Abstract
A previously healthy 67-year-old farmer presented to an outside hospital after a 2-week history of non-specific respiratory symptoms. A certain diagnosis was not initially apparent, and the patient was discharged home on a regimen for presumed chronic obstructive pulmonary disease exacerbation. He re-presented to the emergency department with shock and hypoxaemic respiratory failure requiring prompt intubation and fluid resuscitation. He was then transferred to our institution due to multiorgan failure. On arrival, the patient demonstrated refractory shock and worsening acute kidney injury, severe anaemia and thrombocytopaenia. The peripheral smear revealed absence of microangiopathic haemolytic anaemia. A closer review of the smear displayed red blood cell inclusion bodies consistent with babesiosis. The patient was started on clindamycin and loaded with intravenous quinidine, and subsequently transitioned to oral quinine. A red cell exchange transfusion was pursued with improvement of the parasite load. The patient was discharged home on clindamycin/quinine and scheduled for outpatient intermittent haemodialysis.
Keywords: infectious diseases, intensive care, adult intensive care, mechanical ventilation
Background
Babesiosis is a tick-borne disease caused by obligate intraerythrocytic protozoa. The severity of the infection ranges from an asymptomatic disease to a fulminant illness, primarily depending on the immune status of the host.1 We illustrate an unusual case of severe babesiosis in an immunocompetent host presenting with multiorgan failure and shock, highlighting the potential for severe presentation of babesiosis despite an intact immune system. In addition, this case calls attention to the value of red cell exchange transfusion (ET) among patients with high-grade parasitaemia and severe anaemia. A high degree of suspicion and prompt recognition of this clinical entity lead to timely treatment and avoidance of worsening morbidity.
Case presentation
A previously healthy 67-year-old farmer presented to an outside medical institution after a 2-week history of fatigue, shortness of breath, non-productive cough and intermittent fevers. Despite known exposure through his farm occupation and outdoor lifestyle, the patient did not recall a tick bite prior to the onset of the symptoms. On admission to the emergency department (ED), the patient was alert, in moderate distress and afebrile. Vital signs were notable for a blood pressure (BP) of 140/73 mm Hg, respiratory rate (RR) of 26 breaths per minute, heart rate (HR) of 112 beats per minute (bpm) and SpO2 of 95% on room air. His physical examination was only remarkable for mild expiratory wheezes bilaterally. Laboratory work-up revealed hyperglycaemia (343 mg/dL), anaemia (haemoglobin 11.4 g/dL) and thrombocytopaenia (platelet count 49×109/L). A certain diagnosis was not initially apparent, and because of symptomatic dyspnoea and a CT pulmonary angiogram revealing signs of early emphysema, he was discharged home on a regimen for presumed chronic obstructive pulmonary disease exacerbation.
Over the ensuing days, the patient’s symptoms persisted and he developed progressive worsening in his respiratory status. He re-presented to the ED with shock and hypoxaemic respiratory failure requiring prompt intubation and fluid resuscitation. Laboratory studies were remarkable for worsening anaemia (haemoglobin 8.2 g/dL), thrombocytopaenia (platelet count 53×109/L), hyperglycaemia (668 mg/dL), metabolic acidosis (lactate 6.7 mmol/L; bicarbonate 12 mmol/L; anion gap (AG) 29 mmol/L) and acute kidney injury (creatinine 5.66 mg/dL). The patient was initiated on an insulin drip for presumed diabetic ketoacidosis and emergently transferred to our institution for further care.
On arrival to our intensive care unit (ICU), his vital signs revealed BP of 80/30 mm Hg, HR of 132 bpm, RR of 12 breaths per minute and peripheral capillary oxygen saturation (SpO2) of 99% while on mechanical ventilation (fractional inspired oxygen (FiO2) 60%; positive end-expiratory pressure (PEEP) 10 cmH2O). A central venous access was established and he was initiated on quickly escalating doses of vasopressors for refractory shock. On examination, the patient exhibited generalised pallor, cool skin and dry mucous membranes. His cardiac examination showed tachycardia, but no murmurs, rubs or gallops. The lung auscultation was notable for expiratory wheezes bilaterally, and his abdomen was soft, non-distended, and without signs of splenomegaly and hepatomegaly.
Investigations
Initial laboratory work-up showed persistent metabolic acidosis (arterial blood gas: pH 7.28/partial pressure of oxygen 85 mm Hg/partial pressure of carbon dioxide 29 mm Hg/HCO3− 16, on FiO2 60% and PEEP 10 cmH2O; lactate 3.2 mmol/L; AG 21 mmol/L), acute kidney injury (creatinine 5.6 g/dL), marked hyperglycaemia (459 mg/dL), worsening anaemia (haemoglobin 5.6 g/dL) and thrombocytopaenia (36×109/L). The white cell count (5.6×109/L) and the remaining electrolytes were within normal limits. The liver function test revealed transaminitis (AST 90 U/L; ALT 68 U/L; alkaline phosphatase 124 U/L) and indirect hyperbilirubinaemia (bilirubin: total 2.7 mg/dL; direct 0.5 mg/dL).
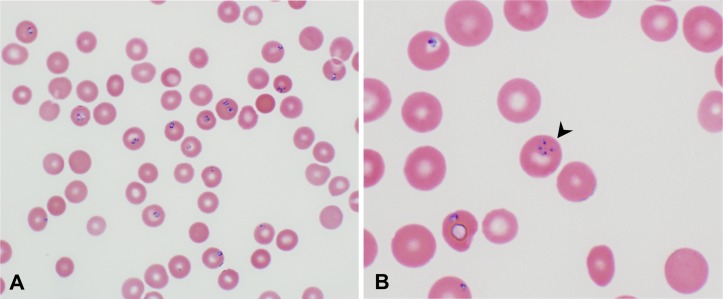
Due to concerns of diabetic ketoacidosis, a beta-hydroxybutyrate was ordered and returned normal (0.1 mmol/L). As the coagulation studies (INR 1.0 and aPTT 29.7 s) were within normal limits and the fibrinogen remained elevated (fibrinogen equivalent units >4.0), the diagnosis of disseminated intravascular coagulation (DIC) was ruled out. Laboratory work-up for severe anaemia was thus initiated. The Coombs test was negative, the lactate dehydrogenase (LDH) was elevated (1616 U/L), the haptoglobin was markedly decreased (<14 mg/dL), and a peripheral smear revealed absence of microangiopathic haemolytic anaemia, suggesting an ongoing non-autoimmune intravascular haemolytic process. Interestingly, a closer review of the smear displayed red blood cell inclusion bodies consistent with babesiosis (figure 1). Further studies revealed a positive Babesia microti DNA real-time PCR. The B. divergens and B. duncani DNA PCR were both negative. Finally, no evidence of coinfection with tick-borne pathogens was seen and the HIV test returned negative.
Figure 1.
Peripheral blood smear showing red blood cells with intracellular ring-shaped inclusions suggestive of Babesia sp (A). Maltese cross formation pathognomonic of babesiosis (black arrowhead) (B).
Differential diagnosis
The differential diagnosis includes diabetic ketoacidosis, thrombotic thrombocytopaenic purpura, haemolytic uraemic syndrome, septic shock, DIC and autoimmune haemolytic anaemia.
Treatment
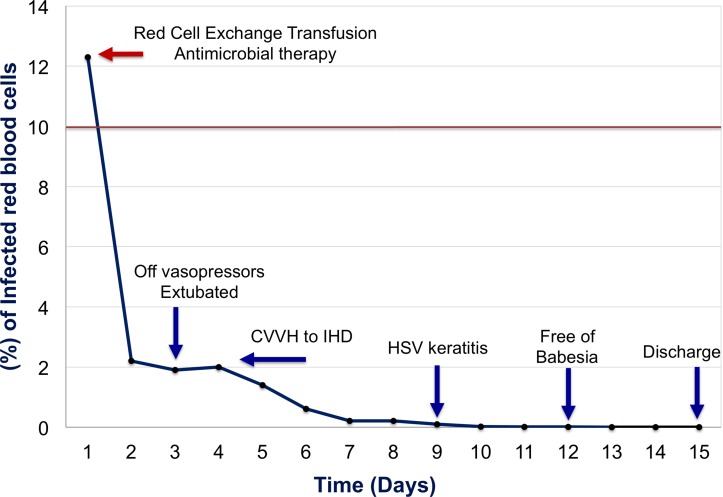
The patient was started on clindamycin and loaded with intravenous quinidine, and subsequently transitioned to oral quinine. As a result of the significant burden of the disease (12.5% of red blood cells infected), a red cell ET was pursued with improvement of the parasite load (1.9%). Two days later, the patient was extubated and weaned off of vasopressors. The ICU course was complicated by oligoanuria requiring continuous venovenous haemodialysis followed by intermittent haemodialysis (figure 2).
Figure 2.
Percentage of infected red blood cells by Babesia microti over time. The solid red line represents the threshold (>10%) for the use of red cell exchange transfusion therapy among patients with babesiosis. CVVH, continuous venovenous haemodialysis; HSV, herpes simplex virus; IHD, intermittent haemodialysis.
Outcome and follow-up
On hospital day 4, the patient was transferred to the general ward. His hospital course was complicated by herpes simplex virus keratitis successfully managed with oral acyclovir. Daily peripheral smears were performed to monitor the parasite load, and on hospital day 12 the patient was noted to be free of Babesia. After 15 days of in-hospital stay and two consecutive peripheral smears negative for babesiosis, the patient was discharged home on a 6-week course of clindamycin/quinine and scheduled for outpatient intermittent haemodialysis due to persistent oligoanuria (figure 2). Following completion of the antimicrobial therapy, the patient underwent a repeat B. microti DNA real-time PCR which returned negative.
Discussion
Human babesiosis is a zoonotic infection transmitted by the Ixodes scapularis tick. Several Babesia species have been implicated in human infections worldwide; however, the major public health burden lies in North America and is due to B. microti. Within the USA, the disease is endemic in the North-East and upper Midwest, and the primary causative agent is B. microti.2 Clinical severity varies from a subclinical presentation to a fulminant illness, and primarily depends on the immune status of the host.1 The overwhelming majority of Babesia infections occur among healthy individuals and manifest as an asymptomatic or self-limited condition. Nevertheless, the severe form of the disease commonly appears among immunocompromised or splenectomised patients, with only few reports in the literature in immunocompetent individuals.3–6 Here, we illustrate a rare case of severe B. microti infection presenting as multiorgan failure and shock in an immunocompetent host successfully treated with antimicrobials and red cell ET therapy.
The severe clinical presentation in our patient was unexpected given the lack of immunocompromising condition, lack of major comorbidities and no history of splenectomy. Nevertheless, few reports in the literature indicate that B. microti infection can manifest as severe disease in older individuals (>60 years old).3 5 7 Therefore, the age of our patient might have played a role in this severe clinical presentation. Common clinical features of severe babesiosis include high-grade fever (40°C–42°C), night sweats, chills, myalgia, haemoglobinuria and haemolytic anaemia. Nearly half of patients with severe babesiosis also develop complications such as disseminated intravascular coagulopathy and acute respiratory distress syndrome. Liver or renal failure, splenic rupture, congestive heart failure and coma may also occur.1 8 9 Our patient presented with multiorgan failure with refractory shock, renal failure, severe haemolysis, acute respiratory distress syndrome and early signs of liver damage.
Among hospitalised immunocompetent patients, the fatality rates range between 6% and 9%, and up to 20% among immunocompromised individuals.1 A high degree of clinical suspicion is thus paramount for appropriate work-up, early diagnosis, institution of appropriate treatment and successful outcomes in patients with severe forms of the disease. Overall, a definitive diagnosis of babesiosis is commonly made by microscopic identification of the parasite on thin blood smears with Wright or Giemsa staining. However, among patients with low-grade parasitaemia, PCR assay represents a more sensitive diagnostic tool.10 In the present case report, the diagnosis of babesiosis was made by identification of the parasite in the peripheral smear. Further characterisation of the Babesia species was conducted by PCR assay. Typically, the severity of the infection is related to the degree of parasitaemia. Severe presentations of babesiosis are defined as ≥4% of red blood cells infected by the parasite.7 9 11 In our case, around 12.5% of red blood cells were infected by the parasite; thus, our patient depicted a severe clinical presentation of the disease.
The common antimicrobial regimen for mild to moderate babesiosis includes a short course (7–10 days) of azithromycin and atovaquone. Treatment of severe infection requires a combination of clindamycin and quinine (oral) or quinidine (intravenous).10 12 A prolonged course of antimicrobial therapy (4–6 weeks) is typically preferred among patients with severe forms of the disease to avoid persistence or relapse of the infection.10 12 A 6-week therapy of clindamycin and atovaquone was decided in our patient. No evidence of persistence or relapsing of the disease was noted during or after completion of the antimicrobial therapy.
The additive measure of red cell ET is warranted among patients with high parasite burden (≥10%), severe anaemia, or pulmonary, renal or hepatic compromise.12 The aim of this therapy is to remove the infected erythrocytes as well as proinflammatory cytokines and vasoactive compounds derived from the intraerythrocytic infection and the immune host response.13 As Babesia species do not exhibit an exoerythrocytic phase, the removal of the infected cells is almost curative. The value of the ET therapy among non-splenectomised and immunocompetent patients with severe babesiosis is based on a limited number of reports.6 7 14 15 A case series of 34 patients diagnosed with severe babesiosis in Long Island revealed a significant reduction in the parasite load and subsequent clinical improvement among 7 cases treated with ET.7 Likewise, a review of 139 cases of babesiosis in New York State between 1982 and 1993 revealed that 6 cases were successfully treated with red blood cell exchange.9 Dorman et al and Genda et al presented two cases of severe babesiosis among immunocompetent hosts, residents from endemic areas of the parasite who demonstrated a rapid clinical improvement after whole ET therapy.6 14 We presented a unique case of a non-splenectomised, immunocompetent patient from a non-endemic area of Babesia who displayed a severe clinical presentation of the disease and responded successfully to ET. Following completion of ET, the degree of parasitaemia in our patient decreased to less than 2%, and a substantial clinical improvement was noted over the course of the next 48 hours. Among patients with severe babesiosis, prompt initiation of ET in conjunction with antimicrobial therapy could represent a life-saving intervention.
Learning points.
Babesia microti is the most common strain associated with human infection.
Although severe clinical presentation (>4% parasitaemia) is more common among immunocompromised or asplenic patients, severe babesiosis might occur in immunocompetent hosts.
Treatment with clindamycin and quinine is frequently used in patients with severe forms of the disease.
Red cell exchange transfusion is warranted for patients with high-grade parasitaemia (≥10%).
Although more frequent in North-East and upper Midwestern USA, endemic areas of Babesia are expanding.
Therefore, a high degree of clinical suspicion is paramount for prompt recognition, early treatment and avoidance of complications.
Footnotes
Contributors: JGR and MSR: concept, literature review and drafting of the manuscript. RLK and CED: drafting and critical review of the manuscript. All the authors approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Vannier E, Krause PJ. Human babesiosis. N Engl J Med 2012;366:2397–407. 10.1056/NEJMra1202018 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Babesiosis surveillance - 18 States, 2011. MMWR Morb Mortal Wkly Rep 2012;61:505–9. [PubMed] [Google Scholar]
- 3.Cunha BA, Cohen YZ, McDermott B. Fever of unknown origin (FUO) due to babesiosis in a immunocompetent host. Heart Lung 2008;37:481–4. 10.1016/j.hrtlng.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 4.Panduranga V, Kumar A. Severe babesiosis presenting as acute respiratory distress syndrome in an immunocompetent patient. Conn Med 2014;78:289–91. [PubMed] [Google Scholar]
- 5.Martínez-Balzano C, Hess M, Malhotra A, et al. Severe babesiosis and Borrelia burgdorferi co-infection. QJM 2015;108:141–3. 10.1093/qjmed/hcs100 [DOI] [PubMed] [Google Scholar]
- 6.Genda J, Negron EA, Lotfipour M, et al. Severe Babesia microti Infection in an Immunocompetent Host in Pennsylvania. J Investig Med High Impact Case Rep 2016;4:232470961666377 10.1177/2324709616663774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatcher JC, Greenberg PD, Antique J, et al. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis 2001;32:1117–25. 10.1086/319742 [DOI] [PubMed] [Google Scholar]
- 8.Meldrum SC, Birkhead GS, White DJ, et al. Human babesiosis in New York State: an epidemiological description of 136 cases. Clin Infect Dis 1992;15:1019–23. 10.1093/clind/15.6.1019 [DOI] [PubMed] [Google Scholar]
- 9.White DJ, Talarico J, Chang HG, et al. Human babesiosis in New York State: Review of 139 hospitalized cases and analysis of prognostic factors. Arch Intern Med 1998;158:2149–54. [DOI] [PubMed] [Google Scholar]
- 10.Ord RL, Lobo CA. Human babesiosis: pathogens, prevalence, diagnosis and treatment. Curr Clin Microbiol Rep 2015;2:173–81. 10.1007/s40588-015-0025-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mareedu N, Schotthoefer AM, Tompkins J, et al. Risk Factors for Severe Infection, Hospitalization, and Prolonged Antimicrobial Therapy in Patients with Babesiosis. Am J Trop Med Hyg 2017;97:1218–25. 10.4269/ajtmh.17-0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis: A Review. JAMA 2016;315:1767–77. 10.1001/jama.2016.2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcus LC, Valigorsky JM, Fanning WL, et al. A case report of transfusion-induced babesiosis. JAMA 1982;248:465–7. 10.1001/jama.1982.03330040053031 [DOI] [PubMed] [Google Scholar]
- 14.Dorman SE, Cannon ME, Telford SR, et al. Fulminant babesiosis treated with clindamycin, quinine, and whole-blood exchange transfusion. Transfusion 2000;40:375–80. 10.1046/j.1537-2995.2000.40030375.x [DOI] [PubMed] [Google Scholar]
- 15.Spaete J, Patrozou E, Rich JD, et al. Red cell exchange transfusion for babesiosis in Rhode Island. J Clin Apher 2009;24:97–105. 10.1002/jca.20197 [DOI] [PubMed] [Google Scholar]