |
| Female EARTH Study participants | BPA | Decreased number of antral follicles measured on Day 3 of an unstimulated cycle | Souter et al. (2013) |
| Female EARTH Study participants | BPA | Increased maternal blood glucose levels | Chiu et al. (2017) |
| Male EARTH Study participants | DBP | Decreased sperm concentration | Hauser et al. (2006) |
| Studies on nutrition | |||
|---|---|---|---|
| Study participant | Dietary factor | Key finding | Reference |
| Women undergoing ART | Folate | Increased live birth rate | Gaskins et al. (2014) |
| Women undergoing ART | Vitamin B12 | Increased live birth rate | Gaskins et al. (2015) |
| Women undergoing ART | Whole grains | Increased live birth rate | Gaskins et al. (2016) |
| Women undergoing ART | Soy product | Increased live birth rate | Vanegas et al. (2015) |
| Women undergoing ART | Vitamin D | Increased fertilization rate | Abadia et al. (2016) |
| Male EARTH Study participants | Caffeine | Decreased live birth rate | Abadia et al. (2017) |
| Male EARTH Study participants | Soy | Decreased sperm concentration | Chavarro et al. (2008) |
| Male EARTH Study participants | Saturated fats | Decreased sperm concentration | Attaman et al. (2012) |
| Male EARTH Study participants | Trans fatty acids | Decreased sperm concentration | Chavarro et al. (2011) |
| Male EARTH Study participants | Fish and omega fatty acids | Increased percent of morphologically normal sperm | Attaman et al. (2012) |
| Male EARTH Study participants | Processed meat | Decreased percent of morphologically normal sperm | Afeiche et al. (2014) |
| Male EARTH Study participants | High pesticide residue fruit and vegetables | Decreased total sperm count and decreased percent morphologically normal sperm | Chiu et al. (2015) |
| Studies on lifestyle factors | |||
|---|---|---|---|
| Study participant | Lifestyle factor | Key finding | Reference |
| Women undergoing ART | Vigorous exercise | Increased live birth rate among women with normal BMI | Gaskins et al. (2016) |
| Female EARTH Study participants | Heavy lifting/moving heavy objects at work | Fewer total and mature oocytes and decreased number of antral follicles | Minguez-Alarcon et al. (2017) |
| Male EARTH Study Participants | Physical activity | Higher sperm concentration | Chavarro et al. (2010) |
| Male EARTH Study Participants | BMI | Men with BMI≥35 kg/m2: decreased total sperm count | Chavarro et al. (2010) |
EDC, Endocrine Disrupting Chemical; DEHP, di-(2-ethylhexyl) phthalate; DOP, Di-n-octyl phthalate; DiNP, Di-isononyl phthalate; DBP, Di-n-butyl phthalate; BPA, Bisphenol A.
Environmental chemicals
Among women in the EARTH Study undergoing ART, higher urinary concentrations of metabolites of di-(2-ethylhexyl) phthalate (DEHP) were associated with reduced oocyte yields, lower likelihood of clinical pregnancy, increased risk of pregnancy loss and lower likelihood of live birth following infertility treatment (Hauser et al., 2016; Messerlian et al., 2016b). Certain urinary phthalate metabolite concentrations among men were also associated with decreased odds of implantation and live birth (Dodge et al., 2015). Maternal soy and folate intake significantly modified the association between bisphenol A (BPA) and IVF outcomes in women (Chavarro et al., 2016; Minguez-Alarcon et al., 2016). We also found that urinary biomarkers of environmental chemicals (BPA and DEHP) were associated with antral follicle count (AFC) measured by ultrasound on Day 3 of the follicular phase of a woman’s unstimulated menstrual cycle (Souter et al., 2013; Messerlian et al., 2016a,b), and BPA was associated with higher second trimester glucose levels (Chiu et al., 2017). Among men, higher monobutyl phthalate concentrations were associated with decreased semen quality in a dose-dependent manner (Hauser et al., 2006).
Nutrition and lifestyle factors
Among women undergoing ART, we found that pre-treatment intake of folate and vitamin B12 (Gaskins et al., 2014, 2015), whole grains (Gaskins et al., 2016a), and soy products (Vanegas et al., 2015) were each independently and positively related to the probability of live birth. Maternal serum vitamin D levels were also positively associated with fertilization rates; however, this did not lead to a higher probability of pregnancy or live birth (Abadia et al., 2016). Paternal habitual caffeine intake was negatively associated with live birth, while maternal caffeine intake was not (Abadia et al., 2017). Maternal vigorous activity prior to ART treatment was positively associated with probability of live birth among women of normal BMI but not among overweight or obese women (Gaskins et al., 2016a,b). Within occupational factors, women who reported lifting/moving heavy objects at work had fewer total and mature oocytes, as well as a small reduction in mean AFC, compared with women who reported never lifting/moving heavy objects (Minguez-Alarcon et al., 2017).
In the EARTH Study, men’s soy food intake was negatively associated with sperm concentration (Chavarro et al., 2008). Saturated (Attaman et al., 2012) and trans fatty acid intake was also inversely associated with sperm concentration (Chavarro et al., 2011). Fish intake and omega 3 fatty acids (Attaman, Toth, Furtado, Campos, Hauser and Chavarro, 2012) were associated with an increase in percentage of morphologically normal sperm (Afeiche et al., 2014), while processed meat was associated with the opposite effect (Afeiche, Gaskins, Williams, Toth, Wright, Tanrikut, Hauser and Chavarro, 2014). High pesticide-residue fruit and vegetable intake was associated with lower total sperm and lower morphologically normal sperm counts (Chiu et al., 2015). Among the lifestyle factors examined, physical activity had a positive effect on sperm concentration, while a BMI ≥35 kg/m2 was associated with lower total sperm count (Chavarro et al., 2010). We found no association between mobile phone use and semen parameters in this cohort (Lewis et al., 2017).
PCP use and exposure
The EARTH Study has also identified determinants of environmental exposures, particularly due to PCP use. We evaluated whether questionnaire-based self-reported use of PCPs predicted urinary biomarkers of phthalates and parabens in men (Supplementary Fig. S1) and women (Supplementary Fig. S2) (Braun et al., 2014; Nassan et al., 2017). Lotion, cosmetic and cologne/perfume use were associated with increases in urinary phthalate metabolite and paraben concentrations, although the magnitude of individual biomarker increases varied by product used. We also found that the total number of PCPs used was predictive of urinary phthalate metabolite and paraben concentrations.
Discussion
The EARTH Study is one of the few cohorts to have repeated exposure measurements-including biospecimen data from men and women from the period before conception, throughout attempted pregnancy cycles, and from each trimester among pregnant participants (Fig. 1). There are several advantages to collecting multiple biospecimens from men and women over an extended time. First, we can identify distinct periods of sensitivity and account for the correlation between exposure windows and within couples. Second, having more than one urine or blood sample for each exposure window reduces the potential for exposure misclassification, particularly for chemicals with short half-lives such as phthalates and phenols. We are also able to study the largely unexplored pre- and peri-conception periods as we have at least one urine sample collected from men and women from this window. The EARTH Study has measured more than 40 different biomarkers of environmental chemical exposures, thus enabling us to investigate the relationships between mixtures of chemicals and endpoints of interest. The study is designed to assess very early pregnancy stages and outcomes for each attempted cycle, allowing for the evaluation of endpoints that are unobservable in most pregnancy cohorts. Documentation of outcomes is also highly accurate as it relies on clinical abstraction of cycle endpoints by trained study staff. We also have comprehensive covariate data collected through self-reported measures as well as from electronic medical records. Finally, owing to the intensive collection of dietary data, the EARTH Study is also one of the few human studies able to assess potential interactions between environmental chemicals and dietary factors, which is an important and emerging area of research.
While the fertility clinic setting provides the opportunity to measure environmental exposures across different windows of vulnerability and evaluate their potential effects on critical early fertility, pregnancy and delivery outcomes, the findings may be less generalizable to naturally conceived pregnancies (Messerlian and Gaskins, 2017). Pregnancies conceived to subfertile couples may also be more vulnerable to exposures and results may be specific to the population under study. However, this potential concern is outweighed by the study strengths—a research design that is internally valid and sufficiently powered to explore previously unstudied paternal and maternal exposures in relation to relevant and measurable endpoints. We further believe that this vulnerable population represents an important public health subpopulation given the growing number of babies born using IVF-based treatment: estimated to be 1.6% of all births or >68 000 births annually in the USA, with even higher proportions in certain European nations. The fraction of births using non-IVF based treatment is even higher at ~4.6% (~191 000 births), totaling >250 000 births per year in the USA (Dyer et al., 2016; Schieve et al., 2009; Sunderam et al., 2017; Zegers-Hochschild et al., 2014).
One particular challenge, however, in studying an infertile subpopulation involves the complexity of disentangling the effects of underlying infertility or its treatment from the exposure—outcome association of interest. The study is limited by the absence of fertile couples as a comparison group that is unconfounded by infertility or its treatment. Nevertheless, we attempt to control for causes of infertility and treatment either through adjustment or stratification (Messerlian et al., 2017). Analytical plans have also relied on the use of directed acyclic graphs to identify potential confounders that are not causal intermediates between exposure and outcomes (Messerlian and Gaskins, 2017). Furthermore, while we can control for many potential confounders, we cannot adjust for some co-exposures to unmeasured environmental chemicals or other unknown determinants of both exposure and health outcomes. Lastly, while the EARTH Study has tested many a priori hypotheses, we have undertaken multiple comparisons and cannot rule out the possibility that some of our findings may be spurious or due to chance.
Where can I find out more?
The EARTH Study has been carried out in collaboration with students, post-doctoral and clinical fellows and visiting scientists, and welcomes the opportunity for new and continued collaborations. All enquiries should be made to Dr. Russ Hauser, Principal Investigator of the EARTH Study, Harvard T.H. Chan School of Public Health (rhauser@hsph.harvard.edu). More information about the study and a complete list of our publications can be found at: https://www.hsph.harvard.edu/earth/
Supplementary Material
Acknowledgements
We would like to acknowledge all past and present research and clinical staff at the Massachusetts General Hospital Fertility Center. We also acknowledge Xiaoyun Ye, Manori Silva, Ella Samandar, Jim Preau and Tao Jia (Centers for Disease Control and Prevention, Atlanta, GA, USA) for their work in measuring urinary biomarkers; Heather Stapleton and lab associates (Duke University, Durham, NC, USA) for their work on measuring flame retardant biomarkers, and Robert Wright and his team (Mount Sinai School of Medicine, New York, NY, USA) for measuring heavy metals and metalloids. Finally, we thank all EARTH Study participants who volunteered their time generously to make this work possible. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Supplementary data
Supplementary data are available at Human Reproduction Open online.
Authors’ roles
R.H. conceived, designed and lead the EARTH Study; C.M. and R.H. conceived and executed the article; C.M. drafted the article, conducted analysis and critically appraised the article; P.L.W. contributed to the design of the cohort, provided statistical leadership, conducted analysis, contributed to the writing and critical appraisal of the article; J.B.F. implemented the cohort and data collection procedures, recruited participants, contributed to the writing and critical appraisal of the article; J.E.C. contributed to the design of the cohort, provided leadership on nutritional components of the study, conducted analysis, contributed to the writing and critical appraisal of the manuscript; R.D. implemented the cohort and collected data, contributed to the writing of the article; L.M.A., J.M.B., A.J.G., J.D.M., T.J.-T., Y.H.C., F.L.N. contributed to the analysis, writing and critical appraisal of the article; I.S., J.P., T.L.T. contributed to the design of the cohort, provided clinical interpretation, contributed to the writing and critical appraisal of the article; M.K. recruited participants and collected data, contributed to the writing of the article; A.M.C. executed laboratory chemical analysis, contributed to the writing and critical appraisal of the article. All authors approved the final article.
Funding
National Institute of Environmental Health Sciences (ES009718, ES022955, K99ES026648, ES000002, K99 ES020346, R00 ES020346), the Electric Power Research Institute (EP-P36221/C16445) and the National Institute for Occupational Safety and Health (OH008578).
Conflict of interest
All authors declare no potential or actual competing interests.
References
- Abadia L, Chiu YH, Williams PL, Toth TL, Souter I, Hauser R, Chavarro JE, Gaskins AJ. The association between pre-treatment maternal alcohol and caffeine intake and outcomes of assisted reproduction in a prospectively followed cohort. Hum Reprod 2017;32:1846–1854. doi:10.1093/humrep/dex237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abadia L, Gaskins AJ, Chiu YH, Williams PL, Keller M, Wright DL, Souter I, Hauser R, Chavarro JE, Environment and Reproductive Health Study Team et al. . Serum 25-hydroxyvitamin D concentrations and treatment outcomes of women undergoing assisted reproduction. Am J Clin Nutr 2016;104:729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afeiche MC, Gaskins AJ, Williams PL, Toth TL, Wright DL, Tanrikut C, Hauser R, Chavarro JE. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. J Nutr 2014;144:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod 2012;27:1466–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman A, Heindel JJ, Jobling S, Kidd KA, Zoeller RT. Organization WH (ed). State of the Science of Endocrine Disrupting Chemicals. Geneva, Switzerland: World Health Organization, 2012. [Google Scholar]
- Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol 2014;24:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Messerlian C, Fathers Matter RH. Why it’s time to consider the impact of paternal environmental exposures on children’s health. Curr Epidemiol Rep 2017;4:46–55. doi:10.1007/s40471-017-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Schisterman EF, Sweeney AM, Wilcosky TC, Gore-Langton RE, Lynch CD, Boyd Barr D, Schrader SM, Kim S, Chen Z et al. . Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development—the LIFE Study. Paediatr Perinat Epidemiol 2011;25:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Longnecker MP, Koch HM, Swan SH, Hauser R, Goldman LR, Lanphear BP, Rudel RA, Engel SM, Teitelbaum SL et al. . Optimal exposure biomarkers for nonpersistent chemicals in environmental epidemiology. Environ Health Perspect 2015;123:A166–A168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin RE, Robbins WA, Schieve LA, Sweeney AM, Tabacova SA, Tomashek KM. Off to a good start: the influence of pre- and periconceptional exposures, parental fertility, and nutrition on children’s health. Environ Health Perspect 2004;112:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Furtado J, Toth TL, Ford J, Keller M, Campos H, Hauser R. Trans-fatty acid levels in sperm are associated with sperm concentration among men from an infertility clinic. Fertil Steril 2011;95:1794–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Minguez-Alarcon L, Chiu YH, Gaskins AJ, Souter I, Williams PL, Calafat AM, Hauser R, Team ES. Soy intake modifies the relation between urinary bisphenol A concentrations and pregnancy outcomes among women undergoing assisted reproduction. J Clin Endocrinol Metab 2016;101:1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Toth TL, Sadio SM, Hauser R. Soy food and soy isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Hum Reprod 2008;23:2584–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril 2010;93:2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Afeiche MC, Gaskins AJ, Williams PL, Petrozza JC, Tanrikut C, Hauser R, Chavarro JE. Fruit and vegetable intake and their pesticide residues in relation to semen quality among men from a fertility clinic. Hum Reprod 2015;30:1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Minguez-Alarcon L, Ford JB, Keller M, Seely EW, Messerlian C, Petrozza J, Williams PL, Ye X, Calafat AM et al. . Trimester-specific urinary bisphenol A concentrations and blood glucose levels among pregnant women from a fertility clinic. J Clin Endocrinol Metab 2017;102:1350–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale: SRS-2, 2nd edn Torrane, California: Western Psychological Services, 2012. [Google Scholar]
- Dodge LE, Williams PL, Williams MA, Missmer SA, Souter I, Calafat AM, Hauser R, Team ES. Associations between paternal urinary phthalate metabolite concentrations and reproductive outcomes among couples seeking fertility treatment. Reprod Toxicol 2015;58:184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer S, Chambers GM, de Mouzon J, Nygren KG, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Adamson GD. International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted Reproductive Technology 2008, 2009 and 2010. Hum Reprod 2016;31:1588–1609. [DOI] [PubMed] [Google Scholar]
- Gaskins AJ, Afeiche MC, Wright DL, Toth TL, Williams PL, Gillman MW, Hauser R, Chavarro JE. Dietary folate and reproductive success among women undergoing assisted reproduction. Obstet Gynecol 2014;124:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Chiu YH, Williams PL, Ford JB, Toth TL, Hauser R, Chavarro JE. Association between serum folate and vitamin B-12 and outcomes of assisted reproductive technologies. Am J Clin Nutr 2015;102:943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Chiu YH, Williams PL, Keller MG, Toth TL, Hauser R, Chavarro JE, Team ES. Maternal whole grain intake and outcomes of in vitro fertilization. Fertil Steril 2016. a;105:1503–1510 e1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Williams PL, Keller MG, Souter I, Hauser R, Chavarro JE, Team ES. Maternal physical and sedentary activities in relation to reproductive outcomes following IVF. Reprod Biomed Online 2016. b;33:513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombok S, Rust J. The measurement of gender role behaviour in pre-school children: a research note. J Child Psychol Psychiatry 1993;34:805–811. [DOI] [PubMed] [Google Scholar]
- Hauser R, Gaskins AJ, Souter I, Smith KW, Dodge LE, Ehrlich S, Meeker JD, Calafat AM, Williams PL, Team ES. Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: results from the EARTH Study. Environ Health Perspect 2016;124:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology 2006;17:682–691. [DOI] [PubMed] [Google Scholar]
- Homan GF, Davies M, Norman R. The impact of lifestyle factors on reproductive performance in the general population and those undergoing infertility treatment: a review. Hum Reprod Update 2007;13:209–223. [DOI] [PubMed] [Google Scholar]
- Lewis RC, Minguez-Alarcon L, Meeker JD, Williams PL, Mezei G, Ford JB, Hauser R. Self-reported mobile phone use and semen parameters among men from a fertility clinic. Reprod Toxicol 2017;67:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis GM, Cooney MA, Lynch CD, Handal A. Periconception window: advising the pregnancy-planning couple. Fertil Steril 2008;89:e119–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Braun JM, Minguez-Alarcon L, Williams PL, Ford JB, Mustieles V, Calafat AM, Souter I, Toth T, Hauser R et al. . Paternal and maternal urinary phthalate metabolite concentrations and birth weight of singletons conceived by subfertile couples. Environ Int 2017;107:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Gaskins AJ. Epidemiologic approaches for studying assisted reproductive technologies: design, methods, analysis, and interpretation. Curr Epidemiol Rep 2017;4:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Souter I, Gaskins AJ, Williams PL, Ford JB, Chiu YH, Calafat AM, Hauser R. Urinary phthalate metabolites and ovarian reserve among women seeking infertility care. Hum Reprod 2016. a;31:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Wylie BJ, Minguez-Alarcon L, Williams PL, Ford JB, Souter IC, Calafat AM, Hauser R. Urinary concentrations of phthalate metabolites and pregnancy loss among women conceiving with medically assisted reproduction. Epidemiology 2016. b;27:879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Gaskins AJ, Chiu YH, Souter I, Williams PL, Calafat AM, Hauser R, Chavarro JE. Dietary folate intake and modification of the association of urinary bisphenol A concentrations with in vitro fertilization outcomes among women from a fertility clinic. Reprod Toxicol 2016;65:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Souter I, Williams PL, Ford JB, Hauser R, Chavarro JE, Gaskins AJ. Occupational factors and markers of ovarian reserve and response among women at a fertility centre. Occup Environ Med 2017;74:426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassan FL, Coull BA, Gaskins AJ, Williams MA, Skakkebaek NE, Ford JB, Ye X, Calafat AM, Braun JM, Hauser R. Personal care product use in men and urinary concentrations of select phthalate metabolites and parabens: results from the Environment And Reproductive Health (EARTH) Study. Environ Health Perspect 2017;125:087012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutrition and reproduction in women. Hum Reprod Update 2006;12:193–207. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. BASC: Behavior Assessment System for Children: Manual. Circle Pines, MN: American Guidance Service, 1998. [Google Scholar]
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–1126. discussion 11271136. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Devine O, Boyle CA, Petrini JR, Warner L. Estimation of the contribution of non-assisted reproductive technology ovulation stimulation fertility treatments to US singleton and multiple births. Am J Epidemiol 2009;170:1396–1407. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Franks S. Environment, lifestyle and infertility—an inter-generational issue. Nat Cell Biol 2002;4:s33–s40. [DOI] [PubMed] [Google Scholar]
- Souter I, Smith KW, Dimitriadis I, Ehrlich S, Williams PL, Calafat AM, Hauser R. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod Toxicol 2013;42:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L, Barfield WD. Assisted Reproductive Technology Surveillance—United States, 2014. MMWR Surveill Summ 2017;66:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas JC, Afeiche MC, Gaskins AJ, Minguez-Alarcon L, Williams PL, Wright DL, Toth TL, Hauser R, Chavarro JE. Soy food intake and treatment outcomes of women undergoing assisted reproductive technology. Fertil Steril 2015;103:749–755 e742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Carlson A, Schwartz JM, Giudice LC. Proceedings of the Summit on Environmental Challenges to Reproductive Health and Fertility: executive summary. Fertil Steril 2008;89:281–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol 2017;185:570–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Mansour R, Ishihara O, Adamson GD, de Mouzon J, Nygren KG, Sullivan EA. International Committee for Monitoring Assisted Reproductive Technology: world report on assisted reproductive technology, 2005. Fertil Steril 2014;101:366–378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.