Abstract
Background: American residents travel to Mexico to purchase medications for a fraction of US cost and frequently without prescription requirements. A previous bioequivalence study found differences in lung function measures between 2 brands of Mexican-manufactured albuterol inhalers (both 100 µg/puff). An investigation of the pharmaceutical performance of different inhalers available may illuminate why different clinical results may be observed and offer insight to consumer and provider expectations of such products. Objective: The purpose of this study is to provide some reasonable expectations for a medical tourist who shops in Mexico for albuterol metered dose inhalers (MDIs) or for their health care providers by comparing pharmaceutical product performance of the consumer-available brands. Methods: Five different albuterol MDI products were purchased in Nogales, Mexico. The albuterol content was quantified through high-performance liquid chromatography. The inhalers were analyzed to determine the amount of the albuterol dose that can be considered respirable and compared with the findings from 2 US innovator products. Results: The mean respirable mass for each brand of albuterol MDI was compared with that of the other 4 brands and the 2 US innovator products using Student’s t test. All evaluations showed significant differences (P < .05) except for 3 comparisons (Sacrusyt vs Assal, P = .89; Xeneric-S vs non-US Ventolin, P = .98; Victory vs US Proventil HFA, P = .06). Conclusion: Since pharmaceutical variability was found among the albuterol MDIs evaluated in this study, consumers and clinicians should appreciate possible differences in product performance of albuterol MDIs obtained in Mexico.
Keywords: asthma, β-2-adrenergic agonists, international medicine/issues, medication safety, pharmaceutics, inhalers
“Medical tourism” is a term describing the international border-crossing for health care products and services.1 The medical tourism industry is estimated to contribute $45 to 95 billion to the global economy, and it accommodates the medical treatment for more than 1.2 million Americans every year.2,3 Mexico is a leading region for medical tourism and attracts American residents for inexpensive, more accessible medical and dental procedures, services, products, and medications.3 Many prescription-only drugs in the United States are available for purchase at border town Mexican pharmacies without prescription requirements and, frequently, for lower cost.
In recent years, the transition in the United States to the more environmentally friendly hydrofluoroalkane (HFA) propellant systems has led to new branded albuterol metered dose inhaler (MDI) products. This transition has also led to an increase in the cost of albuterol inhalers in the United States.4,5 While it is expected that generic products will eventually enter the market, the effect on the consumer price is unknown at this time.6 Indeed, the out-of-pocket cost of albuterol inhalers in Mexico has been reported to be one third of typical US copays (and even less than the cash price for the uninsured or underinsured). Since lower cost is the main reason for medical tourists to purchase medications outside of the United States, it would appear likely that this trend will continue.4,7-9 Yet, the question remains: “What do you get when you cross the border and buy medications, such as albuterol MDIs, for cheaper prices?”
Several studies have compared pharmaceutical quality of medications that were available to patients in other countries and showed varying results. For instance, 2 separate studies performed by Karlage and colleagues, both of which analyzed content uniformity for several products obtained in Mexico, found that while the majority of the products fell within the average US Pharmacopeia (USP) limits for active pharmaceutical ingredient, individual capsules/tablets demonstrated a considerable amount of variability within the products.10,11 Furthermore, 2 of the 12 products tested were found to contain significantly less active pharmaceutical ingredient than the labeled amount.10,11 A product quality study that compared simvastatin tablets purchased from Canadian manufacturers using the Internet with the US innovator products found similar blend uniformity between the 2 groups.12 However, a subsequent Internet simvastatin study that included a variety of international manufacturers discovered nonequivalent drug amount, tablet hardness, and weights among the samples compared with the US standard.13
With respect to albuterol inhalers, a bioequivalence study by Leon-Molina et al of 2 brands of albuterol inhalers available in Mexican pharmacies, Ventolin (“non-US Ventolin”) and Assal, demonstrated clinical differences and bioinequivalence between the 2 brands despite that both were expected to deliver 100 µg of drug per puff.14 Non-US Ventolin exhibited higher forced expiratory volume in 1 second (FEV1) values, higher maximum effect (Emax), higher time to Emax (tmax), and higher area under the percentage of response–time curve (AUC) than Assal. A proposed explanation for the discovered inequivalence in lung function tests was that there may be unaccounted pharmaceutical differences between the 2 products.14 Pertinent pharmaceutical performance factors might include the aerosol particle size difference and actual amount of drug delivered to the lungs.
The purpose of this study is to provide some reasonable expectations for a medical tourist who shops in Mexican border towns for albuterol MDIs or for providers who encounter such a patient by comparing pharmaceutical quality and product performance of these inhalers with one another and with those available in US pharmacies.
Methods
Study Samples and Equipment
This study compared the pharmaceutical performance of albuterol MDIs obtained in the border town of Nogales, Mexico, with each other and with those available in US pharmacies. A variety of brand name products were obtained during multiple visits to Nogales pharmacies including Victory, non-US Ventolin, Assal, Xeneric-S, and Sacrusyt. Two or more units were purchased for each brand, depending on retail obtainability (more than 2 were purchased when they came in multi-packs). The samples included 5 units Xeneric-S, 2 units Victory, 3 units non-US Ventolin, 2 units Assal, and 2 units Sacrusyt, totaling 14 units. For each brand, 2 unique lot numbers were tested, with the exception of Sacrusyt, for which a second lot was unavailable at the purchase times. An evaluation of study sample product labeling is offered in Table 1, and the units are depicted in Figure 1. Two US innovator products, US-purchased Ventolin HFA (“US Ventolin HFA”) and US-purchased Proventil (“US Proventil HFA”), were included for comparison.
Table 1.
Visual Inspection of Sample Albuterol Inhalers Tested.
| Brand (# Units Tested) | Product Manufacturing Country | Pregnancy Warning | Labeled Weight | Labeled Amount of Drug Delivered per Dose (µg) | Out-of-Pocket Unit Cost (US$) |
|---|---|---|---|---|---|
| Victory (2) | China | Yes | 20 g | 100 | 4.25 |
| Non-US Ventolin (3) | Spain | No | 18 g | 100 | 10.00 |
| Assal (2) | Mexico | No | 20 mg | 100 | 4.25 |
| Xeneric-S (5) | India | No | 17 g | 100 | 3.00-4.00 |
| Sacrusyt (2) | China | Yes | 20 mg | 100 | 3.00-5.00 |
| US Proventil (3) | United States | Yes | 6.7 g | 108 of albuterol sulfate; 90 of albuterol | 20.00-50.00 |
| US Ventolin (3) | United Kingdom | Yes | 18 g | 108 of .albuterol sulfate; 90 of albuterol | 20.00-50.00 |
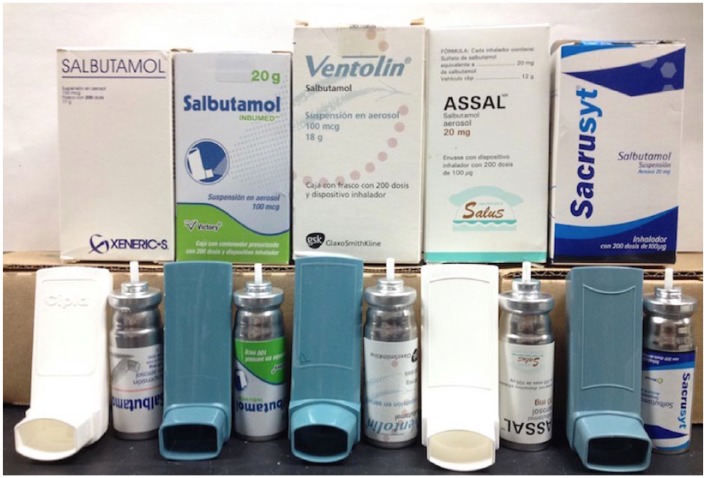
Figure 1.
Images of albuterol (salbutamol) metered dose inhaler products obtained in a Mexican border town.
Left to right, manufacturers: Xeneric, Victory, Ventolin, Assal, Sacrusyt. Shown for each product are the packaging, aerosol medication vial, and actuator.
Mean total dose, respirable mass, and nonrespirable mass were determined using the TSI Model 3306 Impactor Inlet (TSI Inc, Shoreview, MN). In brief, an MDI is actuated into a USP induction port (“throat”), which is coupled to the TSI 3306. The TSI 3306 is a single-stage impactor with a 4.7 µm cutpoint while the USP throat collects particles that likely would deposit in a patient’s throat. A high-performance liquid chromatography (HPLC) system utilized for albuterol analysis was a 2690 separation module combined with a 996 photodiode array (Waters Corporation, Milford, MA). Pharmaceutical parameters used for comparison include the following:
Mean Total Dose is the average albuterol mass that could reach the patient per actuation. This includes all particles deposited on the TSI 3306 filter (which would reach a patient’s lungs) as well as particles deposited in the throat and impactor plate of the TSI 3306 (which would deposit in the patient’s mouth, throat, and trachea).
Mean Respirable Mass is the average mass of albuterol that could reach a patient’s lungs per actuation. Only aerodynamic particles of diameter of 4.7 µm or smaller are likely to reach a patient’s lungs. Mean respirable mass is measured by finding the average albuterol mass deposited on the TSI 3306 filter after each test run.
Mean Nonrespirable Mass is the average mass of albuterol that could not reach a patient’s lungs per actuation. Nonrespirable particles have aerodynamic diameter greater than 4.7 µm diameter and mean nonrespirable mass is measured by finding the average albuterol mass deposited on the TSI 3306 throat and impactor plate after each test run.
Procedure
Every inhaler to be tested was prepared for test runs by priming with 3 actuations, washing the inhaler stem with methanol, and allowing time to dry before recording an initial weight. The TSI 3306 was prepared by adjusting the airflow to reach the manufacturer’s stated calibration flow rate of 28.3 L/min. Three test runs were completed for each MDI unit and the results composited to find mean total dose, mean respirable mass, and mean nonrespirable mass per single actuation. For every test run, a standard protocol was followed in which the inhaler was actuated 5 times (shaken before each actuation) into the throat over 60 seconds. Each component of the inhaler and of the TSI 3306 that could have aerosol on it was rinsed with an appropriate volume of diluent (23:77 methanol–water), and samples were collected for the HPLC assay. These components included the throat, extension, plate, and filter from the TSI 3306 and the stem and actuator from the MDI. HPLC analysis of albuterol was performed using an Apollo C18 column (5 µm, 150 × 4.6 mm) maintained at 30 ± 2°C. The mobile phase consisted of 1% phosphoric acid–methanol (77:23), the flow rate was 0.75 mL/min, and the injection volume was 40 µL. UV detection was set at 225 nm.
Statistical Analysis
To grasp what differences, if any, there were from one consumer-available product to the next, each of the 5 Mexican border town MDI brands were compared one-on-one (Victory vs non-US Ventolin, Victory vs Assal, Victory vs Xeneric-S, etc). The continuous data of total dose and respirable mass included in each one-to-one comparison were analyzed using student’s t tests. To compare the respirable mass data of each of the border town samples against each of the 2 US innovator MDI products, 1-sample t tests were performed.
Results
Visual Inspection
All 5 Mexican border town inhaler brands were sold in a Spanish-labeled box containing a single-page instructions insert. Every inhaler label displayed a visible lot number, expiration date, the international generic drug name for albuterol (salbutamol), and stated a dose of 100 µg. US Ventolin and US Proventil label the dose as 108 µg of albuterol sulfate, which correlates to 90 µg of albuterol per actuation. As illustrated in Figure 1, the package labels of the 5 Mexican border town products exhibit a dose of 100 µg but do not specify if it is albuterol sulfate or not. Some of the inhaler units were short-dated, listing expiration dates that were only 1 month from the purchase date (non-US Ventolin), while other units expired up to 26 months from date of acquisition (Victory). Listed manufacturing locations included China, Mexico, India, and Spain (Table 1). Two brands showed a pictorial pregnancy-related warning label on the box. Most of the MDIs were purchased for about $3 to $5 each, with non-US Ventolin being the most expensive; the non-US Ventolin units used in the study were purchased for $10 each but they were priced as much as $15 or $20 each depending on the pharmacy location. The inhaler labels all exhibited what appeared to be total product weight. Notably, some products listed weight in grams (Victory, Xeneric-S, and non-US Ventolin) while others listed milligrams (Assal and Sacrusyt). It was not always clear on the packaging what component of the product the weight regarded (eg, total canister weight, formulation fill weight, albuterol weight).
Comparison of Total Dose and Respirable Mass Among Mexican Border Town Inhalers
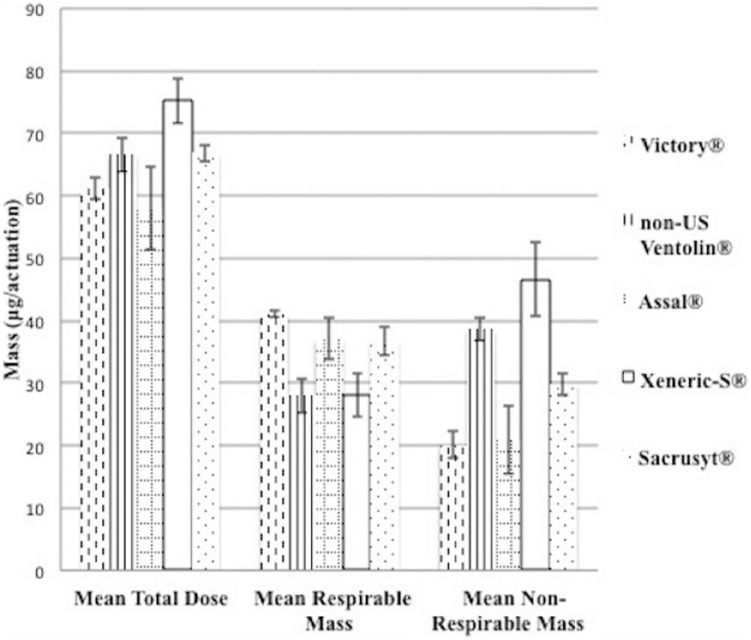
The active drug found in all study samples was albuterol, as labeled on the packaging. However, there were pharmaceutical performance differences discovered between the Mexican border town–purchased inhalers. Figure 2 illustrates the mean total dose, mean respirable mass, and mean nonrespirable mass measured for each of these MDIs. The mean total dose was an area of significant variability among the Mexican border town products. Assal (average total dose of 57.9 µg) tested the lowest among the other 4 Mexican-purchased MDI brands (Xeneric-S 75.2 µg; Sacrusyt 66.9 µg; non-US Ventolin 66.6 µg; Victory 61.1 µg) and exhibited the widest variability (Figure 2). When comparing the mean total dose, only 2 comparisons showed no significant difference (Sacrusyt vs non-US Ventolin, P = .84; Victory vs Assal, P = .28). All remaining comparisons showed significant inconsistencies between the brands, suggesting that patients may encounter total dose differences from one product to the next (P < .05).
Figure 2.
Mean total dose, respirable mass, and nonrespirable mass of sample Mexican border town–purchased albuterol metered dose inhalers, µg/actuation.
Mean total dose for each brand illustrated ranged from 57.9 µg/actuation (Assal) to 75.2 µg/actuation (Xeneric-S). Each brand was compared one-to-one and every evaluation showed differences (P < .05) except for 2 comparisons (Sacrusyt vs non-US Ventolin, P = .84; Victory vs Assal, P = .28). The average respirable mass for each brand measured as high as 41.0 µg/actuation (Victory) and as low as 28 µg/actuation (non-US Ventolin). For this parameter, again, all differences between brands were significant (P < .05) except for 2 comparisons (Sacrusyt vs Assal, P = .89; Xeneric-S vs non-US Ventolin, P = .98).
A key consideration when evaluating inhaler performance is how much of the total dose is respirable (what could reach a patient’s lungs). Differences in respirable mass were also found between the Mexican border town inhaler samples. The mean respirable mass measurements of non-US Ventolin (28.0 µg) and Xeneric (28.1 µg) were the lowest compared with any of the other inhalers (Victory, 41.0 µg; Sacrusyt, 36.8 µg; Assal, 37.0 µg). When the mean respirable mass measurements of the non-US brands of albuterol MDIs were compared with one another (the mean of each brand compared with that of each of the other 4 brands), all comparisons were significantly different (P < .05) except for 2 brands (Sacrusyt vs Assal, P = .89; Xeneric-S vs non-US Ventolin, P = .98). Regarding the amount of albuterol one could expect to reach the lungs in a single puff, most of the brands tested exhibited significant differences from one another, further highlighting another aspect of product variability that a patient may encounter.
Respirable Mass: Comparison Between US Available Products and Study Samples
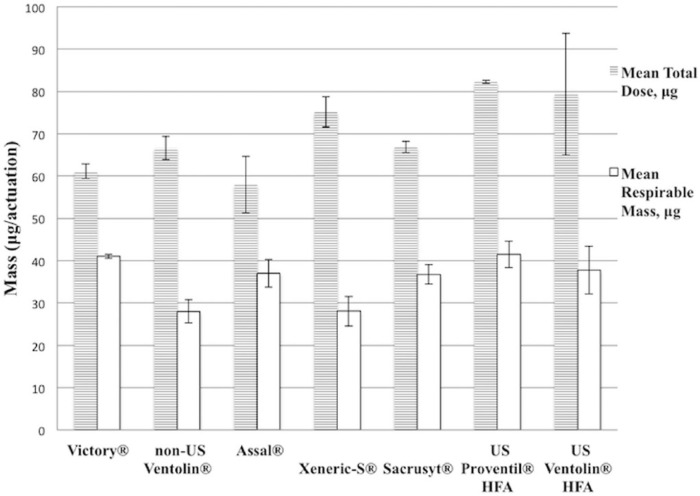
To examine how well a dose from a Mexican border town–purchased albuterol inhaler would reach a patient’s lungs in relation to US counterpart products, respirable mass measurements of each were compared. The mean respirable mass and mean total dose of each Mexican border town–purchased MDI is displayed in conjunction with the measurements from US-purchased MDI products (Figure 3). A 1-sample t test was used to find differences between border town inhaler results and corresponding data from US Ventolin HFA and US Proventil HFA.15 Among the Mexican border town MDIs, all except Victory (41.0 µg) offered lower respirable mass than that of US Proventil HFA (41.5 µg) and US Ventolin HFA (37.8 µg). The respirable mass values of non-US Ventolin, Xeneric-S, and Victory were significantly different from the US Ventolin HFA (P < .001), and the values for all but Victory were different from US Proventil HFA (P < .05). More explicitly, no difference was found between Victory and US Proventil HFA (P = .06), Sacrusyt and US Ventolin HFA (P = .35), or Assal and US Ventolin HFA (P = .61). Furthermore, Victory compared significantly higher in mean respirable mass than a US branded product (Victory > US Ventolin HFA, P < .001).
Figure 3.
Mean total dose and respirable mass of albuterol metered dose inhalers (MDIs) purchased in Mexico compared with US-purchased products, µg/actuation.
The respirable mass data from each of the 5 Mexican border town–purchased MDI brands were compared with that of US Ventolin HFA and US Proventil HFA. Differences were found between US Ventolin HFA and non-US Ventolin, Xeneric-S, and Victory (P < .001); however, no difference was determined between US Ventolin HFA and Sacrusyt (P = .35) nor between US Ventolin HFA and Assal (P = .61). Interestingly, while non-US Ventolin and Xeneric-S performed with significantly lower mean respirable mass per actuation than US Ventolin HFA, Victory measured significantly higher than US Ventolin HFA (P < .001). All of the border town MDIs were different from US Proventil HFA (P < .05) with one exception (Victory vs US Proventil HFA, P = .06).
Clinical Implications
A medical tourist who obtains rescue MDIs from Mexican border towns may face differences from one product to the next or differences between the border town products and the US counterparts. Expiration dates are a particular area of concern since some (but not all) inhaler units acquired in this study were short-dated as low as 1 month from purchase date. Some labeling inconsistencies among Mexican border town–purchased inhalers could be confusing for consumers, that is, some products showing safety warnings and some not. The pharmaceutical product performance examination revealed differences among the Mexican border town MDIs in total dose and respirable mass as well as differences between those products and US brand inhalers.
These differences highlight the fact that even though the labeled dose for the albuterol inhalers may be the same (100 µg for each of the Mexican inhalers), the pharmaceutical product performance is not necessarily the same. This is because the product performance (respirable and nonrespirable mass) is a function of the formulation composition as well as the metered valve size and actuator design.16-18 Differences in these product characteristics will affect the atomization of the formulation and the resulting final aerodynamic particle size distribution. For the products tested in this study, the average weight of an actuation was different (Assal, 59.2 ± 3.8 mg; Sacrusyt, 67.8 ± 1.8 mg; Ventolin, 74.7 ± 0.9 mg; Victory, 83.3 ± 1.2 mg; Xeneric-S, 88.3 ± 3.4 mg). Thus, there are clearly differences for each product with respect to formulation and device attributes, and correspondingly, it is not surprising that there are differences in the product performance between the brands tested.
Some interesting product performance findings were uncovered in this analysis, the most important of which involved the measurement of how much drug per actuation could reach a patient’s lungs (respirable mass). Several border town–purchased products performed comparably in mean respirable mass to the US innovator inhalers (Sacrusyt vs US Ventolin HFA, Assal vs US Ventolin HFA, Victory vs US Proventil HFA; P > .05). Beyond that, the Victory inhaler exhibited significantly higher mean respirable mass than the US Ventolin HFA product. Assal products performed most unpredictably having the widest range of variability in mean total dose. Finally, the non-US Ventolin product was the most expensive but did not perform the best among the border town inhalers in mean total dose and was further detected to actuate the lowest mean respirable mass of all the border town sample products.
What clinical implications these pharmaceutical differences represent are unclear. The statistical differences found seemed to point out that a patient could receive a lower dose or lower amount of respirable drug from one border town product to the next. However, the absolute differences were not so great that any product could be assumed clinically ineffective. In the United States, the 3 branded albuterol MDI products (Proventil HFA, Ventolin HFA, and ProAir HFA) are labeled with the same dose but are not considered interchangeable. If directly compared, product performance and clinical differences could be found among the 3 US brands. Yet any of those products are still considered clinically effective. In the same light, the border town products may still perform within a clinically effective range, even when statistical total dose or respirable mass differences were found.
The question regarding the spirometric differences and bioinequivalence that Leon-Molina et al found between 2 albuterol MDI products available in Mexico remains unresolved.14 The non-US Ventolin demonstrated superior results for clinical efficacy parameters compared with that of Assal, and it was posited that pharmaceutical parameters may explain these differences. However, the pharmaceutical quality results here did not corroborate those findings. While non-US Ventolin actuation provided a higher mean total dose than that of Assal, it also delivered a significantly lower respirable mass than Assal.
Limitations
Due to travel distance, cost, and other logistics, the inhalers were only obtained from Nogales, Mexico, although it is likely that many of the brands would be found in other border towns. It may be worthwhile to test a larger number of sample inhalers, include a variety of Mexican border towns, and include Canadian products, where many Americans living in the northern United States may travel to get cheaper inhalers. In addition, while multiple lot numbers for most of the samples were tested, multiple lots of Sacrusyt inhalers were unable to be obtained due to lack of availability.
Conclusion
Mexican border town inhalers are much more affordable than the US comparatives, and as long as less expensive generic albuterol MDIs remain unavailable in the United States, some Americans are going to continue to travel to get the cheapest medications they can. Although the border town–obtained inhalers varied significantly in pharmaceutical performance from one another and from US products, they still contained albuterol, the expected bronchodilator. Despite labeling differences regarding dose between the Mexican and US products, the mean respirable mass measurements (per actuation) of some of the border town inhalers were generally comparable to the US innovator MDIs. The concern is still, however, the uncertain repercussions in clinical efficacy that these differences in total dose and in respirable mass may imply. Since there was appreciable variability in the Mexican border town–obtained albuterol MDI products, clinicians and patients engaging in medical tourism should be aware of the possible differences in pharmaceutical performance and potentially therapeutic efficacy.
Footnotes
Authors’ Note: This study was presented as a poster at ASHP’s 2014 Midyear Clinical Meeting and Exhibition and abstract accepted for publication on ASHP’s 2014 Midyear Meeting Website.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Baker DE. Has the time come for “medication tourism”? Hosp Pharm. 2014;49:999-1000. doi: 10.1310/hpj4911-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Medical Tourism Association. 2013 MTA Medical Tourism Survey report. http://www.medicaltourismassociation.com/en/2013-mta-survey-report.html. Accessed June 26, 2015.
- 3. Das R. Medical tourism gets a facelift . . . and perhaps a pacemaker. Forbes. August 19, 2014. http://www.forbes.com/sites/reenitadas/2014/08/19/medical-tourism-gets-a-facelift-and-perhaps-a-pacemaker/. Published August 19, 2014. Accessed February 25, 2015.
- 4. Miller-Thayer J. Health migration: crossing borders for affordable health care. Field Actions Science Reports. Special Issue 2. http://factsreports.revues.org/503. Accessed February 1, 2014.
- 5. Rosenthal E. The soaring cost of a simple breath. The New York Times. www.nytimes.com/2013/10/13/us/the-soaring-cost-of-a-simple-breath.html. Published October 3, 2013. Accessed February 1, 2014.
- 6. Teva Pharmaceutical Industries Ltd. Teva reaches settlement in ProAir HFA patent case. http://ir.tevapharm.com/phoenix.zhtml?c=73925p=irol-newsArticleID=1941467. Published June 20, 2014. Accessed September 10, 2014.
- 7. Herrick D. Shopping for Drugs: 2007. Dallas, TX: National Center for Policy Analysis (NCPA Health Policy Report No. 293) http://www.ncpa.org/pub/st293. Published November 16, 2006. Accessed July 1, 2012. [Google Scholar]
- 8. Su D, Richardson C, Wen M, Pagan JA. Cross-border utilization of health care: evidence from a population-based study in South Texas. Health Serv Res. 2011;46:859-876. doi: 10.1111/j.1475-6773.2010.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calvillo JP, Lal L. Pilot study of a survey of US residents purchasing medications in Mexico: demographics, reasons, and types of medications purchased. Clin Ther. 2003;25:561-577. doi: 10.1016/s0149-2918(03)80097-8. [DOI] [PubMed] [Google Scholar]
- 10. Karlage KL, Myrdal PB. Comparison of three pharmaceutical products obtained from Mexico and the United States: a case study. Drug Dev Ind Pharm. 2005;31:993-1000. doi: 10.1080/03639040500306245. [DOI] [PubMed] [Google Scholar]
- 11. Karlage KL, Franklin SJ, Mufich WC, et al. Comparative evaluation of pharmaceutical products obtained in Mexico: augmenting existing scientific data. Drug Dev Ind Pharm. 2012;38:808-814. doi: 10.3109/03639045.2011.628678. [DOI] [PubMed] [Google Scholar]
- 12. Veronin M, Lee E, Lewis E. “Insight” into drug quality: comparison of simvastatin tablets from the US and Canada obtained via the internet. Ann Pharmacother. 2007;41:1111-1115. doi: 10.1345/aph.1h680. [DOI] [PubMed] [Google Scholar]
- 13. Veronin M, Nguyen N. Comparison of simvastatin tablets from the US and international markets obtained via the Internet. Ann Pharmacother. 2008;42:613-620. doi: 10.1345/aph.1K560. [DOI] [PubMed] [Google Scholar]
- 14. Leon-Molina H, Flores-Murrieta FJ, Chapela R. Assessment of comparative bioequivalence of two metered-dose inhaler formulations of salbutamol: measuring bronchodilatory effect in patients with asthma. Clin Drug Investig. 2002;22:435-441. doi: 10.2165/00044011-200222070-00003. [DOI] [Google Scholar]
- 15. Harris JA, Stein SW, Myrdal PB. Evaluation of the TSI aerosol impactor 3306/3321 system using a redesigned impactor stage with solution and suspension metered-dose inhalers. AAPS PharmSciTech. 2006;7(1):E20. doi: 10.1208/pt070120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stein SW, Sheth P, Myrdal PB. A model for predicting size distributions delivered from pMDIs with suspended drug. Int J Pharm. 2012;422:101-115. [DOI] [PubMed] [Google Scholar]
- 17. Myrdal PB, Sheth P, Stein SW. Advances in metered dose inhaler technologies: Formulation development. AAPS PharmSciTech. 2014;15:434-455. doi: 10.1208/s12249-013-0063-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stein SW, Sheth P, Myrdal PB. Advances in metered dose inhaler technologies: Hardware development. AAPS PharmSciTech. 2014;15:326-338. doi: 10.1208/s12249-013-0062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]