Abstract
Groin pain is a frequently occurring complaint in presentations to the Emergency Department. Muscular sprain is often a differential diagnosis, however serious conditions such as pyomyositis should not be ignored. This case report presents a child with atraumatic right groin pain, which was initially diagnosed as a muscular sprain. The patient later re-presented out of hours to the Emergency Department with what was found to be extensive pelvic abscesses. He was subsequently found to have bilateral pneumonia and later developed a pericardial effusion and osteomyelitis of the right iliac bone, sacroiliac joint and sacrum. With multiple surgical interventions and appropriate antibiotics, he made a full recovery and was discharged home after a total admission time of 41 days. The causative organism was found to be Panton-Valentine leucocidin-positive methicillin-susceptible Staphylococcus aureus.
Keywords: orthopaedic and trauma surgery, infections, emergency medicine, bone and joint infections, paediatrics (drugs and medicines)
Background
A 13-year-old boy presented to the Emergency Department (ED) with severe non-traumatic right groin pain. Imaging of the pelvis found multiple abscesses. Panton-Valentine leucocidin methicillin-susceptible Staphylococcus aureus (PVL-MSSA) was isolated from the abscesses and the child required five surgical washout procedures. He developed bilateral pneumonia, pericardial effusion and osteomyelitis, with a total admission duration of 41 days. PVL-MSSA is a cause of recurrent skin and soft tissue infections in otherwise fit and healthy patients.1 Those at risk include those in closed communities with close contact and those who participate in contact sports or through the use of contaminated shared items such as towels.2 PVL-MSSA is also found in invasive infections such as necrotising pneumonia3 and necrotising fasciitis.4 It is associated with osteomyelitis, septic arthritis and pyomyositis.2 This case demonstrates that severe non-traumatic groin pain in children should not be ignored and should be investigated similarly to traumatic hip pain. It also demonstrates the significant comorbidity associated with S. aureus bacteraemia.
Case presentation
A 13-year-old boy presented to the ED with a 5-day history of severe right-sided groin pain. There was no history of trauma. The pain radiated to his right leg and lower abdomen and was exacerbated by movement of the leg. The patient was seen by a general practitioner 4 days prior, who had diagnosed it as tendonitis and discharged him home with analgesia. He became increasingly unwell over the next few days with increased pain, loss of appetite and vomiting. On examination in the ED, he appeared unwell and septic. He was reluctant to move and unable to weight bear. There was no swelling or erythema of the groin on examination. On palpation, his groin was exquisitely tender and there was generalised abdominal tenderness, particularly in the right lower quadrant. Examination of the genitals was unremarkable. The patient was unable to make any active movements of the affected leg and passive movements caused pain, particularly on internal and external rotation. Initial observations revealed a temperature of 36.7°C; heart rate 122 beats/min; blood pressure 130/72 mm Hg; respiratory rate 18 breaths/min and oxygen saturation 100% on room air.
The patient had no medical history or hospital admissions. There was no significant family history of note. He was a keen sportsman and regularly played rugby with the local team.
Differential diagnosis
Septic arthritis of the right hip
Psoas abscess
Appendicitis
Slipped upper femoral epiphysis (SUFE)
Investigations
Blood results on admission:
White cell count: 16.5×109/L (4.0–11.0)
C reactive protein: 316 mg/L (<5)
Lactate: 1.7 mmol/L (0.5–1.3).
Blood culture
S. aureus susceptible to clarithromycin, clindamycin, linezolid and flucloxacillin. Further blood cultures were all negative.
Wound swab
S. aureus susceptible to clarithromycin, clindamycin, linezolid, flucloxacillin and doxycycline.
The S. aureus samples from the culture and wound swab were positive for PVL toxin. Due to a lack of local facilities, the samples were sent to the Antimicrobial Resistance and Healthcare Associated Infections (AMRHAI) reference unit, the national reference laboratory for testing Staphylococcus species in Colindale, London. The sample testing (Illumina sequencing) confirmed detection of PVL genes and the collective data showed that the isolate belonged to multilocus sequence typing (MLST) clonal complex 5 with an allelic profile of 1,4,1,4,12,1,10. The isolates lacked the mecA and mecC genes (which encode methicillin resistance in staphylococci) and also lacked the mupA and mupB genes encoding resistance to mupirocin. The isolates also harboured the seq and sei genes encoding enterotoxins G and I, respectively.
Initial ultrasound abdomen/pelvis: lobular, echogenic mass measuring 7.0 cm x 3.5 cm x 4.0 cm within the iliac muscle, suggestive of an abscess.
MRI as shown in figure 1 confirmed the above findings: an additional collection within the right gluteal compartment abutting the right posterior iliac wing, measuring 8.7×2.4 cm. No signs of osteomyelitis were seen.
Figure 1.
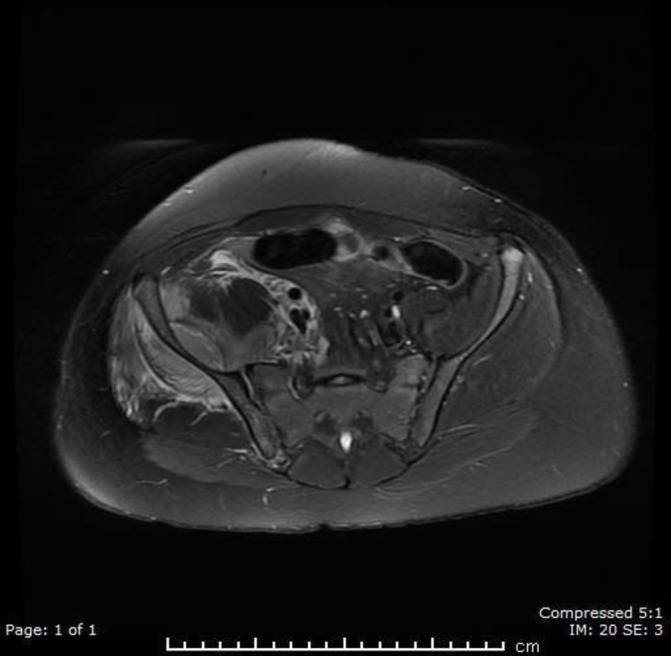
CT of the pelvis showing abscesses anterior and posterior to the iliac crest.
Treatment
After initial assessment, the patient was started on the sepsis pathway and received intravenous morphine. Intravenous flucloxacillin and cefotaxime were commenced empirically as per microbiology advice. It was decided not to perform incision and drainage out of hours by the trauma and orthopaedics team. The patient was taken to theatre for an uneventful incision and drainage of the abscesses on the morning following his presentation. The two abscesses were well defined on either side of the right iliac wing.
Outcome and follow-up
Hours after the procedure, the patients oxygen saturations had dropped to 80% on room air. The arterial blood gas showed a markedly low pO2. A CT pulmonary angiogram was performed to rule out a pulmonary embolism (PE). This showed no evidence of PE, however bibasal consolidation was found (figure 2), and clindamycin was started for pneumonia following consultation with the microbiologist.
Figure 2.
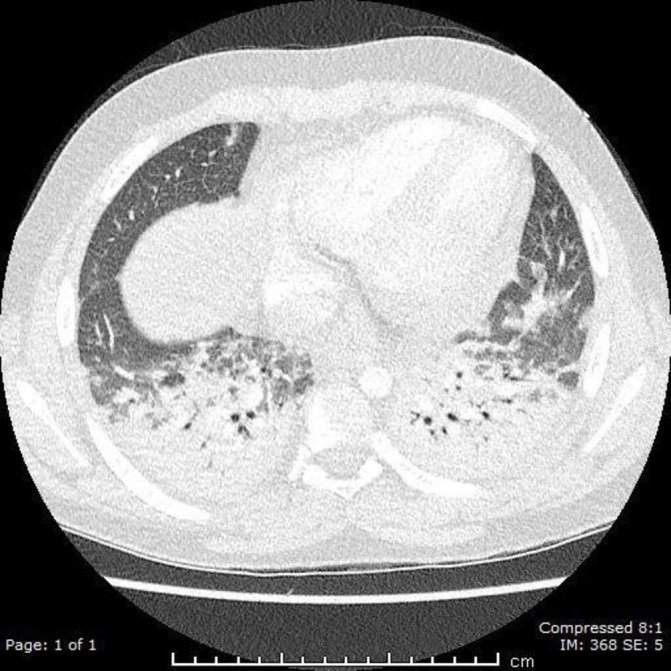
CT of the chest showing bilateral consolidation within the lung fields.
The initial blood cultures detected growth of S. aureus. As advised by the microbiologist, linezolid and clindamycin were commenced. Results from the first intraoperative pus swabs revealed growth of S. aureus and further testing confirmed PVL-MSSA. Only these two samples were tested for PVL. A tissue biopsy taken during surgery also revealed growth of S. aureus. All samples had identical antibiotic susceptibility profiles to previous cultures. Subsequent blood and pus samples sent for culture revealed no growth.
Infective endocarditis was initially thought to be the source of the multifocal infection, and a transthoracic echocardiogram was therefore performed. This showed no evidence of endocarditis, but there was a small pericardial effusion, which was not compromising the cardiac output.
The patient had four subsequent washout procedures. The third procedure found changes suggestive of osteomyelitis of the right iliac bone, which had completely resolved by the fifth and final procedure, as confirmed by MRI.
The overall admission lasted 41 days. The patient remained apyrexial throughout admission. He had clinically improved and was sent home with a course of oral antibiotics. He has remained well after 11 months of follow-up.
Discussion
S. aureus is a gram-positive coccus that is found in skin, nose or respiratory tract of 20%–30% of healthy human beings. It is a leading cause of bacteraemia and can cause a range of complications leading to increased morbidity and may be difficult to recognise initially. Depending on antibiotic susceptibility, it is broadly classified into methicillin-resistant S. aureus (MRSA) and MSSA.5 PVL is a toxin produced by certain strains of S. aureus. The toxin was first described by Panton and Valentine in 1932. The PVL genes are carried by approximately <2% of both MRSA and MSSA according to the data published in 2005,6 and the majority of the isolates were PVL-producing MSSA (62%).7 However, the data from the UK in 2010 demonstrated that 20% of the S. aureus isolated from skin or soft tissue infections was PVL positive.8
PVL infections are mostly community acquired and occur in previously healthy children and young adults.1 Most of the outbreaks of PVL-MSSA have been associated with skin and soft tissue infections. It was found that people living in closed communities with close contact (prisons and military training camps) and those who participate in contact sports (gym, rugby, wrestling and judo) are at risk. In addition, poor personal hygiene and use of contaminated shared items, such as towels, can increase the risk of infection.2 Cutaneous infections are often recurrent. Pain and erythema are often out of proportion to the severity of the signs. PVL is also found in invasive infections such as necrotising pneumonia3 and necrotising fasciitis.4 PVL-MSSA can cause septic shock and could be fatal.9 It is also associated with osteomyelitis, septic arthritis and pyomyositis.2 A systematic review of 32 patients demonstrated that septic shock, influenza like prodrome and absence of previous skin and soft tissue infections are associated with fatal outcome. In multivariate analysis, influenza like prodrome and absence of previous skin and soft tissue infections were significant predictors of death.10 It is recommended that all patients with S. aureus bacteraemia should undergo echocardiography to rule out infective endocarditis11
Within England and Wales PVL infections are not considered as a notifiable disease. It is recommended that cases of PVL should be reported to the local Health Protection Unit, particularly in the case of a PVL-related infection in a closed community, or if there is suspicion of the spread of PVL-associated infection in families, nurseries, schools or sports facilities.12 Topical decolonisation treatments are available, as is advice on reducing the risk of spread suggests maintaining a high level of cleanliness, avoiding use of shared items and covering infected lesions with regularly changed dressings.2
Mild infections may resolve without any antibiotics, however moderate to severe infections should be treated; flucloxacillin, erythromycin or clindamycin can be used. Severe infections may require rifampicin or combination therapy. It is advisable to follow local antibiotic guidelines.12
Learning points.
Severe atraumatic groin pain in a child should be investigated thoroughly.
Disproportionate pain can be caused by more serious pathology than a simple sprain.
Suspicion of a Panton-Valentine leucocidin infection in otherwise unexplained multifocal infections must be considered.
Early involvement of the microbiology team.
Public awareness of the importance of personal hygiene, particularly in association with contact sports.
Footnotes
Contributors: NI, SKGP and JSW-J saw this case in the emergency department. NI wrote the case report and obtained consent from the patient and guardian. SKGP and NI performed the literature search. SKGP and JSW-J critically appraised the article and contributed to make necessary changes in the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol 2010;64:143–62. 10.1146/annurev.micro.112408.134309 [DOI] [PubMed] [Google Scholar]
- 2. Sheikh HQ, Aqil A, Kirby A, et al. Panton-Valentine leukocidin osteomyelitis in children: a growing threat. Br J Hosp Med 2015;76:18–24. ISSN 1750-8460 10.12968/hmed.2015.76.1.18 [DOI] [PubMed] [Google Scholar]
- 3. Haider S, Wright D. Panton-Valentine leukocidin Staphylococcus causing fatal necrotising pneumonia in a young boy. BMJ Case Rep 2013;2013:bcr2012007655 10.1136/bcr-2012-007655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perbet S, Soummer A, Vinsonneau C, et al. Multifocal community-acquired necrotizing fasciitis caused by a Panton-Valentine leukocidin-producing methicillin-sensitive Staphylococcus aureus. Infection 2010;38:223–5. 10.1007/s15010-010-0002-7 [DOI] [PubMed] [Google Scholar]
- 5. Tong SY, Davis JS, Eichenberger E, et al. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015;28:603–61. 10.1128/CMR.00134-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 2002;359:753–9. 10.1016/S0140-6736(02)07877-7 [DOI] [PubMed] [Google Scholar]
- 7. Fogo A, Kemp N, Morris-Jones R. PVL positive Staphylococcus aureus skin infections. BMJ 2011;343:d5343 10.1136/bmj.d5343 [DOI] [PubMed] [Google Scholar]
- 8. Bourigault C, Corvec S, Brulet V, et al. Outbreak of skin infections due to Panton-Valentine Leukocidin-Positive methicillin-susceptible staphylococcus aureus in a French Prison in 2010-2011. PLoS Curr 2014;6 10.1371/currents.outbreaks.e4df88f057fc49e2560a235e0f8f9fea [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akpaka PE, Monecke S, Swanston WH, et al. Methicillin sensitive Staphylococcus aureus producing Panton-Valentine leukocidin toxin in Trinidad & Tobago: a case report. J Med Case Rep 2011;5:157 10.1186/1752-1947-5-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kreienbuehl L, Charbonney E, Eggimann P. Community-acquired necrotizing pneumonia due to methicillin-sensitive Staphylococcus aureus secreting Panton-Valentine leukocidin: a review of case reports. Ann Intensive Care 2011;1:52 10.1186/2110-5820-1-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holland TL, Arnold C, Fowler VG. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 2014;312:1330–41. 10.1001/jama.2014.9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Health Protection Agency. Guidance on the diagnosis and management of PVL-associated Staphylococcus aureus infections (PVL-SA) in England [Internet]. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/322857/Guidance_on_the_diagnosis_and_management_of_PVL_associated_SA_infections_in_England_2_Ed.pdf (cited 6 Oct 2017).


