Abstract
Methotrexate is one of the most commonly used drugs in autoimmune disorders like rheumatoid arthritis. Gastrointestinal symptoms like nausea and stomatitis, skin rashes, alopecia, central nervous system symptoms like headache and confusion, hepatotoxicity and myelosuppression are some of the adverse effects. However, low oral doses on a weekly basis seldom show any signs of toxicity. Leucovorin or folinic acid is given along with methotrexate as rescue to reduce the toxic effects like bone marrow suppression. Non-steroidal anti-inflammatory drugs, like aceclofenac, are also used in chronic inflammatory conditions like rheumatoid arthritis and osteoarthritis. Nephrotoxicity is one of the adverse effects of both methotrexate and non-steroidal anti-inflammatory drugs; and its combined administration should be done with caution. This is a case of an elderly woman, a known case of rheumatoid arthritis, who presented in severe bone marrow suppression due to methotrexate toxicity following aceclofenac-induced acute kidney injury.
Keywords: acute renal failure, rheumatoid arthritis
Background
Methotrexate, in the form of weekly doses, has been commonly used in the treatment of rheumatoid arthritis (RA), and such low dose can rarely cause any toxicity like bone marrow suppression. Aceclofenac is a non-steroidal anti-inflammatory drug (NSAID) with analgesic, antipyretic and anti-inflammatory activity. In comparison with other NSAIDs, it is more gastric friendly. Acute kidney injury (AKI) is a complication of both methotrexate and aceclofenac.
This is a case of an elderly woman, who was on regular treatment for RA with methotrexate. She developed acute bone marrow suppression due to methotrexate toxicity. Further investigation revealed an AKI following aceclofenac consumption as the cause for methotrexate accumulation.
Case presentation
A 65-year-old woman was brought by her relatives with multiple oral ulcers and inability to eat food. She was a known case of RA for the past 10 years; on regular treatment with an outside rheumatologist. Her medication list comprised oral methotrexate (10 mg once weekly), hydroxychloroquine (400 mg once daily), and folic acid and calcium with vitamin D supplementation. Her last complete haemogram, liver and renal functions, which had been tested about 2 weeks prior to presentation, were within normal ranges. She had consumed aceclofenac (100 mg) tablets 2–3 times daily for the past 10 days, as over-the-counter medication, for her knee joint pain. She was a diabetic for the past 10 years, on diet restrictions.
On examination, she was conscious, oriented and afebrile, with a body mass index of 22.67 kg/m2 (height 168 cm, weight 64 kg). She had pallor and bloodshot eyes (figure 1). Her oral cavity showed multiple ulcers (figure 2). Her vitals and systemic examinations were normal. She did not have any small joint pains or swelling. She had bilateral knee osteoarthritis.
Figure 1.
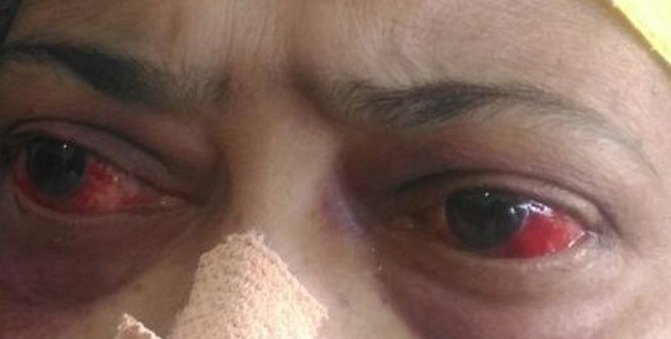
Bloodshot eyes due to subconjunctival haemorrhage.
Figure 2.
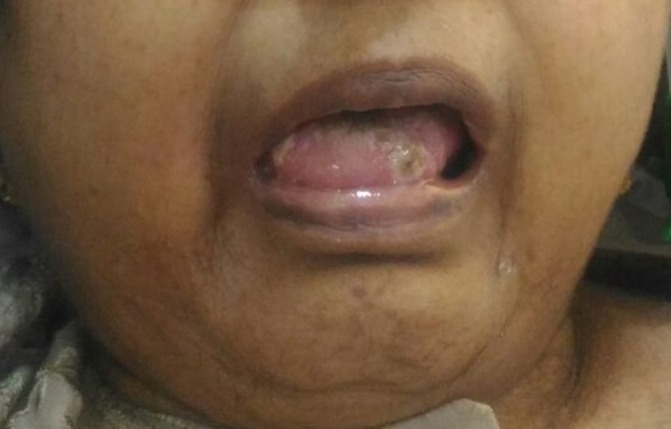
Oral ulcers.
Investigations
Her blood investigations showed pancytopenia with haemoglobin 72 g/L, total white cell count (WCC) 0.9×109/L (neutrophils 30%, lymphocytes 60%, eosinophils 10%) and platelets 7×109/L. Her renal functions were deranged (BUN 12.24 mmol/L and creatinine 683 µmol/L). Her fasting blood glucose was 7.43 mmol/L and haemoglobin A1c was 7.1%. Liver function test showed total bilirubin 49.59 µmol/L, direct bilirubin 13.68 µmol/L, SGOT 99 U/L, SGPT 187 U/L, ALP 364 U/L and albumin 23 g/L. Other investigations like serum electrolytes, TSH, vitamin B12, PT/INR and apTT were normal. Urine microscopy did not show any pus cells or haematuria. Viral markers (HBsAg, anti-HCV and HIV) were negative. Serum ferritin level was mildly elevated (350 ng/mL), ESR was 36 mm/hour, serum triglycerides and fibrinogen were normal, and peripheral smear showed pancytopenia. Serum methotrexate level, after 7 days from the last dose, was 0.1 µmol/L. ECG, ECHO heart and chest X-ray were normal. Stool occult blood was positive (probably due to uraemia or stress ulcers in the setting of thrombocytopenia). Ultrasound of the abdomen showed mild fatty liver.
Treatment
From the history, clinical findings and laboratory investigations, a provisional diagnosis of methotrexate toxicity following aceclofenac-induced AKI was considered. She was started on intravenous leucovorin/folinic acid (15 mg every 6 hours) and subcutaneous filgrastim (G-CSF 300 mcg once daily). She had two episodes of high-grade fever following admission; hence intravenous meropenem (500 mg every 12 hours), levofloxacin (500 mg every 24 hours) and fluconazole (150 mg every 24 hours) were administered empirically. She was also given packed cell and platelet transfusions, along with intravenous fluids and pantoprazole infusion.
Outcome and follow-up
On admission, patient was non-oliguric. By day 3 of admission, her condition started improving. Following hydration, her urine output improved. Conjunctival congestion started reducing. Her haemogram showed a stabilising trend that is, haemoglobin 89 g/L, total WCC 2×109/L and platelets 33×109/L. No further transfusions were given, and filgrastim was stopped. Renal and liver parameters also showed improvement (BUN 7.74 mmol/L and creatinine 424 µmol/L, total bilirubin 35.91 µmol/L, direct bilirubin 13.68 µmol/L, SGOT 79 U/L, SGPT 132 U/L, ALP 304 U/L and albumin 25 g/L). Bone marrow examination was withheld for the time being. On day 6 of admission, the patient had an episode of vomiting, followed by aspiration pneumonia. Her condition started worsening rapidly, requiring ventilator and inotropic support. However, due to the poor prognosis, the patient’s relatives were not willing for aggressive management. The patient went into septic shock and passed away.
Discussion
According to the National Kidney foundation, AKI is characterised by a sudden episode of kidney failure or kidney damage that occurs within a few hours or a few days. It may be due to decreased blood flow to the kidneys (hypotension, burns injury, NSAIDs, myocardial infarction, heart failure) or a direct damage to the kidneys (sepsis, multiple myeloma, vasculitis) or following blockage in the urinary tract (renal calculi, carcinoma, prostatomegaly). There are four major categories of biomarkers for the prediction of AKI: (a) functional markers (serum creatinine, serum cystatin C), (b) up-regulated proteins (neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, liver fatty acid binding protein, interleukin-18), (c) low-molecular weight proteins (urine cystatin C), (d) tubular enzymes (alpha-glutathione S-transferase, pi-glutathione S-transferase, gamma glutamyl transpeptidase, alkaline phosphatase, N-acetyl-β-D-glucosaminidase).1 Mechanical ventilation, elevated urea and creatinine at admission are independent risk factors for AKI; whereas mechanical ventilation, increased lactate, uraemia and hypernatraemia are regarded as independent risk factors for intensive care unit mortality.2
Methotrexate, a folic acid antagonist, has cytotoxic and immunosuppressive actions. Because of these properties, the drug has been used in the treatment of certain cancers, psoriasis and RA. It is metabolised in the liver and excreted largely by the kidneys. Pharmacokinetic studies show that more than 90% of the drug is cleared within the first 24 hours after its administration.3 Toxic effects due to low-dose methotrexate are rare; comprising hepatotoxicity, nephrotoxicity, anaemia, leucopenia, thrombocytopenia, pancytopenia, gastrointestinal side effects and mucocutaneous problems.4–7 However, in cases of renal impairment, these low doses may be enough to cause bone marrow suppression.8 Diabetes may have a role in altering the pharmacokinetics of methotrexate by altering the physiological environment of the body.9 Nephrotoxicity occurs either due to its direct toxic effect on renal tubules or following precipitation of its metabolites, like 7-hydroxy-methotrexate, in the renal tubules.5 10 11 Since methotrexate can decrease red cell folate levels, leucovorin (folinic acid) has been used to diminish the toxicity and counteract the effects of impaired methotrexate elimination.12 13
Nephrotoxicity due to NSAIDs continues to be a matter of concern. These drugs inhibit prostaglandin synthesis from arachidonic acid by non-specific blocking of the enzyme cyclooxygenase, thereby leading to vasoconstriction and reversible mild renal impairment in volume-depleted states. When unopposed, this may progress to acute tubular necrosis (ATN) and AKI. These drugs can also cause acute interstitial nephritis with or without nephrotic syndrome.14 Aceclofenac, an arylacetic acid NSAID, has been reported to cause ATN.15 Drug-induced ATN usually improves after cessation of the offending medication; however, NSAID-induced ATN may result in permanent renal damage. The use of steroid therapy in ATN is controversial.
A differential diagnosis of macrophage activation syndrome (MAS) was considered in our patient. MAS, a form of haemophagocytic lymphohistiocytosis (HLH), is a rare complication associated with several systemic autoimmune disorders like ankylosing spondylitis, RA and systemic lupus erythematosus.16 According to the Histiocyte Society, the diagnosis of HLH requires five out of the eight criteria: (1) prolonged fever; (2) splenomegaly; (3) cytopenias affecting at least two of three lineages in the peripheral blood; (4) hypertriglyceridaemia and/or hypofibrinogenaemia; (5) haemophagocytosis in bone marrow, spleen or lymph; (6) low or absent NK-cell activity; (7) hyperferritinaemia and (8) high levels of soluble CD25. According to the American College of Rheumatology, the European League Against Rheumatism and the Paediatric Rheumatology International Trials Organisation, the classification criteria for MAS (2016) suggests: a febrile patient with known or suspected systemic juvenile idiopathic arthritis is classified as having MAS if the following criteria are met: ferritin >684 ng/mL and any two of the following: platelet count ≤181×109/L, aspartate aminotransferase >48 units/L, triglycerides >156 mg/dL or fibrinogen ≤360 mg/dL. A low or a decreasing trend in ESR is seen in MAS. Our patient did not fulfil the criteria for MAS and had an elevated ESR.
Only a handful of cases have been reported with regard to bone marrow suppression following low-dose methotrexate.16–19 The patient being described was a known case of RA (on low-dose weekly methotrexate) and diabetes mellitus. She was on regular follow-ups and did not have any signs of methotrexate toxicity previously. She had consumed multiple tablets of aceclofenac as self-medication for her knee pain, leading to AKI. The condition was worsened with accumulation of methotrexate in the setting to renal failure, causing further nephrotoxicity and severe bone marrow suppression. Diabetes would have played an additive role to worsen the scenario. This case, therefore, highlights the cautious use of NSAIDs in patients with RA on methotrexate therapy, especially with underlying diabetes.
Learning points.
Low-dose methotrexate can cause toxicity in the setting of renal failure.
Cautious use of non-steroidal anti-inflammatory drugs in patients on methotrexate therapy.
Regular monitoring of liver and renal functions in patients on methotrexate therapy.
Footnotes
Contributors: RGM: concept and design of case report, reviewed the literature, manuscript preparation and treating physician. DP: reviewed the literature and treating medical oncologist. JP: reviewed the literature and treating nephrologist. IKB: reviewed the literature and treating gastroenterologist.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.de Geus HR, Betjes MG, Bakker J. Biomarkers for the prediction of acute kidney injury: a narrative review on current status and future challenges. Clin Kidney J 2012;5:102–8. 10.1093/ckj/sfs008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peres LA, Wandeur V, Matsuo T. Predictors of acute kidney injury and mortality in an Intensive Care Unit. J Bras Nefrol 2015;37:38–46. 10.5935/0101-2800.20150007 [DOI] [PubMed] [Google Scholar]
- 3.Henderson ES, Adamson RH, Oliverio VT. The metabolic fate of tritiated methotrexate. II. Absorption and excretion in man. Cancer Res 1965;25:1018–23. [PubMed] [Google Scholar]
- 4.Gilani ST, Khan DA, Khan FA, et al. Adverse effects of low dose methotrexate in rheumatoid arthritis patients. J Coll Physicians Surg Pak 2012;22:101–4. doi:02.2012/JCPSP.101104 [PubMed] [Google Scholar]
- 5.Methotrexate in rheumatoid arthritis. Health and Public Policy Committee, American College of Physicians. Ann Intern Med 1987;107:418–9. [PubMed] [Google Scholar]
- 6.Mayall B, Poggi G, Parkin JD. Neutropenia due to low-dose methotrexate therapy for psoriasis and rheumatoid arthritis may be fatal. Med J Aust 1991;155:480–4. [DOI] [PubMed] [Google Scholar]
- 7.Trenkwalder P, Eisenlohr H, Prechtel K, et al. Three cases of malignant neoplasm, pneumonitis, and pancytopenia during treatment with low-dose methotrexate. Clin Investig 1992;70:951–5. 10.1007/BF00180446 [DOI] [PubMed] [Google Scholar]
- 8.Strang A, Pullar T. Methotrexate toxicity induced by acute renal failure. J R Soc Med 2004;97:536–7. 10.1177/014107680409701106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iqbal T, Nawaz R, Ilahi A, et al. Disposition kinetics of sulphadiazine in normal and diabetic rabbits. J Pak Med Assoc 1989;39:50–3. [PubMed] [Google Scholar]
- 10.Messmann R, Antifolates AC, In Chabner B, Longo D, et al. Cancer chemotherapy and biotherapy. 10th edn Philadelphia: Lippincott Williams & Wilkins, 2001:139–84. [Google Scholar]
- 11.Smeland E, Fuskevåg OM, Nymann K, et al. High-dose 7-hydromethotrexate: acute toxicity and lethality in a rat model. Cancer Chemother Pharmacol 1996;37:415–22. 10.1007/s002800050406 [DOI] [PubMed] [Google Scholar]
- 12.van Ede AE, Laan RF, Blom HJ, et al. Homocysteine and folate status in methotrexate-treated patients with rheumatoid arthritis. Rheumatology 2002;41:658–65. 10.1093/rheumatology/41.6.658 [DOI] [PubMed] [Google Scholar]
- 13.Ortiz Z, Shea B, Suarez Almazor M, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev 2000;2:CD000951 10.1002/14651858.CD000951 [DOI] [PubMed] [Google Scholar]
- 14.Ejaz P, Bhojani K, Joshi VR. NSAIDs and kidney. J Assoc Physicians India 2004;52:632–40. [PubMed] [Google Scholar]
- 15.Gupta M, Cruz SD, Nada R, et al. Aceclofenac-induced acute tubulointerstitial nephritis in a patient with diabetes. Case Rep Child Meml Hosp Chic 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granata G, Didona D, Stifano G, et al. Macrophage activation syndrome as onset of systemic lupus erythematosus: A case report and a review of the literature. Case Rep Med 2015;2015:1–4. 10.1155/2015/294041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sosin M, Handa S. Low dose methotrexate and bone marrow suppression. BMJ 2003;326:266–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calvo-Romero JM. Severe pancytopenia associated with low-dose methotrexate therapy for rheumatoid arthritis. Ann Pharmacother 2001;35:1575–7. 10.1345/aph.1A052 [DOI] [PubMed] [Google Scholar]
- 19.Laroche F, Perrot S, Menkès CJ. [Pancytopenia in rheumatoid arthritis treated with methotrexate]. Presse Med 1996;25:1144–6. [PubMed] [Google Scholar]


