Abstract
Purpose
The purpose of this study is to evaluate the incidence and treatment of recurrent hemarthrosis after total knee replacement (TKR).
Materials and Methods
Among a total of 5,510 patients who underwent TKR from March 2000 to October 2016, patients who had two or more bleeding 2 weeks after surgery were studied. Conservative treatments were performed for all cases with symptoms. In patients who did not respond to conservative treatment several times, embolization was performed. We retrospectively evaluated the postoperative bleeding time, bleeding frequency, treatment method, and outcome.
Results
Seventeen (0.3%) of the 5,510 patients developed recurrent hemarthrosis. Bleeding occurred at an average of 2 years 3 months after the operation. Joint aspiration was performed 3.5 times (range, 2 to 10 times) on average, and 14 cases (82.3%) were treated with conservative treatment. In 3 patients with severe bleeding and hemorrhage, embolization was performed.
Conclusions
Recurrent hemarthrosis after TKR is a rare disease with a low incidence of 0.3% and usually could be treated by conservative treatment. If recurrences occur repeatedly, embolization through angiography or surgical treatment may be considered, but the results are not satisfactory and careful selection of treatment modalities is warranted.
Keywords: Knee, Arthroplasty, Hemarthrosis, Embolization
Introduction
The prevalence of total knee replacement (TKR) has been increasing in the aging society since 1980s, and development of advanced surgical techniques has contributed to improvement in satisfaction after surgical treatment; however, it is of note that the incidence of complications is also growing as the frequency of TKR increases1,2). Recurrent hemarthrosis is a rare complication of TKR with a prevalence of 0.3%–0.65%3–6). The reported mean interval from TKR to the onset of hemarthrosis is 2 years, which ranges from 2 weeks to 18 years3,4,7,8).
Recurrent bleeding in the joint can result in joint stiffness, poor postoperative function, and, albeit rare, deep joint sepsis9,10). Thus, proper treatment is imperative. The etiology of recurrent hemarthrosis documented in the literature includes repetitive trauma to the hypervascular hypertrophied synovium6), impingement of the hypertrophied synovium between the femoral and tibial components3,4,7), pigmented villonodular synovitis11,12), anticoagulant therapy7), intra- or extra-articular tumor growth, pseudoaneurysm, arteriovenous fistulae10,13–16), a femoral component eroding through a hypertrophied atherosclerotic artery17), bleeding from the arterial branches6,18), bleeding disorders such as hemophilia19), implant malalignment or instability, and polyethylene wear6,17). However, some cases have obscure causes that disrupt effective treatment.
Recurrent hemarthrosis can be treated conservatively (joint aspiration, compression, ice packs, and rest) or surgically (interventional angiographic embolization, radiosynovectomy20), and arthroscopic/open synovectomy). However, a standard treatment method has not yet been established. In this study, we set out to investigate the incidence of recurrent hemarthrosis after TKR and appropriate treatment methods.
Materials and Methods
This study was approved by the Institutional Review Board of the Ethics Committee of the National Health Insurance Service Ilsan Hospital. From March 2000 to October 2016, 5,510 TKRs were performed at our institution by the first author (JHY) of this study. Of those, cases with two or more bleeding episodes from 2 weeks after surgery were included in this study. The surgery was performed using LCS system (DePuy, Warsaw, IN, USA) in 181 shoulders, Maxim complex knee system (Biomet, Warsaw, IN, USA) in 76 shoulders, and Nexgen Legacy Posterior Stabilized Flex Fixed Bearing (LPS Flex Fixed; Zimmer, Warsaw, IN, USA) in 5,253 shoulders. Until July 2004, a conventional approach was used in the surgery in 326 shoulders; thereafter, minimally invasive surgery (MIS) using MIS Quad-Sparing Instrumentation (Zimmer) was performed in 5,184 shoulders.
For symptomatic patients, conservative treatment was the first treatment option, which involved joint aspiration, compression, rest, ice pack application, and splinting, and antithrombotic therapy was discontinued. For recurrent hemarthrosis in spite of three or more joint aspiration sessions, interventional embolization was performed upon confirmation of bleeding with interventional angiography. For bleeding after embolization, arthrotomy was carried out to identify the bleeding source. The interval between TKR and the onset of bleeding, frequency of bleeding, and treatment method and outcome were retrospectively evaluated.
Results
The incidence of recurrent hemarthrosis was 17 of 5,510 shoulders (0.3%) (Table 1). The mean age of the patients with hemarthrosis was 69.1 years (range, 61 to 76 years). There were 3 males and 14 females. The affected shoulder was the right side in 9 cases and left side in 8 cases. At the time of bleeding, 12 patients were on antithrombotic therapy using aspirin in 4, antiplatelets in 2, aspirin+antiplatelets in 5, and warfarin in 1. The preoperative diagnosis was osteoarthritis in all 17 cases. All knees received Nexgen prosthesis in TKR performed via a minimally invasive approach (Table 2). No patient had a history of trauma at the time of bleeding. The femoral and tibial component alignment was normal on radiographs. Component loosening or migration, osteolysis, or joint instability was not observed (Table 3).
Table 1.
Data of Patients
| No. | Sex | Age (yr) | Site | BMI (kg/m2) | Anticoagulant | Interval between TKA and hemarthrosis (wk) | Aspiration | Angio & embolization | Surgical exploration | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 65 | L | 26.3 | - | 70 | 7 | Yes | Yes | Improved |
| 2 | F | 68 | R | 25.4 | - | 238 | 4 | No | No | Improved |
| 3 | F | 70 | R | 26.2 | Aspirin | 310 | 3 | No | No | Improved |
| 4 | F | 71 | L | 27.5 | Arixtra (fondaparinux sodium) | 54 | 2 | No | No | Improved |
| 5 | F | 70 | L | 24.5 | - | 67 | 4 | No | No | Improved |
| 6 | F | 66 | R | 36.7 | Aspirin | 158 | 4 | No | No | Improved |
| 7 | F | 71 | R | 29.9 | Aspirin | 404 | 3 | No | No | Improved |
| 8 | F | 67 | R | 25.4 | Arixtra (fondaparinux sodium) & Ginkgo biloba extract | 98 | 5 | Yes | No | Improved |
| 9 | M | 76 | R | 22.8 | Wafarin | 2 | 2 | No | No | Improved |
| 10 | F | 61 | R | 23.8 | Arixtra (fondaparinux sodium) | 25 | 2 | No | No | Improved |
| 11 | F | 75 | L | 27.2 | Arixtra (rivaroxaban) | 166 | 2 | No | No | Improved |
| 12 | F | 63 | R | 25.4 | Rivaroxaban | 139 | 2 | No | No | Improved |
| 13 | M | 65 | L | 28.0 | Aspirin | 2 | 10 | Yes | Yes | Improved after another embolization |
| 14 | F | 70 | L | 22.1 | - | 2 | 2 | No | No | Improved |
| 15 | M | 76 | R | 21.2 | - | 8 | 4 | No | No | Improved |
| 16 | F | 76 | L | 22.0 | Arixtra (fondaparinux sodium) | 65 | 2 | No | No | Improved |
| 17 | F | 65 | L | 32 | Arixtra (fondaparinux sodium) & aspirin | 79 | 2 | No | No | Improved |
BMI: body mass index, TKA: total knee arthroplasty, L: left, R: right.
Table 2.
Demographics of Patients of Recurrent Hemarthrosis
| Recurrent hemarthrosis | Value |
|---|---|
| No. of cases (%) | 17 (0.3) |
| Gender | |
| Male | 3 |
| Female | 14 |
| Age (yr), mean (range) | 69.1 (61–76) |
| BMI (kg/m2), mean±SD | 26.3±3.9 |
| Preoperative diagnosis | |
| Osteoarthritis | 17 |
| Anticoagulant medication (%) | 12 (70.5) |
| Aspirin | 4 |
| Antiplatelet agent | 2 |
| Aspirin+antiplatelet agent | 5 |
| Warfarin | 1 |
| Implant | |
| Nexgen (case) | 17 |
| Surgical approach | |
| MIS technique (case) | 17 |
BMI: body mass index, SD: standard deviation, MIS: minimally invasive surgery.
Table 3.
Radiological Data
| Characteristic | Value |
|---|---|
| Tibial component alignment angle (°) | 0.8±1.3 varus |
| Tibial component posterior inclination (°) | 2.9±1.9 |
| Femorotibial angle (°) | 5.8±2.0 valgus |
| Tibial component alignment angle in 0°±3° (%) | 94 |
| Femorotibial angle in 6°±3° (%) | 94 |
| Loosening (case) | 0 |
| Osteolysis (case) | 0 |
| Migration (case) | 0 |
The interval between bleeding and TKR was an average of 2 years and 3 months (116 weeks; range, 2 weeks to 7 years and 9 months). Joint aspiration was performed 3.5 times on average (range, 2 to 10 times). Conservative treatment was effective for symptomatic improvement in 14 cases (82.3%). Three cases refractory to conservative treatment and 3 sessions of joint aspiration underwent interventional angiography and embolization. In the first case, angiography revealed bleeding from the superior lateral geniculate artery. In spite of embolization of the artery, bleeding recurred. Through arthrotomy, the source of bleeding was found in the inferior lateral geniculate artery and controlled by hemostasis (Fig. 1). In the second case, recurrence was reported once 2 years after embolization of the superior and inferior lateral geniculate arteries and descending geniculate artery, but it did not lead to another episode of bleeding (Fig. 2). In the third case, bleeding recurred after embolization of the superior and inferior lateral geniculate arteries. During subsequently performed arthrotomy, the source of bleeding was not identified. After discharge, the patient was not available for follow-up at our institution. At another hospital, the patient underwent 3 sessions of joint aspiration due to recurrence. After embolization performed at the hospital, bleeding has not recurred for three years.
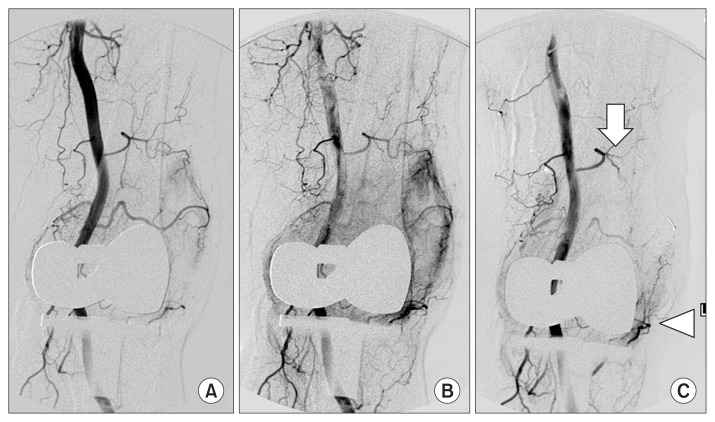
Fig. 1.
(A) Interventional angiography was performed in the left lower limb in a 67-year-old woman with recurrent hemarthrosis. (B) In angiography, a vascular blush was observed in the left superior lateral geniculate artery. (C) Successful embolization was done using 1:4 mixture of glue and lipiodol for the left superior lateral geniculate artery (arrow). However, bleeding from the inferior lateral geniculate artery (arrow head) was neglected.
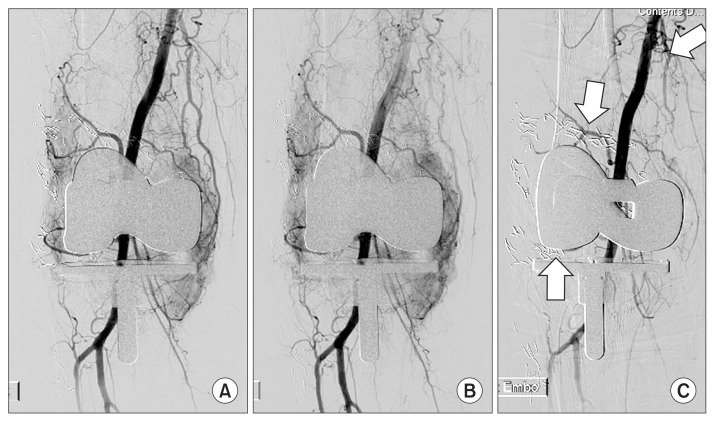
Fig. 2.
(A) Interventional angiography was performed in the right lower limb in a 69-year-old woman with recurrent hemarthrosis. (B) In angiography, a vascular blush was observed in the right superior and inferior lateral geniculate arteries and descending geniculate artery. (C) Gelfoam embolization was performed for the right superior and inferior lateral geniculate arteries and descending geniculate artery (arrows).
Discussion
Recurrent hemarthrosis following TKR is a rare complication; it was observed in only 17 (0.3%) of the total 5,510 TKRs in this study. Thus, it is challenging to determine the cause and prescribe proper treatment. Most hemarthrosis patients complain of acute pain and swelling of the knee with some loss of function without any trauma7,11,21). Due to the difficulty of identifying the cause of such symptoms, conservative treatment is initially employed to halt further hemorrhage, which involves joint aspiration, rest, ice pack application, and splinting. It is also advised to discontinue the use of anticoagulants, unless required for the patient’s medical condition. Further, it is necessary to investigate the presence of coagulation disorder by assessing the prothrombin time, activated thromboplastin time, and bleeding time and to evaluate the platelet function based on the thrombin consumption index, Von Wellebrand factor level, platelet factor 3 availability, and platelet aggregation study9). Conservative treatment resulted in improvement in 9 of 30 patients in a study by Kindsfater and Scott7) and in 3 of 10 patients in a study by Ohdera et al.4). In the current study, 14 (82.3%) of 17 patients obtained improvement with conservative treatment alone. For recurrent hemarthrosis following failing conservative therapy, magnetic resonance angiography22), interventional angiography & embolization, radiosynovectomy (synoviorthesis)20), and arthroscopic/open arthrotomy can be considered. Magnetic resonance angiography22) is a non-invasive modality for the identification of bleeding source and interventional treatment.
Recurrent hemarthrosis after TKR can be characterized as intermittent and acute bleeds in most cases. The most probable cause is bleeding from the peripheral branches of the artery. Interventional angiography & embolization can be the ultimate solution for this, which will produce desirable effects when performed as soon as possible. Angiography allows for identification of “blush-type” hypervascular synovium, vascular abnormality, and vascular damage as well as embolization for treatment. Stent implantation can also be performed for pseudoaneurysm or arteriovenous fistulae. Although it carries the risk of radiation exposure, iatrogenic vascular injury, and contrast-induced nephropathy, it can be performed under local anesthesia, lowers the risk of infection, and expedites postoperative recovery compared to open surgery23). The most common source of bleeding confirmed with angiography is the superior lateral geniculate artery14,15,17,23,24), followed by the superior medial geniculate artery23) and the inferior lateral geniculate artery21,23), and the characteristic feature is “blush-type” synovial hypervascularity. On the treatment outcomes of embolization for hemarthrosis after TKR, Weinder et al.24) reported that 12 (92.3%) of 13 patients obtained resolution of hemarthrosis after one session of embolization, and another session of embolization resulted in resolution in the remaining 1 case. Tat-Sing Law and McClure25) reported improvement in all three cases treated by angiography and embolization.
In our study, angiography performed in the three cases revealed “blush-type” synovial hypervascularity, but vascular malformation or pseudoaneurysm was not observed. In the first case, we initially performed embolization of the superior lateral geniculate artery in angiography, which led to recurrence. With arthrotomy, bleeding from a branch of the inferior lateral geniculate artery was confirmed. Based on a review of the record of previous angiography, the recurrence was attributed to failure to detect the bleeding source and embolize the inferior lateral geniculate artery. In the second case, we performed embolization of the inferior/superior lateral geniculate artery branches. In spite of one episode of bleeding 2 years after surgery, no recurrence has been reported afterwards. In the third case, we performed angiography and embolization of the superior/inferior lateral geniculate arteries after 10 episodes of bleeding, which failed to resolve bleeding. Subsequently, arthrotomy was carried out, but the source of bleeding was not identified during the procedure. Only after angiography and embolization performed at another hospital, the patient could obtain improvement.
Considering that hemorrhage occurs intermittently, rather than continuously, appropriate timing for angiographic embolization is crucial for treatment success of hemarthrosis. In addition, the various possible sources of bleeding including the inferior/superior lateral geniculate artery branches, inferior/superior medial geniculate artery branches, and other areas should be thoroughly examined. For a highly suspected source of bleeding, thorough embolization is advised. One of the common non-surgical treatment options is intra-articular injection of Yttrium 90-citrate to reduce the hypertrophied synovium and control bleeding since hypertrophic synovium is responsible for hemarthrosis in most cases20).
Surgical treatment can be performed arthroscopically, which is advantageous for less soft tissue damage and earlier rehabilitation than open surgery; however, the limited surgical field of view may restrict identification of the source of bleeding and proper treatment, increasing the risk of recurrence7). Arthroscopic arthrotomy can be considered for recurrent hemarthrosis refractory to conservative treatment or angiographic embolization. Common intraoperative findings include hypervascular, proliferative synovium and impingement of synovial tissues between femoral and tibial components. However, it is difficult to locate the exact source of bleeding. Also, the etiology of hypervascular, proliferative synovium has not be clearly elucidated. It has been postulated that intraarticular bleeding stimulates production and activation of inflammatory mediators in the joint, which results in the occurrence of synovitis and proliferation of blood vessels in the synovium, thus rendering it susceptible to trauma and hemorrhage6,26). Symptomatic improvement can be expected with synovectomy alone in many cases even without identification of the source of bleeding in arthrotomy3–5). Worland and Jessup3) reported complete healing in 7 cases after arthrotomy. In the current study, bleeding recurred after angiography in the first case. In the following arthrotomy, the source of bleeding was identified almost at the end of the procedure during probe manipulation around the inferior lateral geniculate artery. Therefore, considering that temporary contraction of blood vessels during arthrotomy may disrupt early detection of the source of bleeding, it is advised to thoroughly examine the suspected region with a probe.
One of the limitations of this study is the lack of comparison of different treatment methods because treatment was carried out without a protocol clearly established. In addition, in spite of the large enrollment, statistical significance was difficult to determine due to the low incidence of recurrent hemarthrosis in the enrolled patients. In an effort to overcome this limitation, we assessed the incidence among a large consecutive number of cases (5,510) performed by a single surgeon over a period of 16 years. Based on our findings, we believe that conservative treatment, interventional angiographic embolization, and surgical treatment can all be effective for the treatment of recurrent hemarthrosis.
Conclusions
Recurrent hemarthrosis following TKR is rare disease with a low incidence of 0.3%. It can be treated conservatively in most cases, for which appropriate pressure application, rest, and sufficient period of immobilization after joint aspiration are essential. Angiography can be considered for recurrent bleeding refractory to conservative treatment for thorough identification of the source of bleeding and embolization. If hemarthrosis recurs even after embolization, arthrotomy may be necessary. However, a cautious approach should be taken with regard to selection of treatment modalities due the difficulty of locating the source of bleeding.
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Dixon T, Shaw M, Ebrahim S, Dieppe P. Trends in hip and knee joint replacement: socioeconomic inequalities and projections of need. Ann Rheum Dis. 2004;63:825–30. doi: 10.1136/ard.2003.012724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Healy WL, Della Valle CJ, Iorio R, Berend KR, Cushner FD, Dalury DF, Lonner JH. Complications of total knee arthroplasty: standardized list and definitions of the Knee Society. Clin Orthop Relat Res. 2013;471:215–20. doi: 10.1007/s11999-012-2489-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worland RL, Jessup DE. Recurrent hemarthrosis after total knee arthroplasty. J Arthroplasty. 1996;11:977–8. doi: 10.1016/S0883-5403(96)80144-6. [DOI] [PubMed] [Google Scholar]
- 4.Ohdera T, Tokunaga M, Hiroshima S, Yoshimoto E, Matsuda S. Recurrent hemarthrosis after knee joint arthroplasty: etiology and treatment. J Arthroplasty. 2004;19:157–61. doi: 10.1016/j.arth.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Oishi CS, Elliott ML, Colwell CW., Jr Recurrent hemarthrosis following a total knee arthroplasty. J Arthroplasty. 1995;10( Suppl):S56–8. doi: 10.1016/S0883-5403(05)80232-3. [DOI] [PubMed] [Google Scholar]
- 6.Saksena J, Platts AD, Dowd GS. Recurrent haemarthrosis following total knee replacement. Knee. 2010;17:7–14. doi: 10.1016/j.knee.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Kindsfater K, Scott R. Recurrent hemarthrosis after total knee arthroplasty. J Arthroplasty. 1995;10( Suppl):S52–5. doi: 10.1016/S0883-5403(05)80231-1. [DOI] [PubMed] [Google Scholar]
- 8.Bagla S, Rholl KS, van Breda A, Sterling KM, van Breda A. Geniculate artery embolization in the management of spontaneous recurrent hemarthrosis of the knee: case series. J Vasc Interv Radiol. 2013;24:439–42. doi: 10.1016/j.jvir.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Malhotra R, Bhan S, Kiran EK. Haemarthroses after total knee arthroplasty caused by an isolated platelet factor 3 availability defect. J Bone Joint Surg Br. 2005;87:1549–52. doi: 10.1302/0301-620X.87B11.16836. [DOI] [PubMed] [Google Scholar]
- 10.Sharma H, Singh GK, Cavanagh SP, Kay D. Pseudoaneurysm of the inferior medial geniculate artery following primary total knee arthroplasty: delayed presentation with recurrent haemorrhagic episodes. Knee Surg Sports Traumatol Arthrosc. 2006;14:153–5. doi: 10.1007/s00167-005-0639-4. [DOI] [PubMed] [Google Scholar]
- 11.Ballard WT, Clark CR, Callaghan JJ. Recurrent spontaneous hemarthrosis nine years after a total knee arthroplasty: a presentation with pigmented villonodular synovitis. J Bone Joint Surg Am. 1993;75:764–7. doi: 10.2106/00004623-199305000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Hamlin BR, Duffy GP, Trousdale RT, Morrey BF. Total knee arthroplasty in patients who have pigmented villonodular synovitis. J Bone Joint Surg Am. 1998;80:76–82. doi: 10.2106/00004623-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Haddad FS, Prendergast CM, Dorrell JH, Platts AD. Arteriovenous fistula after fibular osteotomy leading to recurrent haemarthroses in a total knee replacement. J Bone Joint Surg Br. 1996;78:458–60. doi: 10.1302/0301-620X.78B3.0780458. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim M, Booth RE, Jr, Clark TW. Embolization of traumatic pseudoaneurysms after total knee arthroplasty. J Arthroplasty. 2004;19:123–8. doi: 10.1016/j.arth.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Katsimihas M, Robinson D, Thornton M, Langkamer VG. Therapeutic embolization of the genicular arteries for recurrent hemarthrosis after total knee arthroplasty. J Arthroplasty. 2001;16:935–7. doi: 10.1054/arth.2001.25555. [DOI] [PubMed] [Google Scholar]
- 16.Kalsi PS, Carrington RJ, Skinner JS. Therapeutic embolization for the treatment of recurrent hemarthrosis after total knee arthroplasty due to an arteriovenous fistula. J Arthroplasty. 2007;22:1223–5. doi: 10.1016/j.arth.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham RB, Mariani EM. Spontaneous hemarthrosis 6 years after total knee arthroplasty. J Arthroplasty. 2001;16:133–5. doi: 10.1054/arth.2001.9050. [DOI] [PubMed] [Google Scholar]
- 18.Dhondt E, Vanhoenacker FM, D’ Archambeau O, Snoeckx A, Defreyne L. Angiographic findings and therapeutic embolization of late hemarthrosis after total joint arthroplasty. Skeletal Radiol. 2009;38:31–6. doi: 10.1007/s00256-008-0569-6. [DOI] [PubMed] [Google Scholar]
- 19.Silva M, Luck JV., Jr Long-term results of primary total knee replacement in patients with hemophilia. J Bone Joint Surg Am. 2005;87:85–91. doi: 10.2106/JBJS.C.01609. [DOI] [PubMed] [Google Scholar]
- 20.Kapetanos GA, Papavasiliou KA, Makris V, Nikolaides AP, Kirkos JM, Symeonides PP. Recurrent spontaneous hemarthrosis after total knee arthroplasty successfully treated with synoviorthesis. J Arthroplasty. 2008;23:931–3. doi: 10.1016/j.arth.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Rukavina A, Kerkhoffs GM, Schneider P, Kuster MS. Recurrent hemarthrosis after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2010;18:898–900. doi: 10.1007/s00167-009-1031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hash TW, 2nd, Maderazo AB, Haas SB, Saboeiro GR, Trost DW, Potter HG. Magnetic resonance angiography in the management of recurrent hemarthrosis after total knee arthroplasty. J Arthroplasty. 2011;26:1357–61. doi: 10.1016/j.arth.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Maheshwari R, Kelley SP, Langkamer VG, Loveday E. Spontaneous recurrent haemarthrosis following unicompartmental knee arthroplasty and its successful treatment by coil embolisation. Knee. 2004;11:413–5. doi: 10.1016/j.knee.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Weidner ZD, Hamilton WG, Smirniotopoulos J, Bagla S. Recurrent hemarthrosis following knee arthroplasty treated with arterial embolization. J Arthroplasty. 2015;30:2004–7. doi: 10.1016/j.arth.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 25.Tat-Sing Law M, McClure DN. Therapeutic embolization in the treatment of recurrent haemarthrosis following knee arthroplasty. ANZ J Surg. 2010;80:247–9. doi: 10.1111/j.1445-2197.2010.05244.x. [DOI] [PubMed] [Google Scholar]
- 26.Pritsch T, Pritsch M, Halperin N. Therapeutic embolization for late hemarthrosis after total knee arthroplasty: a case report. J Bone Joint Surg Am. 2003;85:1802–4. doi: 10.2106/00004623-200309000-00022. [DOI] [PubMed] [Google Scholar]