Abstract
Background. Lapatinib is an oral small molecule dual tyrosine kinase inhibitor that has been shown to improve time to progression versus capecitabine in women with HER2+ metastatic breast cancer (MBC) previously treated with trastuzumab. Objective. To describe extent, predictors, and consequences of nonadherence with lapatinib in women with MBC who were previously treated with trastuzumab. Methods. This was a retrospective observational study using data from a large health insurance claims databases spanning January 2000 to March 2010. Measures of lapatinib adherence included medication possession ratio (MPR), time to discontinuation (end of supply), time to first treatment interruption (gap during treatment of 30 days without supply), and duration of continuous therapy (time to gap of 30 days without supply or end of supply). Predictors of nonadherence to lapatinib and the association between nonadherence and outcomes, utilization, and costs were examined using multiple regression analysis. Results. A total of 666 patients met all inclusion criteria. Mean initial lapatinib dosage was 1161 mg daily; 63% received index lapatinib in combination with capecitabine. Mean MPR was 87%; 22% of patients had MPR < 80%. Median time to lapatinib discontinuation was 9.1 months (95% confidence interval = 8.0-10.2). Twenty-seven percent of patients had one or more treatment interruptions during follow-up. Median duration of continuous therapy was 5.9 months (95% confidence interval = 5.1-6.1). Concomitant therapy with a taxane was a predictor of nonadherence (odds ratio for MPR < 80% = 10.30; P < .001). There was a statistically significant association between nonadherence to lapatinib and greater number of outpatient visits (P = .028). Conclusions. In women with MBC who were previously treated with trastuzumab, mean adherence to lapatinib in typical clinical practice is relatively high overall, although there is a small group of patients with high nonadherence. Targeted efforts to improve adherence to lapatinib in this subgroup may be warranted.
Keywords: metastatic breast cancer, lapatinib, patient adherence, retrospective studies
Introduction
Lapatinib (Tykerb; GlaxoSmithKline, Research Triangle Park, NC) is an oral small molecule dual tyrosine kinase inhibitor that binds intracellularly to the ATP binding site of the epidermal growth factor receptor and human epidermal growth factor 2 (HER2) receptors.1 In the United States, lapatinib is indicated in combination with capecitabine for the treatment of patients with advanced or metastatic breast cancer (MBC) whose tumors overexpress HER2 (HER2+) and who have received prior therapy including an anthracycline, a taxane, and trastuzumab. It is also indicated in combination with letrozole for the treatment of postmenopausal women with hormone receptor positive MBC that overexpresses the HER2 receptor for whom hormonal therapy is indicated.
While the benefits of adding lapatinib to capecitabine have been established in the setting of a controlled clinical trial,2-5 the generalizability of findings from such studies to typical clinical practice can sometimes be uncertain, as patients seen in clinical practice may be more heterogeneous than study subjects in clinical trials.6 Moreover, adherence with prescribed treatment in clinical practice may be less than that in controlled trials, as patients are likely to be monitored less frequently and/or intensively in the former setting than the latter.7 Although oral anticancer therapies, such as lapatinib, may have advantages in terms of patient convenience and savings in administration costs compared with medications that are not orally administered, oral therapies may pose particular challenges with respect to adherence.
Previous studies have examined nonadherence with oral anticancer therapies and its clinical and economic consequences.8,9 For example, in a study of patients with early breast cancer receiving tamoxifen, those who filled fewer than 70% of their tamoxifen prescriptions were found to have an increased risk of death.10 In a study of an intensified multidisciplinary pharmaceutical care program on adherence among cancer patients receiving capecitabine, subjects in the intervention group had significantly higher adherence than those in the control group (mean daily adherence, 96.8% vs 87.2%, respectively; P = .029). There was no statistically significant difference in survival, however (97.9% vs 90.5%, P = .069).11 In 2 retrospective studies in adult patients with chronic myelogenous leukemia receiving imatinib, treatment interruptions and nonadherence with imatinib were prevalent and were associated with increased health care utilization and costs.12,13
Comparatively little is known about the prevalence, predictors, and economic consequences of nonadherence to lapatinib in women with MBC who were previously treated with trastuzumab. Only 2 prior studies have reported such information.14,15 In a study of 1816 patients participating in a patient assistance program (TYKERB CARES), mean medication possession ratio (MPR), a common measure of adherence in claims database analyses, was 91%; median time to end of continuous therapy (treatment interruption or discontinuation) was 3.8 months.14 These data may not be representative of patients receiving lapatinib outside of such a program. A prospective study of 69 Italian patients that assessed lapatinib adherence–based patient-completed medication diaries, self-report during the physician interview, and the pharmacy control of the drug box reported that adherence to lapatinib was 82% across all cycles.15 Although informative, these data may not be representative of patients receiving lapatinib in the United States. The objective of this study was to examine the prevalence, predictors, and clinical and economic consequences of nonadherence with lapatinib in women with MBC who were previously treated with trastuzumab, using data from a large US health insurance claims database.
Methods
Data Source
Data for this project were obtained from the MarketScan Commercial Claims and Encounters (CCAE) Database and the Medicare Supplemental and Coordination of Benefits (MDCR) Database (Truven Health Analytics, Ann Arbor, MI). These databases contain information on the health insurance claims (both medical and pharmacy claims) of employees of large, self-insured corporations and their dependents, along with a few commercial health plans, and for Medicare-eligible persons (mainly retirees) who are also covered by self-insured employers. Over the study period, the 2 databases contained health care claims data for over 50 million persons. Information available for each person in the databases includes age, sex, type of employment, and location (state and zip code). Data used in this study spanned the period from January 2000 to March 2010 (“study period”). Although lapatinib was approved in March 2007, claims data from before that point were used to help characterize patients’ baseline characteristics such as time since last trastuzumab claim.
Study Subjects
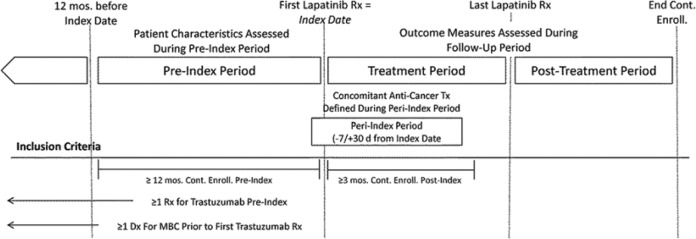
Detailed descriptions of all administrative codes used to identify the sample, patient characteristics, and outcomes are reported in the appendix (available online at http://pmt.sagepub.com/content/by/supplemental-data). Study subjects included all patients in the data set meeting the following criteria: one or more claims for lapatinib during the study period (dispensing date of first claim = “index date”), one or more claims for trastuzumab during the study period, one or more claims with a diagnosis of breast cancer during the study period, and one or more claims with a diagnosis of distant metastases during the study period. Patients meeting any of the following criteria were excluded: male sex, age less than 18 years as of index date, less than 12 months of continuous eligibility prior to index date, less than 3 months from index date to end of study period, no claims for trastuzumab prior to first claim for lapatinib, no claims for trastuzumab after first claim for distant metastases, less than 2 claims for lapatinib (on different days), or missing or ambiguous data on claims or enrollment information required to calculate patient characteristics, lapatinib dosage, or adherence. For patients meeting all eligibility requirements, the pre-index period was defined as the period beginning 12 months prior to the index date (Figure 1). The follow-up period was calculated for each patient as the time from index date to end of last day of health plan enrollment. The peri-index period was defined as the period beginning 7 days prior to the index date and ending 30 days after index date. The lapatinib treatment period was defined as the period beginning with the index date and ending with the last day of lapatinib treatment. The post-lapatinib treatment period was defined as the period beginning the day after the last day of the lapatinib treatment period and ending with the end of follow-up (the last day of health plan enrollment or end of study).
Figure 1.
Study design.
Abbreviations: Dx, diagnosis; MBC, metastatic breast cancer; Mos., months; Rx, prescription; Tx, treatment.
Patient Characteristics
For each patient in the study sample, age, region, and plan type were obtained from enrollment files. Diagnosis codes on medical claims during the pre-index period also were scanned to ascertain selected comorbidities. The Charlson–Deyo comorbidity index was calculated for all patients.16 For each patient, the treatment regimen associated with the index prescription for lapatinib was identified based on hormonal (aromatase inhibitors, tamoxifen), chemotherapy (anthracyclines, taxanes, capecitabine, vinorelbine, gemcitabine, carboplatin, ixabepilone, other), and targeted drugs received during the peri-index period. The number of specific anticancer medications received during the pre-index period also was calculated for each patient. When identifying anticancer therapies during the pre-index period, treatments received during the peri-index period were excluded. Time since last trastuzumab claim was calculated for each patient. Also, history of prior taxanes, prior anthracyclines, and prior taxanes and prior anthracyclines was calculated for each patient based on all claims prior to the index date.
A variety of measures of health care utilization and costs during the 12-month pre-index period also were calculated for each patient. The initial daily dosage of lapatinib (in mg) was also ascertained based on information from the index (ie, first) claim for lapatinib by multiplying the mg per tablet for lapatinib (250) by the prescribed tablets per days for the index claim. The prescribed tablets per day were calculated as the ratio of the quantity supplied and the days supplied on the index claim. The patient contribution (co-pay, coinsurance amount, and deductible) for the index lapatinib prescription was also calculated.
Outcome Measures
Adherence to lapatinib was assessed using information on number of days supplied from outpatient pharmacy claims. Measures of adherence to lapatinib included the MPR, time to treatment interruption, time to treatment discontinuation, and time to end of continuous treatment. MPR for lapatinib was defined as the maximum of 1.0 and the ratio of total days of lapatinib supplied divided by the lapatinib treatment period.17,18
A lapatinib treatment interruption was defined as a failure, during the lapatinib treatment period, to refill a prescription for lapatinib within 30 days of the end of supply from a prior prescription. Time to lapatinib discontinuation was calculated as the time between the index date and the last day with supply of lapatinib on hand. Time to end of continuous lapatinib therapy was calculated as the time between the index date and the minimum of the time to treatment interruption or the time to discontinuation. Because discontinuation may be due to disease progression, whereas treatment interruption is not likely due to disease progression, we report these separately. We report time to end of continuous therapy (the combination of treatment interruption and discontinuation) as this measure is comparable to time to discontinuation in prior studies of adherence.19
Measures of utilization and costs were calculated separately for the pre-index period, the lapatinib treatment period, and the post-lapatinib treatment period. Measures of health care utilization included the number of physicians’ office or outpatient hospital visits, number of hospitalizations, and number of inpatient days. Measures of health care costs included the cost of lapatinib, trastuzumab, chemotherapy, chemotherapy administration, all other costs (those costs not captured in previous categories), and total costs. To control for duration of each period (the lapatinib treatment period and post-lapatinib treatment period could differ across patients), measure of health care utilization and cost were calculated on a monthly basis by dividing total utilization and costs during the period by the number of months of observation during the period. Time to change in treatment was defined as discontinuation of lapatinib or receipt of new hormonal, chemotherapy, or anti-HER2 therapy. New hormonal therapy, chemotherapy, and anti-HER2 therapies were defined as any hormonal therapy, chemotherapy, or anti-HER2 therapy received during the follow-up period that was not received during the peri-index period. For patients with no change in treatment, censoring time was the time between index day and end of study.
Analyses
Time to dose reduction, treatment interruption, treatment discontinuation, end of continuous treatment, and change in treatment were analyzed using Kaplan–Meier methods. In Kaplan–Meier analyses, time to treatment interruption was censored at the end of the treatment period for patients with no gap of 30 days during the treatment period. Time to treatment discontinuation and time to end of continuous lapatinib therapy were censored at the end of the treatment period for patients with supply of medication on hand less than 30 days from the end of the study period. Nonadherence was analyzed as a continuous variable using ordinary least squares regression with ln (100 × (1 − MPR) + 1) as the dependent variable and as a binary variable using logistic regression with patients alternatively classified as nonadherent based on nonadherence of 5% or more (MPR < 95%) and nonadherence of 20% or more (MPR < 80%). Covariates included in these models were population (commercial or Medicare), age (nested within population), region, plan type, comorbidity index, taxane and anthracycline use pre-index, number of physicians’ office or hospital outpatient visits pre-index, total health care costs pre-index, anticancer therapies received during the peri-index period (none, capecitabine only, trastuzumab only, hormonal therapy only, taxanes only, capecitabine and hormonal therapy, capecitabine and trastuzumab, trastuzumab and hormonal therapy, and other), patient contribution on index prescription ($0, >$0 to <$50, $50 to <$100, and ≥$100), and daily dosage of lapatinib on index prescription (<1250 mg vs ≥1250 mg). We also conducted similar analyses of predictors of treatment interruption using Cox proportion hazard regression. Models were generated alternatively using all covariates and stepwise selection.
Analyses of the association between nonadherence and health care utilization and costs were conducted on the numbers of physicians’ office and outpatient hospital visits and total health care costs excluding the costs of lapatinib. These variables were selected based on a power analysis suggesting that power to detect an association would be greatest for these measures. The analysis of costs excluding lapatinib costs is reasonable because it is elementary that lapatinib costs will increase with better adherence. An analysis of the association between nonadherence and total costs including the costs of lapatinib was included for completeness. Analyses of the association between nonadherence and these measures were conducted using generalized linear regression (GLM) models. For visits, log link and negative binomial error terms were employed. For costs, log link and gamma error terms were used. These analyses were conducted with nonadherence (100 × (1 − MPR)) first included as continuous variable, then as a categorical variable (MPR < 95% vs ≥95%; MPR < 80% vs ≥80%; MPR < 80%, 80% ≤ MPR < 80% vs MPR ≥ 95%). There were no formal study hypotheses to be tested in the study. All analyses were hypothesis generating only. All analyses were conducted using SAS, Release 9.0 (SAS Institute Inc, Cary, NC).
Results
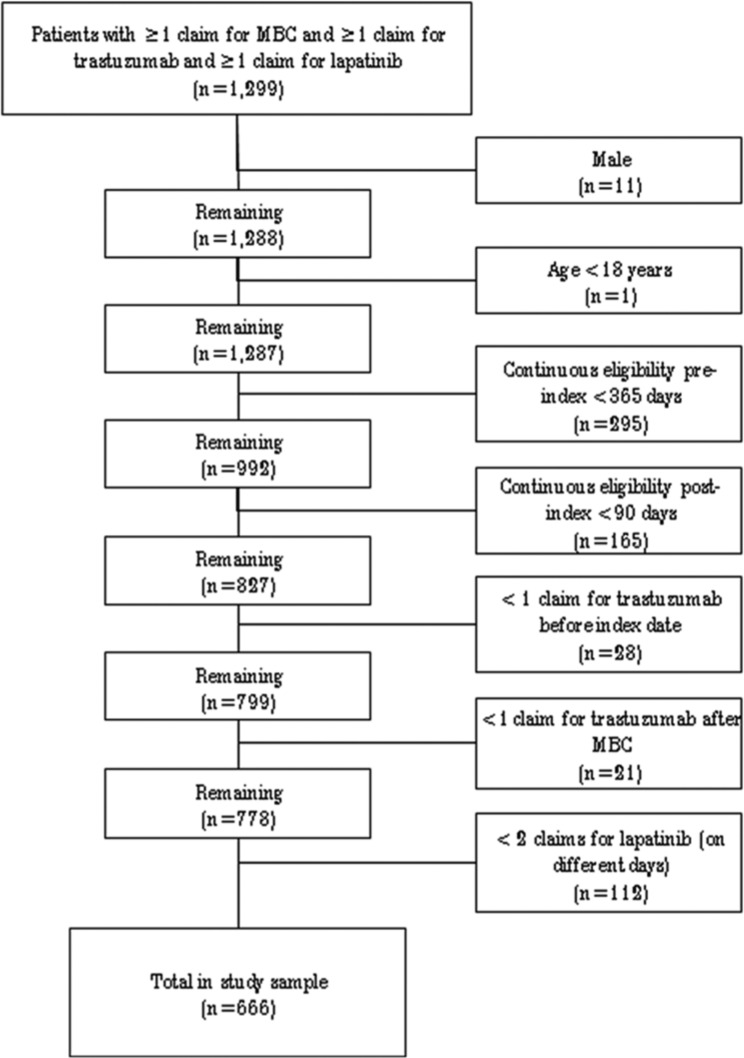
A total of 1299 patients had at least 1 claim with a diagnosis of breast cancer, at least 1 claim with a diagnosis of distant metastases, at least 1 claim for trastuzumab, and at least 1 claim for lapatinib. Of these, 666 met all inclusion criteria (572 commercial and 94 Medicare; Figure 2). Mean (SD) follow-up was 14.0 (8.2) months. Mean (SD) age was 52 (8) years in commercially insured patients and 72 (6) years in Medicare patients and 54 (10) years overall (Table 1). During the 12-month pre-index period, 35.6% of all patients received hormonal therapy, 88.7% received chemotherapy, and 90.2% received trastuzumab (9.8% of patients received their last pre-index dose of trastuzumab more than 12 months prior to the index date). Among all patients, 72.8% had claims history of prior taxanes, 26.7% had prior anthracyclines, and 24.2% had prior taxanes and prior anthracyclines.
Figure 2.
Selection of study subjects.
Abbreviation: MBC, metastatic breast cancer.
Table 1.
Baseline Characteristics.
| Characteristic | Commercial (N = 572) | Medicare (N = 94) | Combined (N = 666) |
|---|---|---|---|
| Age, years, Mean (SD) | 52 (8) | 72 (6) | 54 (10) |
| Geographic region, n (%) | |||
| Northeast | 56 (9.8) | 13 (13.8) | 69 (10.4) |
| North central | 169 (29.5) | 38 (40.4) | 207 (31.1) |
| South | 262 (45.8) | 34 (36.2) | 296 (44.4) |
| West | 81 (14.2) | 9 (9.6) | 90 (13.5) |
| Unknown | 4 (0.7) | 0 (0.0) | 4 (0.6) |
| Insurance type, n (%) | |||
| Health maintenance organization | 92 (16.1) | 3 (3.2) | 95 (14.3) |
| Preferred provider organization | 354 (61.9) | 43 (45.7) | 397 (59.6) |
| Point of service plan | 72 (12.6) | 6 (6.4) | 78 (11.7) |
| Indemnity plan | 32 (5.6) | 40 (42.6) | 72 (10.8) |
| Other/missing | 22 (3.8) | 2 (2.1) | 24 (3.6) |
| Pre-index comorbidity index, mean (SD) | 6.7 (0.9) | 7.1 (1.3) | 6.6 (0.9) |
| Time since last trastuzumab claim (days), mean (SD) | 108 (176) | 112 (185) | 109 (177) |
| Anticancer therapy during pre-index period, n (%) | |||
| Hormonal therapy | 195 (34.1) | 42 (44.7) | 237 (35.6) |
| Chemotherapy | |||
| Taxanes | 269 (47.0) | 44 (46.8) | 313 (47.0) |
| Anthracyclines | 51 (8.9) | 7 (7.4) | 58 (8.7) |
| Capecitabine | 144 (25.2) | 24 (25.5) | 168 (25.2) |
| Carboplatin | 93 (16.3) | 13 (13.8) | 106 (15.9) |
| Gemcitabine | 79 (13.8) | 15 (16.0) | 94 (14.1) |
| Vinorelbine | 124 (21.7) | 28 (29.8) | 152 (22.8) |
| Any chemotherapy | 511 (89.3) | 80 (85.1) | 591 (88.7) |
| Trastuzumab | 521 (91.1) | 80 (85.1) | 601 (90.2) |
Abbreviation: SD, standard deviation.
While the mean initial lapatinib dose was 1161 mg/d, 84% initiated on 1250 mg/d. Lapatinib was initiated in combination with capecitabine in 62.6% of patients, in combination with trastuzumab in 21.3% of patients, and in combination with hormonal therapy in 10.2% of patients; 64.9% of all patients received lapatinib in combination with 1 other anticancer therapy, 17.9% in combination with 2 other anticancer therapies, and 3.2% in combination with ≥3 other anticancer therapies (Table 2).
Table 2.
Number (%) of Patients Receiving Anticancer Drugs Received During the Peri-Index Period.
| Commercial (N = 572) | Medicare (N = 94) | Combined (N = 666) | |
|---|---|---|---|
| Number of specific anti-cancer medications received | |||
| 0 | 68 (11.9) | 26 (27.7) | 94 (14.1) |
| 1 | 380 (66.4) | 52 (55.3) | 432 (64.9) |
| 2 | 106 (18.5) | 13 (13.8) | 119 (17.9) |
| 3 | 17 (3.0) | 2 (2.1) | 19 (2.9) |
| 4+ | 1 (0.2) | 1 (1.1) | 2 (0.3) |
| Anticancer drugs received (any combination) | |||
| Capecitabine | 376 (65.7) | 41 (43.6) | 417 (62.6) |
| Paclitaxel | 21 (3.7) | 6 (6.4) | 27 (4.1) |
| Docetaxel | 7 (1.2) | 1 (1.1) | 8 (1.2) |
| Anthracyclines | 4 (0.7) | 0 (0.0) | 4 (0.6) |
| Vinorelbine | 21 (3.7) | 3 (3.2) | 24 (3.6) |
| Gemcitabine | 15 (2.6) | 3 (3.2) | 18 (2.7) |
| Any hormonal therapy | 59 (10.3) | 9 (20.2) | 68 (10.2) |
| Trastuzumab | 123 (21.5) | 19(20.2) | 142 (21.3) |
| Other chemotherapy | 17 (3.0) | 4 (4.3) | 21 (3.2) |
| Specific anticancer drug combinations received | |||
| None | 68 (11.9) | 26 (27.7) | 94 (14.1) |
| Capecitabine | 297 (51.9) | 31 (33.0) | 328 (49.2) |
| Trastuzumab | 41 (7.2) | 8 (8.5) | 49 (7.4) |
| Capecitabine + trastuzumab | 40 (7.0) | 5 (5.3) | 45 (6.8) |
| Capecitabine + hormone therapy | 20 (3.5) | 1 (1.1) | 21 (3.2) |
| Hormone therapy | 15 (2.6) | 5 (5.3) | 20 (3.0) |
| Taxane | 11 (1.9) | 2 (2.1) | 13 (2.0) |
| Trastuzumab + hormone therapy | 11 (1.9) | 1 (1.1) | 12 (1.8) |
| Capecitabine + trastuzumab + vinorelbine | 6 (1.0) | 0 (0.0) | 6 (0.9) |
| Vinorelbine | 6 (1.0) | 2 (2.1) | 8 (1.2) |
| Gemcitabine | 6 (1.0) | 2 (2.1) | 8 (1.2) |
| Trastuzumab + taxane | 8 (1.4) | 1 (1.1) | 9 (1.4) |
| Other | 43 (7.5) | 10 (10.6) | 53 (8.0) |
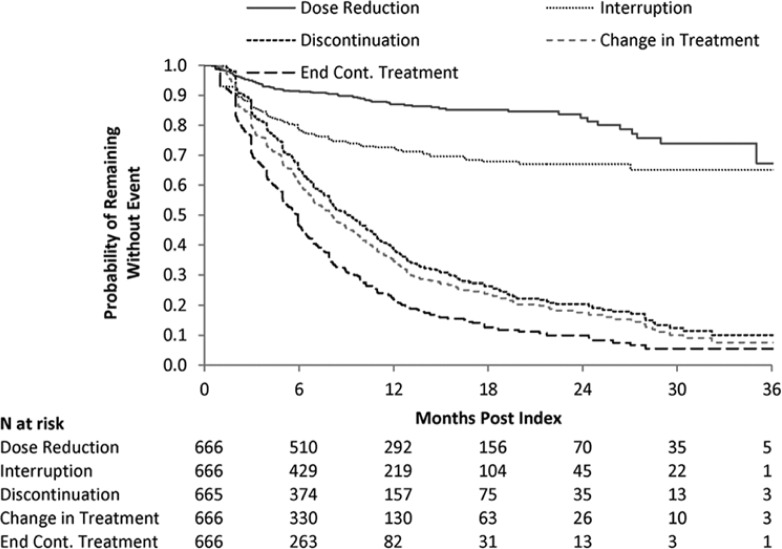
The mean number of lapatinib claims was 7.1. Mean MPR was 87%; Median MPR was 96%. Twenty-two percent of patients had MPR < 80%. Twenty-seven percent of patients had a treatment interruption during follow-up; median time to first treatment interruption was not reached (Figure 3).
Figure 3.
Kaplan–Meier curves for time to event measures.
In these analyses, patients were censored at the end of the study period; disenrollment was not considered a censoring event. This is consistent with a cumulative incidence approach with disenrollment considered a competing risk. Medians were not reached for time to dose reduction and time to interruption.
Kaplan–Meier estimated median time to discontinuation of lapatinib was 9.1 months (95% confidence interval [CI] = 8.0-10.2). Median time to end of continuous lapatinib treatment was 5.9 months (95% CI = 5.1-6.1). Median time to change in anticancer therapy (discontinuation or receipt of new anticancer agent) was 8.0 (95% CI = 7.0-8.9) months. Fourteen percent of patients had a lapatinib dose reduction. Median time to first dose reduction was not reached.
Descriptive statistics on health care utilization and costs are reported in Table 3. Results were similar for commercial and Medicare patients and were therefore combined. The mean number of physicians’ office and hospital outpatient visits per month declined from 6.09 during the 12 months pre-index to 5.45 during the lapatinib treatment period and to 4.55 during the post-lapatinib treatment period. The mean numbers of hospitalizations increased progressively over time from 0.05 during the pre-index period to 0.09 during the lapatinib treatment period and 0.15 during the post-lapatinib treatment period.
Table 3.
Mean (SD) Monthly Health Care Cost in the 12 Months Pre-Index and During Lapatinib Treatment Period and Post-Lapatinib Treatment Period (N = 666).
| Follow-Up Period |
||||
|---|---|---|---|---|
| Measure | 12-Month Pre-Index Period | Lapatinib Treatment | Post Lapatinib Treatment | All |
| Utilization | ||||
| No. of physicians’ office or outpatient hospital visits | 6.09 (3.04) | 5.45 (3.49) | 4.55 (3.90) | 5.22 (3.12) |
| No. of hospitalizations | 0.05 (0.08) | 0.09 (0.19) | 0.15 (0.32) | 0.10 (0.17) |
| No. of inpatient days | 0.24 (0.52) | 0.53 (1.39) | 0.96 (2.52) | 0.64 (1.30) |
| Costs, $ | ||||
| Lapatinib | 0 (0) | 2097 (877) | 0 (0) | 1469 (1031) |
| Trastuzumab | 2762 (2020) | 722 (1731) | 1166 (2134) | 924 (1556) |
| Chemotherapy | 1355 (1898) | 1496 (1698) | 1437 (2729) | 1543 (1805) |
| Chemotherapy administration | 508 (447) | 189 (298) | 326 (516) | 249 (343) |
| Other | 5446 (4680) | 5564 (7198) | 6880 (9726) | 5973 (5765) |
| Total | 10 071 (6056) | 10 067 (7695) | 9809 (10 853) | 10 158 (6468) |
| Total excluding lapatinib | 10 071 (6056) | 7970 (7734) | 9809 (10 853) | 8689 (6526) |
Abbreviation: SD, standard deviation.
Mean lapatinib costs were $2097 per month during the treatment period. Mean trastuzumab costs declined from the pre-index period to the lapatinib treatment period and then increased during the post-lapatinib treatment period. Chemotherapy costs were relatively stable over time. Mean monthly chemotherapy administration costs declined from $508 per month during the pre-index period to $189 per month during the lapatinib treatment period and then increased to $326 during the post-lapatinib treatment period. Mean total monthly costs excluding lapatinib costs declined from $10 071 per month during the pre-index period to $7970 during the lapatinib treatment period and then increased to $9809 during the post-treatment period. Mean total monthly costs including lapatinib costs were virtually unchanged in the lapatinib treatment period versus the pre-index period.
Predictors of Nonadherence
In the multivariate GLM regression model predicting the log of 100 × (1 − MPR) and logistic regression models of MPR < 95% and MPR < 80%, there were statistically significant associations between receipt of concomitant taxanes and receipt of concomitant trastuzumab and hormonal therapy and lower adherence (nonadherence rate ratio 3.26; 95% CI = 1.50-7.09; P = .003; odds ratio for MPR < 80% = 10.30; P < .001). In Cox regression analysis, there was a statistically significant association between receipt of concomitant taxanes and reduced time to treatment interruption (hazard ratio [HR] = 3.98; 95% CI = 1.93-8.22; P < .001). Patient contribution on lapatinib index prescription >$0 to <$50 (vs $0) was associated with improved adherence in all models (HR for time to treatment interruption = 0.59; 95% CI = 0.37-0.93; P = .025). However, patient contribution of $50 to <$100 and ≥$100 were not associated with lower adherence in any model. The multiple linear regression on the log of 100 × (1 − MPR) had a very low R2 (0.05), suggesting that the models explained relatively little of the variability in nonadherence.
Association Between Nonadherence and Outcomes, Utilization, and Costs
In multivariate GLM regression models, there was a statistically significant association between nonadherence to lapatinib (measured as 1 − MPR) and increased physicians’ office/outpatient visits after controlling for baseline characteristics (Table 4; P = .028). There were no statistically significant associations between nonadherence and total health care costs (P = .870) or total health care costs excluding the costs of lapatinib (P = .146). When binary and categorical measures of adherence were entered as predictors of visits and costs, there were no statistically significant associations between measures of adherence and number of visits or costs.
Table 4.
GLM Regressions on Association Between Measures of Adherence and Number of Physicians’ Office/Hospital Outpatient Visits, per Month (N = 666).
| Model No. | Independent Variable Representing Adherence | eCoefficient | 95% CI | P (χ2) |
|---|---|---|---|---|
| 1 | (1 − MPR) | 1.256 | 1.025-1.540 | .028 |
| 2 | MPR ≥ 95% | 1.000 | ||
| MPR < 95% vs MPR ≥ 95% | 1.044 | 0.965-1.130 | .283 | |
| 3 | MPR ≥ 80% | 1.000 | ||
| MPR < 80% vs MPR ≥ 80% | 1.037 | 0.948-1.133 | .430 | |
| 4 | MPR ≥ 95% | 1.000 | ||
| MPR < 80% vs MPR ≥ 95% | 1.049 | 0.954-1.152 | .323 | |
| 80% ≤ MPR < 95% vs MPR ≥ 95% | 1.039 | 0.940-1.147 | .455 |
Abbreviations: CI, confidence interval; GLM, generalized linear model; MPR, medication possession ratio.
Other covariates in regressions included the following (all class variables): population/age (commercial, age ≤ 55 years, commercial, age > 55 years, Medicare, age ≤ 70 years, Medicare, age > 70 years), region (West, Northeast, North central, South), Plan type (HMO, PPO, point of service, independent, other), Charlson Index (<6, ≥6), anthracycline or taxane prior to lapatinib (no, yes), number of visits during pre-index period (<60, ≥60), total payments during pre-index period (<$100 000, ≥$100 000), anticancer therapies received during peri-index period (capecitabine, trastuzumab, hormonal, taxane, capecitabine + hormonal therapy, capecitabine + trastuzumab, trastuzumab + hormonal therapy, none, other), patient contribution on index lapatinib prescription ($0 >$0 to <$50 $50 to <$100 ≥$100), lapatinib dose (<1250 vs ≥1250 mg/d).
Discussion
In women with MBC who were previously treated with trastuzumab, mean adherence to lapatinib in typical clinical practice is relatively high (87%). However, 22% of patients had MPR < 80% and 27% of patients had a treatment interruption during follow-up. In multivariate regression analysis, concomitant receipt of taxanes was significantly associated with reduced adherence in all models. This association may reflect toxicities associated with taxane therapy, or unmeasured confounding factors associated with taxane therapy and adherence. Patient contribution on lapatinib index prescription >$0 to <$50 (vs $0) was associated with improved adherence in all models. However, patient contribution of $50 to <$100 and ≥$100 was not associated with adherence in any models. The absence of an association between the higher categories of co-payment and lapatinib adherence may relate to the use of co-payment assistance programs for lapatinib. Adherence to lapatinib was associated with fewer physicians’ office and outpatient visits. Whether this association reflects a positive effect of treatment or increased toxicities resulting in more visits and worse adherence is uncertain. There was a statistically nonsignificant association between lapatinib nonadherence and increased total health care costs excluding the costs of lapatinib. It may be worthwhile to explore this association again once more patients receiving lapatinib are available in the database. A statically nonsignificant positive association was also observed between higher lapatinib dosage and nonadherence in all models. This might reflect that patients with higher dosages have greater toxicities, which leads to greater nonadherence. Conversely, it might be that toxicities lead to both reduced compliance and lower doses. Further research is needed to assess the effects of lapatinib dose modification on patient outcomes.
Mean MPR in this study (87%) is somewhat lower than that in a recent evaluation of a cohort of patients with MBC enrolled in a lapatinib patient assistance and adherence program (91%; Tykerb CARES).14 Median time to end of continuous therapy (treatment interruption or discontinuation) was greater in this study (5.9 months) than the corresponding value in Tykerb CARES (114 days or 3.8 months). The reason for these differences in findings is uncertain. Median time to treatment discontinuation in this study was 9.1 months. This compares with median time to progression (TTP) by independent review of 6.2 months in the pivotal trial of lapatinib.5
As in the Tykerb CARES study, patient co-pay was not significantly associated with MPR < 80%. Patients in the Tykerb CARES study were participating in a patient assistance program, so the lack of an association between co-pay and adherence is not unexpected. Some patients in the study reported here may also have been participating in this program although it was not possible to identify such patients based on information in the data set. These findings differ from those of a recent retrospective health insurance claims–based study of approximately 8000 women older than age 50 years who were taking aromatase inhibitors for resected breast cancer that found that higher prescription co-payments were associated with both increased nonpersistence and nonadherence to aromatase inhibitors.20 The fact that there was not a consistent association between co-pay and adherence in this study of patients receiving lapatinib may relate to the more severe prognosis for patients in this study (metastatic vs early breast cancer), which may increase patient motivation to be adherent to their medications.
Limitations of this study should be noted. First, this analysis used health insurance claims data, which are subject to coding errors and incomplete claims history in addition to a complete lack of mortality data. As with any claims analysis examining adherence, a claim for payment for a prescription does not ensure that the patient is actually taking the medication as prescribed. Also, it was not feasible to distinguish between patient nonadherence and physician-directed treatment interruption due to toxicities. In examining predictors of adherence, we only examined baseline covariates and did not examine post-index factors that might be affected by treatment such as response to treatment, adverse events, or total pill burden.
An analysis of the association between adverse events and adherence is a potential area for future research. Also, this analysis focused only on patients receiving lapatinib after trastuzumab, and it would be interesting to explore adherence to lapatinib in patients receiving it in other settings (eg, first-line treatment for MBC). Further research to identify the barriers to adherence in women receiving oral chemotherapeutics for MBC may help in the design of programs and strategies to improve adherence in these patients.
Conclusion
In this large insurance claims database study, adherence to lapatinib treatment was relatively high, although there was a subgroup of patients with less than optimal adherence. Predictors of nonadherence in our study were limited to concomitant use of taxanes, with an inconsistent association of co-payment amounts on nonadherence. The consequences of nonadherence were limited to an association with increased outpatient visit utilization. Further research is needed to better understand the reasons for nonadherence in the minority of patients so that these factors may be targeted in future adherence assistance programs.
Supplementary Material
Footnotes
Supplementary material for this article is available on the Journal of Pharmacy Technology Web site at http://pmt.sagepub.com/content/by/supplemental-data.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Thomas Delea and Alex Kartashov are employees of PAI (Policy Analysis Inc), which has received research funding and consulting fees from GlaxoSmithKline (GSK). Puza Sharma is a former employee of GSK and owns GSK stock/stock options.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by GlaxoSmithKline.
References
- 1. Tykerb (lapatinib) tablets [Prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; March 2012 [Google Scholar]
- 2. Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733-2743. [DOI] [PubMed] [Google Scholar]
- 3. Cameron D. Lapatinib plus capecitabine in patients with HER2-positive advanced breast cancer. Clin Adv Hematol Oncol. 2007;5:456-458. [PubMed] [Google Scholar]
- 4. Cameron D, Casey M, Oliva C, Newstat B, Imwalle B, Geyer CE. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist. 2010;15:924-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533-543. [DOI] [PubMed] [Google Scholar]
- 6. Flather M, Delahunty N, Collinson J. Generalizing results of randomized trials to clinical practice: reliability and cautions. Clin Trials. 2006;3:508-512. [DOI] [PubMed] [Google Scholar]
- 7. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497. [DOI] [PubMed] [Google Scholar]
- 8. Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56-66. [DOI] [PubMed] [Google Scholar]
- 9. Wood L. A review on adherence management in patients on oral cancer therapies. Eur J Oncol Nurs. 2012;16:432-438. [DOI] [PubMed] [Google Scholar]
- 10. Thompson AM, Dewar J, Fahey T. Association of poor adherence to prescribed tamoxifen with risk of death from breast cancer. Paper presented at: America Society of Clinical Oncology Breast Cancer Symposium; 2007; San Francisco, CA. [Google Scholar]
- 11. Simons S, Ringsdorf S, Braun M, et al. Enhancing adherence to capecitabine chemotherapy by means of multidisciplinary pharmaceutical care. Support Care Cancer. 2011;19:1009-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu EQ, Johnson S, Beaulieu N, et al. Healthcare resource utilization and costs associated with non-adherence to imatinib treatment in chronic myeloid leukemia patients. Curr Med Res Opin. 2010;26:61-69. [DOI] [PubMed] [Google Scholar]
- 13. Darkow T, Henk HJ, Thomas SK, et al. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: a retrospective analysis among managed care patients with chronic myelogenous leukaemia. Pharmacoeconomics. 2007;25:481-496. [DOI] [PubMed] [Google Scholar]
- 14. Kerrigan M, Shah M, Eaddy M, Juhasz M. Patient adherence to lapatinib for the treatment of breast cancer. Paper presented at: ASCO 2009 Breast Cancer Symposium; October 8-10, 2009; San Francisco, CA. [Google Scholar]
- 15. Addeo R, Vincenzi B, Riccardi F, et al. Multicenter observational study on adherence and acceptance of lapatinib treatment in patients with HER2+ metastatic breast cancer. J Clin Oncol. 2011;29:e11102 [abstract]. [Google Scholar]
- 16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613-619. [DOI] [PubMed] [Google Scholar]
- 17. Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3-12. [DOI] [PubMed] [Google Scholar]
- 18. Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records. Description and validation. Med Care. 1988;26:814-823. [DOI] [PubMed] [Google Scholar]
- 19. Kerrigan M, Shah M, Eaddy M, Juhasz M. Patient adherence to lapatinib for the treatment of breast cancer. Paper presented at: ASCO 2009 Breast Cancer Symposium; October 8-10, 2009; San Francisco, CA; Abstract No. 162. [Google Scholar]
- 20. Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29:2534-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.