Abstract
Background: Target-specific oral anticoagulants (TSOAs) have advantages and disadvantages over warfarin, and they differ by indication, dosage adjustment, and drug interactions. Objective: The purpose of this retrospective cohort study is to determine use patterns and unlabeled uses of TSOAs. Methods: From July 1, 2012, to April 30, 2014, orders for warfarin, dabigatran, rivaroxaban, or apixaban in a tertiary medical center were included. Electronic medical records were reviewed to collect information regarding characteristics of the patients receiving these medications. Unlabeled use was defined as an indication or dose not approved by the US Food and Drug Administration. The percentage of orders for each study drug per month and the percentage of orders for each TSOA that contained an unlabeled use were calculated. Results: Of a total of 869 orders, 140 (16.1%) were for TSOAs (13 dabigatran, 97 rivaroxaban, and 30 apixaban orders). Compared with the first 4 months of the study period, the monthly percentage of orders for a TSOA increased by 2.5-fold in the last 4 months. Of the 3 TSOAs, only orders for dabigatran decreased (5.6% in July 2012 vs 0% in April 2014). Of the 140 TSOA orders, 28 (20.0 %) were unlabeled uses (3 unlabeled indications and 25 unlabeled dose), which included 16 unlabeled renally adjusted doses. The percentage of unlabeled uses did not significantly differ by TSOA. Conclusion: The use of TSOAs has increased and unlabeled uses are common. These data provide opportunities for quality improvement in the use of TSOA in clinical practice.
Keywords: anticoagulants, prescribing pattern, drug utilization, dosing, warfarin
Background
In the past few years, target-specific oral anticoagulants (TSOAs) have emerged as a promising alternative to warfarin in clinical practice. They have been shown to be superior to or noninferior to warfarin for treatment or prevention of thromboembolism in clinical trials.1-3 In addition, they share many desirable features as an oral anticoagulant—rapid onset and offset of anticoagulation effect, predictable pharmacokinetic–pharmacodynamic relationship, fewer drug–drug and drug–food interactions, and as a result, regular coagulation monitoring is not necessary.4 As of January 1, 2015, there are 3 TSOAs approved by the US Food and Drug Administration (FDA) for clinical use: dabigatran, rivaroxaban, and apixaban. All of them have been approved in the United States in the past 5 years: dabigatran in April 2010, rivaroxaban in July 2011, and apixaban in December 2012.5-7
Although the TSOAs share many advantages over warfarin, they have different indications and characteristics that can influence their clinical use. First, FDA-approved indications are subtly different. Although they are all approved for preventing stroke and systemic thromboembolism in nonvalvular atrial fibrillation (AF) and for the treatment of venous thromboembolism (VTE), rivaroxaban and apixaban are also approved for VTE prophylaxis after knee or hip replacement surgery (Table 1).5-7 Second, each drug has a different dose and frequency of administration recommended for each approved indication. Dabigatran and apixaban are administered twice daily whereas rivaroxaban is generally once daily.5-7 Third, adjusting the dose according to kidney function is different for each drug. While dabigatran and rivaroxaban require dose adjustments according to creatinine clearance (CrCl),6,7 apixaban is adjusted based on age, weight, and serum creatinine (Scr) level.5 Fourth, clinically significant drug interactions are different. Dabigatran has a clinically significant interaction with a strong p-glycoprotein inhibitor and inducer whereas rivaroxaban and apixaban have it with a combined strong CYP3A and p-glycoprotein inhibitor and inducer.4-7 Finally, side effect profiles are different. Although all 3 drugs share bleeding as their side effect, dabigatran may have gastrointestinal side effects such as dyspepsia more frequently than the other TSOAs.3 The objective of this study is to determine (a) patterns of the use of oral anticoagulants (ie, dabigatran, rivaroxaban, apixaban, and warfarin) and (b) percentage of unlabeled use of TSOAs in patients who were newly started on an oral anticoagulant in a large medical center.
Table 1.
Comparison of Target-Specific Oral Anticoagulants.
| Dabigatran | Rivaroxaban | Apixaban | |
|---|---|---|---|
| Indication | Nonvalvular AF | Nonvalvular AF | Nonvalvular AF |
| VTE treatment | VTE treatment | VTE treatment | |
| VTE prophylaxisa | VTE prophylaxisa | ||
| Dosing frequency | Twice a day | Once a day | Twice a day |
| Renal dose adjustment | Based on CrClb | Based on CrClc | At least 2 of the followingd: age ≥80, weight ≤60 kg, serum creatinine ≥1.5 mg/dL |
| Clinically significant interaction | Strong p-glycoprotein inhibitors and inducers | Combined strong CYP3A and p-glycoprotein inhibitors and inducers | Combined strong CYP3A and p-glycoprotein inhibitors and inducers |
| Side effect | Bleeding | Bleeding | Bleeding |
| GI side effect (eg, dyspepsia) |
Abbreviations: AF, atrial fibrillation; CrCl, creatinine clearance; CYP3A, cytochrome P450; GI, gastrointestinal; VTE, venous thromboembolism.
After knee or hip replacement surgery.
For nonvalvular AF, reduce the dose if CrCl 15 to 30 mL/min, and avoid the drug if CrCl < 15 mL/min; for VTE treatment and prophylaxis, dosing recommendation unavailable if CrCl < 30 mL/min.
For nonvalvular AF, reduce the dose if CrCl 15 to 50 mL/min, and avoid the drug if CrCl < 15 mL/min; for VTE treatment and prophylaxis, avoid if CrCl < 30 mL/min.
For nonvalular AF, reduce the dose if patient meets at least 2 criteria listed above; for VTE treatment and prophylaxis, no dosage adjustment is necessary.
Methods
Patients
This retrospective review study included patients older than 18 years who were admitted to the University of California San Francisco Medical Center (UCSF MC) from July 1, 2012, to April 30, 2014, and were ordered warfarin, dabigatran, rivaroxaban, or apixaban during admission. Patients were excluded if they used a study drug prior to admission. We collected data from July 1, 2012, because it was the date when a new electronic medical record (EMR) system was fully implemented. The study protocol was approved by the Committee for Human Research Protection.
Institutional Setting of TSOA Use
At UCSF MC, dabigatran, rivaroxaban, and apixaban were added to formulary in February 2011, June 2012, and June 2013, respectively. TSOA use was restricted to nonvalvular AF with the exception of rivaroxaban, which could also be used for VTE prophylaxis in orthopedic patients undergoing hip or knee replacement and acute and chronic treatment of VTE or PE. Although guidelines were developed for when to consider selecting a TSOA over warfarin, selecting a particular TSOA was up to the discretion of the prescriber.
Data Collection
We collected the following data from the EMR: (a) demographic and pertinent laboratory data: age, sex, weight, height, and Scr within 3 days of the first order of a study drug during the admission; (b) comorbidities and concomitant medications including drugs influencing platelet function, anticoagulants, and other medications with potential interactions with TSOAs (ie, amiodarone, clarithromycin, dronedarone, ketoconazole, quinidine, rifampin, ritonavir, and verapamil); (c) information about the study drug: indication, name, dosage, frequency, ordering service, and date ordered. In patients with AF, we calculated CHADS2 risk score (1 point was given for congestive heart failure, hypertension, age more than 75 years, or diabetes mellitus, and 2 points were assigned for prior stroke or transient ischemic attack).8 We calculated CrCl by using the Cockroft–Gault method without rounding the Scr level to 1 mg/dL even if the level was below 1.0 mg/dL.9 In addition, we considered the maximum CrCl as 120 mL/min.
Study Outcomes
There were 2 study outcomes: (a) percentage of orders for each study drug ordered per calendar month and (b) percentage of unlabeled uses. We calculated percentage for each study drug ordered per calendar month by dividing the total number of new orders in a calendar month by the total number for all study drugs ordered in the month. We included only the first new order for a study drug during the index admission. We calculated percentage of unlabeled uses of a TSOA by dividing the total number of new orders for a TSOA with an unlabeled use with the total number of new orders for TSOAs during the study period.
Unlabeled Uses of a TSOA
We defined unlabeled uses of a TSOA as orders containing one of the following: (a) Any non-FDA-approved indications such as prosthetic heart valve replacement. Since the approval dates of dabigatran and apixaban for VTE treatment and prophylaxis were in or after the last month of our study period, we considered the use of these drugs for VTE treatment or prophylaxis during the study period as unlabeled. (b) Any doses for labeled indications that did not follow dose adjustments according to renal function, age, weight, or concomitant medications available on the label. If the use of certain concomitant medications is contraindicated according to the label, we considered their uses as an unlabeled use. We considered an order for apixaban for a patient with CrCl lower than 25 mL/min as a labeled use based on the dose recommendation for a patient on hemodialysis on approved labeling. We classified unlabeled doses into high and low doses: if a dose ordered was higher than approved one for an indication, renal function, or drug interactions, we considered it as a high dose.
Statistical Analysis
We used descriptive statistics to obtain mean, median, range, and standard deviation as evidenced by data. We compared the baseline characteristics between the study drugs with analysis of variance, Kruskal–Wallis, χ2, or Fisher’s exact tests as appropriate. We plotted percentage of orders for each study drug against study months. We obtained Pearson correlation coefficients for monthly percentage of orders for study drugs. We repeated these analyses in only patients with AF. Finally, we used the Fisher’s exact test to compare percentage of unlabeled orders between TSOA orders. We used SAS (version 9.3; Cary, NC) and considered a P value <.05 as statistically significant.
Results
Patterns of the Use of TSOAs
Of a total of 869 orders included during the study period, 729 (83.9%) were for warfarin and 140 for a TSOA: 13 (1.5%) were for dabigatran, 97 (11.2%) were for rivaroxaban, and 30 (3.5%) were for apixaban (Table 2). Compared with patients with an order for a warfarin, those with an order for a TSOA were significantly older, had a higher percentage of AF and VTE prophylaxis as an indication, had a lower percentage of VTE treatment as an indication, had a lower CHADS2 score, and had a higher percentage of nonsteroidal anti-inflammatory drug use. Baseline characteristics were generally comparable among patients who were ordered TSOA. However, those who received apixaban were significantly older, had a higher percentage of AF (100%; P = .004), and concomitant use of amiodarone (23.3%; P = .01) than those with an order for other TSOAs. Patients ordered rivaroxaban had a significantly lower percentage of aspirin use than those receiving the other TSOAs (19.6%; P = .04). Of note, 89 (63.6%) of the TSOA orders came from the cardiology service, whereas 607 (83.3%) of the warfarin orders were issued by noncardiology services.
Table 2.
Characteristics of Patients Who Newly Started on an Oral Anticoagulanta.
| Warfarin (n = 729) | Total TSOAs (n = 140) | P Valueb | Dabigatran (n = 13) | Rivaroxaban (n = 97) | Apixaban (n = 30) | P Valuec | |
|---|---|---|---|---|---|---|---|
| Male (%) | 418 (57.3) | 76 (54.3) | .52 | 10 (76.9) | 52 (53.6) | 14 (46.7) | .17 |
| Age (year) | 63 [19, 99] | 67 [20, 99] | .0002 | 60 [39, 84] | 66 [20, 93] | 72.5 [31, 99] | .03 |
| BMI (kg/m2) | 26.7 [14.2, 69.5] | 26.7 [17.0, 49.9] | .49 | 24.4 [20.4, 33.8] | 28.3 [17.1, 49.9] | 25.7 [17.0, 40.7] | .01 |
| Scr (mg/dL) | 0.9 [0.3, 11.6] | 0.9 [0.4, 1.8] | .45 | 1.0 [0.5, 1.7] | 0.9 [0.4, 1.8] | 0.9 [0.4, 1.8] | .91 |
| CrCl (mL/min) | 73.7 [6.6, 120.0] | 69.6 [14.2, 120.0] | .69 | 96.8 [37.3, 118.5] | 70.3 [22.4, 120.0] | 59.3 [14.2, 120.0] | .21 |
| CrCl category | .73 | .52 | |||||
| ≥50 | 454 (62.3) | 102 (72.9) | 11 (84.6) | 72 (74.2) | 19 (63.3) | ||
| ≥30 and <50 | 111 (15.2) | 24 (17.1) | 2 (15.4) | 16 (16.5) | 6 (20.0) | ||
| ≥15 and <30d | 57 (7.8) | 11 (7.9) | 0 (0) | 8 (8.3) | 3 (10.0) | ||
| <15 | 15 (2.1) | 1 (2.1) | 0 (0) | 0 (0.0) | 1 (3.3) | ||
| NA | 92 (12.6) | 2 (1.4) | 0 (0) | 1 (1.0) | 1 (3.3) | ||
| Dialysis | 30 (4.1) | 1 (0.7) | .05 | 0 (0) | 0 (0) | 1 (3.3) | .30 |
| Comorbidities | |||||||
| AF | 218 (29.9) | 115 (82.1) | <.0001 | 11 (84.6) | 74 (76.3) | 30 (100) | .004 |
| CHADS2 scoree | 2 [0, 6] | 1 [0, 5] | <.0001 | 1 [0,4] | 1 [0,5] | 2 [0,5] | .12 |
| Stroke or TIA | 66 (9.1) | 15 (10.7) | NA | 2 (18.2) | 6 (8.1) | 7 (23.3) | NA |
| CAD/MI history | 62 (8.5) | 28 (20.0) | NA | 3 (27.3) | 16 (21.6) | 9 (30.0) | NA |
| Peptic ulcer | 12 (1.6) | 6 (4.3) | .05 | 1 (7.7) | 2 (2.1) | 3 (10.0) | .08 |
| GERD | 63 (8.6) | 12 (8.6) | 1.0 | 0 (0) | 9 (9.3) | 3 (10.0) | .78 |
| Any bleeding | 96 (13.1) | 15 (10.7) | .49 | 0 (0) | 11 (11.3) | 4 (13.3) | .50 |
| ICH/SAH/SDH | 25 (3.4) | 3 (2.1) | .60 | 0 (0) | 2 (2.1) | 1 (3.3) | .67 |
| GIB | 40 (5.5) | 3 (2.1) | .13 | 0 (0) | 2 (2.1) | 1 (3.3) | .67 |
| Other bleeding | 32 (4.4) | 10 (7.1) | .19 | 0 (0) | 7 (7.2) | 3 (10.0) | .67 |
| Ordering pattern of OAC | |||||||
| Ordering service | |||||||
| Cardiology | 122 (16.7) | 89 (63.6) | <.0001 | 9 (69.2) | 55 (56.7) | 25 (62.5) | <.0001 |
| Noncardiology | 607 (83.3) | 51 (36.4) | 4 (30.8) | 42 (43.3) | 15 (37.5) | ||
| Indication | |||||||
| AF | 218 (29.9) | 115 (82.1) | <.0001 | 11 (84.6) | 74 (76.3) | 30 (100.0) | .004 |
| VTE treatment | 364 (49.9) | 20 (14.2) | <.0001 | 2 (15.4) | 17 (17.5) | 1 (3.3) | .13 |
| VTE prophylaxis | 12 (1.7) | 10 (7.1) | <.0001 | 0 (0) | 10 (10.3) | 0 (0) | .14 |
| Prosthetic heart valve replacementf | 74 (10.2) | 6 (4.3) | .03 | 0 (0) | 3 (3.1) | 3 (10.0) | .21 |
| Other | 130 (17.8) | 3 (2.1) | <.0001 | 1 (7.7) | 1 (1.0) | 1 (3.3) | .13 |
| Concomitant drug | |||||||
| Aspiring | 237 (32.5) | 36 (25.7) | .14 | 5 (38.5) | 19 (19.6) | 12 (40.0) | .04 |
| P2Y12 antagonisth | 30 (4.1) | 7 (5.0) | .65 | 1 (7.7) | 4 (4.1) | 2 (6.7) | .54 |
| DAPT | 20 (2.7) | 3 (2.1) | 1.0 | 1 (7.7) | 1 (1.0) | 1 (3.3) | .13 |
| NSAID | 13 (1.8) | 15 (10.7) | <.0001 | 0 (0) | 13 (13.4) | 2 (6.7) | .41 |
| Amiodaronei | NA | 13 (9.3) | 1 (7.7) | 5 (5.2) | 7 (23.3) | .01 | |
| Dronedaronei | NA | 2 (1.4) | 0 (0) | 1 (1.0) | 1 (3.3) | .52 | |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CrCl, creatinine clearance; DAPT, dual antiplatelet therapy; GERD, gastroesophageal reflux disease; GIB, gastrointestinal bleeding; ICH, intracerebral hemorrhage; MI, myocardial infarction; NA, not available; NSAID, nonsteroidal anti-inflammatory drugs; OAC, oral anticoagulants; SAH, subarachinoid hemorrhage; Scr, serum creatinine; SDH, subdural hemorrhage; TIA, transient ischemic attack; TSOA, target-specific oral anticoagulants; VHD, valvular heart disease; VTE, venous thromboembolism.
Data are expressed as number (%) or median [range] unless specified.
Comparison between warfarin and TSOAs.
Comparison among the 3 TSOAs.
Three patients in apixaban group has CrCl ≥25 and <30 mL/min.
CHADS2 risk score ranged from 0 to 6. Higher score indicates higher risk of stroke or thromboembolism.
All 6 patients in TSOA groups received bioprosthetic valve replacement.
All patients took aspirin 81 mg daily except for 2 patients in rivaroxaban group who took aspirin 325 mg daily.
Ticlopidine, clopidogrel, prasugrel, and ticagrelor.
Warfarin orders were excluded.
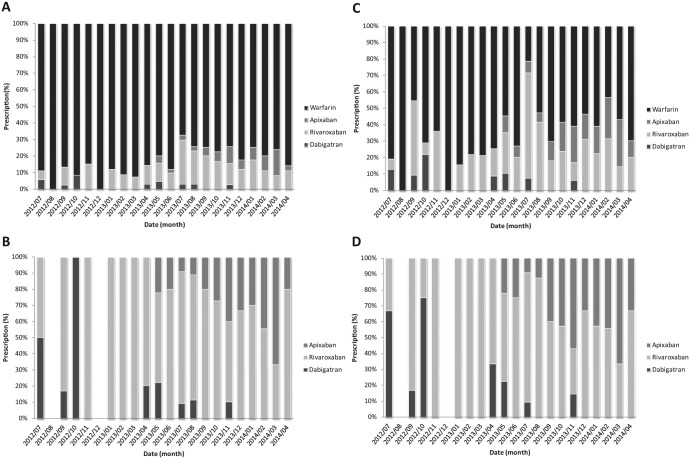
Monthly percentage of orders for a TSOA steadily increased (Figure 1A; Pearson correlation coefficient, r = .73, P < .0001): from July 2012 to October 2012, 8.8% of orders for oral anticoagulants were TSOAs, whereas 20.7% of orders for oral anticoagulants were TSOAs from January 2014 to April 2014. Of a total of 140 orders for a TSOA, 97 (69.3%) were for rivaroxaban, followed by 30 (21.4%) for apixaban and 13 (9.3 %) for dabigatran. Whereas monthly percentage of orders for apixaban significantly increased, that for dabigatran decreased (Figure 1B; apixaban, r = .85; P < .0001; dabigatran, r = −.53; P < .0001). On the other hand, monthly percentage order for rivaroxaban was not significantly changed (r = −0.22; P = .34). Of note, from December 1, 2013, to April 30, 2014, there were no orders for dabigatran.
Figure 1.
(A) Percentage of oral anticoagulant prescriptions. (B) Percentage of prescriptions for target-specific oral anticoagulant. (C) Percentage of oral anticoagulant prescriptions with atrial fibrillation indication. (D) Percentage of prescriptions for a target-specific oral anticoagulant with atrial fibrillation indication.
During the study period, a total of 333 orders for an oral anticoagulant (38.3%) were written for patients with AF. The trends in the use of oral anticoagulants were similar in patients with AF, except for a higher monthly percentage of orders for a TSOA (Figure 1C). Overall, 34.5% of orders for an oral anticoagulant for AF were for a TSOA and the monthly percentage of orders for a TSOA for AF steadily increased during the study period (r = .48; P = .03). Of the 3 TSOAs, rivaroxaban was most often prescribed (69.3%) and only monthly percentage of orders for dabigatran decreased (Figure 1D; r = −.44; P = .04).
Unlabeled Uses of TSOAs
Of the 140 TSOA orders, 28 (20.0%) contained an unlabeled use (Table 3; 4 dabigatran, 16 rivaroxaban, and 8 apixaban). Unlabeled doses were about 8 times more common than unlabeled indications. Of the reasons for unlabeled doses, unlabeled renal doses were most common. The percentage of unlabeled uses was not significantly different among the 3 TSOAs (P = .27) and dabigatran had a trend toward higher percentage of unlabeled indications than the other TSOAs (P = .06). Of note, the vast majority of unlabeled uses of rivaroxaban and apixaban were due to unlabeled doses. Of the unlabeled doses, doses higher than labeled doses for indications, renal function, and drug interaction were more common among orders for rivaroxaban whereas lower doses were more prevalent among orders for the other TSOAs (P = .002).
Table 3.
Unlabeled Uses of Target Specific Oral Anticoagulants.
| Dabigatran (n = 13) | Rivaroxaban (n = 97) | Apixaban (n = 30) | P Value | |
|---|---|---|---|---|
| Total unlabeled use | 4 | 16 | 8 | .27 |
| Unlabeled indicationa | 2 | 1 | 0 | .06 |
| Unlabeled dosea | 2 | 15 | 8 | |
| Unlabeled renal doseb | 1 | 11 | 4 | |
| Unlabeled DDI adjustment | 0 | 0 | 3 | |
| Unlabeled dose for indication | 0 | 2 | 0 | |
| Other unlabeled doses | 1 | 2 | 1 | |
| Unable to evaluate | 0 | 1 | 0 | |
| Distribution of unlabeled dose | .002 | |||
| High | 0 | 10 | 0 | |
| Low | 2 | 5 | 8 |
Abbreviations: DDI, drug–drug interaction; CrCl, creatinine clearance.
Based on total unlabeled use.
Unlabeled renal dose is defined as CrCl 20 to 40 mL/min for dabigatran and 40 to 60 mL/min for rivaroxaban with incorrect renal dose adjustment; for apixaban, defined as adjusting dose with <2 risk factors (age, serum creatinine, and bodyweight), incorrect dose adjustment, or wrong hemodialysis dose.
Some orders were written for patients who did not have characteristics included in large clinical trials on TSOAs. Orders for both rivaroxaban and apixaban were written for 3 patients with bioprosthetic valve replacement and 1 patient with concurrent use of dual antiplatelets. In addition, 2 patients receiving an order for apixaban had valvular heart disease and 1 patient with a rivaroxaban order for VTE treatment had a CrCl lower than 30 mL/min. Apixaban had the highest percentage of unstudied population (20.0%), followed by rivaroxaban (5.2%). Patients with valvular AF were the most common unstudied population (72.7%).
Discussion
In this study, we made 2 major findings. First, orders for a TSOA have steadily increased over time. TSOAs account for an average of 16.1% of new orders for an oral anticoagulant and 34.5% of new orders for the treatment of AF. Of note, orders for a TSOA increased by more than 2-fold from the first 4 months to the last 4 months of the study period. Interestingly, monthly percentage of orders for apixaban has increased whereas that for dabigatran has decreased during the study period. Second, unlabeled uses of a TSOA were common. Specifically, 20.0% TSOA orders were for an unlabeled indication, dose, or both. The most common reason for unlabeled uses of a TSOA was unlabeled renal doses. Although the percentage of orders with unlabeled uses did not differ by TSOA, rivaroxaban and apixaban tended to have a higher percentage of unlabeled doses than dabigatran.
The average utilization rate of TSOAs has been reported from 14.5% to 46% and our data (16.1%) were in the lower part of this range. There are several factors that may explain the reported wide range of TSOA utilization rate. First, since warfarin has wider indications than TSOAs, studies including only nonvalvular AF patients tended to have a higher TSOA utilization rate. For example, the TSOA utilization rate was 42% in a study that included 6893 patients with nonvalvular AF in the United States between October 2010 and June 2013.10 Similarly, another study with 18 611 anticoagulant-naïve nonvalvular AF patients in Denmark reported that a total of 46% oral anticoagulant prescriptions between August 2010 and October 2013 were a TSOA.11 When our analysis was performed only in patients with nonvalvular AF, 34.5% of orders for an oral anticoagulant were a TSOA. Second, the TSOA utilization rate may differ between inpatients and outpatients. The studies reporting the TSOA utilization rate of 42% and 46% included both inpatients and outpatients, and our study evaluated only inpatients.10,11 Future studies should compare the TSOA utilization rate between inpatients and outpatients. Third, the wide range of TSOA utilization rate may reflect variable use patterns of oral anticoagulants among physician practices. While a study using administrative claims data from a national insurer reported that the use of dabigatran accounted for about 30% of the use of total oral anticoagulants between late 2010 and mid-2011, another study using a national registry reported only about 12% accounted for by the use of dabigatran.12
In contrast to a previous study including only patients with AF, patients with a warfarin order were younger than those with a TSOA order in our study.10 This discrepancy is most likely caused by inclusion of patients with non-AF in our study; among AF patients, the mean age was older in those with a warfarin order than in patients with a TSOA order (70.8 ± 12.4 vs 67.6 ± 14.6; P = .036). In contrast, among non-AF patients, those with a warfarin order had a nominally younger mean age than patients with a TSOA (56.2 ± 16.2 vs 61.1 ± 13.1; P = .14). These data suggest that the indication is associated with the use of a TSOA. In addition, TSOA orders were more common in the cardiology service than in other services (41.9% vs 7.9%; P < .001). These data are consistent with those of a previous study reporting cardiologists were more comfortable with prescribing dabigatran than noncardiologists.13
In our study, despite the overall increase in the use of TSOAs, the use of dabigatran decreased over time, a result consistent with that of a previous study.10 Reasons for this decrease in the use of dabigatran may be multifactorial. First, dabigatran may cause gastrointestinal side effect more frequently than the other TSOAs.6 Second, dabigatran requires twice daily dosing whereas rivaroxaban can be given once daily.6,7 Third, compared with warfarin, dabigatran at the currently approved dose may have comparable bleeding risk whereas apixaban has a significantly lower risk of bleeding.2,3 Although there is no randomized controlled trial directly comparing TSOAs, it seems likely that prescribers may extrapolate data comparing a TSOA with warfarin.
The unlabeled use of TSOAs was common in our study. In addition to the unlabeled uses, we found a substantial number of TSOA orders were written for populations who have not been studied in a large clinical trial on a TSOA. These data are consistent with those from previous studies evaluating the use of dabigatran in a physician group practice and a national registry.12,14 Unlabeled uses of a TSOA and uses of a TSOA in an unstudied population carry risks. For example, although warfarin can be used for mechanical heart valve replacement, dabigatran has been shown to increase the risk of thrombosis and bleeding compared with warfarin for this indication.15 In our and previous studies, the majority of unlabeled uses of TSOAs were due to unlabeled renal doses.12,14 Given the dependence on kidney function for excretion of TSOAs and an increased risk of bleeding due to high blood concentrations, doses not adjusted for renal dysfunction are likely to result in harm.16 Interestingly, over 50% of unlabeled doses were lower than recommended doses in our study, and many of these lower doses may have been due to prescribers’ perceived increased risk of bleeding in their patients. However, this practice may increase the risk of thrombosis because of suboptimal blood concentrations of a TSOA. Another source of unlabeled doses is complex recommendations on renal dosage adjustments on the TSOA labels. In our study, apixaban has the largest portion of unlabeled doses, which may be due, in part, to the complex dosage adjustments on apixaban label; they require 2 or more factors (serum creatinine level >1.5 mg/dL, body weight <60 kg, and age ≥80).5 In our study, all of the apixaban orders containing an unlabeled dose had doses lower than labeled doses even though patients with these orders had only one factor for dosage adjustments. These data suggest that many unlabeled uses of TSOAs may have been caused by confusion on labeled doses. Unlabeled doses of a TSOA due to prescriber confusion may be an important clinical issue because the complex dosing recommendations on TSOA labels and these drugs are high risk medications. To promote safe and effective use of TSOAs, providing prescriber education, incorporating prescribing decision support into the computer physician order entry and developing monitoring programs should be considered. In addition, an interdisciplinary approach to include pharmacist and prescribers will improve prescribing patterns of TSOAs and allow more vigilant review of these drugs.
Our study has several strengths. Our study evaluated both patterns and unlabeled uses of all 3 TSOAs at the same time. We reviewed patient medical records to verify such information as medical and medication history including previous use of an oral anticoagulant without relying on administrative claims data. In addition, we did not limit our study to orders written for patients with nonvalvular AF; instead, we included orders written for all patients who received an oral anticoagulant. Therefore, our study may provide a broader picture of how these drugs are used in clinical practice.
We acknowledged the following limitations in our study. First, our study included patients admitted to a single academic tertiary center, which may limit generalizability of our data. In particular, our data may not be applicable to nonacademic or nontertiary centers or outpatient physician practices. However, our study may be useful for other health care institutions to develop a process to evaluate the use of TSOAs in their own institutions as well as to identify potential sources of prescribing error for these drugs. Second, we did not measure clinical outcomes as they were not our study objective. Instead, we evaluated drug use patterns and process outcomes. As a result, our data should not be interpreted as superiority of one TSOA over the others. Third, we were not able to assess use patterns of dabigatran and rivaroxaban from the first date of their availability in our institution because our EMR system started later than the first available dates of these drugs.
In conclusion, the use of TSOAs has increased in clinical practice and unlabeled uses of these drugs are common. These data provide opportunities for quality improvement in the process of TSOA use in clinical practice.
Acknowledgments
We would like to acknowledge Professor Li-Jiuan Shen as well as the Department of Pharmacy of National Taiwan University Hospital, Taipei, Taiwan, for the collaborative support on Ms Lin’s work.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Ms Lin was supported by National Taiwan University, Taipei, Taiwan, for her participation in the international pharmacist exchange program.
References
- 1. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891. [DOI] [PubMed] [Google Scholar]
- 2. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992. [DOI] [PubMed] [Google Scholar]
- 3. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151. [DOI] [PubMed] [Google Scholar]
- 4. Heidbuchel H, Verhamme P, Alings M, et al. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J. 2013;34:2094-2106. [DOI] [PubMed] [Google Scholar]
- 5. Eliquis oral tablets, Apixaban oral tablets [Package insert]. Princeton, NJ: Bristol-Myers Squibb; 2012. [Google Scholar]
- 6. Pradaxa oral capsules, dabigatran etexilate mesylate oral capsules [Package insert]. Ridgefied, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2014. [Google Scholar]
- 7. Xarelto oral tablets, rivaroxaban oral tablets [Package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2012. [Google Scholar]
- 8. Hirsh J, Fuster V, Ansell J, Halperin JL. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. Circulation. 2003;107:1692-1711. [DOI] [PubMed] [Google Scholar]
- 9. Dowling TC, Wang ES, Ferrucci L, Sorkin JD. Glomerular filtration rate equations overestimate creatinine clearance in older individuals enrolled in the Baltimore Longitudinal Study on Aging: impact on renal drug dosing. Pharmacotherapy. 2013;33:912-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desai NR, Krumme AA, Schneeweiss S, et al. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation—quality and cost implications. Am J Med. 2014;127:1075-1082. [DOI] [PubMed] [Google Scholar]
- 11. Olesen JB, Sorensen R, Hansen ML, et al. Non-vitamin K antagonist oral anticoagulation agents in anticoagulant naive atrial fibrillation patients: Danish nationwide descriptive data 2011-2013. Europace. 2015;17:187-193. [DOI] [PubMed] [Google Scholar]
- 12. Steinberg BA, Holmes DN, Piccini JP, et al. Early adoption of dabigatran and its dosing in US patients with atrial fibrillation: results from the outcomes registry for better informed treatment of atrial fibrillation. J Am Heart Assoc. 2013;2:e000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang C, Siu M, Vu L, Wong S, Shin J. Factors influencing doctors’ selection of dabigatran in non-valvular atrial fibrillation. J Eval Clin Pract. 2013;19:938-943. [DOI] [PubMed] [Google Scholar]
- 14. Carley B, Griesbach S, Larson T, Krueger K. Assessment of dabigatran utilization and prescribing patterns for atrial fibrillation in a physician group practice setting. Am J Cardiol. 2014;113:650-654. [DOI] [PubMed] [Google Scholar]
- 15. Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206-1214. [DOI] [PubMed] [Google Scholar]
- 16. Poulsen BK, Grove EL, Husted SE. New oral anticoagulants: a review of the literature with particular emphasis on patients with impaired renal function. Drugs. 2012;72:1739-1753. [DOI] [PubMed] [Google Scholar]