Abstract
Objective
Abnormal engagement of the posterior medial frontal cortex (pMFC) occurs during performance monitoring in obsessive-compulsive disorder (OCD), including in pediatric patients. Yet, the development of pMFC function in OCD-affected youth remains poorly understood.
Method
Sixty-nine patients with pediatric OCD and 72 healthy controls (HC), 8 to 19 years, were scanned during the Multisource Interference Task (MSIT). The effects of group, age, performance and interactions on pMFC response to errors and interference were tested in region of interest ROI) and whole brain analyses. Secondary analyses considered bilateral anterior insula/frontal operculum (aI/fO), given the contribution of these regions with pMFC to a cingulo-opercular network (CON) for task control (e.g., error- and interference-processing).
Results
Error-related pMFC activity was greater for OCD than HC, increased with age in OCD, but decreased with age in HC. Greater pMFC activation associated with better performance in HC, but not OCD. In patients, greater pMFC activation to errors associated with lower OCD severity. Altered error-related activation and performance associations were also observed in right aI/fO in OCD, while left aI/fO response to interference associated with lower OCD severity.
Conclusion
Atypical increase of error-related pMFC activation with age in pediatric OCD suggests altered development of pMFC function during the early course of illness. Greater pMFC activation with better performance in HC, and with age and lower symptom severity in patients suggests an adaptive function of heightened pMFC response to errors that could be further enhanced (e.g., via cognitive training) to improve outcomes in OCD from the early course of illness.
Keywords: pediatric obsessive-compulsive disorder, performance monitoring, task control, cingulo-opercular network, development
Introduction
Abnormal brain response to performance monitoring in obsessive compulsive disorder (OCD) is elicited by simple cognitive tasks not intended to provoke symptoms, suggesting a core process in the pathophysiology of illness.1 Performance monitoring involves detection of errors and interference between competing response options to enable behavioral adjustments,2 and abnormalities of this process could underlie the repetitive thoughts and behaviors of OCD.1 OCD often emerges in childhood or adolescence,3 a period in which neural networks for performance monitoring mature in healthy youth4 and performance monitoring abnormalities can be observed in those affected by OCD.5–7 Yet, despite important implications for early treatment, the development of performance monitoring dysfunction in OCD remains poorly understood.1
In healthy adults, performance monitoring engages posterior medial frontal cortex (pMFC, encompassing dorsal anterior cingulate and pre-supplementary motor area) and bilateral anterior insula/frontal operculum (aI/fO).8 These regions co-activate during cognitively demanding tasks and remain functionally connected at rest, defining a “cingulo-opercular network” (CON) for task control.8 The CON is widely held to mediate selection of salient information from internal and external inputs to guide ongoing behavior, including detection of errors and interference for performance monitoring.8,9 In adults with OCD, several fMRI studies have shown pMFC hyperactivation (dorsal anterior cingulate, dACC and/or pre-supplementary motor area, pre-SMA) during performance monitoring10–15 and electrophysiologic research has consistently demonstrated increased error-related negativity, generated by the dACC (amongst other regions).16 Hyperactivation of the aI/fO to errors also occurs in adult patients,17 implicating broader CON function in OCD.
Neuroimaging studies of performance monitoring in pediatric OCD suggest abnormal pMFC engagement, but are inconsistent regarding the direction of the abnormality, with one study reporting increased activation,5 and another reporting the reverse in patients compared to healthy youth.18 Other studies report no group differences.6,19 These inconsistencies may relate to small sample sizes (n’s < 25 per group), which increase variability and reduce experimental power.20 In addition, pediatric (and adult) OCD cohorts often include patients taking selective serotonin reuptake inhibitors, which impact neural engagement during performance monitoring21 and may alter neurofunctional maturation.22
Overall, this literature implicates atypical engagement of the CON, particularly pMFC, during performance monitoring in OCD. However, no fMRI study has investigated whether the relation of age with CON response to performance monitoring differs between patients with pediatric OCD and healthy youth. Thus, the aim of this study was to test effects of group and group-by-age interactions on activation of the pMFC (primary regions of interest, ROI) and aI/fO (secondary ROIs) during performance monitoring in youth with OCD compared to matched controls. Group-by-performance interactions were assessed to test for differences in the relation of activation with behavioral output. In addition, we sought to clarify whether performance monitoring function relates to symptom severity in young patients, as this has not been the case in adults.10,11,13,17 Finally, we capitalized on our large sample of youth with OCD to explore the effects of medication status on activation.
Method
Participants
Seventy-five patients with pediatric OCD (13.8 ± 2.8 years) and 75 healthy controls (HC, 13.8 ± 3.4 years) were assessed by structured interview with the Kiddie-Schedule for Affective Disorders-Present and Lifetime Version23 and, in patients, the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS).24 Nine participants failed to provide useable data (Supplement 1, available online), yielding 69 patients and 72 HCs for interference analyses (i.e., correct resolution of response competition). For error analyses, at least 5 errors per subject were required,25 leaving 51 patients and 51 HCs. OCD was the primary source of impairment; of the 69 patients, 49 had one to three less severe comorbid diagnoses including anxiety (n = 27), tic (n = 18), and/or attentional (n = 9) disorders or subclinical depressive symptoms (n = 13) (Table S1, available online), consistent with previously described clinical samples.26 Major depressive, autism spectrum, psychotic, or substance use disorders were excluded. HCs had no current or prior history of psychiatric illness and no first degree relatives with OCD. Among patients, thirty-four were medicated, primarily with selective serotonin reuptake inhibitors (see Supplement 1, available online).
Task
Participants performed an event-related version of the MSIT5 (Figure S1, available online). They identified the unique number among three numbers, “1”, “2” and “3” by pressing a button with the first (index), second (middle) and third (ring) fingers, respectively. On incongruent trials (Inc, e.g., “331”), the unique number was positioned incongruently (i.e., “1” in the 3rd position), flanked by distracting numbers (“33”). On congruent trials (Con, e.g., “100”), the unique number was positioned congruently (“1” in the first position), flanked by zeroes. To ensure task understanding and minimize performance variability, participants were trained to achieve 70–90% accuracy on incongruent trials. During scanning, 300 trials (3000 msec/each) were presented over 5 runs for a total of 15 minutes (120 Inc, 120 Con, 60 Fixation) in fixed order.
MRI acquisition and pre-processing
A 3.0 T GE Signa scanner (GE Healthcare, Waukesha, WI) was used to collect T2* reverse spiral images and a low resolution axial T1 for coregistration.27 A high resolution T1-weighted SPGR was acquired for anatomic normalization.27 Functional data were pre-processed in SPM8 (Statistical Parametric Mapping, Wellcome Trust Centre, London, United Kingdom). Raw data were slice-time corrected and realigned to the tenth image acquired. Realignment parameters were retained for inclusion as regressors in first-level analyses and to calculate mean framewise displacement, a summary of subject motion.28 Once coregistered, the high-resolution SPGR was segmented using the VBM8 toolbox {http://dbm.neuro.uni-jena.de/vbm}, normalized using high dimensional DARTEL normalization29, and used to warp functional images into common stereotactic space (Montreal Neurological Institute; MNI). Normalized functional data were smoothed with a 5-mm Gaussian kernel.
Analysis
Behavioral
Linear regression was used to test effects of age, group (OCD, HC), and age-by-group interactions on performance. Separate models were run for incongruent and congruent response times (RT) and accuracy.
Functional MRI
Functional data were analyzed using a standard random effects analysis30 in SPM8. At the first level, incongruent correct, congruent correct, incongruent incorrect and congruent incorrect trials were modeled against fixation trials as implicit baseline; omission errors were regressed from the model. Six realignment parameters and their first derivatives were regressed to remove motion effects. Contrast maps were constructed of incongruent versus congruent correct trials for interference, and incorrect versus correct incongruent trials for errors. Single subject contrasts were entered into second-order random effects analyses31 to test main effects of errors and interference, across subjects at a height threshold of p < 0.05, corrected for family-wise error (FWE) rates (see Table S2, available online, for main effects).
Region of interest (ROI) analyses included pMFC as primary ROI, and aI/fO ROIs as secondary. Primary pMFC ROIs were defined by conjunction of main effects for each contrast (errors, interference across subjects) with a meta-analysis-based medial frontal cortex ROI (Neurosynth MFC, Supplement 1, available online)32; secondary aI/fO ROIs were defined by contrast-specific main effects (Figures 1A, errors; 2A, interference). Secondary ROIs included pMFC subregions, preSMA and dACC, defined by conjunction of primary pMFC ROIs with Neurosynth posterior and middle MFC zones32 (Supplement 1, Figure S2, available online). Parameter estimates were extracted from ROIs for inclusion in stepwise linear regressions, using backwards elimination, to select predictor variables (group, age, performance, and interactions) --- an unbiased method since voxels of interest were orthogonal to predictors.33 Performance variables were incongruent accuracy and response times for error analyses, and overall accuracy and response times (across conditions) for interference. Framewise displacement (FD) was regressed to control for motion effects.28 Continuous variables were mean-centered. Separate analyses tested effects of present symptom severity, as measured by C-YBOCS, in patients. For the primary pMFC ROI, findings were considered significant at p < .05; for the four secondary ROIS (bilateral aI/fO, dACC, preSMA), a more stringent significance threshold was used (p < .013) to correct for multiple comparisons. Finally, given possible medication effects,21,22 exploratory analyses considered unmedicated OCD (uOCD), medicated OCD (mOCD) and the interactions of these patient sub-groups with age and performance, relative to HC. All analyses were carried out in R, version 3.2.5. Partial residual plots were constructed to show the relationship between a given predictor and response variable given that other predictor variables were also in the model.34
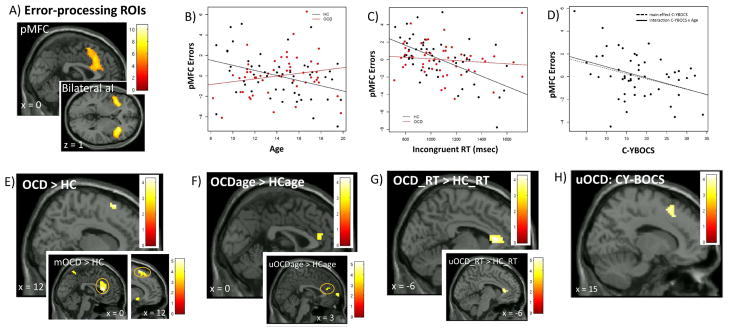
Figure 1. Error-processing in pediatric obsessive-compulsive disorder (OCD).
Note: Error-processing activated posterior medial frontal cortex (pMFC) and bilateral anterior insula (aI/fO) (A). ROI analyses showed greater pMFC activation associated with younger age in HC, but older age in OCD (B); faster response time (RT) in HC, but not OCD (C); and, lower OCD severity (C-YBOCS, Child Yale-Brown Obsessive Compulsive Scale) in patients (D). Whole brain analyses showed greater pMFC activation in OCD than HC (E) and, as with ROI analyses, altered associations of pMFC activation with age (F) and RT (G) in OCD compared to HC. Exploratory analyses suggested the effect of group on pMFC activation was driven by medicated patients (E inset, mOCD > HC), whereas unmedicated patients (uOCD) drove atypical age- (F inset, uOCDage > HCage), altered RT- (G inset, OCD_RT > HC_RT) and inverse OCD severity associations (H) with pMFC activation. Color bars show t-scores, reflecting relative strength of brain activation. Montreal Neurologic Institute coordinates shown (x, y, z).
aI/fO = anterior insula/frontal operculum, C-YBOCS = Child Yale-Brown Obsessive Compulsive Scale, FWE = familywise error, HC = healthy controls, MFC = medial frontal cortex, mOCD = medicated obsessive-compulsive disorder, pMFC = posterior medial frontal cortex, ROIs = regions of interest, RT = response time, uOCD = unmedicated obsessive-compulsive disorder
In addition, whole brain analyses were conducted in SPM8, including the same regressors as ROI analyses. Contrasts were displayed at a peak threshold of p < .001 (uncorrected) and clusters were considered significant at p < .05, corrected for family wise error (FWE) across whole brain and within pMFC, right and left aI/fO ROIs.
Results
Subjects
There were no significant differences between groups on demographics, performance or in-scanner motion (FD) (Table 1). Comparison of uOCD and mOCD (Table S3, available online) showed a trend towards higher present disease severity in uOCD (p = 0.07); nominally but not significantly greater lifetime severity in mOCD; and, greater reduction from lifetime to present severity in mOCD (p = 0.007).
Table 1.
Participant characteristics.
| Error Analysis | Interference Analysis | |||||
|---|---|---|---|---|---|---|
| OCD (N = 51) | HC (N = 51) | Test Statistic (p-value) | OCD (N = 69) | HC (N = 72) | Test Statistic (p-value) | |
| Age | 14.2 ± 2.8 | 14.1 ± 3.2 | t(100) = −.14 (.88) | 13.9 ± 2.8 | 14.0 ± 3.5 | t(139) = .11 (.91) |
| Gender | 28F (55%) | 23F (45%) | χ2(102) =.63 (.43) | 39F (56%) | 33F (46%) | χ2(141) = .34 (.56) |
| SESa | 2.2 ± .45 | 2.3 ± .53 | t(97) = .66 (.51) | 2.2 ± .48 | 2.26 ± .53 | t(136) = .51 (.61) |
| CY-BOCS, Present | 17.8 ± 7.4 | na | na | 18.6 ± 7.7 | na | na |
| CY-BOCS, Lifetime | 27.1 ± 6.6 | na | na | 27.9 ± 6.5 | na | na |
| CY-BOCS, Change | 9.3 ± 7.9 | na | na | 9.3 ± 7.9 | na | na |
| Illness Duration | 6.4 ± 4.3 | na | na | 6.5 ± 4.0 | na | na |
| AAO | 7.7 ± 3.3 | na | na | 7.5 ± 3.0 | na | na |
| Inc RT | 1094 ± 249 | 1060 ± 238 | t(100)= −.71 (.48) | 1114 ± 258 | 1071 ± 220 | t(139) = −1.07 (.29) |
| Con RT | 770 ± 176 | 738 ± 171 | t(100)= −.95 (.35) | 789 ± 192 | 759 ± 162 | t(139) = −.99 (.32) |
| Inc Acc | .87 ± .07 | .88 ± .07 | t(100)= 1.15 (.25) | .89 ± .07 | .91 ± .07 | t(139) = 1.31 (.19) |
| Con Acc | .99 ± .02 | .98 ± .02 | t(100)= −.67 (.50) | .99 ± .02 | .99 ± .02 | t(139) = −.76 (.49) |
| Motion | .20 ± .08 | .19 ± .13 | t(100) = −.11 (.91) | .21 ± .11 | .19 ± .13 | t(139) = −.96 (.34) |
Note: AAO = age of onset, Acc = accuracy, Con = congruent, CY-BOCS = Child Yale-Brown Obsessive Compulsive Scale, HC = Healthy, Inc = incongruent, OCD = Obsessive Compulsive Disorder Control, RT = response time Motion = mean framewise displacement 28. Age, AAO, illness duration in years.
SES missing for 2 HC, 1 OCD.
Behavioral Performance
There were no differences between OCD and healthy youth in performance (Table 1). Older age associated with faster RT on both trial types (p’s < .001), and with higher accuracy, at trend-level, on congruent (p =0.07) but not incongruent (p = 0.23) trials. There were no differences in the effect of age on RT or accuracy between groups (p’s > .16).
Imaging Analyses
Error-processing
Primary pMFC ROI Analysis
ROI-based linear regression analyses showed that error-related activation of the pMFC was greater for OCD relative to HC (β = .98, p = .02); decreased with age in HC, while increasing with age in OCD (β = .39, p =.03, Figure 1B); and, increased with faster RT in HC, but not OCD (β = .005, p = .03, Figure 1C) (Table 2). Exploratory analyses showed that greater pMFC activation in patients was driven by mOCD (Table S4, available online). By contrast, altered associations of pMFC activation with age and RT were present in uOCD, but not mOCD, relative to HC (Figure S3A–B; Table S4, available online).
Table 2.
Cingulo-opercular network function in pediatric obsessive-compulsive disorder (OCD) compared to healthy controls
| ERRORS | ||||||
|---|---|---|---|---|---|---|
| pMFC | Left aI/fO | Right aI/fO | ||||
| Intercept | 1.10 ± .28 | <.001 | 1.48 ± .25 | <.001 | 1.42 ± .28 | <.001 |
| Group | .98 ± .41 | .018 | 0.50 ± .36 | .175 | .58 ± .39 | .139 |
| Age | −.25 ± .13 | .063 | ------------ | --- | −.12 ± .13 | .342 |
| Inc RT | −.006 ± .002 | .001 | ------------ | --- | −0.004 ± .002 | .025 |
| Inc Acc | 10.06 ± 3.07 | .002 | 1.70 ± 3.91 | .665 | 7.1 ± 2.97 | .019 |
| Group × Age | .39 ± .18 | .029 | ------------ | --- | .28 ± .17 | .098 |
| Group × Inc RT | .005 ± .002 | .026 | ------------ | --- | .006 ± .002 | .010* |
| Group × Inc Acc | ------------ | --- | 8.13 ± 5.41 | .136 | ------------ | --- |
| Motion | ------------ | --- | −3.38 ± 1.60 | .037 | ------------ | --- |
| Adjusted R-Square | 0.17 | 0.08 | 0.08 | |||
| Model ANOVA | F(6,95)=4.4 (p<.001) | F(4,97)=3.2 (p=.016) | F (6,95) = 2.5 (p=.03) | |||
| INTERFERENCE | ||||||
| pMFC | Left aI/fO | Right aI/fO | ||||
| Intercept | .60 ± .07 | <.001 | .45 ± .08 | <.001 | .52 ± .08 | <.001 |
| Group | −.02 ± .10 | .847 | −.08 ± .12 | .492 | −.10 ± .12 | .413 |
| Age | ------------ | ---- | ------------ | ---- | ------------ | ---- |
| Overall RT | −.001 ± .0004 | .055 | −.001 ±.0004 | .053 | −.001 ± .0003 | .001* |
| Overall Acc | 1.41 ± 1.76 | .425 | 4.93 ± 2.04 | .017* | 1.77 ± 2.06 | .393 |
| Group × Age | ------------ | ---- | ------------ | ---- | ------------ | ---- |
| Group × overall RT | .001 ± .0005 | .028 | .001 ± .0006 | .100 | ------------ | ---- |
| Group × overall Acc | −5.17 ± 2.51 | .042 | −7.83 ± 2.9 | .008* | −4.90 ± 2.95 | .099 |
| Motion | ------------ | --- | ------------ | --- | ------------ | --- |
| Adjusted R-Square | 0.03 | 0.05 | 0.08 | |||
| Model ANOVA* | F(5,135)= 2.0 (p=.086) | F(5,135)=2.4 (p=.039) | F(4,136)=4.2 (p=.003) | |||
Note: Motion refers to framewise displacement 28. Dashed lines represent predictor variables eliminated during backwards stepwise regression.
Significance levels were p < .05 for pMFC (primary region of interest, bolded) and p < .013 for secondary ROIs (asterisked).
aI/fO = anterior insula/frontal operculum, Acc = accuracy, Inc = incongruent, HC = healthy controls, pMFC = posterior medial frontal cortex, RT = response time
Secondary Left and Right aI/fO ROI Analyses
As with pMFC, the typical increase of right aI/fO activation with faster RT was attenuated in OCD relative to HC (β = .006, p = .01, Table 2). Exploratory analyses showed this attenuation in uOCD, but not mOCD, relative to HC; in addition, right aI/fO activation was greater in mOCD, but not uOCD, compared to HC (Table S4, available online).
Secondary dACC and preSMA ROI Analyses
Analysis of pMFC sub-regions suggested several trend-level effects that did not reach significance after correction (Table S5, available online). Exploratory analyses showed greater activation in dACC (p = .002) and in pre-SMA at trend level (p = .029) in mOCD, but not uOCD, relative to HC, but suggested altered age (trend: p = .031) and RT (p = .011) - activation associations in the pMFC may have been driven by dACC voxels in uOCD (Table S6, available online).
Wholebrain Analyses
Consistent with the ROI analysis, OCD patients compared to HC showed greater error-related activation in the pMFC at the preSMA-dACC border (“preSMA-dACC”, Figure 1E); atypical increase in dACC activation with age (Figure 1F); and, reversal of the typical relation of faster RT with activation in a cluster encompassing dACC, rostral ACC and left caudate (Figure 1G, Table S7, available online). There were no areas in which HC showed greater activation than OCD. Exploratory analyses comparing uOCD and mOCD with HC (Figure 1E–G insets; Table S8, available online) showed greater activation in dACC and, at trend-level, in preSMA in mOCD, and atypical association of age and RT with dACC activation in uOCD.
Interference-processing
Primary pMFC ROI Analysis
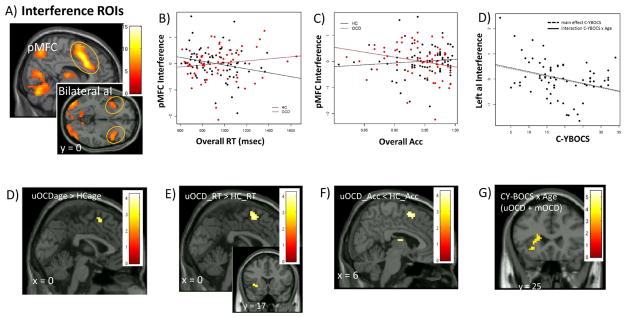
There were no group differences in pMFC response to interference, but there was a reversal of the typical relation of greater interference-related pMFC activation with faster RT and greater accuracy in OCD relative to HC (β = .001 ± .001, p = .03; β = −5.17 ± 2.51, p = .04; Figure 2B–C; Table 2). Exploratory analyses suggested that uOCD predominantly contributed to this effect (Table S4, available online).
Figure 2. Interference-processing in left anterior insula/frontal operculum (aI/fO) and posterior medial frontal cortex (pMFC): Effects of symptom severity in unmedicated patients with pediatric obsessive-compulsive disorder (OCD).
Note: Interference-processing activated posterior medial frontal cortex (pMFC) and bilateral anterior insula (aI/fO) (A). ROI analyses showed associations of greater pMFC activation with better performance (faster response time, RT; higher accuracy, Acc) in HC, but worse performance in OCD (B–C) and greater left aI/fO activation with lower OCD severity (C-YBOCS, Child Yale Brown Obsessive Compulsive Scale) in patients (D). Exploratory wholebrain analyses showed altered associations of pMFC activation with age (E), RT (F) and Acc (G) in unmedicated patients (uOCD) compared to HC. In patients, there was a CY-BOCs-by-age interaction driven by the association of greater left aI/fO activation with lower OCD severity at older ages across uOCD and medicated (mOCD) patients (H). Color bars show t-scores, reflecting relative strength of brain activation. Montreal Neurologic Institute coordinates shown (x, y, z).
Acc = accuracy, C-YBOCS = Child Yale-Brown Obsessive Compulsive Scale, FWE = familywise error, HC = healthy controls, MFC = medial frontal cortex, mOCD = medicated obsessive-compulsive disorder, ROIs = regions of interest, RT = response time, uOCD = unmedicated obsessive-compulsive disorder
Secondary Left and Right aI/fO ROI Analyses
The typical relation of greater left aI/fO activation with greater accuracy was attenuated in OCD (β = −7.83 ± 2.9, p = .008, Table 2). Exploratory analyses suggested this effect may have been driven by uOCD, albeit at a level of significance that fell just below threshold for multiple comparison correction (p = .014, Table S4, available online).
Secondary dACC and preSMA ROI Analyses
Analysis of pMFC sub-regions suggested several trend-level effects across both dACC and pre-SMA that did not reach significance after correction (Tables S5, S6, available online).
Wholebrain Analyses
There were no group or interaction effects on interference-related activation; however, exploratory analyses attenuation of normative increase in preSMA-dACC activation with younger age, faster RT and higher accuracy in uOCD compared to HC (Figure 2E–G; Table S9, available online).
OCD severity: Correlations brain activation to errors and interference
ROI analyses
Lower OCD severity predicted greater activation to errors in pMFC (β = −0.08, p =0.02; Figure 1D) and interference in left aI/fO (β = −0.03, p =0.009; Figure 2D) (Table 3). Exploratory analyses suggested severity associations were driven by uOCD for error-related pMFC activation (Figure S3C, available online), by both patient groups for interference-related aI/fO activation (Figure S4A, available online), and that these associations increased with age (Table S10, available online). In addition, an association between lower OCD severity and greater interference-related pMFC activation at older ages emerged across uOCD and mOCD (Figure S4B available online, Table S10, available online). Analysis of pMFC subregions showed an association between lower OCD severity and greater error-related activation of dACC in uOCD (p = .012, Tables S11 and S12, available online). Associations of OCD severity with CON activation remained after covarying illness duration.
Table 3.
Cingulo-opercular network function and obsessive-compulsive disorder (OCD) severity
| ERRORS | ||||||
|---|---|---|---|---|---|---|
| pMFC | Left aI/fO | Right aI/fO | ||||
| Intercept | 1.70 ± .34 | <.001 | 1.91 ± .23 | <.001 | 1.99 ± .25 | <.001 |
| CYBOCS | −.08 ± .04 | .025 | −.05 ± .03 | .124 | ------------ | --- |
| Age | .14 ± .09 | .139 | .12 ± .08 | .142 | .29 ± .11 | .013* |
| Inc RT | ------------ | --- | ------------ | --- | ------------ | --- |
| Inc Acc | 12.66 ± 3.65 | .001* | 10.09 ± 3.40 | .005* | 7.68 ± 3.59 | .038 |
| CY-BOCS × Age | −.02 ± .01 | .079 | ------------ | --- | ------------ | --- |
| Motion | ------------ | --- | ------------ | --- | 9.91 ± 3.44 | .006* |
| Adjusted R-Square | .30 | .18 | .18 | |||
| Model ANOVA | F(4,46)=6.3 (p<.001) | F(3,47)=4.8 (p=.005) | F (3,47) = 4.6 (.006) | |||
| INTERFERENCE | ||||||
| pMFC | Left aI/fO | Right aI/fO | ||||
| Intercept | .57 ± .07 | <.001 | .35 ± .08 | <.001 | .42 ± .09 | <.001 |
| CY-BOCS | −.007 ± .01 | .478 | −.03 ± .01 | .009* | ------------ | --- |
| Age | .02 ± .03 | .504 | .01 ± .03 | .798 | .07 ± .03 | .043 |
| Overall RT | ------------ | --- | ------------ | --- | ------------ | --- |
| Overall Acc | −3.69 ± 1.81 | .045 | ------------ | --- | ------------ | --- |
| CY-BOCS × Age | −.006 ± .004 | .097 | −.008 ± .004 | .055 | ------------ | --- |
| Motion | 1.43 ± .76 | .065 | ------------ | --- | ------------ | --- |
| Adjusted R-Square | 0.09 | 0.15 | 0.05 | |||
| Model ANOVA | F(5,63)=2.3 (p=.048) | F(3,65)=4.0 (p=.012) | F(1,67)=4.3 (p=.043) | |||
Note: Motion refers to framewise displacement 28. Dashed lines represent predictor variables eliminated during backwards stepwise regression.
Significance levels were p < .05 for pMFC (primary region of interest, bolded) and p < .013 for secondary ROIs (asterisked).
Acc = accuracy, aI/fO = anterior insula/frontal operculum, CY-BOCS = Child Yale- Brown Obsessive Compulsive Scale, Inc = incongruent, pMFC = posterior medial frontal cortex, RT = Response time
Wholebrain Analyses
There were no significant effects of CYBOCS on activation to error or interference in primary analyses (i.e., medication status not modelled). However, when medication status was modelled, lower OCD severity was found to associate with greater error-related preSMA-dACC activation in uOCD (Figure 1H, Table S13, available online) and greater interference-related activation at older ages in left aI/fO across uOCD and mOCD (Figure 2H; Table S14, available online).
Discussion
Aberrant maturation of pMFC-based performance monitoring function has been posited to underlie the early course of OCD, but pMFC development remains to be characterized in young patients. In a large sample of OCD-affected compared to healthy youth, patients exhibited greater error-related activation of the pMFC and atypical increase of pMFC activation to errors with age. These findings provide new evidence of atypical development of pMFC-based error-processing function in pediatric OCD. Importantly, greater pMFC activation to errors associated with better performance in HC and lower OCD severity in patients. Collectively, these findings raise the possibility that ”hyperactive” pMFC response to errors may represent an adaptive response that normally facilitates task performance and, in pediatric OCD, develops with age to help patients control symptoms.
The notion that heightened error-related pMFC activation could serve a compensatory role in pediatric OCD is consistent with the function of this region in signaling for cognitive control to facilitate the flexible adjustment of behavior.2 In the context of OCD, increased pMFC signaling could serve to improve patients’ ability to detect and dismiss obsessive thoughts and compulsive urges as irrelevant (i.e., false alarms or ‘thinking errors’) to move on to other, more appropriate behaviors. Greater interference-related activation in pMFC correlated with better performance in HC and, in left aI/fO, with lower OCD severity in patients, implicating more general performance monitoring function (i.e., errors and interference) of the broader CON (i.e., pMFC and aI/fO). Indeed, recent work suggests that greater engagement of the CON and related networks for task control may enhance performance on cognitive tasks in adults at familial risk of OCD and protect against OCD expression.35,36 Other recent models suggest that increased pMFC signaling may serve to compensate for attentional demands of anxiety during task performance, enabling patients to maintain normal performance but not necessarily reducing anxiety or OCD symptoms.16 These possibilities represent alternatives to prior work in which increased CON response to errors in OCD was interpreted to reflect excessive emotional sensitivity to and/or detection of mistakes that could drive symptoms.10,17
Exploratory analyses showed greater pMFC and right aI/fO response to errors in mOCD than HC or uOCD, raising the possibility that medication, rather than illness, may be responsible for CON hyperactivity. However, prior work in adult OCD shows no effect of SSRIs on CON hyperactivity to errors.17 In our pediatric sample, mOCD were characterized by greater decrease from most severe past to less severe present CY-BOCS, suggesting that medication could induce CON engagement to support symptom suppression. The possibility that medication may help to resolve CON dysfunction aligns with normal association of greater error- and interference-related pMFC and right aI/fO activation with faster RT in mOCD, but attenuation of this relationship in uOCD. Similarly, the normative association of greater pMFC activation to errors (and, at trend level, interference) at younger ages was observed in mOCD, but reversed in uOCD. These findings raise the possibility that medication may normalize developmental trajectories in mOCD. By contrast, in uOCD, greater pMFC response to errors and interference at older ages and with lower OCD severity suggest that pMFC activation could increase naturalistically, with development, to help patients suppress symptoms.
The presence of age-related change in pMFC-based performance monitoring function in pediatric OCD suggests a still-developing system that could be modulated to improve outcomes in affected youth. Performance monitoring capabilities improve dramatically in typically developing adolescents, alongside age-related change in brain activation to performance monitoring demands.37 During adolescence, before CON connectivity reaches maturity,38 the pMFC may be considered less efficient, leading to greater dynamic range in task-related activity. In this light, the association of OCD-related hyperactivity with lower OCD severity may reflect a dynamic pMFC with relevance for improving illness outcomes. Given that pMFC function appears to stabilize in adulthood,39 adolescence may be a critical developmental window during which targeting pMFC is most likely to be successful. Should one try to augment function of the pMFC in pediatric OCD? Findings from the present study cannot answer this question, but justify longitudinal work to determine whether increases in pMFC-based capacity for task monitoring and control associate with reduction in OCD symptoms. In the long term, such work could pave the way for cognitive training to ‘exercise’ pMFC as augmentation and/or an alternative to currently available treatments.
Atypical age-related increase of pMFC (and right aI/fO) response to errors has been previously reported in a smaller sample of OCD compared to health youth.19 However, in contrast to our findings, the prior study showed no association of activation with OCD severity.19 Further, controls from the prior study showed no relation between activation and age,19 contrasting with the age-related decrease in activation observed in our sample of healthy youth. These inconsistencies may relate to smaller sample size19 or different analytic techniques. For example, the prior study assessed correlations of activation with OCD severity without co-varying age or performance which, as shown by our results, significantly impact CON-based performance monitoring function. On the other hand, the prior study found pre- to post-cognitive behavioral therapy (CBT) increase in interference-related pMFC activation associated with decreasing OCD severity. Consistent is the premise that greater pMFC activation during performance monitoring may index an adaptive response in OCD.
Within the pMFC, motor-cognitive compared to cognitive-affective processes have been described as localizing along a neuroanatomical continuum from posterior-dorsal to anterior-rostral areas,32 leading us to consider preSMA and dACC subregions in secondary ROI analyses. Many of the ROI results indicated trend-level effects that generalized across both areas and most results from the wholebrain analysis --- a more precise method for functional neuroanatomic localization --- were observed in an area spanning preSMA and dACC. These findings are consistent with a recent meta-analysis of nearly 10,000 fMRI studies showing task control processes (including errors and interference) to preferentially associate with preSMA and dACC.32 Consequently, we use the term “pMFC” to link to the broader literature on overlapping task control functions in the midline frontal area that encompasses these subregions,32 while also noting instances --- specifically altered associations of age and RT with error-related activation in uOCD --- in which findings may localize to dACC, not preSMA.
Strengths of this work include large sample size, replication of findings across ROI and wholebrain analyses, and consideration of performance and medication effects; however, several important limitations should be noted. OCD was the primary diagnosis but, in line with the clinical presentation of OCD, some subjects had comorbid diagnoses (e.g., anxiety, attentional problems) which may have contributed variance. Due to insufficient errors (< 5), 28% of subjects were excluded from error analyses; nonetheless, the percentage of excluded subjects was nearly the same across OCD (26%) and HC (25%) groups, meaning that observed group differences should not have been biased. In addition, patient age and illness duration were correlated such that persistent illness, rather than developmental effects, may have driven greater pMFC response to errors in older OCD subjects; disentangling age and illness duration will require the recruitment of same-aged patients who vary in OCD chronicity. Finally, we acknowledge that our study was not designed to test medication-induced change in CON function and other factors, including higher rates of CBT exposure in mOCD than uOCD, could have contributed to effects observed in exploratory analyses. Future, longitudinal work should examine CON function in patients, at different ages, before and after treatment with medication, CBT and the combination.
In summary, our study shows reversal of age-related decrease of pMFC response to errors in pediatric OCD compared to healthy youth, suggesting atypical development of neural substrate for performance monitoring during the early course of illness. In addition, error-related pMFC activity was greater in patients than controls, increased with better performance in controls and lower OCD severity in patients. Collectively, we have interpreted findings to suggest that greater pMFC engagement may serve a compensatory role in pediatric OCD. Future studies using longitudinal designs are needed to characterize maturational trajectories of pMFC and broader CON function in pediatric OCD and to determine whether increases in activation lead to reduction in illness severity after treatment and/or naturalistically over time.
Supplementary Material
Acknowledgments
None
Funding: This research was supported by the National Institute of Mental Health (NIMH) grants K23-MH082176 (K.D.F.) and R01-MH102242 (K.D.F./S.F.T.), the Dana Foundation (K.D.F.), NARSAD (K.D.F.), and the Todd Ouida Memorial Children’s Fund (K.D.F.).
Footnotes
This study was presented in symposia at the American Academy of Child and Adolescent Psychiatry’s 63rd Annual Meeting in New York, NY, October 24–29, 2016; the American College of Neuropsychopharmacology’s 55th Annual Meeting in Hollywood, FL, December 4–8, 2016; and the Society of Biological Psychiatry’s 72nd Annual Meeting in San Diego, CA, May 18–20, 2017.
Dr. Johnson served as the statistical expert for this research.
Disclosures:
Dr. Taylor has received research support through Otsuka/Vanguard Research Group and Boehringer-Ingelheim.
Drs. Fitzgerald, Liu, Johnson, Moser, Marsh, and Hanna report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Kate Dimond Fitzgerald, University of Michigan School of Public Health.
Dr. Yanni Liu, University of Michigan School of Public Health.
Dr. Timothy Johnson, University of Michigan School of Public Health.
Dr. Jason Moser, Michigan State.
Dr. Rachel Marsh, Columbia University, NY.
Dr. Gregory L. Hanna, University of Michigan School of Public Health.
Dr. Stephan F. Taylor, University of Michigan School of Public Health.
References
- 1.Fitzgerald KD, Taylor SF. Error-processing abnormalities in pediatric anxiety and obsessive compulsive disorders. CNS spectrums. 2015;20(4):346–354. doi: 10.1017/S1092852915000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 4.Rubia K, Smith AB, Woolley J, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald KD, Stern ER, Angstadt M, et al. Altered function and connectivity of the medial frontal cortex in pediatric obsessive-compulsive disorder. Biol Psychiatry. 2010a;68(11):1039–1047. doi: 10.1016/j.biopsych.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald KD, Liu Y, Stern ER, et al. Reduced error-related activation of dorsolateral prefrontal cortex across pediatric anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(11):1183–1191. e1181. doi: 10.1016/j.jaac.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna GL, Carrasco M, Harbin SM, et al. Error-related negativity and tic history in pediatric obsessive-compulsive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(9):902–910. doi: 10.1016/j.jaac.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dosenbach NU, Visscher KM, Palmer ED, et al. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol Sci. 2003;14(4):347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- 11.Maltby N, Tolin DF, Worhunsky P, O’Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24(2):495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald KD, Welsh RC, Gehring WJ, et al. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol Psychiatry. 2005;57(3):287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 13.Yucel M, Harrison BJ, Wood SJ, et al. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Arch Gen Psychiatry. 2007;64(8):946–955. doi: 10.1001/archpsyc.64.8.946. [DOI] [PubMed] [Google Scholar]
- 14.Grutzmann R, Endrass T, Kaufmann C, Allen E, Eichele T, Kathmann N. Presupplementary Motor Area Contributes to Altered Error Monitoring in Obsessive-Compulsive Disorder. Biol Psychiatry. 2016;80(7):562–571. doi: 10.1016/j.biopsych.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Agam Y, Greenberg JL, Isom M, et al. Aberrant error processing in relation to symptom severity in obsessive-compulsive disorder: A multimodal neuroimaging study. NeuroImage Clinical. 2014;5:141–151. doi: 10.1016/j.nicl.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moser JS, Moran TP, Schroder HS, Donnellan MB, Yeung N. On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Front Hum Neurosci. 2013;7:466. doi: 10.3389/fnhum.2013.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern ER, Welsh RC, Fitzgerald KD, et al. Hyperactive Error Responses and Altered Connectivity in Ventromedial and Frontoinsular Cortices in Obsessive-Compulsive Disorder. Biol Psychiatry. 2011;69:583–591. doi: 10.1016/j.biopsych.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woolley J, Heyman I, Brammer M, Frampton I, McGuire PK, Rubia K. Brain activation in paediatric obsessive compulsive disorder during tasks of inhibitory control. Br J Psychiatry. 2008;192(1):25–31. doi: 10.1192/bjp.bp.107.036558. [DOI] [PubMed] [Google Scholar]
- 19.Huyser C, Veltman DJ, Wolters LH, de Haan E, Boer F. Developmental aspects of error and high-conflict-related brain activity in pediatric obsessive-compulsive disorder: a fMRI study with a Flanker task before and after CBT. Journal of child psychology and psychiatry, and allied disciplines. 2011;52(12):1251–1260. doi: 10.1111/j.1469-7610.2011.02439.x. [DOI] [PubMed] [Google Scholar]
- 20.Button KS, Ioannidis JP, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5):365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 21.Jocham G, Ullsperger M. Neuropharmacology of performance monitoring. Neurosci Biobehav Rev. 2009;33(1):48–60. doi: 10.1016/j.neubiorev.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Andersen SL, Navalta CP. Annual Research Review: New frontiers in developmental neuropharmacology: can long-term therapeutic effects of drugs be optimized through carefully timed early intervention? J Child Psychol Psychiatry. 2011;52(4):476–503. doi: 10.1111/j.1469-7610.2011.02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Scahill L, Riddle MA, McSwiggin-Hardin M, et al. Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36(6):844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Steele VR, Claus ED, Aharoni E, et al. A large scale (N=102) functional neuroimaging study of error processing in a Go/NoGo task. Behav Brain Res. 2014;268:127–138. doi: 10.1016/j.bbr.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geller D, Biederman J, Jones J, et al. Is juvenile obsessive-compulsive disorder a developmental subtype of the disorder? A review of the pediatric literature. J Am Acad Child Adolesc Psychiatry. 1998;37(4):420–427. doi: 10.1097/00004583-199804000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Stenger VA, Boada FE, Noll DC. Three-dimensional tailored RF pulses for the reduction of susceptibility artifacts in T(*)(2)-weighted functional MRI. Magn Reson Med. 2000;44(4):525–531. doi: 10.1002/1522-2594(200010)44:4<525::aid-mrm5>3.0.co;2-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaren DG, Kosmatka KJ, Kastman EK, Bendlin BB, Johnson SC. Rhesus macaque brain morphometry: a methodological comparison of voxel-wise approaches. Methods. 2010;50(3):157–165. doi: 10.1016/j.ymeth.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worsley KJ, Poline JB, Friston KJ, Evans AC. Characterizing the response of PET and fMRI data using multivariate linear models. Neuroimage. 1997;6(4):305–319. doi: 10.1006/nimg.1997.0294. [DOI] [PubMed] [Google Scholar]
- 31.Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10(4):385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- 32.de la Vega A, Chang LJ, Banich MT, Wager TD, Yarkoni T. Large-Scale Meta-Analysis of Human Medial Frontal Cortex Reveals Tripartite Functional Organization. J Neurosci. 2016;36(24):6553–6562. doi: 10.1523/JNEUROSCI.4402-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12(5):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Draper NR, Smith H. Applied Regression Analysis. 3. John Wiley; 1998. [Google Scholar]
- 35.de Wit SJ, de Vries FE, van der Werf YD, et al. Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am J Psychiatry. 2012;169(10):1100–1108. doi: 10.1176/appi.ajp.2012.12010073. [DOI] [PubMed] [Google Scholar]
- 36.de Vries FE, de Wit SJ, Cath DC, et al. Compensatory frontoparietal activity during working memory: an endophenotype of obsessive-compulsive disorder. Biol Psychiatry. 2014;76(11):878–887. doi: 10.1016/j.biopsych.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 37.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fair DA, Dosenbach NU, Church JA, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104(33):13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fitzgerald KD, Perkins SC, Angstadt M, et al. The development of performance-monitoring function in the posterior medial frontal cortex. Neuroimage. 2010;49(4):3463–3473. doi: 10.1016/j.neuroimage.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.