Abstract
Ciprofloxacin, a very common antibiotic used in our day-to-day practice can cause adverse cutaneous reactions in 1–2% of patients. Photosensitivity, urticaria and maculopapular rash are the usual skin reactions. Fixed drug eruption (FDE) is an uncommon side effect of ciprofloxacin. Ciprofloxacin-induced generalised bullous FDEs have been very rarely reported in the literature. We report one such case of a young man who developed generalised non-bullous FDEs after treatment with ciprofloxacin.
Keywords: foodborne infections, unwanted effects / adverse reactions, skin
Background
Fixed drug eruptions (FDEs) occur as well-circumscribed round to oval macules with a dusky red centre and a pale periphery with or without associated bulla. They disappear after discontinuing the drug leaving a residual hyperpigmentation. Recurrence at the same site after reinstitution of the drug with worse and increased number of lesions is very characteristic. It is very alarming to the patients and their care takers. The usual sites of occurrence are the genitals typically glans penis, oral mucosa and the extremities. Trunk and neck are less commonly involved. Our patient had more lesions spread over the trunk and few lesions in the extremities sparing the genitals and oral mucosa. The lesions in FDE are usually few in number limited to one particular part of the body but our patient had 12 lesions in a generalised distribution. Generalised bullous FDEs are reported with ciprofloxacin but the non-bullous variant mimicking erythema multiforme which can easily misguide the physician to look for an infectious cause are rarely reported in the literature. This case is reported because of its rarity and alarming concern to the patient.
Case presentation
A 19-year-old man was admitted with a history of high grade fever for a week, generalised body pain and dysphagia. He had around 10 episodes of diarrhoea for 2 days before the onset of fever. There was no history of bleeding or drug allergy. On examination, he was febrile (1040 F), with pulse rate 96/min, BP 110/80 mm Hg and respiratory rate 14/min. Pharyngeal congestion was present. There were no rash on admission. Systemic examination was normal. Routine blood investigations were normal on the day of admission. A provisional diagnosis of acute pharyngitis was made. He was started on intravenous cefotaxime. Following 2 days of treatment, he remained febrile. Since the culture reports were pending, intravenous ciprofloxacin was administered empirically for enteric fever. Twenty-four hours later, he complained of itching over the chest.
Multiple round well-circumscribed erythematous lesions with a dark red centre and paler periphery over his chest, abdomen, neck, back, left shoulder (figure 1) and both thighs around 12 in number were present. The largest of the lesion was of size 6×5 cm in the right infra-axillary region (figure 2). There was no orogenital lesion. Ciprofloxacin was immediately discontinued. Twenty-four hours after discontinuing the drug, the lesions decreased in size and started to fade. He was started on topical betamethasone. Five days after discontinuing ciprofloxacin, all the erythematous rashes got replaced by hyperpigmented macules (figures 3 and 4). He was receiving cefotaxime when ciprofloxacin was discontinued. He was advised to continue the topical steroid for 1 week.
Figure 1.
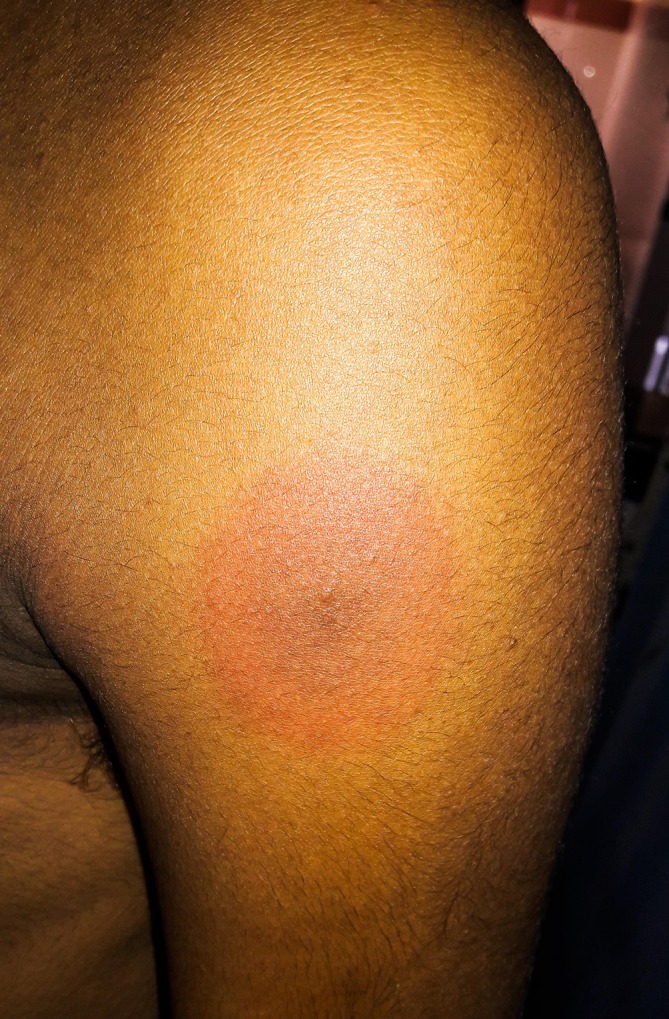
Left deltoid. A patch of 4×4 cm size with a dusky red centre surrounded by a pale pink periphery.
Figure 2.
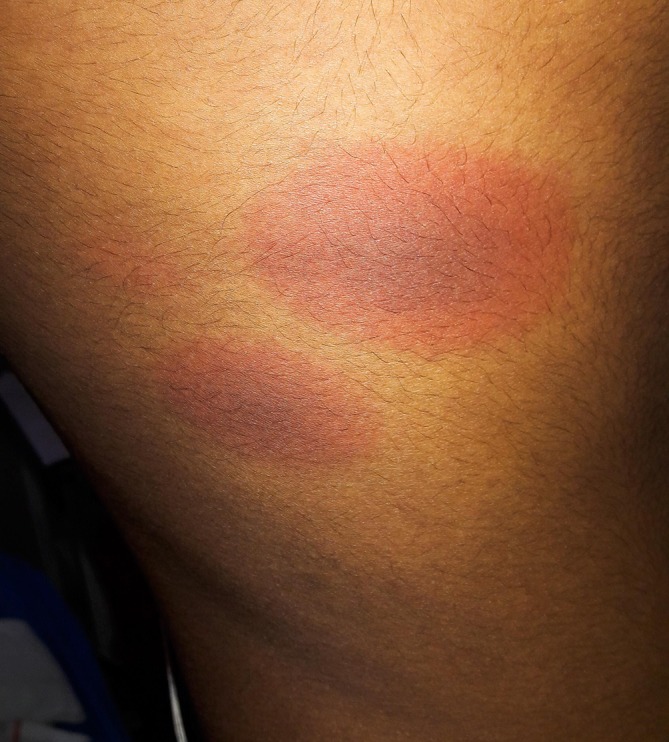
Right infraaxillary area. Two patches, the largest one measuring 6×5 cm. The lesions are similar as depicted in figure 1.
Figure 3.
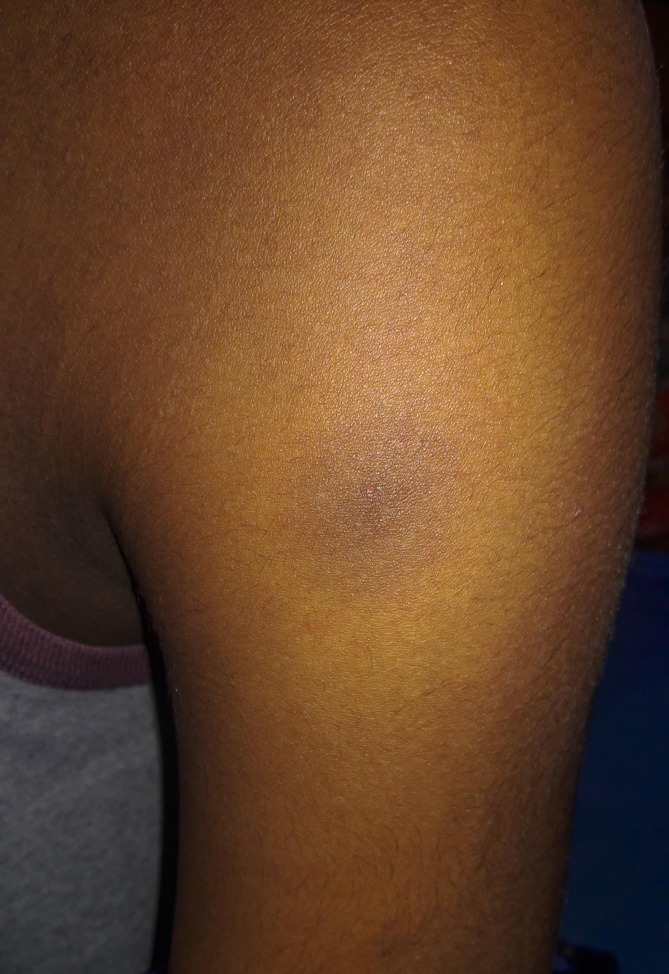
Left deltoid. Lesion in figures 1, 5 days after discontinuing ciprofloxacin. The primary erythematous lesion has disappeared leaving a hyperpigmented residue.
Figure 4.
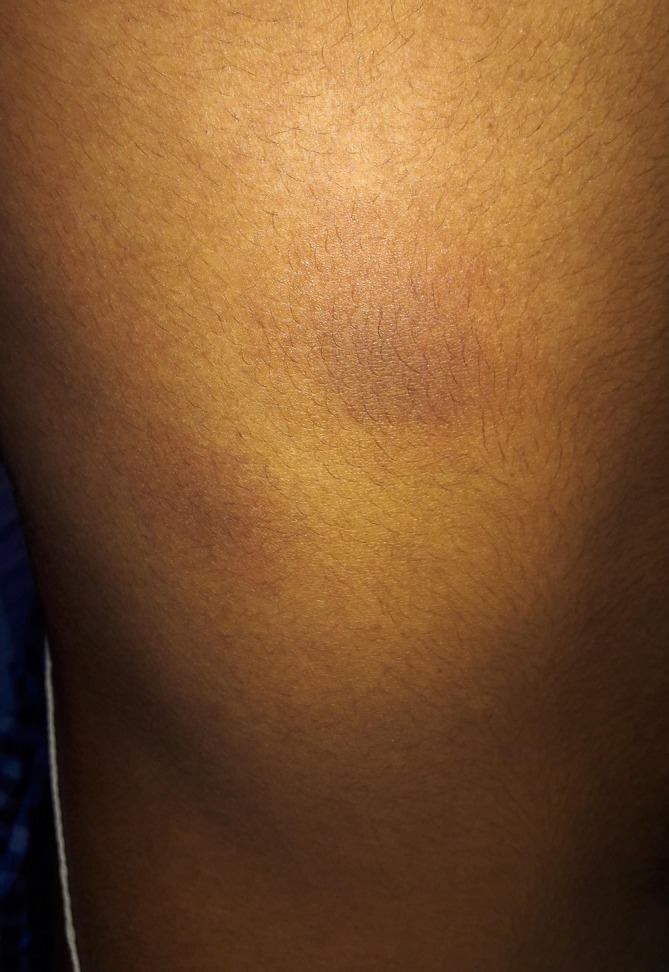
Right infra-axillary area. The lesions in figures 2, 5 days after discontinuing ciprofloxacin. The primary erythematous lesions have disappeared leaving hyperpigmented residues.
As the patient denied to undergo an oral challenge test, we had used Naranjo et al’s algorithm to determine the causal relationship between the drug exposure and adverse reaction (ADR).1 There are previous conclusive reports on this reaction (+1), the adverse event appeared after administering ciprofloxacin (+2), the ADR improved immediately after discontinuing the drug (+1) and the reaction was less severe when the dose decreased (+1). The patient had an overall score of 5, labelling the adverse reaction as ‘Probable’. Based on Hartwig et al scale, the ADR was moderate in severity (level 4).2
Investigations
Total WBC: 11 000/cubic mm.
Differential count: neutrophils 82%, lymphocytes 16%, eosinophils 4%.
Platelets: 2.2 lakh/cubic mm.
INR: 1.1.
WIDAL: Salmonella typhi O – 1:40, H – 1:20.
Salmonella paratyphi AH – 1:20, BH – 1:20.
Differential diagnosis
Erythema multiforme, Lyme disease and Toxic epidermal necrolysis.
Outcome and follow-up
On discharge from the hospital, the patient had hyperpigmented macules over the sites of primary lesions. The patient and his parents were warned about the recurrence of the rash on subsequent exposure to ciprofloxacin and to inform the physicians in future consultations. He was advised to avoid ciprofloxacin during the rest of his life.
Discussion
In 1884, Brocq first described FDEs associated with antipyrines. It is thought to be a result of lymphocyte CD8-mediated delayed type of hypersensitivity reaction against the drug which may induce reactivation of memory T-cell lymphocytes that are localised in epidermal and dermal tissues.3 4 A biopsy of the lesions reveal infiltration of superficial and deep epidermal plexuses by neutrophils, lymphocytes and eosinophils. Severe form of FDE should be distinguished from toxic epidermal necrolysis (TEN). Both may behave as same clinically and histologically. After discontinuing the drug FDE usually resolves, while TEN may continue to progress fatally.5 Drugs commonly causing FDE are one among the antiepileptics, non-steroidal anti-inflammatory drugs or antibiotics. Antibiotics commonly encountered with FDE are cotrimoxazole, amoxicillin, doxycycline, tetracycline, metronidazole, norfloxacin and ciprofloxacin. Most commonly involved site for FDE in men is the orogenital mucosa and in women, it is the trunk and extremities.
At the first glance, the skin lesions resembled target lesions of erythema multiforme (EM). Our patient’s lesion was a macule with two concentric rings, the outer paler ring and central dusky red ring. In EM, the characteristic target lesion also called as iris lesion has three rings comprising the central dark red or dusky area which may have blisters, surrounded by a pale pink ring which is raised due to oedema and the outermost bright red ring. Erythema multiforme usually starts as papules evolving into plaques. Our patient had an acute presentation, all the lesions appeared over 24 hours and there was no progression. There were no papules. EM lesions are predominantly spread over the extremities. Smaller satellite lesions around the primary lesion are seen in EM. There were no satellite lesions seen in our patient, so erythema multiforme was ruled out of diagnosis.
Ciprofloxacin is a well-tolerated drug used very commonly in our day-to-day practice. Ciprofloxacin can cause both delayed type and IgE-mediated hypersensitivity reactions.6 Extremities are the usual sites of ciprofloxacin-induced FDE. Our patient had eight lesions in the trunk and four on the extremities. Differential diagnosis considered in our patient were bullous disorders, erythema multiforme, Lyme disease and ecchymoses due to thrombocytopaenia. Acute onset of the rash 24 hours following ciprofloxacin exposure, disappearance of the rash after the discontinuation of ciprofloxacin and residual hyperpigmentation, no other bleeding manifestations, all favoured to come to a diagnosis of ciprofloxacin-induced generalised non-bullous FDE. The possibility of cefotaxime as a cause of this reaction is very unlikely since the patient was receiving cefotaxime from the day of admission till he was discharged.
Patient’s perspective.
I had come to see the physician for my fever some days back. Few days after admission I saw these itchy big rings all over my body. I was shocked and showed my parents and the doctor. The doctor explained me that it may be due to the new drug he added the previous day. He stopped that drug. The next day I saw the rings decreasing in size and I think in 2 or 3 days all of them disappeared. The doctor had told me that this is the first time he had seen something like this for that particular medicine which he had been using very often. I thank the doctor and his team for the efforts they took to treat me.
Learning points.
Though ciprofloxacin is not a common drug to cause fixed drug eruption, this adverse cutaneous reaction is alarming to patients and the physician particularly when it is generalised as in our case.
Immediate discontinuation of the drug, topical steroids and avoidance of the drug in the future are the simple treatment measures to be followed.
Labelling this possible adverse effect by the drug manufacturers and warning the physicians should be considered by the pharmaceutical industry.
There is no such drug which is completely safe. As William Osler once said ‘One of the first duties of a physician is to educate the masses not to take medicines’.
Footnotes
Contributors: MI: treating physician; case diagnosis, management and manuscript writing. MRSR: literature review. UD: proofreading and corrections.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Naranjo CA, Busto U, Sellers EM, et al. . A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45. 10.1038/clpt.1981.154 [DOI] [PubMed] [Google Scholar]
- 2. Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm 1992;49:2229–32. [PubMed] [Google Scholar]
- 3. Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by self-inflicted responses of intraepidermal T cells. Eur J Dermatol 2007;17:201–8. 10.1684/ejd.2007.0149 [DOI] [PubMed] [Google Scholar]
- 4. Shiohara T, Tetsuo S. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol 2009;9:316–21. 10.1097/ACI.0b013e32832cda4c [DOI] [PubMed] [Google Scholar]
- 5. Butler DF, Ilse RI. Fixed drug eruptions, E-medicine Dermatology, 2010. [Google Scholar]
- 6. Sánchez-Morillas L, Rojas Pérez-Ezquerra P, González Morales ML, et al. . Fixed drug eruption due to norfloxacin and cross-reactivity with other quinolones. Allergol Immunopathol 2013;41:60–1. 10.1016/j.aller.2011.10.004 [DOI] [PubMed] [Google Scholar]


