There is an incomplete understanding in the pathogenesis of oropharyngeal squamous cell carcinoma (OPSCC) and cancer broadly. It is essential to discover new pathways of normal cell-cycle regulation and oncogenesis. We identified that the loss of inositol polyphosphate-5-phosphatase (INPP5A) may play a role in the development and progression of cutaneous squamous cell carcinoma (SCC).1 Loss of INPP5A has been previously linked to cancer development and progression.2 Inositol signaling pathways are involved in intracellular calcium release, membrane trafficking, chemotaxis, ion channel activity, and many other nuclear functions.3 Inositol signaling is highly conserved throughout the animal kingdom and plays a key role in gene regulation.
We hypothesized that, like cutaneous SCC, INPP5A expression will be decreased in OPSCC and that progressive reduction of INPP5A will occur with the transition from primary to metastatic disease. To test this hypothesis, we assessed INPP5A protein levels, using immunohistochemistry (IHC), in patients with primary OPSCC with matched lymph node metastasis.
Mayo Clinic Institutional Review Board approved this study. A prospective database of head and neck cancer patients treated from 1994–2014 with OPSCC at Mayo Clinic Arizona and Rochester was reviewed. A total of 40 cases were identified with both archived primary OPSCC tissue and matched metastatic lymph node tissue. One case had inadequate lymph node tissue. Stained slides were evaluated with the normal mucosa acting as the internal control. A scoring system was used to assess the intensity of staining (0–3) with a score of 0 representing no staining and score of 3 as intense staining.1
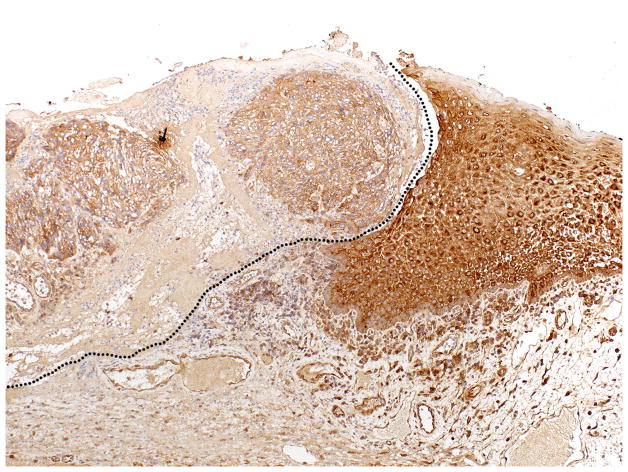
INPP5A expression by IHC demonstrated diffuse cytoplasmic staining in normal tissue with a diminished staining within the tumor (Fig. 1). A comparison of INPP5A staining intensity between primary OPSCC samples and the internal controls showed a relative reduction of INPP5A staining in 22/40 (55%) cases (Table 1). To assess the role of INPP5A loss in the process of disease progression, we measured the IHC staining of 39 primary OPSCC with matched lymph node metastasis. IHC analysis of these paired tissues detected a further reduction of INPP5A staining in 13/39 (33.3%) cases, no relative change in 20/39 (51%) cases, and an increase in 6/39 (15%) cases respectively. There were no cases in which the primary or the metastatic disease had higher staining intensity than the matched normal mucosa.
Figure 1.
Inositol polyphosphate-5-phosphatase (INPP5A) Expression in Oropharyngeal Squamous Cell Carcinomas (OPSCC) – The dotted black line delineates the decreased staining in the invasive tumor (left) compared to the normal mucosa (right).
Table 1.
Comparison of INPP5A protein levels in normal mucosa, primary oropharyngeal squamous cell carcinoma (OPSCC), and metastatic SCC
| INPP5A staining intensity | Frequency |
|---|---|
| Primary OPSCC compared with matched normal mucosa | |
| Normal mucosa > primary OPSCC | 22/40 (55%) |
| Normal mucosa = primary OPSCC | 18/40 (45%) |
| Normal mucosa < primary OPSCC | 0/40 (0%) |
| Metastatic SCC compared with matched normal mucosa | |
| Normal mucosa > metastatic SCC | 23/39 (59%) |
| Normal mucosa = metastatic SCC | 16/39 (41%) |
| Normal mucosa < metastatic SCC | 0/39 (0%) |
| Primary OPSCC compared with matched metastatic SCC | |
| Primary OPSCC > Metastatic SCC | 13/39 (33%) |
| Primary OPSCC = Metastatic SCC | 20/39 (51%) |
| Primary OPSCC < Metastatic SCC | 6/39 (15%) |
We have identified decreased expression of INPP5A in primary and metastatic OPSCC. This data indicates an association of INPP5A loss with OPSCC development and disease progression.
Our findings strengthen previously reported observations that implicate INPP5A’s role in human cancers. The loss of the chromosomal region 10q26, which encodes INPP5A, is associated with brain tumors and decreased INPP5A activity is associated with human leukemias.4–8 Our prior study in cutaneous SCC found that the INPP5A gene was lost in 24% of tumors and had decreased protein expression in 72% of primary tumors.1 Additionally, primary cutaneous SCC with metastasis showed a loss in 92% of cases.1
The precise mechanism(s) of INPP5A loss in SCC is unknown and a better understanding between INPP5A and uncontrolled cellular proliferation in cutaneous and mucosal SCC may provide novel insights into the pathogenesis of SCC and result in novel therapies. For example, murine models of cutaneous carcinogenesis found that bypassing the loss of INPP5A with IP6 reduced tumor formation at the initiation phase of carcinogenesis.9 In vitro studies of other cancers, such as breast, have shown the efficacy of IP6 as monotherapy and in combination with chemotherapy in resistant cell lines.10
In conclusion, INPP5A expression is commonly decreased in OPSCC, correlates with disease progression, and represents a potential prognostic and therapeutic target.
Acknowledgments
Funding Sources: NIH Grant 5R01CA179157 for the support of Dr. Sekulic; Dermatology Foundation Career Development Award for the support of Dr. Mangold
NIH Grant for the support of Dr. Sekulic
Dermatology Foundation Career Development Award for the support of Dr. Mangold
Footnotes
Conflicts of Interest: The authors declare that they have no conflict of interest and no financial disclosure
References
- 1.Sekulic A, Kim SY, Hostetter G, Savage S, Einspahr JG, Prasad A, et al. Loss of inositol polyphosphate 5-phosphatase is an early event in development of cutaneous squamous cell carcinoma. Cancer Prev Res (Phila) 2010;3(10):1277–83. doi: 10.1158/1940-6207.CAPR-10-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Speed CJ, Little PJ, Hayman JA, Mitchell CA. Underexpression of the 43 kDa inositol polyphosphate 5-phosphatase is associated with cellular transformation. EMBO J. 1996;15(18):4852–61. [PMC free article] [PubMed] [Google Scholar]
- 3.York JD. Regulation of nuclear processes by inositol polyphosphates. Biochim Biophys Acta. 2006;1761(5–6):552–9. doi: 10.1016/j.bbalip.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Fults D, Pedone C. Deletion mapping of the long arm of chromosome 10 in glioblastoma multiforme. Genes Chromosomes Cancer. 1993;7(3):173–7. doi: 10.1002/gcc.2870070311. [DOI] [PubMed] [Google Scholar]
- 5.Lee SH, Davison JA, Vidal SM, Belouchi A. Cloning, expression and chromosomal location of NKX6B TO 10Q26, a region frequently deleted in brain tumors. Mamm Genome. 2001;12(2):157–62. doi: 10.1007/s003350010247. [DOI] [PubMed] [Google Scholar]
- 6.Mengubas K, Jabbar SA, Nye KE, Wilkes S, Hoffbrand AV, Wickremasinghe RG. Inactivation of calcium ion-regulating inositol polyphosphate second messengers is impaired in subpopulations of human leukemia cells. Leukemia. 1994;8(10):1718–25. [PubMed] [Google Scholar]
- 7.Milinkovic V, Bankovic J, Rakic M, Stankovic T, Skender-Gazibara M, Ruzdijic S, et al. Identification of novel genetic alterations in samples of malignant glioma patients. PLoS One. 2013;8(12):e82108. doi: 10.1371/journal.pone.0082108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nye KE, Riley GA, Poulter LW, Porfiri E, Hoffbrand AV, Wickremasinghe RG. Impaired degradation of Ca(2+)-regulating second messengers in myeloid leukemia cells. Implications for the regulation of leukemia cell proliferation. Leukemia. 1992;6(8):801–5. [PubMed] [Google Scholar]
- 9.Ishikawa T, Nakatsuru Y, Zarkovic M, Shamsuddin AM. Inhibition of skin cancer by IP6 in vivo: initiation-promotion model. Anticancer Res. 1999;19(5A):3749–52. [PubMed] [Google Scholar]
- 10.Tantivejkul K, Vucenik I, Eiseman J, Shamsuddin AM. Inositol hexaphosphate (IP6) enhances the anti-proliferative effects of adriamycin and tamoxifen in breast cancer. Breast Cancer Res Treat. 2003;79(3):301–12. doi: 10.1023/a:1024078415339. [DOI] [PubMed] [Google Scholar]