Abstract
Purpose
This aim of this study was to evaluate the rate of surgical site infection (SSI) in patients undergoing Total Knee Arthroplasty (TKA), to improve our understanding of the associations between infection rate and obesity.
Methods
Data was reviewed for 839 primary TKA procedures performed at a National Arthroplasty Centre over one year (April 2007–March 2008). SSI data was collected at 30 days and one year post-operatively. Patients were grouped guided by the WHO classifications of obesity; normal (BMI < 25.0), overweight (BMI 25.00–29.99), obese class I (BMI 30.00–34.99), obese class II (BMI 35.00–39.99), obese class III (BMI ≥ 40.00). Statistical significance was assessed by Fisher’s Exact Test.
Results
When grouped by BMI, 30.9% of patients were obese class I, 19.0% obese class II and 8.7% obese class III. Of the total cohort, 22 patients (2.6%) had superficial SSI and 13 (1.5%) had deep SSI. When comparing the obese class III cohort to all other cohorts (non-obese class III), the odds ratios for superficial SSI was 4.20 (95% CI [1.59, 11.09]; p = 0.009) and deep SSI was 6.97 (95% CI [2.22, 21.89]; p = 0.003). In the obese class III cohort, superficial SSI rate was higher in females (8.9%) than males (5.9%), yet deep SSI demonstrated the opposite, with a higher occurrence in males (11.8%) compared to females (5.4%).
Conclusion
This study suggests that obese class III TKA patients are at increased odds of superficial and deep SSI compared to other BMI cohorts. Interestingly, male obese class III patients demonstrated a higher rate of deep infection compared to their female counterparts. However, it must be noted that study findings are limited as confounders were unable to be accounted for in this retrospective study design.
Keywords: Total knee arthroplasty, Obesity, Surgical site infection, Body mass index
1. Introduction
Overweight and obesity are worldwide health epidemics, placing burden on healthcare systems. According to figures published by the Scottish government, in 2015, 65% of Scottish adults aged 16 years or older were overweight, and 29% of these adults were obese.1 Equally as alarming, recent Australian reports have suggested increasing rates of overweight and obesity with age, displaying an increase from 39% of those aged 18–24, to 74% of people aged 65–74 years.2
Obesity has been shown to closely align with the incidence of osteoarthritis,3,4 whereby obese individuals are more likely to require total joint arthroplasty at a younger age and have a higher revision rate.[5], [6], [7] Total joint replacement, although associated with excellent gains in quality of life, is a highly invasive surgery that is not devoid of complications, such as those associated with superficial or deep surgical site infection (SSI). The most common cause of SSI appears to be Staphylococcus aureus,8,9 however some coagulase-negative Staphlococci have been reported in studies at similar or greater rates than S. aureus.10,11 The bacterial origin of SSI is usually identified as a patient’s own skin flora12 and nasal carriage of S. aureus is a major factor for SSI.13
There are two types of commonly reported SSI; superficial infection which typically occurs up to 30 days after surgery; and deep infection, which can occur up to one year after surgery. Superficial infection is typically treatable with a course of antibiotics and occasionally debridement, which often resolves with minimal complications.14 However, deep infection can require one or more surgeries, with revision of part or the complete prostheses, and occasionally arthrodesis.15,16 Such surgeries come with significant morbidity to the patient. Furthermore, revision surgeries are expensive and complex, with infection responsible for 22.5% of primary total knee revisions.17
Obesity is considered a risk factor for SSI, demonstrating the paradigm that not only are obese patients more likely to require arthroplasty, they are at a greater risk of complications, which should be considered before surgery.12,[18], [19], [20] However, the literature remains contentious,21 as studies have illustrated both no significant increases in infection risk,[22], [23], [24], [25] or highly significant increases in risk.[26], [27], [28], [29] These controversies exist, partly, as the link between obesity and infection remains unclear.30 As obesity is typically associated with underlying co-morbidities such as Diabetes Mellitus and components of metabolic syndrome,31 these additional complications may be contributors to poor surgical outcomes such as SSI.32,33 In addition, the use of body mass index (BMI) as a sole measure of obesity may not always accurately illustrate these complexities. Well-acknowledged limitations including the inability to distinguish between adipose tissue and lean mass, or account for body fat type and distribution result in the potential for misdiagnosis of obesity when using BMI.34 Nevertheless BMI remains a widely adopted measure for categorisation of obesity.
Obesity is a pro-inflammatory state of increased chronic and low-grade inflammation, with the potential to augment the post-operative inflammatory response.35 This altered inflammatory state has the potential to alter the way in which obese individuals respond to infection.[36], [37], [38] However, to better understand the molecular link between obesity and SSI, there needs to be improved clarity on the correlation between incidence and type of SSI in obese TKA patients. Therefore, this study retrospectively evaluated BMI and the incidence of SSI in 839 patients who underwent routine primary TKA at the Golden Jubilee National Hospital of Glasgow, to assess any potential correlation between obesity and SSI, to improve early identification of high risk cohorts.
2. Methods
2.1. Patient recruitment
In this study, 839 patients who had previously undergone routine primary TKA at the Golden Jubilee National Hospital of Glasgow were retrospectively evaluated for BMI levels and incidence of SSI over a one year period, April 2007 to March 2008. SSI data was collected from two sources. Infection data was collected prospectively by the independent hospital Infection Control Team for in-patient stays and post-discharge up to 30 days post-operatively. This data was available for all patients and issued in monthly reports, which was used to identify superficial SSI up to 30 days post-operatively. Data was also sourced from ISD (Information Services Division of NHS Scotland) who independently collected all re-admissions to any hospital in Scotland for infection within one year of operation. This data was used to identify all cases of deep SSI within one year.
Patient records were obtained and the results recorded for SSI and BMI. Patients were grouped based on the World Health Organisation (WHO) classifications of adult obesity according to BMI: normal (BMI < 25.0), overweight (BMI 25.00–29.99), obese class I (BMI 30.00–34.99), obese class II (BMI 35.00–39.99) and obese class III (BMI ≥ 40.00).39 BMI is expressed in units of kg/m2.
2.2. Data collection
Data was analysed by comparing SSI occurrence during the follow-up period of one year post-operative and the type of SSI that was found. Deep SSI (DSSI) was classed as any infection to the operative area that required a re-admission to hospital, up to one year following surgery, as per the Scottish Arthroplasty Project. Superficial SSI (SSSI) was classed as any infection that did not require re-hospitalisation and occurred during the first 30 days post-operative. Patients were grouped by BMI cohort as described in Section 2.1. Additionally, for data analyses SSSI and DSSI was assessed between the obese class III (BMI ≥ 40.00) and non-obese class III, which was inclusive of all cohorts with a BMI < 40.00. Analyses were also performed between the obese cohort (all patients with a BMI ≥ 30.00) and the non-obese cohort (all patients with a BMI < 30.00).
2.3. Statistical analyses
Statistical analyses were performed using GraphPad Prism (Version 6). Comparisons were made between obese class III and non-obese class III using Fisher’s Exact Test (2-tailed) to determine the odds ratio (OR) of SSSI or DSSI incidence. Comparisons were also made between obese and non-obese cohorts to determine the odds ratio of SSSI or DSSI incidence. Where the confidence interval (CI) did not cross the y-axis, significance was achieved indicating p < .05.
3. Results
3.1. Patient characteristics and BMI
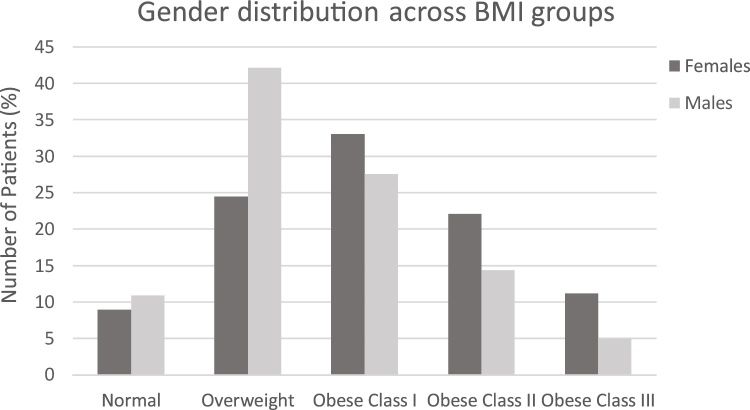
There were a total of 839 TKAs performed over the selected study period and BMI measurements were available for all 839 patients, with a mean BMI of 31.9. When grouped by BMI class, 9.8% of patients had normal BMI, 31.7% were overweight, 30.9% were obese class I, 19.0% were obese class II and 8.7% were obese class III. Of this cohort of patients, 498 were female and 341 were male, with females found to have a higher mean BMI. A breakdown of gender distribution across BMI groups is provided in Fig. 1.
Fig. 1.
Gender distribution across BMI cohorts.
3.2. Infection rate with BMI
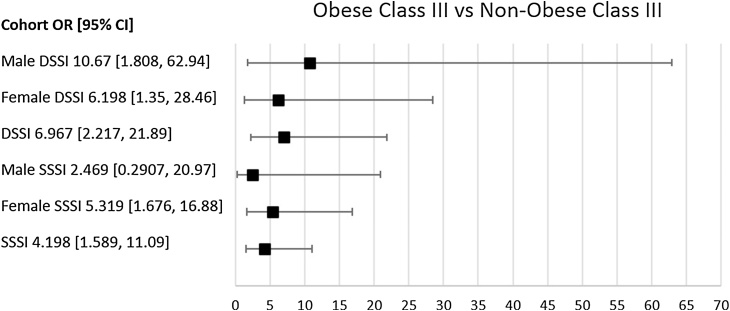
There were 22 patients with SSSI at 30 days and 13 with DSSI at one year, with an overall SSSI rate of 2.6% and DSSI rate of 1.5%. The obese class III group had higher rates of both SSSI (8.2%) and DSSI (6.8%) when compared to all other BMI groups (Table 1). Furthermore, the obese class III group were at 4.20 times greater odds of SSSI (95% CI [1.59, 11.09]; p = 0.009) and 6.97 times greater odds of DSSI (95% CI [2.22, 21.89; p = 0.003), when compared to all other cohorts (denoted as non-obese class III) (Table 1, Fig. 2). However, for the cohort divided as either obese (all-inclusive BMI ≥ 30.00) vs. non-obese (all-inclusive BMI < 30.00) there were no significant differences in OR for either SSSI (1.54 [95% CI [0.62, 3.81, p = 0.389) or DSSI (1.14 [95% CI [0.37 to 3.50, p = 1.000).
Table 1.
Total number of TKA patients grouped by obese class and infection type. Infection rate is expressed as a percentage (%).
| BMI | Normal | Overweight | Obese class I | Obese class II | Obese class III |
|---|---|---|---|---|---|
| Patients% (n) | 9.8 (82) | 31.7 (266) | 30.9 (259) | 19.0 (159) | 8.7 (73) |
| 30 day SSSI% (n) | 1.2 (1) | 2.3 (6) | 1.5 (4) | 3.1 (5) | 8.2 (6) |
| 1 year DSSI% (n) | 1.2 (1) | 1.5 (4) | 0.8 (2) | 0.6 (1) | 6.8 (5) |
SSSI: Superficial Surgical Site Infection; DSSI: Deep Surgical Site Infection.
Fig. 2.
Forrest Plot demonstrating the odds ratio between obese class III and non-obese class III, for SSSI and DSSI. Error bars show 95% confidence interval (CI). Infections with a 95% CI that crosses the y-axis have a non-significant odds ratio.
3.3. Association between gender, obesity and SSI
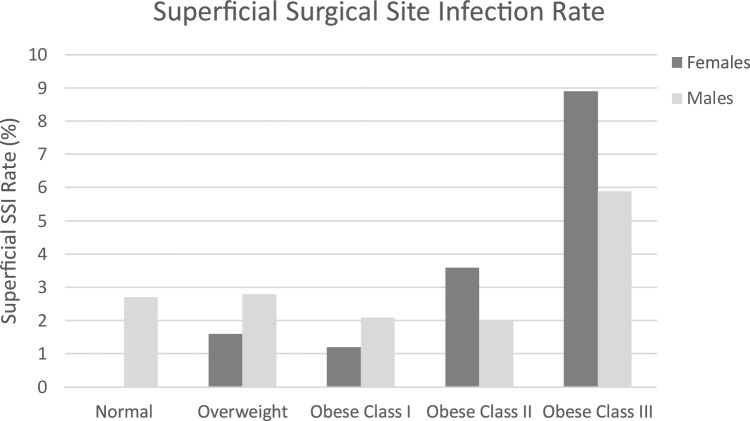
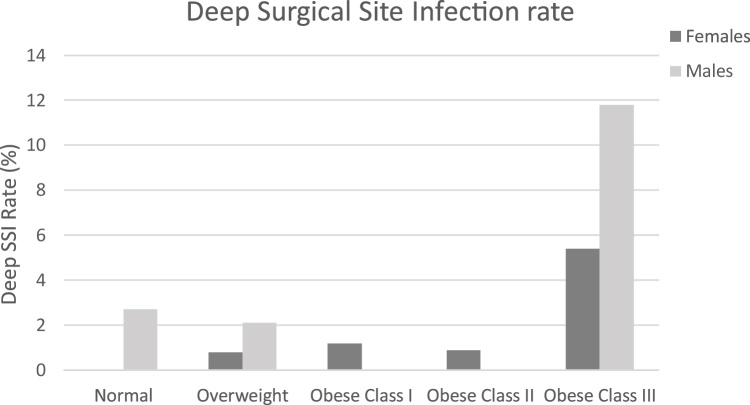
While there were no significant changes in infection rate between males and females (data not shown), there tended to be more females constituting the obese class III cohort (11.2%) than males (5.0%) (Table 2, Fig. 1). In the obese class III cohort, SSSI rate was higher in females (8.9%) than males (5.9%) (Fig. 3), yet DSSI was lower in females (5.4%) than males (11.8%) (Fig. 4). For females the obese class III group had higher ORs for SSSI (OR = 5.32) and DSSI (OR = 6.20) (Table 2, Fig. 2). In males, the obese class III group were at 10.67 times greater odds of DSSI, compared to patients with a BMI of less than 40 (Table 2, Fig. 2). No statistically significant difference was found for odds of SSSI in obese class III males, in comparison to non-obese class III males. It should be noted that 95% CIs of all ORs were wide, which limits findings.
Table 2.
Obese class III (BMI ≥ 40) vs. Non-obese class III (BMI < 40) infection rates expressed by gender and infection type. Infection rates for SSSI and DSSI are expressed as a percentage (%). Odds ratio (OR) and 95% confidence interval (95% [CI]) are calculated for obese class III versus non-obese class III groups.
| Female |
Male |
|||
|---|---|---|---|---|
| BMI < 40 | Obese class III | BMI < 40 | Obese class III | |
| n | 442 | 56 | 324 | 17 |
| SSSI% (n) | 1.81 (8) | 8.92 (5) | 2.50 (8) | 5.88 (1) |
| OR (95%[CI]) | 5.32 [1.68, 16.88] | 2.47 [0.29, 20.97] | ||
| p value | 0.0096 | 0.372 | ||
| DSSI% (n) | 0.90 (4) | 5.35 (3) | 1.20 (4) | 11.76 (2) |
| OR (95%[CI]) | 6.198 [1.35, 28.46] | 10.67 [1.81, 62.94] | ||
| p value | 0.034 | 0.031 | ||
SSSI: Superficial Surgical Site Infection; DSSI: Deep Surgical Site Infection; Obese class III: BMI ≥ 40.00.
Fig. 3.
Superficial SSI rate (%) by gender distribution across BMI cohorts.
Fig. 4.
Deep SSI rate (%) by gender distribution across BMI cohorts.
When cohorts were categorised as either obese (all-inclusive BMI ≥ 30.00) vs. non-obese (all-inclusive BMI < 30.00) and grouped by gender, there were no significant differences in either SSSI or DSSI observed (Table 3).
Table 3.
Infection rates for SSSI and DSSI by gender are expressed as a percentage (%). Odds ratio (OR) and confidence interval (CI) are calculated for Obese (BMI ≥ 30.00) vs. Non-obese (BMI < 30.00) groups.
| Female |
Male |
||||
|---|---|---|---|---|---|
| BMI < 30.00 | BMI ≥ 30.00 | BMI < 30.00 | BMI ≥ 30.00 | ||
| n | 167 | 331 | 181 | 160 | |
| SSSI% (n) | 1.19 (2) | 3.32 (11) | 2.76 (5) | 2.50 (4) | |
| OR SSSI (95%[CI]) | 2.84 [0.621, 12.95] | 0.903 [0.238, 3.422] | |||
| p value | 0.236 | 1.000 | |||
| DSSI% (n) | 0.59 (1) | 1.81 (6) | 2.21 (4) | 1.25 (2) | |
| OR DSSI (95%[CI]) | 3.07 [0.366, 25.68] | 0.560 [0.101, 3.10] | |||
| p value | 0.433 | 0.688 | |||
SSSI: Superficial Surgical Site Infection; DSSI: Deep Surgical Site Infection.
4. Discussion
Overweight and obesity are worldwide health epidemics with serious implications on the global health system. Obesity has been shown to be closely aligned with the incidence of osteoarthritis,3,4 whereby obese individuals of a younger age are more likely to require total joint arthroplasty and have a higher revision rate.[5], [6], [7] Total joint replacement, although associated with improved quality of life outcomes, is a highly invasive surgery with risks of complication, such as those associated with SSSI or DSSI. As the incidence of obesity increases in the developed world, coupled with the ageing population, the demand for replacement surgeries is also on the rise.17 One common causative factor of prosthesis failure and revision is infection.17 Rather than assessing infection as an endpoint, studies involving obesity and TKA focus on outcomes of physical function scores or longevity of the prostheses.40 This highlights the need for an improved understanding of the incidence and risk factors associated with infection as a result of joint replacement surgery.
This study demonstrated that overweight, obese class I and obese class II patients do not appear to have an increase in SSI rate compared to patients with normal or low BMI, demonstrated by assessing the potential association between SSSI and DSSI incidence in the obese vs. non-obese TKA population. However, when assessing morbidly obese patients, the obese class III cohort demonstrated a significant association with SSI incidence consistent with the current literature, where morbid obesity has been shown to be associated with a higher incidence of post-surgery infection.41 This was particularly obvious in the female class III cohort with an increased incidence of SSSI and DSSI however the male class III cohort demonstrated an increased incidence in DSSI only. Interestingly there was increased odds of deep infection in the male cohort, yet not superficial infection. This presents an interesting point, however the wide confidence intervals reported limits this finding. Subcutaneous fat tissue depth has also been demonstrated as a contributing factor to SSI rate following cervical spine fusion42 and fat tissue depth around the knee has been suggested to be a potential measure of obesity.43 However, as male obese patients have reduced subcutaneous fat thickness around the knee joint compared to females, fat tissue depth may not be a contributing factor for increased deep infection in this instance, however requires further investigation.43 This finding may therefore indicate more complex systemic or metabolic factors, whereby increased postoperative pain and complication risk associated with obesity has been attributed to the presentation of a systemic pro-inflammatory state.35
Interestingly, there have been a number of recent studies investigating the prevalence of multiple components of metabolic syndrome (MetS) and increased incidence of post-operative complications including SSI.44,45 MetS, a diagnostic grouping of Diabetes Mellitus, dyslipidema, hypertension and obesity, has been of great interest with regard to its potential role in the pathogenesis of osteoarthritis and the associated systemic inflammatory state.[46], [47], [48] In addition, a recent study of the immune response in a rat model of MetS, found rats challenged with S. aureus had higher systemic levels of bacteria, pro-inflammatory cytokines and chemokines 48 h after infection compared to non-MetS.49 Together these studies suggest that MetS may contribute to an altered systemic inflammatory state, increasing risk of a number of post-surgical complications including infection. However, an improved understanding of the molecular link between obesity, MetS and infection is still required. Furthermore, S. aureus nasal carriage is more likely in the obese population50 and this may partially influence the increase in SSI among obese patients, as S. aureus is a common pathogen in SSI. Another potential confounding factor is under-dosing of prophylactic antibiotics in obese patients, as a recent review of antibiotic dosing in an emergency department illustrated that doses given to obese patients were as low as 2–8% compliant with the hospital’s established protocol.51
There are also increased costs and burden placed on the healthcare system associated with increased infection rates that need to be considered. While not as serious as deep infection, superficial infection may increase the cost and length of hospital stay, readmission rates and additional surgeries.52 This presents the complex question of whether obese or morbidly obese patients should have joint replacement surgery, as infection is only one of a number of potential complications that these patients may face,53 however further discussion of this concept is not within the scope of the present study.
There are a number of limitations to consider, most significantly due to retrospective nature of this study. Firstly, confounding variables such as comorbidities including Diabetes Mellitus were unable to be accounted for, limiting the conclusions that can be drawn from this study. The retrospective study design also provides the potential for SSI to have been underreported. In addition, underweight individuals were not explicitly defined and it is unclear if any such patients were included in the normal body weight cohort. Previous studies have identified underweight or malnourished patients are at a higher risk of morbidity and mortality than those with a BMI of above 20 but below 40.54,55
Furthermore, BMI was the only identifier of obesity, thus additional measures should be considered for accurate classification of obese patients in future assessments.
5. Conclusion
The present study demonstrated a higher incidence of SSI, both superficial and deep, in primary TKA obese class III patients and an unchanged rate of infection in overweight, obese class I and obese class II patients when compared to the normal weight cohort. Interestingly male obese class III patients had the highest rate of deep SSI infection suggesting the systemic effects of obesity are more important than gender specific body habitus, however this requires further investigation.
Furthermore, confounders such as comorbidity were unable to be accounted for, limiting plausible inference. Infection in obesity remains a challenge, and an improved understanding of the relationship and the mechanism by which morbid obesity in particular, appears to contribute to SSI requires further investigation. Additionally, the clinical management of orthopaedic surgery should continue to take into account patient obesity class in relation to surgical risk factors.
Conflict of interest
The authors have none to declare.
Each author certifies that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangements etc.) that might pose a conflict of interest in connection with the submitted article.
Ethics approval was not required in Scotland at the time of the study for a retrospective review of data not altering patient care. The study was carried out under the clinical governance procedures of the hospital.
Data were collected by Dr Wilson, Dr Oburu and Dr Deakin at the Golden Jubilee National Hospital, a public elective arthroplasty center in Glasgow.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributions
Dr Christopher John Wilson: Study development, data collection and interpretation of clinical findings and manuscript review.
Dr Kristen Renee Georgiou: Data analysis, interpretation and manuscript write-up and review.
Dr Ezekiel Oburu: Study development, data collection and manuscript review.
Ms Annika Theodoulou: Manuscript write-up, review and preparation for journal submission.
Dr Angela H Deakin: Data collection, data analysis and manuscript review.
Professor Jeganath Krishnan: Manuscript review.
Contributor Information
Christopher John Wilson, Email: Christopher.Wilson@sa.gov.au.
Kristen Renee Georgiou, Email: kgeorgiou@bionomics.com.au.
Ezekiel Oburu, Email: Oburue@yahoo.com.
Annika Theodoulou, Email: annika.theodoulou@flinders.edu.au, Annika.Theodoulou@imri.org.au.
Angela H. Deakin, Email: ahdeakin7@gmail.com.
Jeganath Krishnan, Email: krishnanadmin@sahi.org.au.
References
- 1.Scottish Government. Obesity indicators monitoring progress for the prevention of obesity route map – December 2016 Report. http://www.gov.scot/Publications/2016/12/3526/0, Accessed 10 August 2017.
- 2.Australian Institute of Health and Welfare . AIHW; Canberra: 2016. Australian Institute of Health and Welfare annual report 2015–16. Cat. no. AUS 209. [Google Scholar]
- 3.Crowson C.S., Matteson E.L., Davis J.M., 3rd, Gabriel S.E. Contribution of obesity to the rise in incidence of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65(1):71–77. doi: 10.1002/acr.21660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y.C., McPherson K., Marsh T., Gortmaker S.L., Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 5.Franklin J., Ingvarsson T., Englund M., Lohmander L.S. Sex differences in the association between body mass index and total hip or knee joint replacement resulting from osteoarthritis. Ann Rheum Dis. 2009;68(4):536–540. doi: 10.1136/ard.2007.086868. [DOI] [PubMed] [Google Scholar]
- 6.Changulani M., Kalairajah Y., Peel T., Field R.E. The relationship between obesity and the age at which hip and knee replacement is undertaken. J Bone Joint Surg Br. 2008;90(3):360–363. doi: 10.1302/0301-620X.90B3.19782. [DOI] [PubMed] [Google Scholar]
- 7.Pfefferle K.J., Gil K.M., Fening S.D., Dilisio M.F. Validation study of a pooled electronic healthcare database: the effect of obesity on the revision rate of total knee arthroplasty. Eur J Orthop Surg Traumatol. 2014;24(8):1625–1628. doi: 10.1007/s00590-014-1423-2. [DOI] [PubMed] [Google Scholar]
- 8.Ridgeway S., Wilson J., Charlet A., Kafatos G., Pearson A., Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844–850. doi: 10.1302/0301-620X.87B6.15121. [DOI] [PubMed] [Google Scholar]
- 9.Lilani S.P., Jangale N., Chowdhary A., Daver G.B. Surgical site infection in clean and clean-contaminated cases. Indian J Med Microbiol. 2005;23(4):249–252. [PubMed] [Google Scholar]
- 10.Gardlund B., Bitkover C.Y., Vaage J. Postoperative mediastinitis in cardiac surgery − microbiology and pathogenesis. Eur J Cardiothorac Surg. 2002;21(5):825–830. doi: 10.1016/s1010-7940(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 11.Nickinson R.S., Board T.N., Gambhir A.K., Porter M.L., Kay P.R. The microbiology of the infected knee arthroplasty. Int Orthop. 2010;34(4):505–510. doi: 10.1007/s00264-009-0797-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mangram A.J., Horan T.C., Pearson M.L., Silver L.C., Jarvis W.R. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27(2):97–132. quiz 133–134; discussion 196. [PubMed] [Google Scholar]
- 13.Levy P.Y., Ollivier M., Drancourt M., Raoult D., Argenson J.N. Relation between nasal carriage of Staphylococcus aureus and surgical site infection in orthopedic surgery: the role of nasal contamination. A systematic literature review and meta-analysis. Orthop Traumatol Surg Res. 2013;99(6):645–651. doi: 10.1016/j.otsr.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Tsukayama D.T., Goldberg V.M., Kyle R. Diagnosis and management of infection after total knee arthroplasty. J Bone Joint Surg Am. 2003;85-A(Suppl. 1):S75–S80. doi: 10.2106/00004623-200300001-00014. [DOI] [PubMed] [Google Scholar]
- 15.Darouiche R.O. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 16.Abudu A., Sivardeen K.A., Grimer R.J., Pynsent P.B., Noy M. The outcome of perioperative wound infection after total hip and knee arthroplasty. Int Orthop. 2002;26(1):40–43. doi: 10.1007/s00264-001-0301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Australian Orthopaedic Association National Joint Replacement Registry . AOA; Adelaide: 2016. Annual Report. [Google Scholar]
- 18.Malone D.L., Genuit T., Tracy J.K., Gannon C., Napolitano L.M. Surgical site infections: reanalysis of risk factors. J Surg Res. 2002;103(1):89–95. doi: 10.1006/jsre.2001.6343. [DOI] [PubMed] [Google Scholar]
- 19.Canturk Z., Canturk N.Z., Cetinarslan B., Utkan N.Z., Tarkun I. Nosocomial infections and obesity in surgical patients. Obes Res. 2003;11(6):769–775. doi: 10.1038/oby.2003.107. [DOI] [PubMed] [Google Scholar]
- 20.Kirby J.P., Mazuski J.E. Prevention of surgical site infection. Surg Clin North Am. 2009;89(2):365–389. doi: 10.1016/j.suc.2009.01.001. viii. [DOI] [PubMed] [Google Scholar]
- 21.Jamsen E., Nevalainen P., Eskelinen A., Huotari K., Kalliovalkama J., Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101. doi: 10.2106/JBJS.J.01935. [DOI] [PubMed] [Google Scholar]
- 22.Dindo D., Muller M.K., Weber M., Clavien P.A. Obesity in general elective surgery. Lancet. 2003;361(9374):2032–2035. doi: 10.1016/S0140-6736(03)13640-9. [DOI] [PubMed] [Google Scholar]
- 23.Birkmeyer N.J., Charlesworth D.C., Hernandez F. Obesity and risk of adverse outcomes associated with coronary artery bypass surgery: Northern New England Cardiovascular Disease Study Group. Circulation. 1998;97(17):1689–1694. doi: 10.1161/01.cir.97.17.1689. [DOI] [PubMed] [Google Scholar]
- 24.Jackson R.S., Black J.H., 3rd, Lum Y.W. Class I obesity is paradoxically associated with decreased risk of postoperative stroke after carotid endarterectomy. J Vasc Surg. 2012;55(5):1306–1312. doi: 10.1016/j.jvs.2011.11.135. [DOI] [PubMed] [Google Scholar]
- 25.Turrentine F.E., Hanks J.B., Schirmer B.D., Stukenborg G.J. The relationship between body mass index and 30-day mortality risk, by principal surgical procedure. Arch Surg. 2012;147(3):236–242. doi: 10.1001/archsurg.2011.310. [DOI] [PubMed] [Google Scholar]
- 26.Pikarsky A.J., Saida Y., Yamaguchi T. Is obesity a high-risk factor for laparoscopic colorectal surgery? Surg Endosc. 2002;16(5):855–858. doi: 10.1007/s004640080069. [DOI] [PubMed] [Google Scholar]
- 27.Wigfield C.H., Lindsey J.D., Munoz A., Chopra P.S., Edwards N.M., Love R.B. Is extreme obesity a risk factor for cardiac surgery? An analysis of patients with a BMI> or =40. Eur J Cardiothorac Surg. 2006;29(4):434–440. doi: 10.1016/j.ejcts.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Silber J.H., Rosenbaum P.R., Kelz R.R. Medical and financial risks associated with surgery in the elderly obese. Ann Surg. 2012;256(1):79–86. doi: 10.1097/SLA.0b013e31825375ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y., Wu L., Li X., Wu X., Li B. Outcome of laparoscopic colorectal surgery in obese and nonobese patients: a meta-analysis. Surg Endosc. 2012;26(3):783–789. doi: 10.1007/s00464-011-1952-2. [DOI] [PubMed] [Google Scholar]
- 30.Huttunen R., Syrjanen J. Obesity and the risk and outcome of infection. Int J Obes (Lond) 2013;37(3):333–340. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- 31.Shin D. Association between metabolic syndrome, radiographic knee osteoarthritis, and intensity of knee pain: results of a national survey. J Clin Endocrinol Metab. 2014;99(9):3177–3183. doi: 10.1210/jc.2014-1043. [DOI] [PubMed] [Google Scholar]
- 32.Latham R., Lancaster A.D., Covington J.F., Pirolo J.S., Thomas C.S., Jr. The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol. 2001;22(10):607–612. doi: 10.1086/501830. [DOI] [PubMed] [Google Scholar]
- 33.Dowsey M.M., Choong P.F. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577–1581. doi: 10.1007/s11999-008-0551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoner L., Cornwall J. Did the American Medical Association make the correct decision classifying obesity as a disease? Australas Med J. 2014;7(11):462–464. doi: 10.4066/AMJ.2014.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motaghedi R., Bae J.J., Memtsoudis S.G. Association of obesity with inflammation and pain after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(5):1442–1448. doi: 10.1007/s11999-013-3282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanneganti T.D., Dixit V.D. Immunological complications of obesity. Nat Immunol. 2012;13(8):707–712. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- 37.Ferrante A.W., Jr. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262(4):408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 38.Milner J.J., Beck M.A. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71(2):298–306. doi: 10.1017/S0029665112000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization. BMI classification. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html. Accessed 10 August 2017.
- 40.Foran J.R., Mont M.A., Rajadhyaksha A.D., Jones L.C., Etienne G., Hungerford D.S. Total knee arthroplasty in obese patients: a comparison with a matched control group. J Arthroplasty. 2004;19(7):817–824. doi: 10.1016/j.arth.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Falagas M.E., Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6(7):438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 42.Mehta A.I., Babu R., Sharma R. Thickness of subcutaneous fat as a risk factor for infection in cervical spine fusion surgery. J Bone Joint Surg Am. 2013;95(4):323–328. doi: 10.2106/JBJS.L.00225. [DOI] [PubMed] [Google Scholar]
- 43.Kok H.K., Donnellan J., Ryan D., Torreggiani W.C. Correlation between subcutaneous knee fat thickness and chondromalacia patellae on magnetic resonance imaging of the knee. Can Assoc Radiol J. 2013;64(3):182–186. doi: 10.1016/j.carj.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Gage M.J., Schwarzkopf R., Abrouk M., Slover J.D. Impact of metabolic syndrome on perioperative complication rates after total joint arthroplasty surgery. J Arthroplasty. 2014;29(9):1842–1845. doi: 10.1016/j.arth.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Zmistowski B., Dizdarevic I., Jacovides C.L., Radcliff K.E., Mraovic B., Parvizi J. Patients with uncontrolled components of metabolic syndrome have increased risk of complications following total joint arthroplasty. J Arthroplasty. 2013;28(6):904–907. doi: 10.1016/j.arth.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 46.Hussain S.M., Cicuttini F.M., Bell R.J. Incidence of total knee and hip replacement for osteoarthritis in relation to circulating sex steroid hormone concentrations in women. Arthritis Rheumatol. 2014;66(8):2144–2151. doi: 10.1002/art.38651. [DOI] [PubMed] [Google Scholar]
- 47.King L.K., Henneicke H., Seibel M.J., March L., Anandacoomarasmy A. Association of adipokines and joint biomarkers with cartilage-modifying effects of weight loss in obese subjects. Osteoarthr Cartil. 2015;23(3):397–404. doi: 10.1016/j.joca.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 48.Gandhi R., Woo K.M., Zywiel M.G., Rampersaud Y.R. Metabolic syndrome increases the prevalence of spine osteoarthritis. Orthop Surg. 2014;6(1):23–27. doi: 10.1111/os.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng X., Maze M., Koch L.G., Britton S.L., Hellman J. Exaggerated acute lung injury and impaired antibacterial defenses during staphylococcus aureus infection in rats with the metabolic syndrome. PLoS One. 2015;10(5):e0126906. doi: 10.1371/journal.pone.0126906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olsen K., Danielsen K., Wilsgaard T. Obesity and Staphylococcus aureus nasal colonization among women and men in a general population. PLoS One. 2013;8(5):e63716. doi: 10.1371/journal.pone.0063716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roe J.L., Fuentes J.M., Mullins M.E. Underdosing of common antibiotics for obese patients in the ED. Am J Emerg Med. 2012;30(7):1212–1214. doi: 10.1016/j.ajem.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 52.Vince K., Chivas D., Droll K.P. Wound complications after total knee arthroplasty. J Arthroplasty. 2007;22(4 Suppl 1):39–44. doi: 10.1016/j.arth.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 53.Saleh K., Olson M., Resig S. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506–515. doi: 10.1016/S0736-0266(01)00153-X. [DOI] [PubMed] [Google Scholar]
- 54.Davenport D.L., Xenos E.S., Hosokawa P., Radford J., Henderson W.G., Endean E.D. The influence of body mass index obesity status on vascular surgery 30-day morbidity and mortality. J Vasc Surg. 2009;49(1):140–147. doi: 10.1016/j.jvs.2008.08.052. 147, e141, discussion 147. [DOI] [PubMed] [Google Scholar]
- 55.Pessaux P., Atallah D., Lermite E. Risk factors for prediction of surgical site infections in clean surgery. Am J Infect Control. 2005;33(5):292–298. doi: 10.1016/j.ajic.2004.12.005. [DOI] [PubMed] [Google Scholar]