Abstract
Purpose
The aim of the study was to examine clinical and radiographic results of a cementless humeral surface and to evaluate prognostic parameters for implant failure.
Methods
34 shoulders were examined preoperatively and after a mean 2.7 years. Radiographic parameters, Constant scores (CS) and complications were recorded.
Results
The mean CS improved from 27 to 51 points. Eight patients (24%) had an implant revision for secondary glenoid erosion. In the revision group was an increase of the LGHO of 8.4%.
Conclusions
The study shows a high revision-rate (24%). Predictor for an implant failure was an operative changing of the LGHO.
Keywords: Cemenless humeral head resurfacing, Shoulder arthroplasty, Degenerative glenohumeral osteoarthritis, Shoulder replacement, Shoulder hemiarthroplasty
1. Introduction
Osteoarthritis of the shoulder is common and can cause significant pain and functional limitation.1 For advanced glenohumeral osteoarthritis resistant to nonoperative management, a stemmed nonconstrained shoulder replacement, is a viable treatment option.1,2 Since the first humeral resurfacing procedures, performed in the late 1970s by Copeland,3 the idea of a cementless surface replacement arthroplasty was to restore the patient’s individual anatomy and to preserve bone to facilitate a possible further revision.4,5 Today, humeral resurfacing procedures are used for the treatment of a variety of shoulder pathologies as osteoarthritis, rheumatoid arthritis, avascular necrosis, instability arthropathy, post-traumatic arthropathy, and cuff arthropathy, with predominant good functional results.4,6,7 In the literature, there is less data about clinical and radiographic results of a cementless humeral surface replacement arthroplasty in patients with primary degenerative osteoarthritis.1,8 Therefore, the aim of the present study was to examine clinical and radiographic results of a cementless humeral surface replacement arthroplasty, in patients with primary osteoarthritis with an intact rotator cuff and to evaluate if there are any prognostic parameters. A second aim was to examine the survival of the prosthesis.
2. Materials and methods
2.1. Subjects
In this study we analyzed 34 patients (34 shoulders) with glenohumeral osteoarthritis with an intact rotator cuff, treated with a cementless humeral surface replacement hemiarthroplasty. All clinical and radiographic patient data were analyzed retrospectively. Inclusion criteria were a cementless humeral surface replacement arthroplasty for the treatment of primary glenohumeral osteoarthritis, and an intact rotator cuff. Exclusion criterias were rheumatoid arthritis, osteonecrosis of the humeral head, neural injuries, and bone defects of the glenoid. 20 women and 14 men were included with a mean age of 63.7 (±11.2). The mean duration follow-up was 33 months [range, 5 to 68 months] for the clinical and radiographical follow-up. Radiographs of the shoulder were made in the true anteroposterior and axillary projections for all patients preoperatively and postoperatively (Fig. 1). One of the authors (F.Z.) did the radiological assessment. Implant loosening on the immediate postoperative and most recent follow-up radiographs was compared by analyzing implant inclination, the distance above the greater tuberosity, the vertical humeral head height, and the perpendiculars to the observed humeral implant diameter running to the humeral shaft axis or the lateral cortex of the greater tuberosity for measurable migration of the implant in relation to the proximal part of the humerus as described by Rydholm and Sjögren9 (Fig. 2). All patients were evaluated preoperatively with use of the Constant score (CS),10 adjusted for age and sex. Additionally, active range of motion was recorded for shoulder flexion, abduction, and rotation, with the hanging arm in a neutral position and the elbow flexed to 90°. The morphology of the glenoid was classified according to the classification of Walch et al.11 An A1 glenoid was found in 23 cases, and an A2 glenoid was found in 11 cases. Postoperatively, all patients were routinely evaluated after six and twelve months and at the time of the most recent follow-up examination with the same standardized clinical and radiographic protocol.
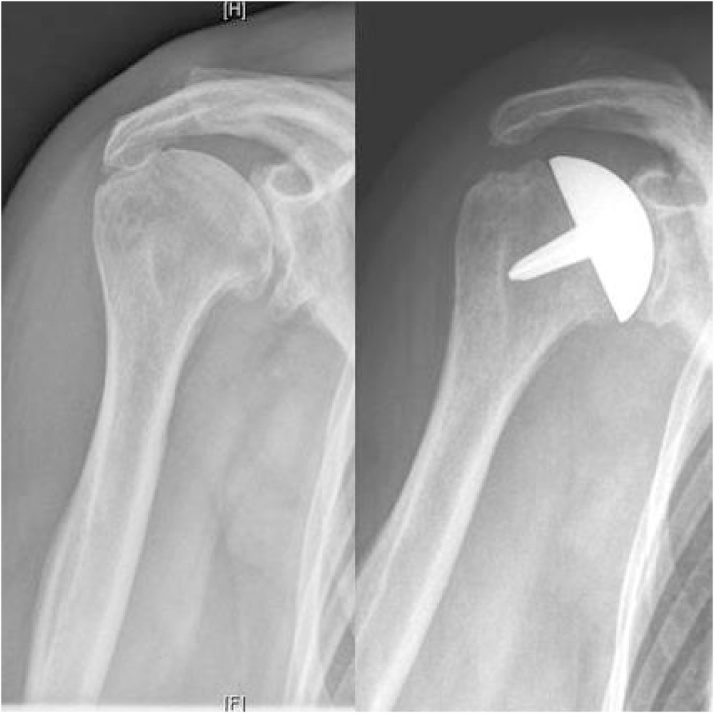
Fig. 1.
Preoperative anteroposterior radiograph of a 74-year old female with degenerative osteoarthritis and follow-up radiograph of the same patient twelve months after implantation of the cementless surface replacement arthroplasty.
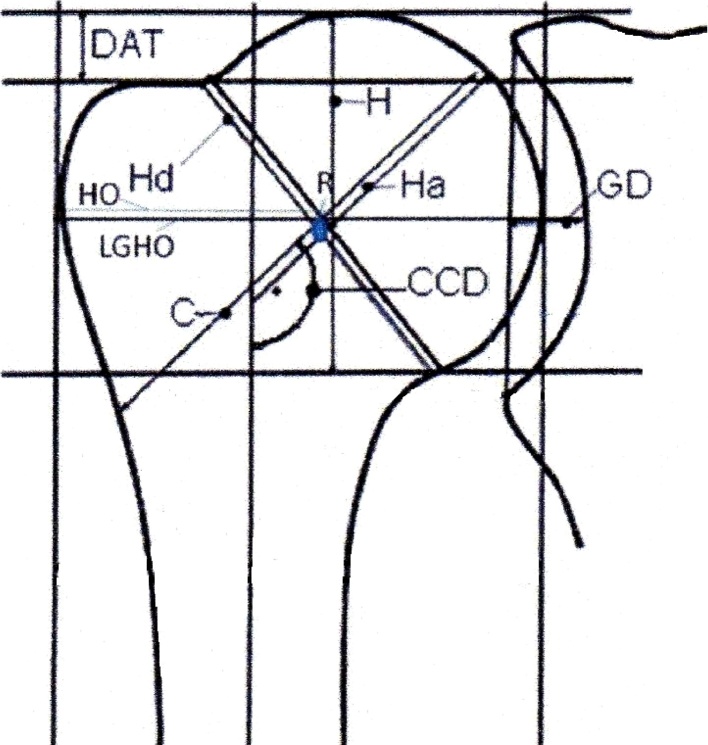
Fig. 2.
Illustration showing the parameters that were measured on true anteroposterior radiographs immediately after surgery and at the time of the most recent follow-up.
HO = humeral offset; H = humeral head height, GD = glenoid depth, LGHO = lateral glenohumeral offset, Hd = observed implant diameter for calculating magnification effects; CCD = inclination angle of implant; H = vertical humeral head height; Ha = perpendicular to the center of Hd, running to the shaft axis; C = perpendicular to the center of Hd, running through the shaft axis to the lateral aspect of the greater tuberosity; DAT = distance above the greater tuberosity and apex of implant.
2.2. Operative technique and implants
All operations were started by using the deltopectoral approach described by Neer et al.12 A rotator cuff tear was found in no case. The first step was to detach the subscapularis tendon, followed by performing a capsular release. There was no discrepancy between the intraoperative joint status and the radiographic findings. In all cases the biceps tendon was dissected close to its glenoid attachment, followed by tenodesing it in the bicipital groove. The humeral head was reamed with hemispherical reamers of decreasing size. Using the uncemented press-fit technique was the method of choice for placing the resurfacing implant. Primary stability, which was tested manually, could be achieved without exception. In 10 cases, a Copeland Shoulder (Biomet Europe, Dordrecht, The Netherlands) was used; in 21 cases the EPOCA RH Cup (Argomedical, Cham, Switzerland) was used and the remaining 3 patients had surgery with an Aequalis RH (Tornier Warsaw, IN USA). All implants were coated with hydroxyapatite on the inner surface. Common ground of the 3 systems is the possibility of using a spherical joint surface. The primary stability in the humeral epiphysis was achieved in different ways: for the Copeland Shoulder a centered peg, whereas for the EPOCA Cup a conical crown-shaped ring was used. After the placement of the implants, the subscapularis tendon was repaired by using three to five nonabsorbable tendon-to tendon sutures. In 4 cases there was an internal rotation contracture. These patients were treated with a subscapularis release according to the method of Walch et al.13; this procedure was performed when a minimum of 0° of external rotation was not attained after provisional coadaptation of the muscle. In all cases drains where placed in and were removed on the first day after surgery. To protect the reconstructed subscapularis tendon, the arm was placed in internal rotation into a shoulder abduction pillow for four weeks. Postoperatively, the shoulder was mobilized passively by a physiotherapist for six weeks to 60° of flexion and abduction and 0° of external rotation. Patients were asked to support these movements actively. Free range of motion was allowed six weeks after surgery.
2.3. Statistical analysis
The Wilcoxon test was used to compare the preoperative and postoperative Constant scores and subscores as well as to compare the parameters of implant loosening between the immediate postoperative and most recent follow-up radiographs. The Pearson correlation coefficient was measured. The level of significance was set at p < 0.05.
2.4. Source of funding
The non-commercial research fund of the “Deutsche Arthrose-Hilfe e.V.” supports clinical investigations using the “shoulder arthroplasty register” of the Clinic for Orthopedics and Trauma Surgery of the Heidelberg University Hospital.
3. Results
3.1. Survival and complications
In the present study, eight patients (24%) underwent revision surgery (Table 1) after a mean duration of 22.9 months with the earliest being after 8 months and latest after 68 months. All revisions were performed due to glenoid erosion and they all received a stemmed total shoulder prosthesis (Tornier Aequalis shaft and glenoid) as replacement for the CUP hemiprosthesis. No revisions have been performed for aseptic loosening, or trauma to date.
Table 1.
Characteristics of the revision patients.
| Patient | prosthesis | Reason for revision | Revision after x months | Revision procedure |
|---|---|---|---|---|
| 1 | Copeland | Glenoid erosion | 9 | Change to a stemmed cemmented TSA |
| 2 | Epoca | Glenoid erosion | 9 | Change to a stemmed cemmented TSA |
| 3 | Copland | Glenoid erosion | 8 | Change to a stemmed cemmented TSA |
| 4 | Epoca | Glenoid erosion | 12 | Change to a stemmed cemmented TSA |
| 5 | Copland | Glenoid erosion | 27 | Change to a stemmed cemmented TSA |
| 6 | Aequalis | Glenoid erosion | 13 | Change to a stemmed cemmented TSA |
| 7 | Epoca | Glenoid erosion | 35 | Change to a stemmed cemmented TSA |
| 8 | Epoca | Glenoid erosion | 68 | Change to a stemmed cemmented TSA |
3.2. Early clinical results and patient satisfaction
The mean Constant score improved from 27 points (range, 10 to 49 points) preoperatively to 51 points (range, 10 to 83 points) 2.7 years postoperatively (p < 0.0001), and, adjusted by age and sex, from 36% (range, 14% to 61%) to 69% (range, 14% to 123%) (p < 0.0001). Significant differences were also found in terms of pain relief, activity, mobility, shoulder flexion, abduction, and external and internal rotation (p < 0.05). The findings of the preoperative and postoperative clinical examinations are shown in Table 2.
Table 2.
Preoperative and 2.7 years postoperative clinical findings.
| Preoperativea | Postoperativea | P Value | |
|---|---|---|---|
| Constant score (points) | 26.9 ± 10.8 (10–49) | 51.1 ± 24.0 (10–83) | <0.0001 |
| Constant score (%) | 35.7 ± 14.7 (14–61) | 69.1 ± 33.8 (14–123) | <0.0001 |
| Pain (points) | 3.4 ± 2.8 (0–10) | 9.5 ± 5.3 (0–15) | <0.0001 |
| Power (points) | 2.1 ± 3.3 (0–15) | 3.7 ± 3.9 (0–13) | <0.0686 |
| Activity (points) | 8.5 ± 3.0 (4–14) | 15.3 ± 4.3 (7–20) | <0.0001 |
| Mobility (points) | 12.8 ± 5.7 (2–24) | 23.0 ± 11.7 (2–40) | <0.0001 |
| Flexion (deg) | 80.7 ± 29.8 (10–150) | 118.1 ± 47.6 (30–170) | <0.0013 |
| Abduction (deg) | 61.5 ± 24.7 (0–140) | 105.8 ± 51.3 (20–180) | <0.0001 |
| External rotation (deg) | 10.0 ± 14.1 (0–20) | 32.7 ± 25.5 (0–80) | <0.0001 |
The values are given as the mean and the standard deviation, with the range in parentheses.
3.3. Early radiographic results and their clinical correlation
The pre- and 2.7 years postoperative radiographic parameters of the 34 patients are shown in Table 3. The LGHO in the non-revision group didn’t considerably change (preop 51.9, postop 51.7), while the revision group showed an increase in LGHO (preop 48.9, postop 50.4). In the revision group there was a correlation between postoperative CS and preoperative LGHO (r = 0.5), height (r = 0.8), CCD (r = −0.56) and preoperative CS (r = 0.49).
Table 3.
Preoperative and postoperative radiographic parameters.
| Preoperativea | Postoperativea | P Value | |
|---|---|---|---|
| HO (mm) | 22.7 ± 3.6 (15.2–30.1) | 25.6 ± 3.6 (18.8–34.4) | <0.001 |
| H (mm) | 36.3 ± 8.5 (23.3–53.7) | 38.6 ± 4.6 (28.7–49.3) | 0.078 |
| GD (mm) | 4.8 ± 1.9 (1.7–10.6) | 6.7 ± 1.8 (2.9–10.0) | <0.001 |
| LGHO (mm) | 51.3 ± 7.7 (36.8–64.5) | 51.4 ± 4.8 (43.0–61.6) | 0.909 |
| Hd (mm) | 46.7 ± 7.7 (33.1–60.6) | 44.0 ± 4.5 (34.8–51.0) | 0.018 |
| CCD (mm) | 129.2 ± 9.7 (105–150) | 133.7 ± 9.3 (109.8–150.8) | <0.01 |
| Ha (mm) | 27.9 ± 6.9 (13.8–41.9) | 31.6 ± 6.7 (12.9–50.2) | 0.001 |
| C (mm) | 49.4 ± 7.8 (36.5–63.2) | 53.8 ± 6.6 (43.1–69.8) | <0.001 |
| DAT (mm) | 7.7 ± 2.9 (0–12.9) | 9.6 ± 3.4 (0–17.6) | 0.005 |
HO = humeral offset; mm = millimeter; H = vertical humeral head height, GD = glenoid depth, LGHO = lateral glenohumeral offset; Hd = observed implant diameter for calculating magnification effects; CCD = inclination angle of implant; Ha = perpendicular to the center of Hd, running to the shaft axis; C = perpendicular to the center of Hd, running through the shaft axis to the lateral aspect of the greater tuberosity; DAT = distance above the greater tuberosity and apex of implant.
The values are given as the mean and the standard deviation, with the range in parentheses.
4. Discussion
Good clinical outcomes and low revision rates have been described for cementless humeral surface replacement arthroplasty for a variety of shoulder pathologies as rheumatoid arthritis, avascular necrosis, instability arthropathy, post-traumatic arthropathy, and cuff arthropathy.4,6,7,14 While in the treatment of glenohumeral osteoarthritis stemmed unconstrained total shoulder replacement is considered the gold standard1,2,15 there is less and inconsistent data about clinical and radiographic results of a cementless humeral surface replacement arthroplasty used as a hemiprosthesis in patients with primary degenerative osteoarthritis.1,4,8,16 Therefore, the purpose of the present study was to examine clinical and radiographic results of a cementless humeral surface replacement arthroplasty, in patients with primary osteoarthritis with an intact rotator cuff. The results of the present study show disappointing short-term results with a revision-rate of 24% after 2.7 years after cementless humeral surface replacement arthroplasty. As the present study showed, there was a correlation between the revisions and the surgery conditioned LGHO changes. This might be based on technical or implant problems.
In the literature, there are few studies, analyzing the results of a cementless humeral surface replacement arthroplasty in patients with glenohumeral osteoarthritis with inconsistent results. Studies showing good results have been mostly reported by Copelands group.16,17 A non-developer series of Al-Hadithy et al.1 described 53 Mark III Copeland hemiarthroplasties in patients with glenohumeral osteoarthritis. Mean follow-up was 4.2 years. Comparable to our results, the mean age-adjusted Constant scores improved from 38.5 to 75.1. As complications one anterosuperior escape of the humeral head was reported in a patient who had an oversized humeral component due to progressive rotator cuff failure at 2 years. Moderate glenoid erosion was present in 12% and correlated with oversizing of the humeral component. No patient required revision for aseptic loosening, rotator cuff failure, or glenoid erosion. Al-Hadithy reported about one revision to a stemmed cemented hemiarthroplasty for periprosthetic fracture and concluded, that Copeland surface replacement hemiarthroplasty for glenohumeral osteoarthritis could provide functional results similar to modular stemmed prostheses, with a relatively low revision rate (2%) in the short-term follow-up. The present study demonstrates completely different results with a high revision-rate of 24% after 2.7 years.
To evaluate, if there are any prognostic parameters, we analyzed the radiographic parameters and found, that predictors for an implant failure was an operative changing of the lateral glenohumeral offset. This might cause damage of the rotator cuff or impingement. Mansat et al.8 showed in 64 patients with cementless humeral surface replacement arthroplasty for different indications that glenohumeral relationships correlates with the final clinical results. They found a tendency to position the prosthesis in varus because of technical imperfections. With follow-up, medialization of the humerus with glenoid wear was observed and was correlated in some patients with reappearance of pain. Mansat reported a height of the humeral head of 21 ± 4 mm preoperatively and 19 ± 2 mm postoperatively. In a study by Iannotti et al.18 the humeral height was of 19 ± 2 mm. The distance between the superior most point on the top of the prosthesis and the greater tuberosity, or cephalotuberosity index, was 8 ± 5 mm, similar to the results presented by Iannotti et al,15 with 8 ± 3.2 mm in normal glenohumeral joint. Positioning the resurfacing implant related to the top of the greater tuberosity is fundamental to avoid impingement of the greater tuberosity under the acromion if it is placed too low, or overstuffing the cuff tendons with limited range of motion if it is placed too proud.
The current study has its limitations. The number of cases was relatively small and we used three different types of implants for cementless surface replacement. Furthermore, there is no radiological analysis in the axial plane, hence limiting the evaluation of the resurfacing. As Jia et al.19 showed, using a 3-D computed tomography would be more reproducible than plain radiography assessment. Nevertheless, postoperative x-ray imaging of patients undergoing shoulder replacement is a central component of clinical follow-up, why we used it in the present study.
The results of the present study show deflating short-term results with a revision-rate of 24% after 2.7 years after cementless humeral surface replacement arthroplasty.
In literature the CUP-Prosthesis for young patients is often discussed as an adequate option. Young patients being defined as younger than 55 years20, 21, 22, 23 or younger than 50 years.24 In our study, we set the limit for young patients to 55 years for a better comparability. We found that the revision rate after 2.7 years was at 30% for patients aged <55 years. Rasmussen et al.23 already found a high revision rate of 12.1% in young patients, a result which supports our findings.
Using metaphyseal fixed humeral components, in a multicentre study, Churchill et al.25 found good results after a minimum follow-up of 2 years after implantation. The constant score, SST and ASES improved from pre- to post-operative, the ROM and strength were improved to. The complication rate in the study was low. In our practice, we found similar results to Churchill et al. with a metaphyseal fixed humeral prosthesis.26 Therefore, we decided in our practice, that we prefer a metaphyseal fixed anatomical shoulder prosthesis to the CUP-Prosthesis, especially for young patients, considering the dramatic results of the CUP in patients under 55 years.
5. Conclusion
The study shows a high revision rate (24%) after surface replacement arthroplasties. There was a correlation between the revisions and the surgery conditioned LGHO changes, a lower preoperative CS, decreased preoperative LGHO and heights as well as a higher preoperative CCD.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Al-Hadithy N., Domos P., Sewell M.D., Naleem A., Papanna M.C., Pandit R. Cementless surface replacement arthroplasty of the shoulder for osteoarthritis: results of fifty Mark III Copeland prosthesis from an independent center with four-year mean follow-up. J Shoulder Elbow Surg. 2012;21(Dec. (12)):1776–1781. doi: 10.1016/j.jse.2012.01.024. Epub 2012 May 8. [DOI] [PubMed] [Google Scholar]
- 2.Radnay C.S., Setter K.J., Chambers L., Levine W.N., Bigliani L.U., Ahmad C.S. Total shoulder replacement compared with humeral head replacement for the treatment of primary glenohumeral osteoarthritis: a systematic review. J Shoulder Elbow Surg. 2007;16(4):396–402. doi: 10.1016/j.jse.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Copeland S. The continuing development of shoulder replacement: reaching the surface. J Bone Joint Surg Am. 2006;88(4):900–905. doi: 10.2106/JBJS.F.00024. [DOI] [PubMed] [Google Scholar]
- 4.Levy O., Copeland S.A. Cementless surface replacement arthroplasty (Copeland CSRA) for osteoarthritis of the shoulder. J Shoulder Elbow Surg. 2004;13(3):266–271. doi: 10.1016/j.jse.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Burgess D.L., McGrath M.S., Bonutti P.M., Marker D.R., Delanois R.E., Mont M.A. Shoulder resurfacing. J Bone Joint Surg Am. 2009;91(5):1228–1238. doi: 10.2106/JBJS.H.01082. [DOI] [PubMed] [Google Scholar]
- 6.Raiss P., Kasten P., Baumann F., Moser M., Rickert M., Loew M. Treatment of osteonecrosis of the humeral head with cementless surface replacement arthroplasty. J Bone Joint Surg Am. 2009;91(2):340–349. doi: 10.2106/JBJS.H.00560. [DOI] [PubMed] [Google Scholar]
- 7.Pape G., Bruckner T., Loew M., Zeifang F. Treatment of severe cuff tear arthropathy with the humeral head resurfacing arthroplasty: two-year minimum follow-up. J Shoulder Elbow Surg. 2013;22(1):e1–7. doi: 10.1016/j.jse.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Mansat P., Coutie A.S., Bonnevialle N., Rongieres M., Mansat M., Bonnevialle P. Resurfacing humeral prosthesis: do we really reconstruct the anatomy? J Shoulder Elbow Surg. 2013;22(5):612–619. doi: 10.1016/j.jse.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Rydholm U., Sjogren J. Surface replacement of the humeral head in the rheumatoid shoulder. J Shoulder Elbow Surg. 1993;2(6):286–295. doi: 10.1016/1058-2746(93)90074-Q. [DOI] [PubMed] [Google Scholar]
- 10.Constant C.R. An evaluation of the Constant-Murley shoulder assessment. J Bone Joint Surg Br. 1997;79(4):695–696. [PubMed] [Google Scholar]
- 11.Walch G., Badet R., Boulahia A., Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J.Arthroplasty. 1999;14(6):756–760. doi: 10.1016/s0883-5403(99)90232-2. [DOI] [PubMed] [Google Scholar]
- 12.Neer C.S., Watson K.C., Stanton F.J. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319–337. [PubMed] [Google Scholar]
- 13.Walch G., Boileau P., Riand N., Pozzi I. Technical problems encountered with subscapularis and the superior cuff in shoulder arthroplasty. In: Walch G., Boileau P., editors. Shoulder Arthroplasty. Springer; Berlin, Heidelberg, New York, Tokyo: 1999. pp. 101–104. [Google Scholar]
- 14.Thomas S.R., Wilson A.J., Chambler A., Harding I., Thomas M. Outcome of Copeland surface replacement shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(5):485–491. doi: 10.1016/j.jse.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Edwards T.B., Kadakia N.R., Boulahia A. A comparison of hemiarthroplasty and total shoulder arthroplasty in the treatment of primary glenohumeral osteoarthritis: results of a multicenter study. J Shoulder Elbow Surg. 2003;12(3):207–213. doi: 10.1016/s1058-2746(02)86804-5. [DOI] [PubMed] [Google Scholar]
- 16.Levy O., Copeland S.A. Cementless surface replacement arthroplasty of the shoulder. 5- to 10-year results with the Copeland mark-2 prosthesis. J Bone Joint Surg Br. 2001;83(2):213–221. doi: 10.1302/0301-620x.83b2.11238. [DOI] [PubMed] [Google Scholar]
- 17.Levy O., Funk L., Sforza G., Copeland S.A. Copeland surface replacement arthroplasty of the shoulder in rheumatoid arthritis. J Bone Joint Surg Am. 2004;86-A(3):512–518. doi: 10.2106/00004623-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Ianotti J.P. Review: relocation and anterior release tests diagnose shoulder instability in selected patients. J Bone Joint Surg Am. 2005;87(5):1168. doi: 10.2106/JBJS.8705.ebo1. [DOI] [PubMed] [Google Scholar]
- 19.Jia X., Chen Y., Qiang M. Compared to X-ray, three-dimensional computed tomography measurement is a reproducible radiographic method for normal proximal humerus. J Orthop Surg Res. 2016;11(1):82. doi: 10.1186/s13018-016-0417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raiss P., Pape G., Becker S., Rickert M., Loew M. Cementless humeral surface replacement arthroplasty in patients less than 55 years of age. Orthopade. 2010;39(Feb. (2)):201–208. doi: 10.1007/s00132-009-1525-4. [DOI] [PubMed] [Google Scholar]
- 21.Bailie D.S., Llinas P.J., Ellenbecker T.S. Cementless humeral resurfacing arthroplasty in active patients less than fifty-five years of age. J Bone Joint Surg Am. 2008;90(1):110–117. doi: 10.2106/JBJS.F.01552. [DOI] [PubMed] [Google Scholar]
- 22.Bartelt R., Sperling J.W., Schleck C.D., Cofield R.H. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg. 2011;20(1):123–130. doi: 10.1016/j.jse.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen J.V., Polk A., Sorensen A.K., Olsen B.S., Brorson S. Outcome, revision rate and indication for revision following resurfacing hemiarthroplasty for osteoarthritis of the shoulder: 837 operations reported to the Danish Shoulder Arthroplasty Registry. Bone Joint J. 2014;96-B(4):519–525. doi: 10.1302/0301-620X.96B4.31850. [DOI] [PubMed] [Google Scholar]
- 24.Sperling J.W., Cofield R.H., Rowland C.M. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg. 2004;13(6):604–613. doi: 10.1016/S1058274604001296. [DOI] [PubMed] [Google Scholar]
- 25.Churchill R.S., Chuinard C., Wiater J.M. Clinical and Radiographic Outcomes of the Simpliciti Canal-Sparing Shoulder Arthroplasty System: A Prospective Two-Year Multicenter Study. J Bone Joint Surg Am. 2016;98(7):552–560. doi: 10.2106/JBJS.15.00181. [DOI] [PubMed] [Google Scholar]
- 26.Maier M.W., Lauer S., Klotz M.C., Bulhoff M., Spranz D., Zeifang F. Are there differences between stemless and conventional stemmed shoulder prostheses in the treatment of glenohumeral osteoarthritis? BMC Musculoskelet Disord. 2015;16:275. doi: 10.1186/s12891-015-0723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]