Abstract
The relative contributions of the direct and indirect pathways in alloimmune responses have not been fully elucidated. We report a novel murine TCR transgenic system that can simultaneously track the CD4-direct (CD4-d), CD4-indirect (CD4-i), and CD8-direct (CD8-d) pathways after transplantation. Using this system, we have observed a profoundly greater proliferation of CD4-i T cells relative to CD4-d and CD8-d T cells after transplantation. Furthermore, a much larger proportion of CD4-i T cells attain an effector phenotype. We also analyzed endogenous, wild-type T cells using enzyme-linked immunospot analysis. In naive mice, T cells with indirect reactivity were undetectable, but T cells with direct reactivity were abundant. However, 10 days after skin or heterotopic heart transplantation, CD4-i T cells comprised approximately 10% of the CD4+ response. Consistent with increased priming of the CD4-i pathway, we observed that the CD4-i T cells were further enriched in the effector cells migrating to the allograft and in the memory-like T cells persisting after rejection. Thus, priming of the CD4-i pathway is favored after transplantation, allowing a rare population to rapidly become a major component of the CD4+ T cell response in acute allograft rejection. The generalizability of this observation to other models remains to be determined.
Introduction
T cells respond to alloantigen via the direct and indirect pathways (1, 2). T cells with direct specificity recognize donor MHC molecules expressed on donor cells, whereas T cells with indirect specificity recognize donor peptide presented by self-MHC. T cells with direct alloreactivity have a precursor frequency that is many orders of magnitude greater than that for a typical immune response to foreign antigen (Ag). In the mouse, the precursor frequency of T cells with direct reactivity in a full MHC mismatch has been estimated to be as high as 5-10% of the total T cell population (3). This remarkably high precursor frequency, in addition to the preponderance of direct pathway responses in most in vitro assays of alloreactivity, has contributed to the prevailing concept that the direct pathway is the dominant pathway in acute cellular allograft rejection.
The existence of the indirect pathway was postulated based upon in vitro studies; however, its relevance in vivo was initially questioned. The development of MHC knockout mice demonstrated definitively that the indirect pathway is operative in vivo, and that the CD4+ indirect pathway is sufficient to cause allograft rejection in the complete absence of the CD4+ direct pathway (2). Moreover, several groups reported heightened levels of indirect alloresponses in the setting of chronic graft rejection (4, 5). Thus, a paradigm has emerged that direct alloresponses dominate in the early post-transplant period, whereas indirect alloresponses become important in the chronic setting, when the donor–derived Ag presenting cells (APC) have been depleted and self-restricted allopeptides continue to be produced by the allograft (6). This paradigm has been invoked to explain, for example, why allografts that are depleted of donor APC undergo delayed rejection (1, 7, 8), but it does not explain why, in most studies, acute rejection proceeds with normal or near normal kinetics when the CD4+ direct pathway is absent (2, 9).
We have developed a murine TCR transgenic system to simultaneously track the CD4+ direct (CD4-d), CD4+ indirect (CD4-i), and CD8+ direct (CD8-d) allo-responses in the same host. Using this system, we studied the activation kinetics of these pathways after transplantation. Strikingly, proliferation of the CD4-i pathway was dominant at all time points post-transplant. This phenomenon was not an artifact of the transgenic system, as demonstrated by ELISPOT analysis of wild-type, endogenous T cells. Despite an initial precursor frequency that was too low to quantify in the naive population, the CD4-i pathway underwent greater proliferation relative to the CD4-d pathway and comprised approximately 10% of the alloresponse in the peripheral lymphoid organs after 7 days. Consistent with increased priming of the CD4-i pathway, endogenous cells with CD4-i reactivity were further enriched in the T-effector population and in the memory T cell population. Our findings help to refine the paradigm of T cell alloreactivity, and may have an impact on the rational design of immunosuppressive/tolerance induction strategies.
Materials and Methods
Mice
C57BL/6 (B6) and BALB/c mice were purchased from the National Cancer Institute or Jackson Laboratories (Bar Harbor, ME, USA). The following TCR-tg mouse strains were used: 2C (CD8 direct alloreactive)(10) kindly provided by Dr. Faddi Lakkis (Pittsburgh, PA), TCR75 (CD4+ indirect alloreactive) (9) and 4C (CD4+ direct alloreactive) (11). These strains are on the B6 background (H-2b) and have alloreactivity towards BALB/c Ag (H-2d). All TCR-tg mouse strains were backcrossed onto the Rag1-/- background to prevent the formation of hybrid TCRs that may result from combinations of the transgenic and endogenous TCR chains. To facilitate cell identification by FACS analysis, the 2C and 4C mice were maintained on the Ly5.1 congenic background, and the TCR75 was maintained on the Thy1.1 congenic background. Mice were bred and maintained in specific pathogen-free facilities at the University of California San Francisco according to an IACUC approved protocol.
Transplantation
Abdominal, vascularized heterotopic cardiac transplants were performed as previously described (12). Rejection was defined by the cessation of a palpable heartbeat for two successive days, and was confirmed by laparotomy. Full-thickness tail skin grafts (1-1.5 cm2) were transplanted onto the dorsal thorax of recipient mice and sutured in place using 6-0 silk sutures placed at the corners. Grafts were covered with Vaseline gauze and secured with a Band-Aid for 7 days. Rejection was defined as >90% necrosis of graft tissue.
Adoptive transfer and analysis of TCR-tg T cells
CD4+ and CD8+ T cells were purified by positive selection using magnetic bead isolation (Dynal Biotech, Oslo, Norway). Purified T cells were incubated in 5 μM CFSE (Molecular Probes, Carlsbad, CA) for 5 min at 37°C, quenched with cold culture media, rinsed twice, resuspended in sterile PBS, and then injected intravenously into recipient mice by retro-orbital injection. In co-injection experiments, the various TCR-tg T cells were mixed together prior to labeling with CFSE. 1 × 106 T cells from each TCR-tg mouse line were co-injected unless otherwise indicated.
Flow cytometric analysis
Analysis of adoptively transferred cells was done by flow cytometric analysis using a FACS Calibur (BD, Franklin Lakes, NJ). Spleen and lymph nodes were harvested at various time points following adoptive transfer and made into single cell suspensions by mechanical disruption through a fine metal screen. Antibodies to CD4 (GK1.5), CD8a (53-6.7), Ly5.1 (A20), Thy1.1 (HIS51), CD44 (IM7), CD62L (MEL-14), and IFNγ (XMG1.2) were purchased from eBioscience (San Diego, CA). For intracellular staining of IFNγ, T cells were re-activated for 4 hrs at 37°C in vitro using Leukocyte Activating Cocktail with Golgiplug (BD Pharmingen, San Jose, CA) and then fixed with 4% paraformaldehyde, followed by permeabilization with 0.1% saponin prior to Ab staining.
Preparation of graft-infiltrating lymphocytes (GIL)
Cardiac grafts were harvested and diced into 1-2 mm3 pieces with a razor and placed into a single well of a 12-well plate in 3 ml RPMI media with 1 mg/ml collagenase type 2 (265 U/mg; Worthington Biochemical Co, Lakewood, NJ). Samples were then incubated for 30 min at 37°C, agitated by passing through a transfer pipette, and the supernatant was collected. This process was repeated and the supernatants were combined. Splenic and GIL CD4+ T cells were purified using anti-CD4 magnetic beads followed by negative selection of CD8+ T cells with anti-CD8 magnetic beads (Dynal Biotech; Oslo, Norway).
IL-2 and IFNγ enzyme-linked immunospot (ELISPOT) assays
BALB/c skin grafts were placed on the thorax of B6 mice and the draining axillary lymph nodes (LN) were harvested at the time of graft rejection—post-op day 10. CD4+ T cells from the axillary LN were purified using anti-CD4 magnetic beads (Dynal Biotech; Oslo, Norway). Direct CD4+ and indirect CD4+ alloantigen specific T cells were detected by IFNγ and IL-2 ELISPOT assays (R&D, Minneapolis, MN) following the manufacturer’s instructions. To detect direct CD4+ T cells, 5×104 purified axillary LN CD4+ T cells were co-cultured with 5×105 BALB/c irradiated splenocytes (3000 rad) in 200 μl of culture media (RPMI-1640 with 10% FCS and 1% Pen/Strep/L-Glu). CD4-i T cells were assayed as reported by Benichou et. al(13) by co-culturing 5×104 purified axillary LN CD4+ T cells with 5×105 B6 irradiated splenocytes in the presence of 50 μl BALB/c splenocyte lysate in total volume of 200 μl. BALB/c lysate was made by sonicating BALB/c splenocytes resuspended in RPMI-5% FCS at a concentration of 2×107 cells/ml using a Fisher Model 100 Sonic Dismembrator (Fisher Scientific, Pittsburgh, PA) with 20 one-second pulses at level 13. The sonicate was frozen at -80°C, thawed, centrifuged and the supernatant collected. Graft infiltrating CD4+ lymphocytes were isolated as described above and similarly assayed. ELISPOT cultures were incubated at 37°C in a 5% CO2 humidified incubator for 24 h. The spots were counted using an automated ELISPOT plate reader (AID ELISPOT Reader System, Strassberg, Germany. Software version 3.1).
Results
A novel three TCR-tg T cell adoptive transfer model for simultaneous tracking of the CD4-d, CD4-i, and CD8-d pathways
We recently generated and characterized a TCR-transgenic (TCR-tg) mouse with CD4-d alloreactivity in the commonly used BALB/c donor → B6 host strain combination. This mouse, named “4C,” was developed on the B6 background and recognizes I-Ad complexed with an unidentified peptide(s) (11). To analyze the CD4-i response, we used the TCR-tg mouse model, TCR75 (9, 14), which was developed on the B6 background and is specific for a peptide derived from the MHC class I molecules Kd (residues 54-68) presented by I-Ab. To track the CD8-d pathway, we used the 2C TCR-tg model, which also was developed on the B6 background. The 2C TCR-tg mouse has CD8 T cells specific for the BALB/c MHC class I molecule, Ld (10). These TCR-tg mice were bred to express the Ly 5.1 or Thy 1.1 congenic markers to allow simultaneous tracking of the TCR-tg T cells in the same animal.
To assess the dynamics of allospecific T cell responses during acute rejection, we first purified T cells from the 3 types of TCR-tg mice and confirmed their purity and the presence of the transgenic TCR by flow cytometry (Figure 1A). The TCR-tg T cells then were mixed together before being labeled with CFSE and intravenously injected into syngeneic mice one day before transplantation (Figure 1B). Multicolor flow cytometric analysis readily distinguished the transferred cells from non-transgenic host cells based on CD4 and CD8 expression and the congenic markers, Ly5.1 and Thy1.1 (Figure 1C).
Figure 1. Adoptive transfer of TCR-tg T cells allows for simultaneous tracking of three subtypes of alloreactive T cells.
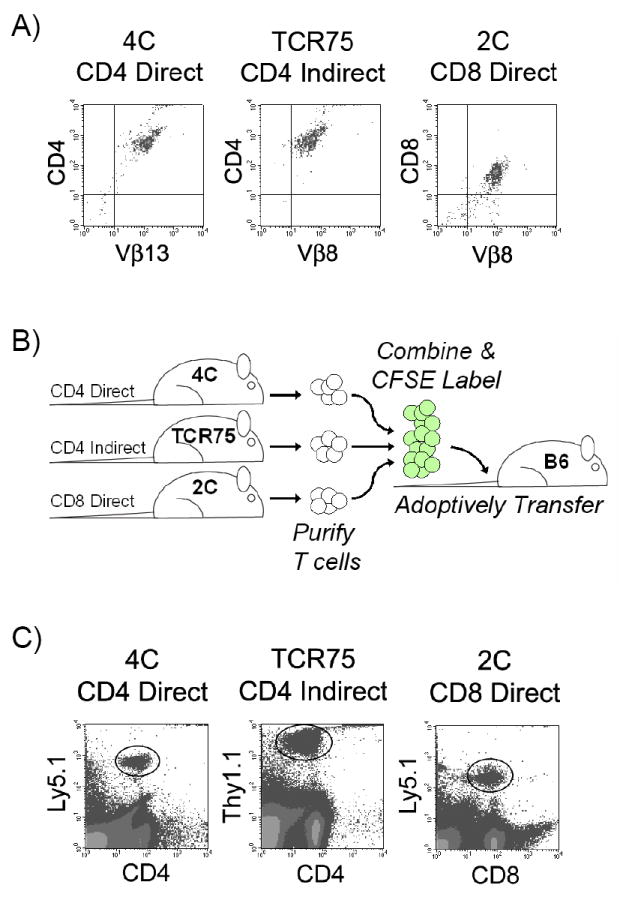
A) TCR-tg T cells representing the CD4-d (4C), CD4-i (TCR75) and CD8-d (2C) pathways of alloantigen recognition were purified by magnetic bead isolation and tested for purity by TCR-Vβ transgene expression and CD4 or CD8 expression using FACS analysis. B) Schematic of T cell purification, labeling with intravital dye (CFSE), and intravenous adoptive transfer into a syngeneic host. C) After transplantation, TCR-tg T cells could be identified amongst host T cells by the congenic cell surface markers, Ly5.1 and Thy1.1.
Equivalent proliferative capacity of TCR-tg CD4-d and CD4-i T cells
TCR transgenic models may have differential TCR expression levels, TCR affinity, and potentially other factors that may influence their ability to proliferate in response to Ag in vivo. To compare the proliferation ability of the three types of TCR-tg T cells in the setting of excess direct and indirect Ag, we adoptively transferred the CD4-d, CD4-i, and CD8-d TCR-tg T cells into BALB/c × B6 (F1) mice. Three days after adoptive transfer, the TCR-tg T cells were analyzed for proliferation by CSFE dilution. All three TCR-tg T cells proliferated vigorously and to a similar degree (Figure 2), and obtained an activated phenotype (not shown).
Figure 2. TCR-tg T cells have equivalent proliferative capabilities in vivo when Ag is abundant.
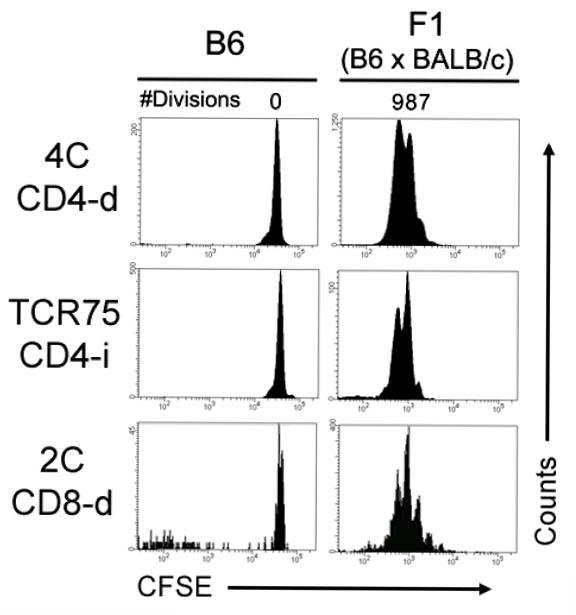
CFSE-labeled TCR-tg T cells representing the CD4-d, CD4-i and CD8-d pathways of alloantigen recognition were co-adoptively transferred into syngeneic (B6) or semi-allogeneic F1 (BALB/c × B6) recipients. After 3 days, recipient splenocytes were harvested and their proliferation was assessed by CFSE dilution detected by FACS. Results are representative of two independent experiments with 2-3 mice per group.
Preferential priming of indirect alloreactive T cells
After BALB/c heart transplantation into B6 hosts, adoptively transferred CD4-i TCR-tg T cells proliferated earlier and more robustly than did either the CD4-d or CD8-d TCR-tg T cells (Figure 3A). As early as 3 days after transplantation (D3), a large proportion of the CD4-i TCR-tg T cells had already undergone 5 or more divisions and by D5, all transferred CD4-i TCR-tg cells within the spleen had undergone more than 8 divisions. In contrast, a substantial proportion of CD4-d and CD8-d TCR-tg T cells remained undivided on D5. The proportion of CD8-d T cells proliferating appeared to be comparable to that of the CD4-d pathway, but the CD8-d T cells that were stimulated to divide appeared to divide many times. The continued division of CD8 T cells after the initial Ag encounter has been previously described (15, 16).
Figure 3. CD4-i TCR-tg T cells are preferentially primed after transplantation.
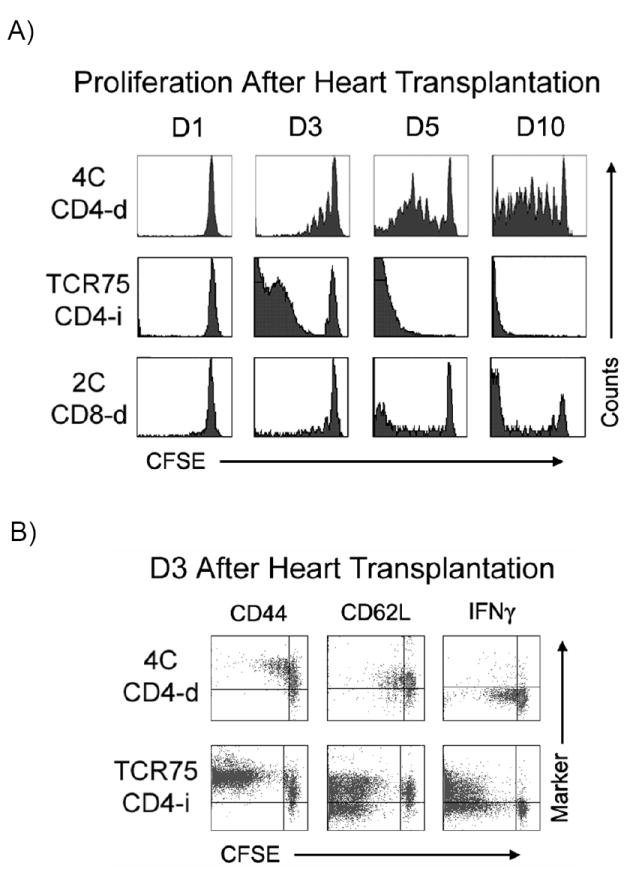
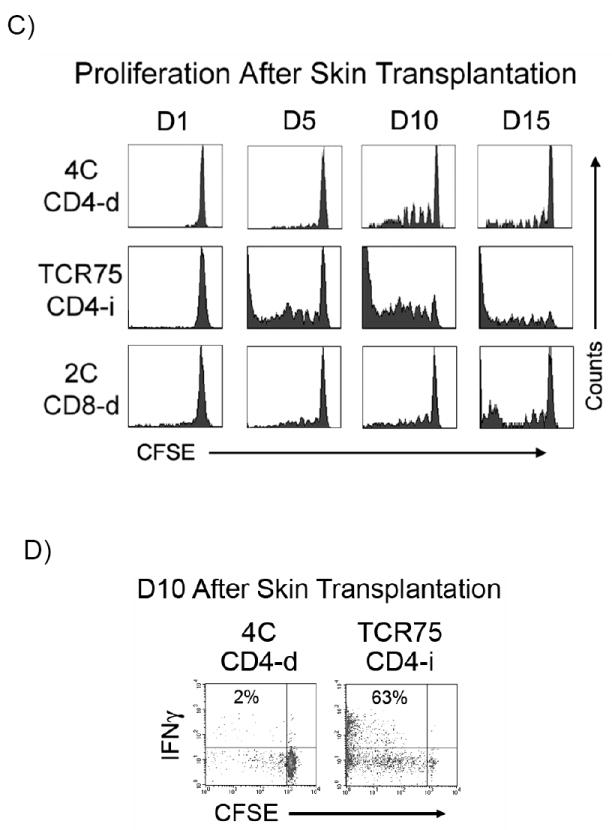
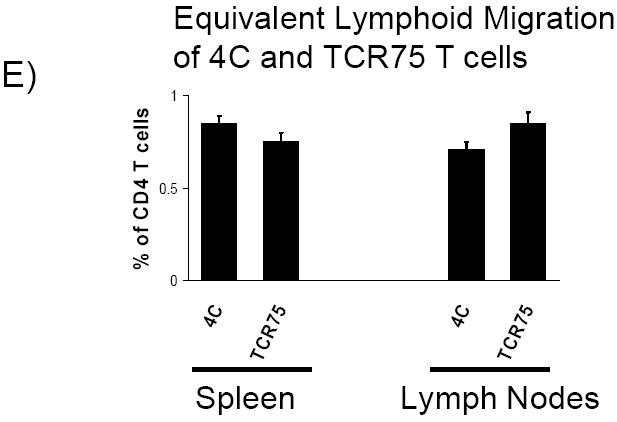
A) At various time-points after BALB/c heart transplantation, recipient splenocytes were isolated and the proliferation of adoptively transferred TCR-tg T cells was determined by CFSE dilution. Data is representative of at least 3 independent experiments, with at least 2-3 mice per experiment. B) CD44, CD62L and IFNγ expression by adoptively transferred CD4-d and CD4-i TCR-tg T cells were assessed by FACS three days (D3) following cardiac allograft transplantation. C) Proliferation of TCR-tg T cells following allogeneic skin transplantation at the draining axillary lymph nodes demonstrated by CFSE dilution. Data are representative of 3 independent experiments, 3 mice per experiment. D) Comparison of intracellular IFNγ production by CD4-d and CD4-i TCR-tg T cells 10 days after BALB/c skin transplantation. E) Equivalent migration of 4C and TCR75 TCR-tg T cells to spleen and lymph node. 2.5 × 106 T cells from each TCR-tg were co-injected intravenously into B6 mice. 7 days later, spleen and lymph node were isolated and analyzed by FACS.
The expression of CD44, CD62L (L-selectin), and IFNγ by CD4-i and CD4-d T cells was examined three days after transplantation (Figure 3B). CD44 was up-regulated on all CD4-d and CD4-i T cells that had undergone division as well as in a portion of the undivided cells. CD62L was down-regulated on approximately half of the T cells that had undergone multiple divisions. IFNγ was generally expressed by T cells that had undergone at least 4 rounds of division, as was previously demonstrated for other TCR-tg T cells (17, 18); hence, IFNγ production was much more prevalent in the CD4-i T cells.
We next examined whether the differential priming of the CD4-d and CD4-i pathways is operative in the skin transplant model, which has classically been considered to be a strong stimulator of the CD4-d pathway due to the large number of skin resident dendritic cells (DC). As shown in Figure 3C, the CD4-i TCR-tg T cells again demonstrated an earlier and more complete proliferative response after transplantation. At the time of skin graft rejection on D10, all of the CD4-i TCR-tg T cells had undergone proliferation, with a large portion having undergone more than 8 divisions. In comparison, the majority of the CD4-d and CD8-d TCR-tg T cells were still undivided with a relatively small population that had undergone more than 3 divisions. Consistent with the proliferation data, a much higher percentage of CD4-i T cells than CD4-d T cells produced IFNγ (Figure 3D). The relative accumulation of TCR-tg T cells in the lymph nodes was proportional to the relative proliferation by CFSE. At day 10, the percentage of 4C T cells was essentially unchanged, while the percentage of TCR75 T cells increased by six-fold (data not shown). These differences were not due to differential homing of the TCR-tg T cells to lymphoid organs (Figure 3E).
Differential priming of endogenous, non-transgenic CD4-d and CD4-i T cells
Although the above results are striking, it is possible that they are artifacts of clonal differences such as TCR affinity. To determine whether our results in the TCR-tg system reflect those of a normal alloimmune response, we studied endogenous T cell responses in wild-type mice using ELISPOT analysis. CD4+ T cells were isolated and stimulated in vitro with BALB/c splenocytes (direct pathway), or with B6 splenocytes pulsed with a lysate of BALB/c splenocytes (indirect pathway), as previously reported by Benichou et al (13).
The precursor frequency of CD4-d and CD4-i T was determined using the IL-2 ELISPOT assay. In naive mice, CD4-d T cells were abundant, but CD4-i T cells were not detectable (Figure 4A). As expected, no IFNγ–producing T cells were detected for either pathway in naive mice (data not shown). Ten days after allogeneic skin transplantation, the CD4-d T cell frequency increased modestly (approximately 2-fold) as determined by IL-2 and IFNγ ELISPOT (Figures 4B & 4C). In contrast to the findings in naive mice, CD4-i T cells producing IL-2 and IFNγ were readily detectable 10 days after skin transplant and comprised approximately 10% of the alloreactive CD4+ T cells (Figures 4B & 4C). The fold-expansion of the CD4-i T cells could not be calculated because the frequency of CD4-i T cells in the naive mice was below the limit of detection of our ELISPOT assay, which in our experiments was approximately 3-5 cells/million, consistent with previous studies (19). Consistent with previous reports(13), direct responses to third party APC (Figure 4E) or syngeneic APC (not shown) were not significantly induced by skin transplantation. Indirect responses to third party splenocyte lysates were detectable(figure 4F), as previously shown by Heeger’s group(13, 20), likely due to common alloreactive peptides as well as the development of autoreactive T cells(20). In summary, endogenous CD4-i T cells expanded more than the CD4-d T cells after skin transplantation, and eventually comprised a significant proportion of alloreactive T cells.
Figure 4. ELISPOT analysis of non-transgenic CD4-d and CD4-i T cells.
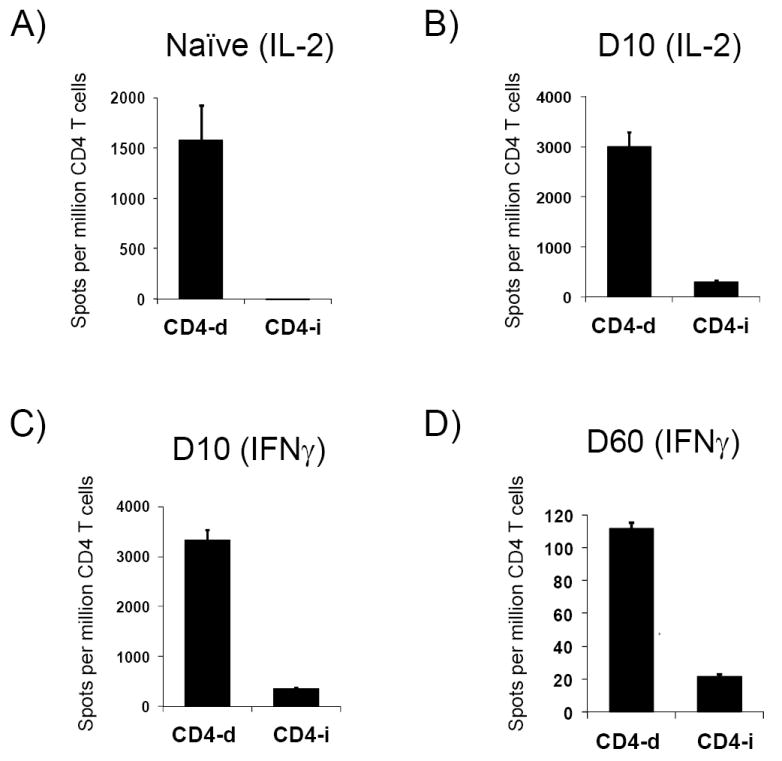
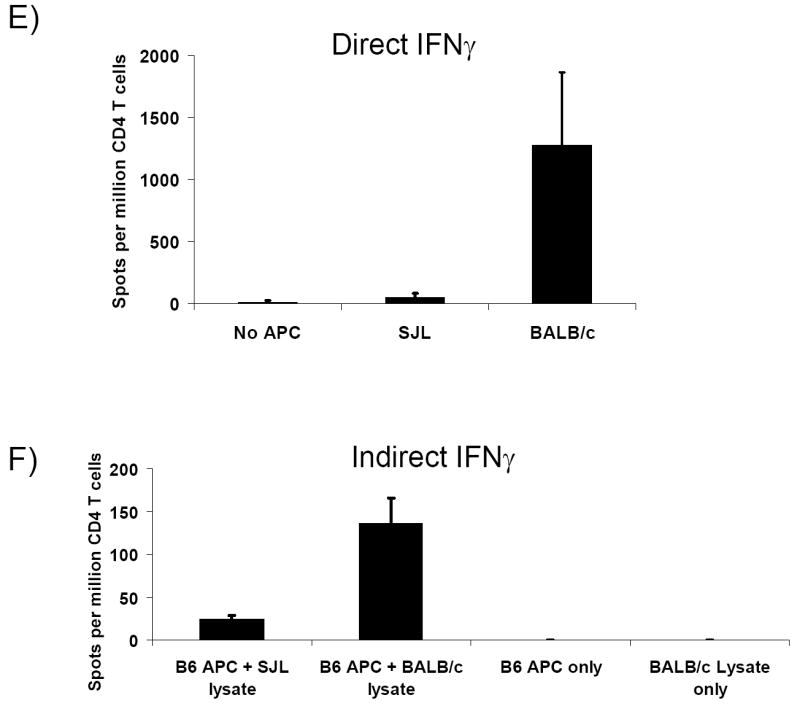
After BALB/c → B6 skin transplantation, CD4+ T cells were purified from recipient axillary LN. CD4-d and CD4-i T cells were then identified by IL-2 and IFNγ ELISPOT assays by their response to direct or indirect alloantigen presentation. No TCR-tg T cells were utilized in these experiments. A) Naive B6 mouse (no transplant), IL-2; B) day (D) 10 after skin transplant, IL-2; C) D10 after skin transplant, IFNγ; D) D60 after skin transplant, IFNγ. Data are representative of two independent experiments with 2-3 mice per data point. E, F) IFNγ ELISPOT of draining lymph node CD4+ T cells 10 days after skin transplant with 3rd party controls.
CD4-i T cells comprise a large proportion of allospecific memory T cells and graft infiltrating lymphocytes
The development of memory T cells may be linked to the magnitude and quality of T cell stimulation during the immunizing event (21, 22). Our results therefore predicted that CD4-i T cells would be enriched in memory-like T cells. To test this prediction, we analyzed CD4+ T cells from B6 mice 60 days after BALB/c skin transplantation (approximately 50 days after complete rejection). As expected, the absolute numbers of both the CD4-d and the CD4-i T cells producing IFNγ had greatly decreased by day 60 compared to day 10 (Figure 4D). Notably, CD4-i T cells comprised about 20% of the IFNγ producing CD4+ T cells, a 2-fold increase in relative reactivity compared to day 10 after transplant.
Taken together, analysis of TCR-tg T cell responses and endogenous T cell responses suggests that, compared to CD4-d T cells, a larger proportion of CD4-i T cells undergo vigorous proliferation and attain an effector phenotype during acute rejection. Thus, we hypothesized that the CD4-i T cells should be enriched in the population of T cells that infiltrate the graft undergoing rejection. To test this hypothesis, we utilized the vascularized cardiac transplant model. Seven days after a BALB/c heart transplant was placed into a B6 host, we isolated CD4+ T cells from the spleen as well as from the allograft. We then examined the pathway of alloreactivity using the ELISPOT assay. The indirect pathway accounted for approximately 6% of IFNγ producing CD4+ T cells in the spleen (Figure 5A); strikingly, over one-third of the alloreactive GIL CD4+ T cells responded via the indirect pathway (Figure 5B).
Figure 5. CD4-i T cells are enriched in graft infiltrating lymphocytes.
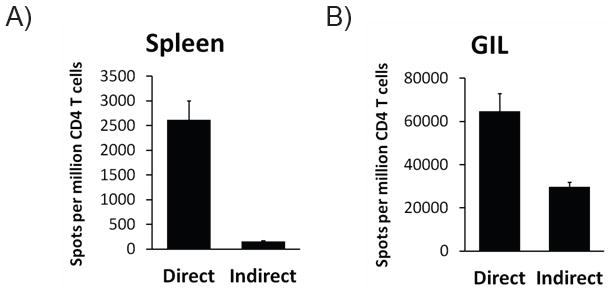
On postoperative day 7 after BALB/c → B6 heart transplantation, cardiac allografts were excised and CD4+ T cells were isolated from the graft infiltrating lymphocytes (GIL) using magnetic beads. Purified CD4+ T cells from GIL and spleen were tested for direct and indirect alloreactivity by IFNγ ELISPOT assay. Data represent 3 heart transplant recipients.
Preferential priming of CD4-i T cells by healed skin grafts
A longstanding hypothesis has been that CD4-d priming should wane after transplant, whereas CD4-i priming should be maintained. We and others have previously shown that TCR-tg CD4-d T cells could acutely reject skin grafts (11, 23). TCR-tg CD4-i T cells have also been shown to acutely reject heart grafts (9). To further understand the dynamics of priming in long-term grafts, we transplanted BALB/c skin grafts onto immunodeficient B6 Rag1-/- mice and allowed the grafts to heal for 100 days. Adoptively transferred CD4-d T cells were not able to reject healed skin grafts. In contrast, CD4-i T cells were able to reject healed and fresh skin grafts with rapid kinetics (Figure 6). To test whether the failure of CD4-d T cells to reject healed skin was due to lack of priming or to a fundamental resistance of healed skin grafts to rejection by CD4-d T cells, we provided exogenous priming by co-transferring BALB/c DC at the time of T cell transfer. Augmented priming caused rapid rejection of healed skin by CD4-d T cells. Thus, healed grafts were able to productively prime CD4-i T cells but not CD4-d T cells, consistent with the concept that priming of the indirect pathway is longer lived than that of the direct pathway.
Figure 6. Differential priming of CD4-d and CD4-i pathways in fresh vs. healed grafts.
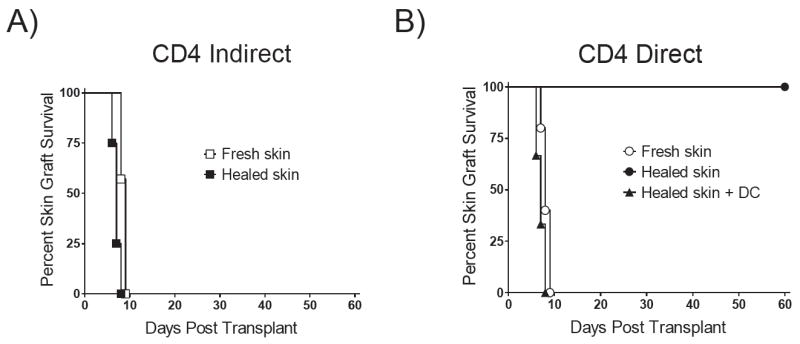
Kaplan-Meier survival plot of BALB/c skin transplants on B6 Rag1-/- hosts. A) Two million CD4-i TCR-tg T cells were adoptively transferred at the time of skin transplantation (fresh skin) or 100 days after skin transplantation (healed skin) (p = NS). B) Similarly, two million CD4-d TCR-tg T cells were adoptively transferred into recipients of fresh skin or healed skin transplants. 107 BALB/c DCs were injected at the time of CD4-d TCR-tg T cell transfer in recipients with healed BALB/c allografts.
Discussion
The results of our study reveal dramatically more priming of CD4-i T cells than of CD4-d T cells following transplantation. This fundamental difference allows the CD4-i T cells to become a sizeable proportion of the acute T cell response to allografts despite their initially lower frequency. Our results are consistent with a previous report by Benichou et. al in the B10.A donor→BALB/c recipient strain combination(13). In their study, the CD4-i response was not detectable by ELISPOT 5 days after skin transplant, but reached approximately 5% of the CD4-d response by the time of graft rejection. Our results with the TCR-tg adoptive transfer model provide a novel framework for understanding these observations.
Previous studies have shown that, in general, differentiation into effector and possibly memory cells depends on the level of priming (17). Consistent with this concept, we found further enrichment of CD4-i T cells in the effector T cells that were able to home to the allograft. Similarly, we found relative enrichment of CD4-i T cells in long-lived “memory-like” T cells sixty days after transplantation.
The mechanisms underlying this differential priming merit further study. Several potential explanations exist. First, competition for Ag has been shown to play an important role in the degree of T cell proliferation, possibly by preventing stable interactions with Ag-bearing DC (24). In mice, the precursor frequency of the CD4-d pathway has been estimated to be approximately 5-10% of all T cells against a single haplotype (3). This supraphysiologic precursor frequency is at least 3-4 orders of magnitude greater than that for conventional Ag (21, 24). The exact precursor frequency of the CD4-i pathway has not been determined, but the preponderance of experimental data suggests that it is at least 2-3 orders of magnitude lower than for the CD4-d pathway. The comparatively low precursor frequency of the CD4-i pathway makes competition for Ag less likely, favoring the proliferation of CD4-i T cells relative to CD4-d T cells.
The second potential explanation is that the abundance and persistence of Ag are likely to differ between the CD4-d and CD4-i pathways. CD4-d T cell priming is dependent on donor tissue-derived APC that have migrated out of the graft and into the lymphoid compartment. Donor-derived DC are limited in number and lifespan. Theoretically, exosomes from donor DC or intercellular transfer of MHC from donor cells and self-APC could serve to extend direct Ag presentation. However, Morelli’s group has shown that donor-DC derived exosomes exclusively stimulate CD4-i T cells (25) and there is no evidence to date that intercellular MHC transfer is immunologically relevant in vivo (26). In contrast, CD4-i T cell priming is dependent on ubiquitous and renewable self-APC that may share Ag via apoptosomes and by direct sharing of membrane fragments (26, 27). Moreover, live donor DC may serve as important carriers of indirect alloantigen via the shedding of exosomes (25), and DC dying via apoptosis and necrosis can efficiently deliver Ag to host DC (28). In addition to allopeptides that may be derived from donor APC, the graft itself is likely shed Ag for processing by host APC even after donor APC have been depleted. Consistent with this concept, CD4-d TCR-tg T cells cannot reject a 100-day-old allograft in Rag-/- recipients, whereas CD4-i TCR-tg T cells reject the healed grafts with near normal kinetics. The relative contributions of donor APC and donor parenchymal cells to CD4-i Ag production have not been elucidated. However, our results clearly demonstrate that when productive priming of the direct pathway is absent, the indirect pathway can be productively primed by long-term grafts. Thus, we speculate that indirect Ag is more abundant and longer-lived, again favoring priming of the CD4-i pathway.
It is notable that two previous studies using TCR-tg models of alloreactivity have suggested that Ag are more efficiently presented through the indirect pathway. Chen et al. demonstrated that the male minor transplantation Ag preferentially activates CD4+ T cells through the indirect pathway(29). Elimination of the indirect pathway by using MHCII deficient hosts demonstrated minimal priming by the direct pathway, although a potential caveat is that CD4+ T cells may not demonstrate optimal function in MHCII deficient hosts (30). Baratin et al. used the 2.102 TCR transgenic system that has direct reactivity to IEp and indirect reactivity to an immunodominant peptide of the mouse hemoglobin β chain (31). Although the 2.102 TCR could rapidly reject skin grafts via either pathway, proliferation and activation of 2.102 T cells in the lymph node and spleen was much more vigorous in response to skin grafts that expressed the indirect Ag (31). Our studies of TCR-tg as well as endogenous T cell responses using conventional alloantigens complement and extend these previous studies.
These two potential mechanisms underlying the disparate priming of the CD4-i and CD4-d pathways are not mutually exclusive and indeed would act in synergy. The relative scarcity of CD4-d Ag will amplify the competition amongst the high-frequency CD4-d T cells. Another potential mechanism may be related to the differential TCR-MHC interactions between the CD4-i and CD4-d pathways; however, this complex topic is beyond the scope of this work.
This study helps to reconcile the longstanding paradox that elimination of the CD4-d pathway does not significantly change the kinetics of transplant rejection. The CD4-i pathway forms a substantial portion of the CD4 T cell response to allografts and, like the CD4-d pathway, is able to provide help for CD8+ alloreactive T cells (32). Moreover, it is now clear that CD4-i T cells can damage the allograft independently of CD8 T cells through as yet incompletely elucidated mechanisms (9).
A revised paradigm of alloreactivity that includes greater levels of indirect priming relative to direct priming may help shape our understanding and design of immunosuppressive and tolerance induction strategies. For example, regulatory T cells (Tregs) are known to require TCR stimulation for function (33). Our findings predict that Tregs and effector T cells with CD4-i specificity will receive greater and more persistent TCR stimulation than for the direct pathway. Hence, Tregs with indirect specificity are likely to be important in the acute and the chronic setting. Indeed, there is experimental evidence for the efficacy of CD4-i Tregs in preventing acute rejection, and donor-specific CD4-i Tregs have been found in long-term renal allograft recipients (34). The differential priming may also affect the responses of the two pathways to immunosuppressive agents as well as to immunomodulatory agents. Of particular clinical relevance, the CD4-i pathway has been shown to uniquely help produce alloantibodies (35, 36).
In conclusion, the TCR-tg model presented here allows for the simultaneous tracking of the T cell alloresponse in the CD4-d, CD4-i, and CD8-d pathways. This model appears to reasonably reflect the endogenous response to allografts, and should be useful for studying these and other questions in alloimmunity.
Acknowledgments
We thank Drs. Abul Abbas and Jeffrey Bluestone for helpful discussions. We thank Pamela Derish and Susannah McCormick for assistance with preparation and review of the manuscript.
This work was supported by grants from: The UCSF Nicholas Fund, the American College of Surgeons (T.V.B), ASTS-Roche Award (T.V.B.), ASTS Faculty Development Award (S.M.K.), the National Institutes of Health (NIDDK K08 DK61970, R03 DK75431) (S.M.K.), and the UCSF Liver Center (NIDDK P30DK26743).
References
- 1.Rogers NJ, Lechler RI. Allorecognition. Am J Transplant. 2001 Jul;1(2):97–102. [PubMed] [Google Scholar]
- 2.Auchincloss H, Jr, Lee R, Shea S, Markowitz JS, Grusby MJ, Glimcher LH. The role of “indirect” recognition in initiating rejection of skin grafts from major histocompatibility complex class II-deficient mice. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3373–7. doi: 10.1073/pnas.90.8.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001 Jan 15;166(2):973–81. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 4.Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Cook HT, Lechler RI. Loss of direct and maintenance of indirect alloresponses in renal allograft recipients: implications for the pathogenesis of chronic allograft nephropathy. J Immunol. 2001 Dec 15;167(12):7199–206. doi: 10.4049/jimmunol.167.12.7199. [DOI] [PubMed] [Google Scholar]
- 5.Vella JP, Vos L, Carpenter CB, Sayegh MH. Role of indirect allorecognition in experimental late acute rejection. Transplantation. 1997 Dec 27;64(12):1823–8. doi: 10.1097/00007890-199712270-00033. [DOI] [PubMed] [Google Scholar]
- 6.Heeger PS. T-cell allorecognition and transplant rejection: a summary and update. Am J Transplant. 2003 May;3(5):525–33. doi: 10.1034/j.1600-6143.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 7.Faustman DL, Steinman RM, Gebel HM, Hauptfeld V, Davie JM, Lacy PE. Prevention of rejection of murine islet allografts by pretreatment with anti-dendritic cell antibody. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3864–8. doi: 10.1073/pnas.81.12.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechler RI, Batchelor JR. Immunogenicity of retransplanted rat kidney allografts. Effect of inducing chimerism in the first recipient and quantitative studies on immunosuppression of the second recipient. J Exp Med. 1982 Dec 1;156(6):1835–41. doi: 10.1084/jem.156.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honjo K, Xu X, Bucy RP. CD4+ T-cell receptor transgenic T cells alone can reject vascularized heart transplants through the indirect pathway of alloantigen recognition. Transplantation. 2004 Feb 15;77(3):452–5. doi: 10.1097/01.TP.0000112937.12491.42. [DOI] [PubMed] [Google Scholar]
- 10.Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Selective expression of an antigen receptor on CD8-bearing T lymphocytes in transgenic mice. Nature. 1988 Sep 15;335(6187):271–4. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 11.Brennan TV, Hoang V, Garrod KR, Liu FC, Hayden T, Kim J, et al. A new T-cell receptor transgenic model of the CD4+ direct pathway: level of priming determines acute versus chronic rejection. Transplantation. 2008 Jan 27;85(2):247–55. doi: 10.1097/TP.0b013e31815e883e. [DOI] [PubMed] [Google Scholar]
- 12.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973 Oct;16(4):343–50. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999 Jan 1;162(1):352–8. [PubMed] [Google Scholar]
- 14.Honjo K, Xu XY, Kapp JA, Bucy RP. Activation and migration of allo-peptide specific TCR transgenic T cells in cardiac allograft rejection. Cell Immunol. 2004 Jul;230(1):44–55. doi: 10.1016/j.cellimm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J Immunol. 2002 Feb 15;168(4):1528–32. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 16.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001 May;2(5):415–22. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gudmundsdottir H, Wells AD, Turka LA. Dynamics and requirements of T cell clonal expansion in vivo at the single-cell level: effector function is linked to proliferative capacity. J Immunol. 1999 May 1;162(9):5212–23. [PubMed] [Google Scholar]
- 18.Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, et al. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998 Aug;9(2):229–37. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 19.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999 Aug 15;163(4):2267–75. [PubMed] [Google Scholar]
- 20.Valujskikh A, Fedoseyeva E, Benichou G, Heeger PS. Development of autoimmunity after skin graft rejection via an indirect alloresponse. Transplantation. 2002 Apr 15;73(7):1130–7. doi: 10.1097/00007890-200204150-00021. [DOI] [PubMed] [Google Scholar]
- 21.Whitmire JK, Benning N, Eam B, Whitton JL. Increasing the CD4+ T cell precursor frequency leads to competition for IFN-gamma thereby degrading memory cell quantity and quality. J Immunol. 2008 May 15;180(10):6777–85. doi: 10.4049/jimmunol.180.10.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med. 2005 May 16;201(10):1555–65. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rulifson IC, Szot GL, Palmer E, Bluestone JA. Inability to induce tolerance through direct antigen presentation. Am J Transplant. 2002 Jul;2(6):510–9. doi: 10.1034/j.1600-6143.2002.20604.x. [DOI] [PubMed] [Google Scholar]
- 24.Garcia Z, Pradelli E, Celli S, Beuneu H, Simon A, Bousso P. Competition for antigen determines the stability of T cell-dendritic cell interactions during clonal expansion. Proc Natl Acad Sci U S A. 2007 Mar 13;104(11):4553–8. doi: 10.1073/pnas.0610019104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montecalvo A, Shufesky WJ, Stolz DB, Sullivan MG, Wang Z, Divito SJ, et al. Exosomes As a Short-Range Mechanism to Spread Alloantigen between Dendritic Cells during T Cell Allorecognition. J Immunol. 2008 Mar 1;180(5):3081–90. doi: 10.4049/jimmunol.180.5.3081. [DOI] [PubMed] [Google Scholar]
- 26.Smyth LA, Afzali B, Tsang J, Lombardi G, Lechler RI. Intercellular transfer of MHC and immunological molecules: molecular mechanisms and biological significance. Am J Transplant. 2007 Jun;7(6):1442–9. doi: 10.1111/j.1600-6143.2007.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001 Mar 15;166(6):3717–23. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 28.Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med. 1998 Dec 7;188(11):2163–73. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Demir Y, Valujskikh A, Heeger PS. The male minor transplantation antigen preferentially activates recipient CD4+ T cells through the indirect presentation pathway in vivo. J Immunol. 2003 Dec 15;171(12):6510–8. doi: 10.4049/jimmunol.171.12.6510. [DOI] [PubMed] [Google Scholar]
- 30.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002 Nov 28;420(6914):429–34. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 31.Baratin M, Bonin K, Daniel C. Frontline: Peripheral priming of alloreactive T cells by the direct pathway of allorecognition. Eur J Immunol. 2004 Dec;34(12):3305–14. doi: 10.1002/eji.200425309. [DOI] [PubMed] [Google Scholar]
- 32.Lee RS, Grusby MJ, Glimcher LH, Winn HJ, Auchincloss H., Jr Indirect recognition by helper cells can induce donor-specific cytotoxic T lymphocytes in vivo. J Exp Med. 1994 Mar 1;179(3):865–72. doi: 10.1084/jem.179.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang SM, Tang Q, Bluestone JA. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am J Transplant. 2007 Jun;7(6):1457–63. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 34.Salama AD, Najafian N, Clarkson MR, Harmon WE, Sayegh MH. Regulatory CD25+ T cells in human kidney transplant recipients. J Am Soc Nephrol. 2003 Jun;14(6):1643–51. doi: 10.1097/01.asn.0000057540.98231.c1. [DOI] [PubMed] [Google Scholar]
- 35.Steele DJ, Laufer TM, Smiley ST, Ando Y, Grusby MJ, Glimcher LH, et al. Two levels of help for B cell alloantibody production. J Exp Med. 1996 Feb 1;183(2):699–703. doi: 10.1084/jem.183.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sauve D, Baratin M, Leduc C, Bonin K, Daniel C. Alloantibody production is regulated by CD4+ T cells’ alloreactive pathway, rather than precursor frequency or Th1/Th2 differentiation. Am J Transplant. 2004 Aug;4(8):1237–45. doi: 10.1111/j.1600-6143.2004.00520.x. [DOI] [PubMed] [Google Scholar]


