Abstract
Network dynamics can reveal information about the adaptive function of social behaviour and the extent to which social relationships can flexibly respond to extrinsic pressures. Changes in social networks occur following changes to the social and physical environment. By contrast, we have limited understanding of whether changes in social networks precede major group events. Permanent evictions can be important determinants of gene flow and population structure and are a clear example of an event that might be preceded by social network dynamics. Here we examined the social networks of a group of rhesus macaques, Macaca mulatta, in the 2 years leading up to the eviction of 22% of adult females, which are the philopatric sex. We found that females engaged in the same amount of aggression and grooming in the 2 years leading up to the eviction but that there were clear changes in their choice of social partners. Females that would eventually be evicted received more aggression from lower-ranking females as the eviction approached. Evicted females also became more discriminating in their grooming relationships in the year nearer the split, showing a greater preference for one another and becoming more cliquish. Put simply, the females that would later be evicted continued to travel with the rest of the group as the eviction approached but were less likely to interact with other group members in an affiliative manner. These results have potential implications for understanding group cohesion and the balance between cooperation and competition that mediates social groups.
Keywords: group fission, Macaca mulatta, network dynamics, social bonds, social network, social stability
Animals that live in groups are faced with the challenge of balancing the benefits of group living with the costs of conflicting interests between groupmates (Krause & Ruxton, 2002; Silk, 2007). Balancing these costs and benefits may be especially difficult for individuals that live in groups composed of both kin and nonkin (Seyfarth & Cheney, 2012). Much theoretical and empirical research has focused on how individuals may use aggression, social status, cooperation and social bonds to cope with intragroup conflict. Yet a great deal about the origins and maintenance of group living remains unclear (Brent, Chang Gariépy, & Platt, 2014; Krause & Ruxton, 2002; Nowak, Tarnita, & Wilson, 2010; Shultz, Opie, & Atkinson, 2011). Network dynamics within groups can reveal the processes that underpin the structuring of animal societies and can uncover information about the adaptive functions of social behaviours and relationships (Berger-Wolf & Saia, 2006; Bode, Wood, & Franks, 2011a, b; Pinter-Wollman et al., 2014). Describing dynamic shifts in social networks and determining when and why these shifts occur is therefore an important route to understanding the maintenance of social groups, and hence the evolution of sociality.
A growing number of studies have documented network dynamics within groups that have followed changes to the physical environment. For example, association networks become more tightly connected when resources are scarce in killer whales, Orcinus orca (Foster et al., 2012). This finding is in accordance with the hypothesis that prosocial relationships are more valuable during times of hardship because they help individuals to cope with intragroup competition (Barrett, Henzi, Weingrill, Lycett, & Hill, 1999; van Schaik, 1989). In contrast, a negative relationship between network connectedness and the level of resource competition, as measured by group size, suggests competition rather than cooperation shapes sociality in wild chimpanzees, Pan troglodytes (Lehmann & Boesch, 2009). In sleepy lizards, Tiliqua rugosa, the number and strength of network connections do not change in response to changes in climate, although the nature of social connections differs with fewer intersexual associations in drier years (Godfrey, Sih, & Bull, 2013). In contrast, the social networks of some populations do not appear to respond to changes in the physical environment; although guppies, Poecilia reticulata, from areas with low levels of predation showed more social mixing than their high-predation counterparts, no changes to social networks occurred within populations following experimental manipulation of habitat complexity or predation risk (Edenbrow et al., 2011).
In addition to changes in the physical environment, network dynamics following changes in social factors, such as reproductive seasonality (Brent, Maclarnon, Platt, & Semple, 2013; Hamede, Bashford, McCallum, & Jones, 2009) and group composition, have revealed important information about social processes. For instance, network dynamics following the simulated, experimental or natural loss of individuals from groups suggests that some individuals are more important to group cohesion than others (Kanngiesser, Sueur, Riedl, Grossmann, & Call, 2010; Lehmann, Andrews, & Dunbar, 2010; Manno, 2008) and can occupy specific social roles (Flack, Girvan, de Waal, & Krakauer, 2006). Following experimental manipulation of the sex ratio of guppy groups, a breakdown in female–female associations in populations with a greater number of males, and hence a greater level of sexual harassment, suggests that repeated social interactions are needed to establish individual recognition between groupmates (Darden, James, Ramnarine, & Croft, 2009). Wild chacma baboon, Papio ursinus, females compensate for the death of a close relative by broadening and strengthening their grooming networks (Engh et al., 2006), particularly by extending their social relationships to unrelated groupmates. This apparent compensatory behaviour suggests that social relationships are valuable to female baboons, and provides preliminary evidence regarding the differential value of social relationships with kin compared to nonkin. Finally, changes to social networks have been observed in response to changes in the social hierarchy. The grooming networks of female chacma baboons were less diverse in the weeks following a period of instability in the alpha male position in their group (Wittig et al., 2008). Females that contracted their grooming networks the most showed a less dramatic rise in faecal glucocorticoid metabolite levels and returned to baseline levels more quickly (Wittig et al., 2008). Taken together, these findings suggest that affiliative bonds with a small number of preferred partners help these animals to cope with social instability.
Network dynamics not only occur in response to changes to the environment but also may precede or even provoke such changes. Understanding the links between network dynamics that occur in advance of shifts in the physical or social environment can therefore also have important implications for our understanding of social processes and relationships, and may even allow scientists to predict the occurrence of major events. Instances where we might expect network dynamics to occur in advance of social or physical perturbations include: seasonally predictable changes in climate or resource abundance; the joining/splitting of subgroups in species with high levels of fission–fusion sociality (Sueur & Maire, 2014); large outbreaks of intragroup aggression; and the dispersal, death (i.e. in cases where death is preceded by a gradual decline in condition) or permanent eviction of groupmates. However, few studies have documented network dynamics prior to major events because the occurrence of these events can be difficult to anticipate and studies of this nature must often rely on coincidental collection of behavioural data.
Here we evaluated network dynamics preceding the permanent mass eviction of many females from a group of rhesus macaques, Macaca mulatta. Rhesus macaques, like many primates, live in social groups composed of multiple adult males and females (Thierry, 2007). Females are the philopatric sex and membership of females in rhesus macaque groups is ‘closed’ (i.e. females do not disperse in/out of groups; they must be born into them). Nevertheless, rhesus macaque groups are characterized by a mixed relatedness structure, containing both related and unrelated females (Brent, Maclarnon, et al., 2013; Missakian, 1972). Affiliative relationships are often the strongest and most stable between kin, but social bonds between unrelated females are also common (Beisner, Jackson, Cameron, & McCowan, 2011; Cheney, 1992). In addition to high rates of affiliative interactions, social life in female rhesus macaques is characterized by high rates of aggression that is unidirectional (i.e. aggression is typically directed from high- to low-ranking animals) and that occurs within strict, linear and relatively stable dominance hierarchies (Datta, 1988). Females inherit the rank immediately beneath their mother and thus closely related females tend to be of similar dominance rank (Brent, Heilbronner, et al., 2013; Missakian, 1972). Permanent evictions of females have been documented in this species but are rare (Chepko-Sade & Sade, 1979; Ehardt & Bernstein, 1986; Widdig et al., 2006). Because of the relatively stable social structure that characterizes female rhesus macaque life, it is reasonable to assume that social markers of instability would be detectable prior to a mass eviction, but this has not yet been described.
The eviction that is the focus of this study occurred in a group of 55 adult females from three separate ancestral lines and resulted in the removal of the 13 highest-ranking females. We examined the aggression and grooming networks of all adult females during two periods preceding the eviction, the year immediately before the eviction (2011) and the year before that (2010). We determined whether network dynamics occurred in advance of the eviction by examining three aspects of social networks: (1) the rate at which individuals engaged in social interactions; (2) individuals’ choice of social partners and the nature of their interactions with those partners; and (3) the clustering of local subgroups.
METHODS
Study Population and Eviction Event
Our subjects were rhesus macaques living in the semifree-ranging colony on Cayo Santiago Island, Puerto Rico (18°09′N, 65°44′W; Rawlings & Kessler, 1986). Monkeys are provisioned daily at this site with commercial feed and with water supplied ad libitum. There are no predators present. Population control takes the form of annual removal of mostly juveniles. Beyond these measures, the monkeys are free to roam and to self-organize into groups and there is no medical intervention or contraceptive use.
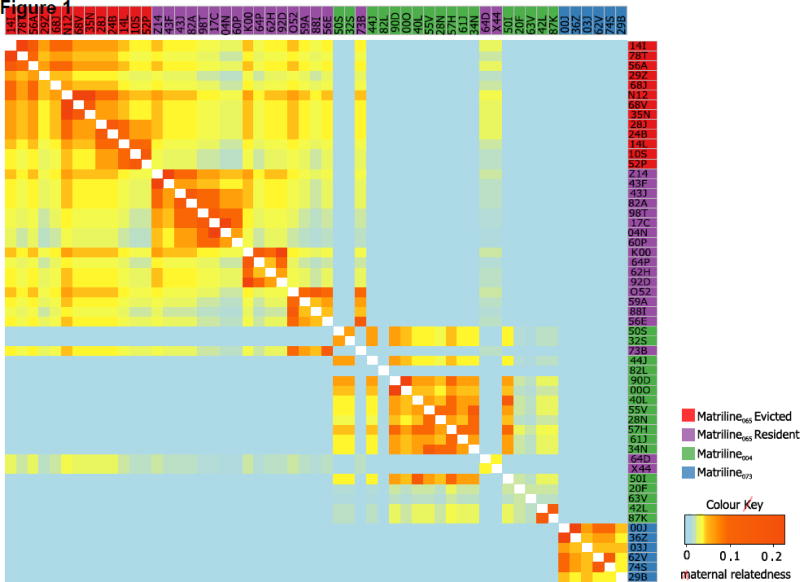
We studied animals in a single social group (‘F’), which at the time of study was the largest of the six groups on the island (N = 55 adult females). Group F was made up of three separate female ancestral lines, or matrilines, where all females in a matriline are descendants of a single unique female, and where maternal relatedness between members of different matrilines is typically zero (Fig. 1). The three matrilines were named after their founding females, 065, 004 and 073, which were first documented ranging together in group F over 50 years ago (Caribbean Primate Research Center, n.d.) and varied in size (065: N = 32; 004: N = 17; 073: N = 6). Owing to the linear nature of dominance hierarchies and the maternal inheritance of dominance rank, rhesus macaque matrilines can also generally be categorized according to rank: matriline 065 contained the highest-ranking females, females from matriline 004 were the next highest in rank (apart from three members of matriline 065, which were lower in rank than some members of matriline 004; two of these females did not have many close relatives in the group and may therefore have lacked the social support needed to maintain high rank), and matriline 073 contained the lowest-ranking females (Fig. 1).
Figure 1.
Maternal relatedness structure for the adult females in group F. Female names are listed along the top and right-hand edge and are coloured by matriline membership. Matriline 065 is partitioned into females that were evicted and those that remained in the parent group (‘Resident’) Females are ordered by descending dominance rank. Cells represent the maternal relatedness coefficient for each pair of individuals.
At the beginning of 2012, we observed a sudden outbreak of aggression that resulted in the death of the alpha female and the permanent eviction of 12 of group F’s highest-ranking females (22% of all adult females; Fig. 1). Although we could not collect systematic behavioural data during the aggressive outbreak, we opportunistically recorded cuts and wounds on the bodies of these members of matriline 065. The injuries sustained by the alpha female were especially severe and she died 2 weeks later, presumably from sepsis. The remaining 12 females began to range independently from the group along with their offspring and a few males. First, they ranged separately in two daughter groups then, approximately 8 months later, as one consolidated group.
Data Collection
As part of an unrelated study we collected behavioural data on the adult females in group F for 2 years prior to the eviction during two temporally similar periods: May–December 2010 and April–December 2011. These two periods were divided by a halt in behavioural data collection that takes place annually in the colony. All subjects were individually recognized and habituated to observer presence. We collected a total of 843.70 h of continuous data using 10 min focal animal samples with means (SD) per individual of 4.07 (0.39) and 5.02 (0.11) h in 2010 and 2011, respectively. We balanced observations of individuals across time to control for daily as well as monthly variation. We recorded all instances of aggression, submissive gestures and grooming. We used agonistic win/loss interactions to construct dominance hierarchies for the females independently in each year, although female ranks were stable across years. We limited our analyses to females that were present for the entirety of the 2 years, which excluded two females that died and nine initially juvenile females.
Social Network Analysis
We used social network analysis to explore social dynamics. Social network analysis comprises a suite of statistics that describe various levels of a network: individualized scores that describe properties of a node (e.g. a node’s centrality); metrics that describe dyadic interactions (e.g. the probability of an edge between two individuals); and metrics that describe global network properties (e.g. size, shape, connectedness). It is thus suitable for addressing the variation between individuals within a network and between networks at a subgroup, group, population or species level (Brent, 2015; Krause, James, Franks, & Croft, 2014; Wasserman & Faust, 1994).
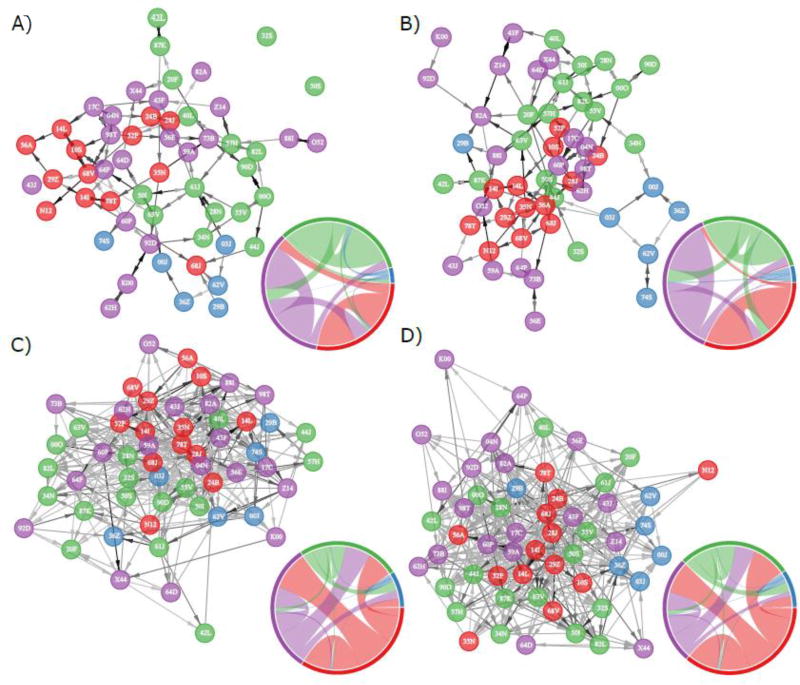
To determine whether changes to networks occurred as the eviction approached, we compared the females’ grooming and aggression networks from 2010 to those in 2011. We created one grooming and one aggression network for each year, resulting in four networks in total (Fig. 2). Edges in these networks represented all observed grooming or aggressive interactions recorded within a given dyad. We treated networks as directed (i.e. the donor and recipients of an interaction are defined) and weighted (i.e. the rate at which a dyad interacted is represented rather than the simple presence/absence of an interaction). For grooming networks, edges were weighted by the seconds of grooming per hour that took place within each dyad; for aggression networks, edges were weighted by the frequency of aggressive interactions per hour per dyad. Within years, our grooming and aggression networks were not significantly related to one another (2010: r = −0.025, P = 0.052; 2011: r = 0.026, P = 0.086) and thus we treat them separately in analyses.
Figure 2.
Grooming and aggression networks. The (a, b) grooming and (c, d) aggression networks for (a, c) 2010 and (b, d) 2011. Node colour represents partition membership where Evicted females are red, Resident females purple, matriline 004 females green and matriline 073 females blue. Colour intensity of the edge arrows indicates the relative weight of the interaction, with darker edges indicating greater intensity. Each network is force-directed using the Fruchterman–Reingold algorithm. Inset chord diagrams: width of chords represents the summation of interactions initiated by a given partition and directed at other partitions. Chords take the colour of the initiating partition.
Changes in rates of social interaction
We first determined whether the general tendency for all females to engage in social interactions changed as the eviction event approached by comparing grooming network and aggression network densities across years. We performed this analysis using the paired nodes density function in UCINET v6.588 (Borgatti, Everett, & Freeman, 2002). Assessing changes to network density is an important first step before analysing differences in network structure because apparent structural changes can be brought about by changes to density alone (Brent, Maclarnon, et al., 2013) and so the impact of density on structural changes must be taken into account.
Changes in social partner identity and the nature of social relationships
We next explored whether the identity of social partners and/or the nature of social relationships changed in the year nearer to the eviction. Owing to the maternal relatedness structure that underpins aggressive and affiliative interactions in this species (Brent, Heilbronner, et al., 2013; Missakian, 1972), we divided females according to their three ancestral matrilines in order to explore changes in social partnerships that occurred within and between related partitions of females. We further divided matriline 065 into two partitions, ‘Evicted’ and ‘Resident’, to reflect the fact that the eviction was localized within this matriline and to allow us to examine any social changes that occurred between these females.
We evaluated the extent to which social interactions were directed within and between partitions in each study period using a joint-count analysis. This procedure starts by calculating the ratio of the observed edge weights that occurred within or between a partition(s) and the expected edge weights, which are generated from networks of similar size and density, and for which the edge weights are the median of the observed values. The ratio of observed to expected edge weights therefore describes the extent to which observed edge weights differ from those that would be observed if individuals interacted at random (that is, a model in which our chosen partitions were not meaningful). We then simulated 5000 random graphs in which the edges were reshuffled randomly between nodes (Erdős–Rényi networks). For each permuted network, we calculated the observed to expected edge weight ratio. We evaluated the statistical significance of our observed edge weights by determining the proportion of permuted values that met or exceeded the observed value, a technique that is akin to traditional P values (Croft, Madden, Franks, & James, 2011). We also compared the ratio of observed to expected edge weights across study periods to assess how partner choice changed as the eviction approached.
We predicted that the nature of aggressive interactions would change the year nearer to the eviction in a manner that would indicate instability in the dominance hierarchy. We therefore determined whether females from lower-ranking partitions were more likely to direct aggression at higher-ranking partitions in the year closer to the eviction. We additionally explored changes to aggression within partitions, as instability could also be localized to more closely related females. For affiliative interactions, we predicted that grooming would be more focused onto related partners (i.e. within partitions) in the year nearer the eviction, as an additional indicator of social instability (Beisner et al., 2011) and in accordance with previous findings in Old World monkeys that suggest that kin-based relationships are more valuable during times of hardship (e.g. Engh et al., 2006).
Changes to clustering of local networks
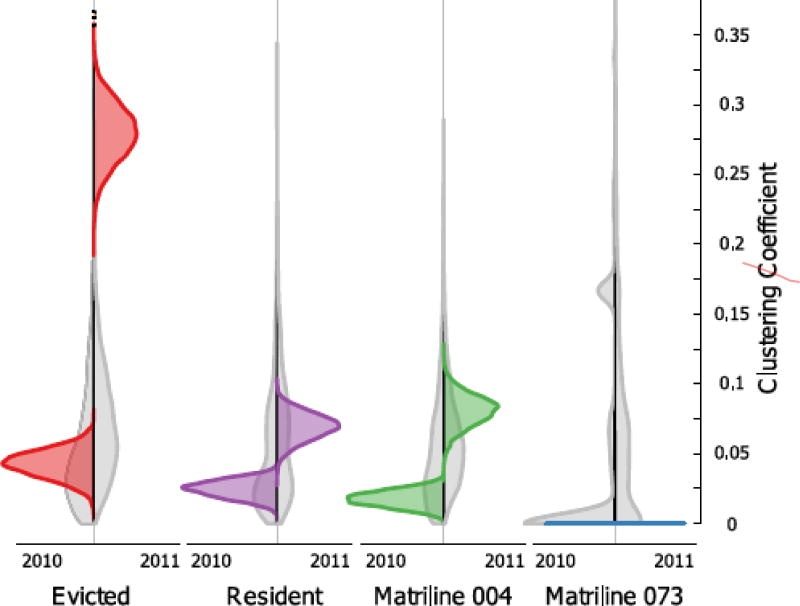
Finally, we determined whether there were changes to the nature of local grooming networks across years. To do this, we compared the mean clustering coefficient for each partition in each study period. The clustering coefficient measures the degree to which an individual’s social partners are connected to each other (Newman, 2003). The mean of this measure is therefore an indicator of the degree to which a partition is structured into tightly knit cliques or clusters. We explored clustering coefficients of the grooming networks only, owing to the linear, nontriadic, nature of aggressive interactions in this species (Datta, 1988). We calculated a weighted version of the clustering coefficient using the tnet package in R (Opsahl, 2009), which first necessitated converting our directed networks to undirected networks. We evaluated the statistical significance of observed clustering coefficients in two ways. First, we compared the clustering coefficient of a given partition within each study period to the clustering coefficient derived from a model of random association. To create random models, we generated 5000 Erdős–Rényi graphs of similar size and density to the observed networks and calculated the mean weighted clustering coefficient in each partition for each permutation. We determined the proportion of these permuted values that met or exceeded observed values as a measure of statistical significance. To compare clustering coefficients across partitions, we performed a two-sample bootstrapping test. Here, we took the difference in mean clustering coefficients of the two partitions being compared (either the same partition across years or different partitions within a year). Then, we pooled the clustering coefficients for each female in each partition. We resampled from this pool with replacement sets of equal size 5000 times, and calculated the difference in the clustering coefficients that were generated to create a null distribution. We calculated P values as the proportion of differences in clustering coefficients between bootstrapped partitions that were more extreme than observed differences. To visualize differences in clustering across years, we generated 5000 random graphs in which the edge weights from a given partition were permuted but the positions of the edges were held constant and we created violin plots of the resulting values.
Ethical Note
This research complied with protocols approved by the Institutional Animal Care and Use Committee of the University of Puerto Rico (protocol no. A6850108) and by the University of Exeter School of Psychology’s Ethics Committee.
RESULTS
Rates of Social Interactions Were Static across Years
We found no evidence for changes between 2010 and 2011 in the overall rate of aggression (2010: 0.02; 2011: 0.02; tstat=0.49, P=0.31) or grooming (2010: 1.20; 2011: 1.17; tstat=0.13, P = 0.43), as indicated by network densities. Any other structural differences in the observed networks (e.g. differences in clustering) cannot therefore be due to differences in network density.
Aggression Directed Up the Hierarchy Was More Likely in the Year Nearer the Eviction
Aggressive interactions generally reflected the dominance hierarchy, with most aggression performed by higher-ranking females and directed at lower-ranking females in both years (Table 1). However, there were changes from 2010 to 2011 in the extent to which aggression was directed up the hierarchy, which may indicate instability in the dominance hierarchy that was largely localized to matriline 065. In particular, females from low-ranking matrilines 004 and 073 were more likely to be aggressive towards the Evicted females in 2011 than in 2010. Females from matriline 004 were also more likely to be aggressive towards the Resident females in 2011 than in 2010 (Table 1). Although these increases represent only a small absolute number of aggressive interactions, reflecting the smaller number of females that belonged to the lower-ranking matrilines (Fig. 2), they are notable due to the typically unidirectional nature of aggression in rhesus macaques. The probability of aggressive interactions also increased among Evicted females from 2010 to 2011. However, there were decreases in the probability of aggression being directed from the Evicted females to the Resident females, and from the Resident females to the Evicted females.
Table 1.
Observed and expected rates of grooming and aggression within and between females
| Donor | Recipient | 2010 | 2011 | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| E | O | O/E (P) | E | O | O/E (P) | ||
| Grooming networks | |||||||
| Evicted | Themselves | 142 | 712 | 5.02 (0.001) | 140 | 904 | 6.45 (<0.001) |
| Resident | 207 | 201 | 0.96 (0.25) | 205 | 69 | 0.34 (0.01) | |
| Matriline 004 | 185 | 3 | 0.02 (<0.001) | 183 | 19 | 0.10 (0.001) | |
| Matriline 073 | 65 | 0 | 0.00 (0.05) | 65 | 0 | 0.00 (0.03) | |
| Resident | Evicted | 207 | 290 | 1.40 (0.48) | 205 | 176 | 0.86 (0.17) |
| Themselves | 303 | 825 | 2.73 (0.01) | 299 | 983 | 3.29 (0.001) | |
| Matriline 004 | 271 | 147 | 0.54 (0.03) | 268 | 168 | 0.63 (0.04) | |
| Matriline 073 | 96 | 0 | 0.00 (0.02) | 95 | 25 | 0.27 (0.05) | |
| Matriline 004 | Evicted | 185 | 48 | 0.26 (0.01) | 183 | 177 | 0.97 (0.24) |
| Resident | 271 | 244 | 0.90 (0.17) | 268 | 245 | 0.91 (0.15) | |
| Themselves | 242 | 890 | 3.67 (<0.001) | 240 | 666 | 2.78 (0.01) | |
| Matriline 073 | 86 | 72 | 0.84 (0.32) | 85 | 14 | 0.17 (0.04) | |
| Matriline 073 | Evicted | 65 | 32 | 0.49 (0.22) | 65 | 5 | 0.08 (0.04) |
| Resident | 96 | 0 | 0.00 (0.01) | 95 | 21 | 0.22 (0.04) | |
| Matriline 073004 | 86 | 68 | 0.79 (0.29) | 85 | 7 | 0.08 (0.02) | |
| Themselves | 30 | 45 | 1.49 (0.30) | 30 | 104 | 3.49 (0.08) | |
| Aggression networks | |||||||
| Evicted | Themselves | 2.43 | 8.00 | 3.30 (<0.001) | 2.18 | 8.37 | 3.84 (<0.001) |
| Resident | 3.56 | 11.73 | 3.29 (<0.001) | 3.18 | 8.98 | 2.82 (<0.001) | |
| Matriline 004 | 3.18 | 7.94 | 2.50 (<0.001) | 2.85 | 9.56 | 3.35 (<0.001) | |
| Matriline 073 | 1.12 | 2.50 | 2.23 (0.04) | 1.01 | 2.93 | 2.92 (0.004) | |
| Resident | Evicted | 3.56 | 0.59 | 0.17 (<0.001) | 3.18 | 0.40 | 0.13 (<0.001) |
| Themselves | 5.20 | 6.21 | 1.19 (0.38) | 4.65 | 5.26 | 1.13 (0.19) | |
| Matriline 004 | 4.65 | 7.77 | 1.67 (0.08) | 4.16 | 5.76 | 1.38 (0.48) | |
| Matriline 073 | 1.64 | 2.56 | 1.56 (0.27) | 1.47 | 1.48 | 1.01 (0.15) | |
| Matriline 004 | Evicted | 3.18 | 0.25 | 0.08 (<0.001) | 2.85 | 0.49 | 0.17 (<0.001) |
| Resident | 4.65 | 1.13 | 0.24 (<0.001) | 4.16 | 1.20 | 0.29 (<0.001) | |
| Themselves | 4.16 | 4.35 | 1.05 (0.19) | 3.72 | 5.16 | 1.38 (0.37) | |
| Matriline 073 | 1.47 | 2.80 | 1.92 (0.09) | 1.32 | 2.75 | 2.09 (0.08) | |
| Matriline 073 | Evicted | 1.12 | 0.00 | 0.00 (<0.001) | 1.01 | 0.10 | 0.10 (<0.001) |
| Resident | 1.64 | 0.12 | 0.07 (<0.001) | 1.47 | 0.10 | 0.07 (<0.001) | |
| Matriline 004 | 1.47 | 0.12 | 0.08 (<0.001) | 1.32 | 0.10 | 0.07 (<0.001) | |
| themselves | 0.51 | 0.95 | 1.82 (0.14) | 0.46 | 1.17 | 2.52 (0.04) | |
The observed (O) and expected rates (E) of interaction, and the ratio of observed to expected for each network within and between the four partitions (Evicted, Resident, matrilines 004 and 073). Interactions are initiated by ‘donors’ and are received by ‘recipients’. Pval is calculated as the proportion of simulated networks in which the O/E value exceeded or met the observed O/E value. Values in bold differed significantly from chance.
Females Changed Grooming Partners as the Eviction Approached
We found that, as expected, females were more likely to engage in grooming with members of their own partition. The Evicted, Resident and matriline 004 females were more likely to groom members of their own partition than members of other partitions in both 2010 and 2011 (Table 1). This pattern was not significant for females from the small 073 matriline. Females also tended to groom females outside their own partition at rates either expected by chance or significantly lower than chance in both years. Yet there were notable differences in the identities of grooming partners both within and between partitions across years (Fig. 2). For example, the tendency for females to groom members of their own partition increased from 2010 to 2011 for Evicted, Resident and matriline 004 females, with the Evicted females showing the largest increase in within-partition grooming (2010: 5.02 observed/expected, P < 0.01; 2011: 6.45, P < 0.01). In addition, the amount of grooming that occurred between Evicted and Resident females did not differ from chance levels in 2010 but was less than expected in 2011 (2010: 0.96, P = 0.25; 2011: 0.34, P = 0.01). In other words, in the year nearer to the eviction, Evicted females were more likely to groom one another and less likely to groom the Resident members of their matriline.
Evicted Females Formed Tighter Grooming Clusters in the Year Before Their Eviction
The mean clustering coefficient of the grooming network of Evicted females was significantly greater than expected in 2011 but not in 2010 (Table 2). The mean clustering coefficient of no other partition differed from expected values in either year. In other words, the grooming relationships of Evicted females were more cliquish than expected based on random association in the year directly before their eviction, whereas no such differences were observed in the other partitions, including the Resident members of this matriline. The grooming relationships of the Evicted females were also significantly more clustered in 2011 than in 2010, and were significantly more clustered in 2011 than any other partition examined (Fig. 3). Although there were small increases in clustering from 2010 to 2011 for the Resident and matriline 004 females, this was only significant for the latter (Table 2). The clustering coefficient for matriline 073 was zero because there were no closed triads within the network and thus no amount of edge weight reshuffling could produce a result other than zero. We found relative similarities between our random graphs across years (Fig. 3). Because changes in network densities were the central drivers of differences between the random graphs, it is unlikely that differences across time in our observed clustering coefficients were driven by differences in density alone.
Table 2.
Clustering of grooming relationships: observed compared to randomized networks, comparisons between partitions of females and comparisons within partitions of females across years
| Year | Observed clustering coefficient |
Randomized networks |
Evicted | Resident | Matriline 004 | Matriline 073 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||
| 2010 | 2011 | 2010 | 2011 | 2010 | 2011 | 2010 | 2011 | ||||
| Evicted | 2010 | 0.04 | 0.01 (0.49) | 0.23 (<0.01) | −0.01 (0.31) | −0.02 (0.23) | 0.03 (0.08) | 0.05 (0.05) | 0.05 (0.09) | 0.05 (0.09) | |
| 2011 | 0.28 | 0.21 (<0.01) | 0.24 (<0.01) | 0.21 (<0.01) | 0.26 (<0.01) | 0.18 (<0.01) | 0.28 (<0.01) | 0.28 (<0.01) | |||
| Resident | 2010 | 0.02 | 0.02 (0.35) | 0.03 (0.13) | 0.02 (0.15) | 0.06 (0.03) | 0.04 (0.17) | 0.04 (0.17) | |||
| 2011 | 0.07 | 0.001 (0.43) | 0.04 (0.01) | 0.03 (0.15) | 0.06 (0.02) | 0.06 (0.02) | |||||
| Matriline 004 | 2010 | 0.02 | 0.03 (0.22) | 0.08 (<0.01) | 0.02 (0.14) | 0.02 (0.14) | |||||
| 2011 | 0.08 | 0.02 (0.24) | 0.09 (0.01) | 0.09 (0.01) | |||||||
| Matriline 073 | 2010 | 0.00 | 0.04 (0.69) | 0.00 (1.00) | |||||||
| 2011 | 0.00 | 0.06 (0.29) | |||||||||
P values for the difference in observed and random networks are calculated as the proportion of random networks that produced values as extreme the observed value. P values for the difference of observed values across partitions and study periods are based on bootstrap two-sample permutation tests. Values in bold differ significantly from chance.
Figure 3.
Mean clustering coefficients by partition. Violin plots showing estimates of the mean clustering coefficient for each partition in each study period. Grey plots show estimates for the given partition and year based on Erdős–Rényi random graphs. Coloured densities represent mean clustering coefficients from 5000 permuted graphs in which we shuffled weights across edges while holding the positions of edges constant.
DISCUSSION
The study of dynamic social networks is an area of rapidly growing research interest (Bode, Wood, & Franks, 2011a, b; Ilany, Booms, & Holekamp, 2015; Pinter-Wollman et al., 2014). Although social networks appear to be able to respond flexibly to changes in the social and physical environment, whether changes to social networks also precede major events is less clear. Here we report network dynamics in advance of the mass eviction of members of the philopatric sex. Prior to the eviction, researchers studying the group reported no conspicuous signs of social instability. Therefore, the changes to the networks of these animals occurred in advance of a major event but were subtle and revealed only through subsequent analysis. Permanent evictions can have serious consequences for individuals; intragroup aggression prior to evictions can result in fatal injuries (Ehardt & Bernstein, 1986; Gygax, Harley, & Kummer, 1997; Samuels & Henrickson, 1983) and decreased reproduction (Dettmer, Woodward, & Suomi, 2015), while smaller posteviction daughter groups can suffer higher risks of predation and reduced foraging efficiency (Krause & Ruxton, 2002). There is some evidence that reproductive competition is the trigger for evictions in cooperative-breeding species (Thompson et al., 2016), but it is unclear whether similar factors would be at play in a primate such as the rhesus macaque, which is highly polygynous and has only moderate levels of reproductive skew (Dubuc, Ruiz-Lambides, & Widdig, 2014). Although we do not know whether there are causal links between changes to the social networks in this study and the eviction, a consistent patterning of network dynamics prior to evictions would nevertheless allow evictions to be predicted in the future, which could have implications for the management of captive groups (Beisner et al., 2011) and the design of naturalistic experimental studies.
A number of theories have been put forward regarding the maintenance of group cohesion and the balance of competition and cooperation between unrelated groupmates. For instance, group cohesion may be limited by the amount of time individuals have available to spend engaged in social interactions. This ‘time constraints’ model predicts that groups break apart once individuals can no longer maintain or keep track of relationships with all other groups members (Dunbar, 1991, 1992). Prior to the mass eviction in this study, we did not detect any changes in the amount of time individuals dedicated to grooming or aggressive interactions. Although these animals are provisioned and may not easily suffer from restrictions in their daily time budgets, our results nevertheless suggest that the breakdown in group cohesion did not follow from reductions in social effort.
Group cohesion may depend not only on the amount of time individuals engage in social interactions but also on with whom they interact. For example, pay-to-stay mechanisms, whereby individuals ‘pay’ their groupmates with affiliative interactions, have been proposed as a means to maintain groups of cooperative breeders with highly skewed reproductive success (Bergmüller & Taborsky, 2005; Gaston, 1978; Johnstone & Cant, 1999), as well as groups of unrelated animals faced with intense between-group competition (Radford, 2008; van Schaik, 1989; Wrangham, 1980). In the latter instance, dominant animals would use social interactions, for example grooming, to establish alliances with their lower-ranking groupmates to ensure they will help in contests with other groups (Cheney, 1992; van Schaik, 1989). A meta-analysis of data from cercopithecine primates suggests the link between grooming relationships, intragroup contest and the maintenance of group cohesion is weak or nonexistent (Cheney, 1992; although see Majolo, de Bortoli Vizioli, & Lehmann, 2016). In the present study, an increase in cliquishness in the local grooming networks of evicted females suggests that grooming relationships among kin and nonkin of divergent social status may indeed play a role in the cohesion of rhesus macaque groups. However, cause and consequence cannot be disentangled here and, just as the reduced diversity of grooming relationships may have caused the eviction, the pending eviction may have resulted in the reduction of diversity in grooming relationships.
Changes to affiliative relationships leading up to a mass eviction also reveal more direct information about the patterns and processes that underpin social relationships in these animals. Biologists’ understanding of the evolution of social bonds in animals has grown rapidly in recent years (Archie, Tung, Clark, Altmann, & Alberts, 2014; Brent, Heilbronner, et al., 2013; Chang et al., 2013; Seyfarth & Cheney, 2012; Silk et al., 2009). Affiliative tendencies have been shown to be heritable (Brent, Heilbronner, et al., 2013; Brent, Semple, et al., 2014; Lea, Blumstein, Wey, & Martin, 2010), and a positive association between affiliative relationships and proxies of fitness have been found in a small range of species, including baboons (Archie et al., 2014; Silk et al., 2009; Cheney et al., 2016) and rhesus macaques (Brent, Heilbronner, et al., 2013; Brent et al., 2017). Yet despite these advances, the adaptive functions of social bonds remain unclear (Brent, Chang, et al., 2014). A growing number of studies have shown that affiliative social relationships between members of the philopatric sex are more flexible in nonhuman primates than traditionally believed (e.g. Barrett, Gaynor, & Henzi, 2002; Barrett & Henzi, 2002; Engh et al., 2006; Wittig et al., 2008). In accordance with this work, we found evidence for dynamic shifts in affiliative relationships in this study. Together, these findings may reflect the use of social relationships to cope with the vicissitudes of life such as death, disease and shifts in social status, as well as other short-term social, environmental and demographic events.
Our results may also hint that some social bonds are more valuable than others. Previous work has shown that instability in primate groups can be followed by shifts in social partners. Following the death of the alpha male in wild chimpanzees, individuals became more socially discriminating of grooming partners that failed to reciprocate (Kaburu & Newton-Fisher, 2013). In cercopithecines, social relationships are most common among related females (Cheney, 1992). Relatedness may be a useful cue for reliable cooperative partners because of the ability to gain inclusive fitness benefits via these relationships. Female baboons focused their grooming networks onto close kin following instability in the male hierarchy (Wittig et al., 2008). In the current study, grooming relationships largely collapsed along kin lines prior to the mass eviction, with the females that would be evicted focusing their relationships onto their closest kin; in times of social instability, affiliative relationships with nonrelatives may become too risky for rhesus macaque females.
The adaptive role of social relationships in variable contexts begs an understanding of how individuals of variable phenotypes integrate to form certain group dynamics. Here, we focused on rates of interactions and the formation of clusters as indicators of changes in network structure and partner choice. Other network metrics with alternative properties might elucidate social dynamics differently (Brent, 2015). For example, eigenvector centrality, which uses direct and indirect connections to distinguish socially integrated from marginal individuals, was found to positively correlate with proxies of fitness in wild baboons (Cheney et al., 2016) and in the Cayo Santiago rhesus macaques (Brent et al., 2013). As our current analyses indicated the emergence of distinct subgroupings over time without any changes in the overall rates of interactions, we felt eigenvector centrality would be of limited analytical power (although will nevertheless continue to be important to consider in future studies focused on revealing information about differences in social connectedness between individuals) and we instead performed a joint-count analysis to explore not just how involved the different subgroups were in social life, but with whom.
The stability of a group is not attributable to the phenotype of any one individual, but it is nevertheless likely to impact upon individual fitness. Research in group-living species suggests that the interplay between group stability and individual fitness is complex (Muir, 2005; Saltz, 2013; WolfWolf, Brodie, & Cheverud, 1998). A more thorough understanding of how the metagenome (i.e. the influence of one individual’s genotype and phenotype on another’s) influences network dynamics will also be useful for behavioural ecologists approaching these questions.
Network dynamics have the power to reveal the function of social behaviours.
We explored network dynamics leading up to the fissioning of a social group.
Females that would be evicted became less likely to interact with others.
Network changes were subtle and revealed only through analysis.
Our results align with the idea that social bonds contribute to group cohesion.
Acknowledgments
We thank the Caribbean Primate Research Center (CPRC) for logistical and technical support as well as Bonn Aure, Jacqueline Buhl, Monica Carlson and Elizabeth Maldonado for research support. We were supported by National Institute of Mental Health grants R01-MH089484 and R01-MH096875, and an Incubator Award from the Duke Institute for Brain Sciences. L.J.N.B. was supported by a Duke Center for Interdisciplinary Decision Sciences Fellowship and by an Early Career Fellowship from the Leverhulme Trust. The CPRC is supported by grant 8-P40 OD012217-25 from the National Center for Research Resources (NCRR) and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archie EA, Tung J, Clark M, Altmann J, Alberts SC. Social affiliation matters: both same-sex and opposite-sex relationships predict survival in wild female baboons. Proceedings of the Royal Society B: Biological Sciences. 2014;281:20141261. doi: 10.1098/rspb.2014.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L, Gaynor D, Henzi SP. A dynamic interaction between aggression and grooming reciprocity among female chacma baboons. Animal Behaviour. 2002;63:1047–1053. [Google Scholar]
- Barrett L, Henzi SP. Constraints on relationship formation among female primates. Behaviour. 2002;139:263–289. [Google Scholar]
- Barrett L, Henzi SP, Weingrill T, Lycett JE, Hill RA. Market forces predict grooming reciprocity in female baboons. Proceedings of the Royal Society B: Biological Sciences. 1999;266:665–670. [Google Scholar]
- Beisner BA, Jackson ME, Cameron AN, McCowan B. Detecting instability in animal social Networks: Genetic fragmentation is associated with social instability in rhesus macaques. PLoS One. 2011;6:e16365. doi: 10.1371/journal.pone.0016365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Wolf TY, Saia J. A framework for analysis of dynamic social networks; Paper presented at the Proceedings of the 12th ACM SIGKDD international conference on Knowledge discovery and data mining; Philadelphia, PA, USA. 2006. [Google Scholar]
- Bergmüller R, Taborsky M. Experimental manipulation of helping in a cooperative breeder: Helpers ‘pay to stay’ by pre-emptive appeasement. Animal Behaviour. 2005;69:19–28. [Google Scholar]
- Bode NWF, Wood AJ, Franks DW. Social networks and models for collective motion in animals. Behavioral Ecology and Sociobiology. 2011a;65:117–130. [Google Scholar]
- Bode NWF, Wood AJ, Franks DW. The impact of social networks on animal collective motion. Animal Behaviour. 2011b;82:29–38. [Google Scholar]
- Borgatti SP, Everett MG, Freeman LC. Ucinet for Windows: Software for network analysis. Harvard, MA: Analytic Technologies; 2002. [Google Scholar]
- Brent LJN. Friends of friends: Are indirect connections in social networks important to animal behaviour? Animal Behaviour. 2015;103:211–222. doi: 10.1016/j.anbehav.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent LJN, Chang SWC, Gariépy J-F, Platt ML. The neuroethology of friendship. Annals of the New York Academy of Sciences. 2014;1316:1–17. doi: 10.1111/nyas.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent LJN, Heilbronner SR, Horvath JE, Gonzalez-Martinez J, Ruiz-Lambides A, Robinson AG, et al. Genetic origins of social networks in rhesus macaques. Scientific Reports. 2013;3:1042. doi: 10.1038/srep01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent LJN, Maclarnon A, Platt ML, Semple S. Seasonal changes in the structure of rhesus macaque social networks. Behavioral Ecology and Sociobiology. 2013;67(3):349–359. doi: 10.1007/s00265-012-1455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent LJN, Ruiz-Lambides A, Platt ML. Family network size and survival across the lifespan of female macaques. Proceedings of the Royal Society B: Biological Sciences. 2017;284:20170515. doi: 10.1098/rspb.2017.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent LJN, Semple S, Maclarnon A, Ruiz-Lambides A, Gonzalez-Martinez J, Platt ML. Personality traits in rhesus macaques (Macaca mulatta) are heritable but do not predict reproductive output. International Journal of Primatology. 2014;35(1):188–209. doi: 10.1007/s10764-013-9724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caribbean Primate Research Center. Demographic data for the Cayo Santiago rhesus macaques. Unpublished raw data; (n.d.) [Google Scholar]
- Cheney DL. Intragroup cohesion and intergroup hostility: The relation between grooming distributions and intergroup competition among female primates. Behavioral Ecology. 1992;3:334–345. [Google Scholar]
- Cheney DL, Silk JB, Seyfarth RM. Network connections, dyadic bonds, and fitness in wild female baboons. Royal Society Open Science. 2016;3(7):160255. doi: 10.1098/rsos.160255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepko-Sade BD, Sade DS. Patterns of group splitting within matrilineal kinship groups. Behavioral Ecology and Sociobiology. 1979;5:67–86. [Google Scholar]
- Croft DP, Madden JR, Franks DW, James R. Hypothesis testing in animal social networks. Trends in Ecology & Evolution. 2011;26:502–507. doi: 10.1016/j.tree.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Darden SK, James R, Ramnarine IW, Croft DP. Social implications of the battle of the sexes: Sexual harassment disrupts female sociality and social recognition. Proceedings of the Royal Society B: Biological Sciences. 2009;276:2651–2656. doi: 10.1098/rspb.2009.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. The acquisition of dominance among free-ranging rhesus monkey siblings. Animal Behaviour. 1988;36:754–772. [Google Scholar]
- Dettmer AM, Woodward RA, Suomi SJ. Reproductive consequences of a matrilineal overthrow in rhesus monkeys. American Journal of Primatology. 2015;77:346–352. doi: 10.1002/ajp.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc C, Ruiz-Lambides A, Widdig A. Variance in male lifetime reproductive success and estimation of the degree of polygyny in a primate. Behavioral Ecology. 2014;25(4):878–889. doi: 10.1093/beheco/aru052. https://doi.org/10.1093/beheco/aru052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar R. Functional significance of social grooming in primates. Folia Primatologica. 1991;57:121–131. [Google Scholar]
- Dunbar R. Time: A hidden constraint on the behavioural ecology of baboons. Behavioral Ecology and Sociobiology. 1992;31:35–49. [Google Scholar]
- Edenbrow M, Darden SK, Ramnarine IW, Evans JP, James R, Croft DP. Environmental effects on social interaction networks and male reproductive behaviour in guppies, Poecilia reticulata. Animal Behaviour. 2011;81:551–558. [Google Scholar]
- Ehardt CL, Bernstein IS. Matrilineal overthrows in rhesus monkey groups. International Journal of Primatology. 1986;7:157–181. [Google Scholar]
- Engh AL, Beehner JC, Bergman TJ, Whitten PL, Hoffmeier RR, Seyfarth RM, et al. Behavioural and hormonal responses to predation in female chacma baboons (Papio hamadryas ursinus) Proceedings of the Royal Society B: Biological Sciences. 2006;273:707–712. doi: 10.1098/rspb.2005.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flack JC, Girvan M, de Waal FBM, Krakauer DC. Policing stabilizes construction of social niches in primates. Nature. 2006;439:426–429. doi: 10.1038/nature04326. [DOI] [PubMed] [Google Scholar]
- Foster EA, Franks DW, Morrell LJ, Balcomb KC, Parsons KM, van Ginneken A, et al. Social network correlates of food availability in an endangered population of killer whales, Orcinus orca. Animal Behaviour. 2012;83:731–736. [Google Scholar]
- Gaston AJ. The evolution of group territorial behavior and cooperative breeding. American Naturalist. 1978;112:1091–1100. [Google Scholar]
- Godfrey SS, Sih A, Bull CM. The response of a sleepy lizard social network to altered ecological conditions. Animal Behaviour. 2013;86:763–772. [Google Scholar]
- Gygax L, Harley N, Kummer H. A matrilineal overthrow with destructive aggression in Macaca fascicularis. Primates. 1997;38:149–158. [Google Scholar]
- Hamede RK, Bashford J, McCallum H, Jones M. Contact networks in a wild Tasmanian devil (Sarcophilus harrisii) population: using social network analysis to reveal seasonal variability in social behaviour and its implications for transmission of devil facial tumour disease. Ecology Letters. 2009;12:1147–1157. doi: 10.1111/j.1461-0248.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- Ilany A, Booms AS, Holekamp KE. Topological effects of network structure on long-term social network dynamics in a wild mammal. Ecology letters. 2015;18:687–695. doi: 10.1111/ele.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone RA, Cant MA. Reproductive skew and the threat of eviction: A new perspective. Proceedings of the Royal Society B: Biological Sciences. 1999;266:275–279. [Google Scholar]
- Kaburu SSK, Newton-Fisher NE. Social instability raises the stakes during social grooming among wild male chimpanzees. Animal Behaviour. 2013;86(3):519–527. [Google Scholar]
- Kanngiesser P, Sueur C, Riedl K, Grossmann J, Call J. Grooming network cohesion and the role of individuals in a captive chimpanzee group. American Journal of Primatology. 2010;73(8):758–767. doi: 10.1002/ajp.20914. [DOI] [PubMed] [Google Scholar]
- Krause J, James R, Franks DW, Croft DP. Animal social networks. New York, NY: Oxford University Press; 2014. [Google Scholar]
- Krause J, Ruxton GD. Living in groups. New York, NY: Oxford University Press; 2002. [Google Scholar]
- Lea AJ, Blumstein DT, Wey TW, Martin JGA. Heritable victimization and the benefits of agonistic relationships. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21587–21592. doi: 10.1073/pnas.1009882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Andrews K, Dunbar RIM. Social networks and social complexity in female-bonded primates. In: Dunbar RIM, Gamble C, Gowlett JA, editors. Social brain, distributed mind. Oxford, U.K: Oxford University Press; 2010. pp. 57–82. [Google Scholar]
- Lehmann J, Boesch C. Sociality of the dispersing sex: The nature of social bonds in West African female chimpanzees, Pan troglodytes. Animal Behaviour. 2009;77:377–387. [Google Scholar]
- Majolo B, de Bortoli Vizioli A, Lehmann J. The effect of intergroup competition on intragroup affiliation in primates. Animal Behaviour. 2016;114:13–19. [Google Scholar]
- Manno TG. Social networking in the Columbian ground squirrel, Spermophilus columbianus. Animal Behaviour. 2008;75:1221–1228. [Google Scholar]
- Missakian EA. Genealogical and cross-genealogical dominance relations in a group of free-ranging rhesus monkeys (Macaca mulatta) on Cayo Santiago. Primates. 1972;13:169–180. [Google Scholar]
- Muir W. Incorporation of competitive effects in forest tree or animal breeding programs. Genetics. 2005;170:1247–1259. doi: 10.1534/genetics.104.035956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MEJ. The structure and function of complex networks. Siam Review. 2003;45:167–256. [Google Scholar]
- Nowak MA, Tarnita CE, Wilson EO. The evolution of eusociality. Nature. 2010;466(7310):1057–1062. doi: 10.1038/nature09205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opsahl T. Ph.D. thesis. London, U.K: Queen Mary University of London; 2009. Structure and evolution of weighted networks. [Google Scholar]
- Pinter-Wollman N, Hobson EA, Smith JE, Edelman AJ, Shizuka D, de Silva S, et al. The dynamics of animal social networks: Analytical, conceptual, and theoretical advances. Behavioral Ecology. 2014;25:242–255. [Google Scholar]
- Radford AN. Duration and outcome of intergroup conflict influences intragroup affiliative behaviour. Proceedings of the Royal Society B: Biological Sciences. 2008;275:2787–2791. doi: 10.1098/rspb.2008.0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings R, Kessler M. The Cayo Santiago macaques: History, behavior & biology. New York, NY: State University of New York Press; 1986. [Google Scholar]
- Saltz JB. Genetic composition of social groups influences male aggressive behaviour and fitness in natural genotypes of Drosophila melanogaster. Proceedings of the Royal Society B: Biological Sciences. 2013;280:20131926. doi: 10.1098/rspb.2013.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels A, Henrickson RV. Brief report: Outbreak of severe aggression in captive Macaca mulatta. American Journal of Primatology. 1983;5:277–281. doi: 10.1002/ajp.1350050314. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM, Cheney DL. The evolutionary origins of friendship. Annual Review of Psychology. 2012;63:153–177. doi: 10.1146/annurev-psych-120710-100337. [DOI] [PubMed] [Google Scholar]
- Shultz S, Opie C, Atkinson QD. Stepwise evolution of stable sociality in primates. Nature. 2011;479:219222. doi: 10.1038/nature10601. [DOI] [PubMed] [Google Scholar]
- Silk JB. The adaptive value of sociality in mammalian groups. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:539–559. doi: 10.1098/rstb.2006.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, et al. The benefits of social capital: Close social bonds among female baboons enhance offspring survival. Proceedings of the Royal Society B: Biological Sciences. 2009;276:3099–3104. doi: 10.1098/rspb.2009.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueur C, Maire A. Modelling animal group fission using social network dynamics. PLoS One. 2014;9:e97813. doi: 10.1371/journal.pone.0097813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry B. Unity in diversity: Lessons from macaque societies. Evolutionary Anthropology: Issues, News, and Reviews. 2007;16:224–238. [Google Scholar]
- Thompson FJ, Marshall HH, Sanderson JL, Vitikainen EIK, Nichols HJ, Gilchrist JS, et al. Reproductive competition triggers mass eviction in cooperative banded mongooses. Proceedings of the Royal Society B: Biological Sciences. 2016;283:20152607. doi: 10.1098/rspb.2015.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik CP. The ecology of social relationships amongst female primates. In: Standen V, Foley RA, editors. Comparative socioecology: The behavioural ecology of humans and other mammals. Oxford, U.K: Wiley Blackwell; 1989. pp. 195–218. [Google Scholar]
- Wasserman S, Faust K. Social network analysis: Methods and applications. Vol. 8. Cambridge, U.K: Cambridge University Press; 1994. [Google Scholar]
- Wittig RM, Crockford C, Lehmann J, Whitten PL, Seyfarth RM, Cheney DL. Focused grooming networks and stress alleviation in wild female baboons. Hormones and Behavior. 2008;54:170–177. doi: 10.1016/j.yhbeh.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig A, Nürnberg P, Bercovitch FB, Trefilov A, Berard JB, Kessler MJ, et al. Consequences of group fission for the patterns of relatedness among rhesus macaques. Molecular Ecology. 2006;15:3825–3832. doi: 10.1111/j.1365-294X.2006.03039.x. [DOI] [PubMed] [Google Scholar]
- Wolf JB, Brodie ED, III, Cheverud JM. Evolutionary consequences of indirect genetic effects. Trends in Ecology & Evolution. 1998;13:64–69. doi: 10.1016/s0169-5347(97)01233-0. [DOI] [PubMed] [Google Scholar]
- Wrangham RW. An ecological model of female-bonded primate groups. Behaviour. 1980;75:262–300. [Google Scholar]