Abstract
Adult neural stem cells (NSCs) play important roles in learning and memory and are negatively impacted by neurological disease. It is known that biochemical and genetic factors regulate self-renewal and differentiation, and it has recently been suggested that mechanical and solid-state cues, such as extracellular-matrix (ECM) stiffness, can also regulate the functions of NSCs and other stem cell types. However, relatively little is known of the molecular mechanisms through which stem cells transduce mechanical inputs into fate decisions, the extent to which mechanical inputs instruct fate decisions versus select for or against lineage-committed blast populations, or the in vivo relevance of mechanotransductive signaling molecules in native stem cell niches. Here we demonstrate that ECM-derived mechanical signals act through Rho GTPases to activate the cellular contractility machinery in a key early window during differentiation to regulate NSC lineage commitment. Furthermore, culturing NSCs on increasingly stiff ECMs enhances RhoA and Cdc42 activation, increases NSC stiffness, and suppresses neurogenesis. Likewise, inhibiting RhoA and Cdc42 or downstream regulators of cellular contractility rescues NSCs from stiff matrix- and Rho GTPase-induced neurosuppression. Importantly, Rho GTPase expression and ECM stiffness do not alter proliferation or apoptosis rates indicating that an instructive rather than selective mechanism modulates lineage distributions. Finally, in the adult brain, RhoA activation in hippocampal progenitors suppresses neurogenesis, analogous to its effect in vitro. These results establish Rho GTPase-based mechanotransduction and cellular stiffness as biophysical regulators of NSC fate in vitro and RhoA as an important regulatory protein in the hippocampal stem cell niche.
MeSH Keywords: Neural Stem Cells, Cellular Mechanotransduction, rho GTP-Binding Proteins, Hippocampus, Extracellular Matrix, Elastic Modulus
Introduction
Neural stem cells (NSCs) in the adult mammalian brain generate new neurons, astrocytes, and oligodendrocytes throughout life. One population of NSCs resides in the subgranular zone of the hippocampus [1, 2], and NSC-mediated adult hippocampal neurogenesis has been specifically implicated in learning and memory, mood regulation, and neurological disorders [3–7]. Thus, a deeper cellular and molecular mechanistic understanding of the regulation of NSC self-renewal and differentiation may lend new insights into the roles of NSCs in these important biological processes.
In pursuit of these mechanisms, the field has focused primarily on the important roles of soluble cues and how biochemical signaling and epigenetics process these cues [8–15]. However, microenvironments also contain diverse biophysical inputs such as specific geometric and mechanical characteristics of both the cell and extracellular matrix (ECM) that have been shown previously to strongly regulate a variety of processes in non-stem cells including gene expression [16], cellular signaling [17], proliferation [18], and migration [19]. Biophysical cues may also be in a position to influence NSC behavior, as suggested by the findings that there are stiffness gradients in the hippocampus [20] and that brain tissue softens with increasing age [21]. Furthermore, the higher stiffnesses associated with glial scars and brain tumors compared to surrounding healthy tissue have been shown to modulate the behavior of cultured neurons [22] and glioblastoma cells [18] and may also affect NSC homeostasis.
Our initial study demonstrated that ECM stiffness does indeed modulate NSC behavior [23]. Although potential mechanisms have not been investigated, analogous studies with mesenchymal stem cells (MSCs) would suggest that ECM stiffness modulates cellular tension, which in turn biases the composition of differentiated cultures [24]. However, many key questions remain to be explored, most pertinently: Might mechanotransductive proteins represent a new class of molecules that may regulate neural stem cells in vitro and in vivo? Do NSCs process ECM stiffness signals by adapting their own intrinsic mechanical properties? If so, is this mechanoadaptation necessary to bias differentiation, and which signaling pathways are responsible for transducing extracellular mechanical cues into intracellular biophysical responses (e.g. changes in cellular stiffness) and functional phenotypes (e.g. lineage commitment)? Furthermore, can biophysical signals impact stem cell differentiation in the complete absence of the strong soluble differentiation cues that have been included in previous NSC and MSC studies [23, 24]? Finally, does the effect of ECM stiffness on stem cell differentiation operate via a selective mechanism in which cells or precursors of one derivative lineage or another apoptose or proliferate preferentially as a function of stiffness, or an instructive mechanism in which ECM stiffness biases lineage commitment of multipotent stem cells? Here we have integrated biophysical, genetic, biomaterials, and animal model approaches to address these important questions.
Materials and Methods
Neural Stem Cell Culture
Neural stem cells were isolated from the hippocampus of adult female Fischer 344 rats as described here [25] and in Supplemental Data. For experiments, NSCs were seeded at a density of 15,000 cells/cm2. For BrdU treatments, cells were cultured with 10 μM BrdU (5-Bromo-2′-deoxyuridine, Sigma) from hours 0–12, 12–96, or 96–144 prior to fixation. Finally, GLISA™ assays were performed according to manufacturer’s instructions (Cytoskeleton, Inc., Denver, CO).
Rho GTPase Constructs
Dominant negative and constitutively active small Rho GTPase constructs (pcDNA3 myc CA RhoA Q63L, pcDNA3 myc DN RhoA T19N, pcDNA3 EGFP Cdc42 Q61L, pcDNA3 EGFP Cdc42 T17N, pcDNA3 EGFP Rac1 Q61L, pcDNA3 EGFP Rac1 T17N) were kind gifts of Dr. G.S. Martin, UC Berkeley. See Supplemental Data for details of viral production and in vitro and in vivo delivery.
Immunofluorescence Staining
Cells and tissue sections were immunostained as described previously [7, 23] and in Supplemental Data.
Polyacrylamide Substrate Preparation
Using a protocol similar to that described previously [19], polyacrylamide gels (70 μm nominal thickness) were synthesized on 12 mm glass coverslips using solutions composed of varying concentrations of acrylamide monomer and bisacrylamide crosslinker (Table 1). 100 μg/ml laminin was linked to the surface through sulfo-SANPAH (Thermo-Fisher, Waltham, MA) chemistry.
Table 1.
Acrylamide and bisacrylamide concentrations used to make polyacrylamide gels with different Young’s moduli as measured by AFM. Values are means and 95% confidence intervals, n = 14.
Polyacrylamide gel formulations and corresponding Young’s Modulus
| Acrylamide v/v % | Bisacrylamide v/v % | Young’s Modulus (Pa) |
|---|---|---|
| 3 | 0.025 | 102 ± 2 |
| 3 | 0.04 | 207 ± 16 |
| 3 | 0.1 | 528 ± 11 |
| 4 | 0.05 | 692 ± 2 |
| 4 | 0.075 | 1498 ± 80 |
| 4 | 0.1 | 2123 ± 215 |
| 4 | 0.2 | 4018 ± 307 |
| 5 | 0.2 | 13365 ± 103 |
| 8 | 0.3 | 30567 ± 979 |
| 10 | 0.3 | 72904 ± 159 |
| 12 | 0.6 | 151002 ± 1498 |
| 15 | 1.2 | 292300 ± 528 |
Atomic Force Microscopy
An Asylum MFP-3D atomic force microscope (Asylum Research, Santa Barbara, CA) was used to probe single cells in contact mode. Silicon nitride pyramidal AFM tips (MLCT-ANUM, Veeco Metrology, Inc., Santa Barbara, CA) with spring constants of 10–30 pN/nm were calibrated by the thermal resonance method. All measurements were made at a constant velocity of 2 μm/s. Elastic moduli reported are Young’s moduli calculated using the Hertz model [26] modified for a pyramidal tip geometry [27], assuming a Poisson ratio of 0.45. Cells were probed on the cell body and not on process extensions to minimize the impact of regional variations within cells. Cells were chosen randomly by rastering the sample stage blind to the location of cells and probing the nearest cell to the AFM tip after each location change. 50–100 cells were probed per culture. Force curves were fitted to the first 500 nm of indentation to minimize mechanical contributions from the underlying substrate or of the nucleus [26].
Results
ECM stiffness biases NSC differentiation
It was recently demonstrated that the lineage distributions of NSCs can be controlled by varying the stiffness (i.e. elastic modulus or modulus) of hydrogel scaffolds [23, 28–30]. In this work, we employed a polyacrylamide (PA) ECM system that is tunable over a broad range of stiffnesses that readily encompasses the stiffness of brain tissue. Furthermore, these surfaces can be covalently conjugated with full-length ECM proteins, as well as resist non-specific protein adsorption, yielding a well-defined substrate for cell adhesion. We conjugated full-length laminin protein, which is abundant in native NSC niches and supports NSC self-renewal and differentiation in vitro [31, 32], to PA hydrogel surfaces ranging from 100 – 75,000 Pa in stiffness. We then tested the capacity of ECM stiffness to drive cell differentiation under soluble conditions that induce differentiation into mixtures of neurons, astrocytes, and, to a small extent, oligodendrocytes (1 μM retinoic acid and 1 v/v% fetal bovine serum, or “mixed conditions”) [23, 33], as well as under minimal growth factor conditions that promote cell survival but not proliferation (0.1 ng/ml fibroblast growth factor-2, or “survival conditions”).
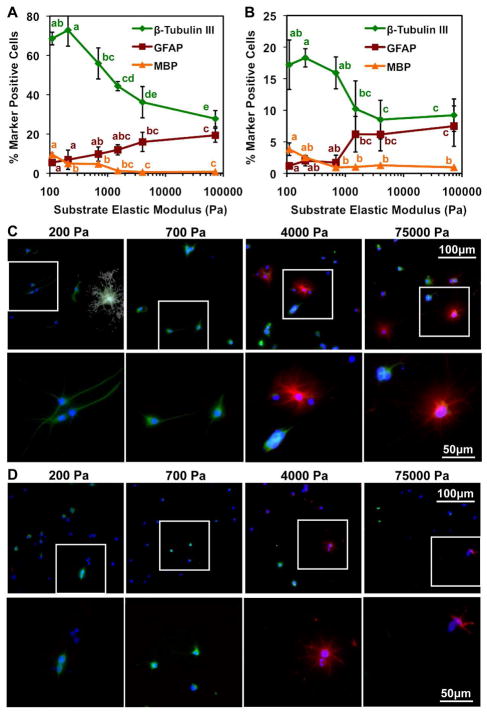
After 6 days in culture under mixed conditions, immunostaining for lineage markers (neuronal β-tubulin III, astrocytic glial fibrillary acidic protein-GFAP, oligodendrocytic myelin basic protein-MBP) showed that relatively compliant ECM substrates (100 – 700 Pa) biased lineage distributions towards neuron-rich populations (60% neurons, 10% astrocytes, 5% oligodendrocytes), whereas stiffer substrates (1,500 – 75,000 Pa) yielded cultures with roughly equal proportions of neurons and astrocytes (~30% neurons, 20% astrocytes, 0% oligodendrocytes) (Figure 1A and 1C). The proportion of oligodendrocytes was generally very low but increased on softer substrates. It should be noted that the NSCs used in this study correspond to Type IIa neural progenitors that are GFAP negative as seen previously, and GFAP specifically labels astrocytes in this system [1, 25, 33–36]. Also, the remaining marker negative cells were largely undifferentiated, Nestin-positive cells [34] and 10–20% were partially differentiated Nestin-negative cells (Figure S1). Interestingly, stiffer substrates yielded higher levels of undifferentiated cells than softer substrates.
Figure 1.
ECM elastic modulus biases relative proportions of neurons versus astrocytes. (A)(C) mixed and (B)(D) survival conditions. Error bars are 95% confidence intervals, n = 6. Means compared by analysis of variance, Tukey-Kramer post hoc (ANOVA-TK), p < 0.05. (C)(D) Neurons, β-tubulin III (green); astrocytes, GFAP (red); nuclei, DAPI (blue); oligodendrocytes, MBP (white). Insets (white boxes) are shown in bottom rows.
In parallel, under survival conditions, neuronal, astrocytic, and oligodendrocytic differentiation were observed at lower levels and with less mature morphologies than under mixed conditions, consistent with the absence of strong soluble factors to induce maturation following lineage commitment. However, ECM stiffness again strikingly biased lineage distributions towards neurons on soft ECMs and towards equal proportions of neurons and astrocytes on stiff ECMs (Figure 1B and 1D). We observed few oligodendrocytes, but as in mixed conditions their percentage slightly increased on the softest ECMs. These data demonstrate that ECM stiffness can strongly influence and drive NSC lineage commitment even in the absence of exogenous soluble differentiation cues.
NSCs sense and respond biomechanically to ECM stiffness through RhoA and Cdc42
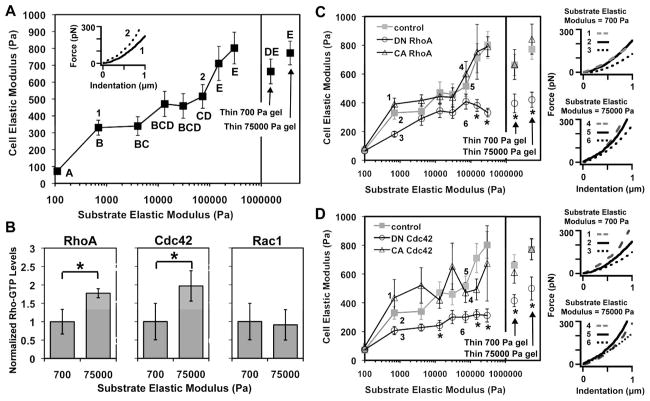
NSCs can sense mechanical information encoded within the ECM; however, it is unclear how they process these cues to modulate differentiation. One may anticipate that the most direct response to changes in ECM stiffness is for NSCs to adapt their own intrinsic mechanical properties. To test this hypothesis, we used atomic force microscopy (AFM) [37] to measure the elastic modulus of individual NSCs cultured on ECMs of defined stiffness. We seeded NSCs in mixed conditions, then probed them with AFM after 12 hours, a duration sufficient for cells to maximally adhere and spread but likely not for lineage commitment to occur [38]. Interestingly, by this early time point, cellular elastic modulus varied strongly and monotonically with increasing ECM stiffness, such that cells on the stiffest matrices exhibited elastic moduli nearly 8-fold greater than those cultured on the most compliant ECMs (Figure 2A). Prior work has indicated that ECM ligand density and presentation remain constant over this range of PA formulations [39]. However, to preclude the possibility that such surface biochemical differences may contribute to stem cell stiffness differences, we repeated these experiments on highly compliant and highly rigid gel formulations cast as very thin layers (<7 μm) on top of glass, such that the stiffness of the cell-ECM interface is dictated by the underlying hard substrate rather than the intrinsic properties of the gel [40] (Figure 2A). Both thin gels yielded NSC stiffnesses in the range of 700–800 Pa, similar to that observed on stiff gels, confirming that ECM stiffness modulates NSC stiffness.
Figure 2.
NSCs are mechanically and biochemically responsive to ECM stiffness through Rho GTPase activity. (A)(C)(D) NSC stiffnesses measured by AFM. Error bars are 95% confidence intervals for n = 14–50 cells. Means compared by ANOVA-TK, p < 0.05. (Insets) Representative AFM force-indentation curves for NSCs. (B) Rho-GTP levels of NSCs normalized to the soft gel (700 Pa) value. *p < 0.05, Student’s unpaired two-tailed t-test.
The observation that cells stiffen in response to increasing ECM modulus indicates that cellular mechanotransductive signaling pathways may sense and process extracellular mechanical information into intracellular mechanical responses. The Rho family of GTPases – including RhoA, Cdc42, and Rac1 – have been extensively studied in somatic cells and are known to regulate the assembly and activity of cytoskeletal processes needed for the establishment of cell shape and the generation of contractile forces [41]. These proteins cycle between active GTP-bound and inactive GDP-bound states, and levels of the active, GTP-bound form can be measured using enzyme-linked immunosorbent assays (ELISA) [42]. We cultured NSCs on two ECM stiffnesses (700 and 75,000 Pa) and found that the cellular activities of RhoA and Cdc42, measured at an early time point (12 hours post seeding) in mixed conditions, were nearly two-fold higher on the stiff vs. soft ECMs (Figure 2B), whereas Rac1 activity remained unchanged, indicating that ECM stiffness preferentially activates specific Rho GTPases. Together with the AFM results, these experiments confirm the hypotheses that NSCs respond to increasing ECM stiffness both by altering their intrinsic mechanical properties (stiffness) and by activating mechanotransductive signals (RhoA and Cdc42).
To determine whether RhoA and Cdc42 activity mediate the effect of ECM modulus on NSC mechanoadaptation, we retrovirally transduced NSCs to stably express dominant negative (DN) and constitutively active (CA) mutants of RhoA and Cdc42 [43] (Figure S2), cultured them on a range of ECM moduli in mixed conditions, and measured NSC stiffnesses by AFM 12 hours after seeding. Compared to control cells transduced with an empty retroviral vector, the stiffnesses of NSCs expressing DN RhoA and DN Cdc42 were lower and less sensitive to changes in ECM modulus, whereas CA RhoA and CA Cdc42 retained normal mechanoadaptation and even increased cell stiffness for some ECM moduli (Figures 2C and D, respectively). These differences in stiffness, readily apparent from representative force-indentation curves (Figures 2C and D, right) and analysis of covariance in trends (Figures S2A and B), demonstrate that RhoA and Cdc42 activation are necessary for NSC stiffening in response to increasing ECM modulus.
RhoA and Cdc42 modulate the effect of ECM stiffness on NSC differentiation
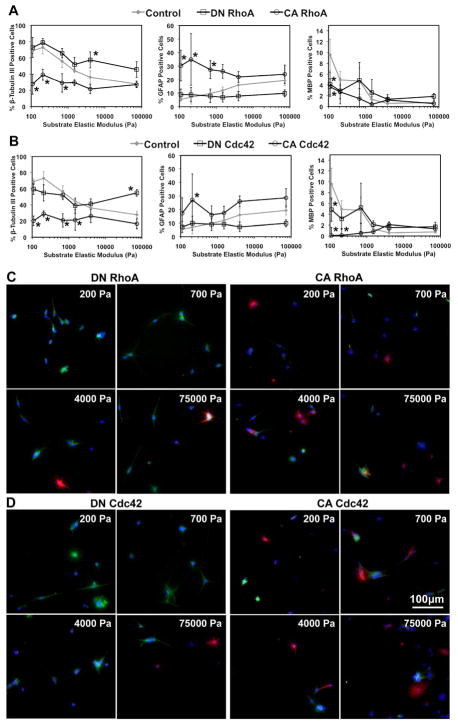
Given that ECM stiffness modulates NSC lineage distributions (Fig. 1), cell stiffness (Fig. 2A), and RhoA and Cdc42 activities (Fig. 2B), and that direct manipulation of RhoA and Cdc42 activity modulates cell mechanoadaptation (Fig. 2C and D), we reasoned that RhoA and Cdc42 may be responsible for transducing the effects of variable ECM stiffness on NSC differentiation (Figure 1). To test this hypothesis, we cultured NSCs expressing DN and CA RhoA and Cdc42 on ECMs of different stiffnesses in mixed conditions and immunostained for lineage markers after 6 days. On soft (<1000 Pa) ECMs, expression of DN RhoA did not further increase the percentage of neurons observed compared to control cells (Figure 3A and C); however, on rigid (>4000 Pa) ECMs it rescued neuronal differentiation up to levels approaching 50%. In contrast, increasing RhoA activity had the opposite effect, reducing the fraction of neurons on compliant ECMs compared to control cells, but not appreciably changing the percentage of neurons on the stiffest substrate, thereby resulting in ~30% neurons on all ECM stiffnesses. Astrocytic differentiation followed complementary trends, with CA RhoA increasing astrocytic differentiation on soft substrates and DN RhoA decreasing astrocytic differentiation on stiffer substrates. Similar results were obtained with NSCs expressing DN or CA Cdc42 (Figure 3B and D). These results and further statistical analysis of covariance in trends (Figure S4) indicate that expression of DN RhoA or Cdc42 mimics phenotypes observed on soft gels, whereas expression of CA RhoA or Cdc42 mimics differentiation observed on stiff gels. Interestingly, these trends persisted in survival conditions. DN RhoA and Cdc42 rescued neuronal differentiation (~ 20% neurons), while CA RhoA and Cdc42 slightly suppressed neuronal differentiation on all ECM stiffnesses (Figure S5). In addition, with the lone exception of DN Cdc42-expressing cells on soft ECMs, astrocytic differentiation was suppressed by DN RhoA and Cdc42, and largely unaffected by expression of CA RhoA and Cdc42.
Figure 3.
Rho GTPases modulate the effect of ECM elastic modulus on the proportions of neurons and astrocytes in mixed conditions. Error bars are 95% confidence intervals, n = 5–6. *p < 0.05 for comparisons to control for each substrate elastic modulus (control data previously shown in Figure 1A) (ANOVA-TK). β-tubulin III (green), GFAP (red), DAPI (blue), MBP (white). See Figure S4 for higher power images of (C) and (D).
The effects of RhoA and Cdc42 on differentiation in both mixed and survival conditions were also observed on traditional glass substrates (most similar to the stiffest hydrogel ECMs) by QRT-PCR (Figure S6) and immunostaining (Figure S7). It should be noted that cell populations expressing mutant Rho GTPases in mixed conditions all displayed classical neuronal, astrocytic, and oligodendrocytic morphologies, with no differences from differentiated control NSCs (Figure S7A) [33]. Furthermore, expression of DN and CA Rho GTPases as well as culture on soft and stiff polyacrylamide gels did not compromise later stages of neuronal maturation and subtype marker expression, with GABAergic and glutaminergic neurons detectable across all ECM stiffnesses and all RhoA/Cdc42 genotypes (Figure S8). Finally, expression of DN or CA Rac1 GTPase interestingly did not bias lineage distributions (Figure S7), consistent with our earlier finding that the activities of RhoA and Cdc42, but not Rac1, are regulated by ECM stiffness (Figure 2B). Collectively, these results under mixed and survival conditions show that RhoA and Cdc42 serve as important transducers of ECM stiffness into downstream cell fate decisions.
Comparative longitudinal apoptosis and proliferation measurements strongly support an instructive mechanism
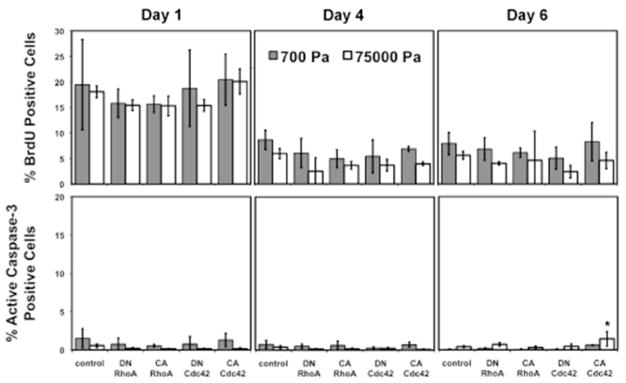
While RhoA and Cdc42 modulated the ECM stiffness effect on lineage distributions, it was unclear whether these changes in lineage distributions were due to instructive biasing of NSC lineage commitment or selection for specific populations via modulation of proliferation and/or apoptosis of lineage-committed cells. The initial homogeneity of the culture was assessed by single-cell sorting clonal analysis, which revealed that ~82% of clonal populations were capable of giving rise to neurons, astrocytes, and oligodendrocytes (tripotent), indicating that cells seeded at the beginning of the experiment were predominantly multipotent NSCs. Our previous work has also shown that unipotent soluble conditions were capable of generating almost pure neuronal or astrocytic cultures, providing further evidence of the NSC culture homogeneity [23, 24]. We then measured proliferation (BrdU) and apoptosis (active caspase 3) at early (0–12 hours), middle (12–96 hours), and late (96–144 hours) time points for NSCs on soft (700 Pa) and stiff (75,000 Pa) ECMs under mixed conditions (Figure 4). For all cultures, proliferation was moderate during the first 12 hours and thereafter decreased to minimal levels (~5% for BrdU pulse durations), and active caspase 3 levels were low across all conditions throughout the experiment (<1.5%). Furthermore, cells expressing the mutant Rho GTPases exhibited similar proliferation rates to control cells on soft and stiff ECMs. Importantly, all experiments were conducted at low initial seeding densities (<20000 cells/cm2) to minimize confounding effects of cell-cell adhesion (e.g. contact inhibition). Repeating the experiment at initial cell densities ranging from 5000 to 25000 cells/cm2 did not affect lineage distributions (data not shown). The overall low proliferation and apoptosis levels throughout the experiment strongly indicate that ECM stiffness and Rho GTPase regulate NSC lineage distributions through an instructive rather than selective mechanism.
Figure 4.
Rho GTPases and ECM stiffness do not affect proliferation and apoptosis rates during differentiation. Error bars are 95% confidence intervals, n = 3–6. *p < 0.05 for comparisons to control for each substrate elastic modulus for each day (ANOVA-TK).
Inhibition of contractile proteins rescues neuronal differentiation
While RhoA and Cdc42 can change NSC mechanoadaptation in response to extracellular stiffness (Figure 2) and instruct NSC differentiation decisions (Figure 3), it is not clear whether the former is necessary for the latter. While there are no clear means to directly manipulate cellular contractile properties in isolation, it is possible to inhibit the activity of cellular mechanotransducers and motors, such as the downstream Rho GTPase effectors Rho Kinase (ROCK) and myosin II whose activities directly underlie cellular stiffening, as well as other mechanotransductive proteins such as Myosin Light Chain Kinase (MLCK), Src, and Focal Adhesion Kinase (FAK). Furthermore, transient inhibition of these pathways can help address the question of whether they instruct cell fate during a critical, early time window. We therefore investigated whether inhibition of these contractility-related proteins early during lineage commitment could rescue neuronal differentiation under conditions that would otherwise instruct astrocytic fates.
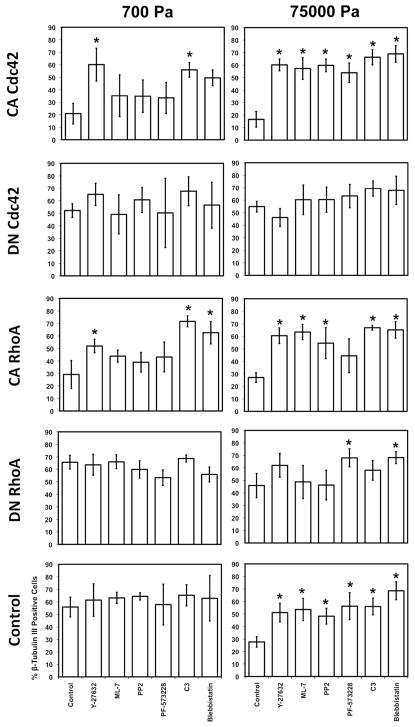
NSCs expressing CA and DN RhoA and Cdc42 were cultured in mixed conditions on compliant (700 Pa) and stiff (75,000 Pa) ECMs and immunostained for lineage markers after 6 days. Inhibitors of ROCK (10 μM Y-27632), Myosin II (1 μM Blebbistatin), Src (0.5 μM PP2), FAK (0.5 μM PF-573228), RhoA/B/C (0.17 μg/mL C3), and MLCK (0.5 μM ML-7) were pulsed in the medium for the first 2 days, when lineage commitment decisions are most likely made, then washed out for the remaining 4 days to minimize any potential effects on later steps of cell differentiation. For CA RhoA- and Cdc42-expressing NSCs on soft ECMs, inhibition of RhoA/B/C and downstream effectors ROCK and Myosin II (for CA RhoA-expressing NSCs) strikingly rescued neuronal differentiation to control levels (Figure 5). By contrast, MLCK, Src, and FAK inhibition had no effect. Interestingly, all six inhibitors reduced astrocytic differentiation of CA RhoA-expressing cells (Figure S9), suggesting that this process may utilize additional Rho GTPase-independent mechanotransductive signaling machinery.
Figure 5.
Inhibition of proteins that regulate cellular contractility rescues neuronal differentiation in mixed conditions on soft and stiff ECMs. Error bars are 95% confidence intervals, n = 5–6. *p < 0.05 for comparisons to NSCs in control media conditions expressing the same Rho GTPase mutant and on the same stiffness (control data previously shown in Figure 1A) (ANOVA-TK).
On stiff ECMs, all inhibitors restored neuronal differentiation for all NSC populations to levels found on compliant ECMs (~60% neurons) (Figure 5) and reduced astrocytic differentiation (Figure S9). Similar trends were observed in survival conditions, except that the rescue of neuronal differentiation on soft ECMs was not as pronounced (Figure S10A). Finally, under all conditions, oligodendrocytic differentiation was not appreciably affected (Figure S10B and S9C). These results strongly indicate that cellular contractility mediates the effects of instructive ECM stiffness cues on NSC lineage commitment.
RhoA Activity Suppresses Neurogenesis In Vivo
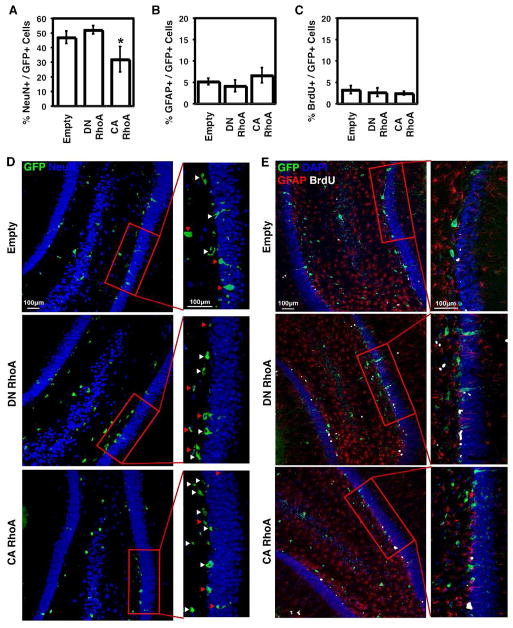
We investigated whether RhoA also regulates NSC behavior in vivo. Retroviral vectors encoding either GFP only, DN RhoA and GFP, or CA RhoA and GFP (all driven by a CAG promoter) were stereotaxically injected into the hippocampal dentate gyrus of adult rats. Retrovirus is known to infect dividing cells, specifically neural progenitors in the subgranular zone of the dentate gyrus [7], which we identified by GFP expression. At 1, 2, and 3 weeks post-injection, BrdU was administered to monitor potential differences in proliferation throughout the experiment. After 4 weeks, brain sections were immunostained for the neuronal marker neuronal nuclei (NeuN), GFAP, and BrdU (Figure 6). The percentages of mature, post-mitotic NeuN+ neurons [44] derived from neural progenitors infected with retroviral vectors (GFP+) (Figure 6A) strikingly followed the same trend as that on soft ECMs (Figure 3A), consistent with the fact that hippocampal tissue is also relatively soft at <1 kPa [20]. Specifically, CA RhoA reduced neuronal differentiation from ~50 to 30% of GFP+ cells, while DN RhoA produced a slight trend towards increased neuronal differentiation. Astrocytic differentiation was low as expected given that neural progenitors in the hippocampus are strongly biased towards neuronal differentiation (Figure 6B) [45]. Finally, proliferation was low and similar across all conditions, suggesting RhoA activity does not modulate NSC proliferation. These results show that RhoA regulates neurogenesis in vivo.
Figure 6.
RhoA suppresses neurogenesis in vivo in the adult rat hippocampus. Error bars are 95% confidence intervals, n = 4 rats. *p < 0.05 for comparison to empty vector control (ANOVA-TK). Red arrows indicate cells double positive for GFP and NeuN. White arrows indicate cells positive for GFP only.
Discussion
We have demonstrated that stiffness cues encoded in the ECM directly bias NSC lineage commitment through RhoA- and Cdc42-regulated changes in actomyosin contractility and cellular stiffness. These results elucidate several novel features of NSC mechanotransduction that contribute to our understanding of stem cell biology, neuroscience, and mechanobiology: (1) Changes in cellular mechanics – which precede expression of lineage markers by several days – potently regulate ECM stiffness-dependent NSC differentiation. (2) This mode of regulation depends strongly on RhoA and Cdc42 activation, and other mechanotransductive pathways may be mobilized to control this response, including MLCK- and FAK-based signaling, depending on the mechanical properties of the ECM. To our knowledge, several of these molecules – including Cdc42, MLCK, and Src – have not previously been implicated in stem cell mechanosensitivity. (3) Transient inhibition of specific mechanotransducers during a critical developmental window is sufficient to profoundly alter lineage distributions that only declare themselves days after the inhibition is removed. (4) Along with early transient inhibition of mechanotransducers, similarly low proliferation and apoptosis levels for Rho-GTPase mutant-expressing cells on soft and stiff ECMs strongly indicate substrate mechanical properties can directly instruct, rather than select for, neural stem cell lineage commitment. In conjunction with clonal analysis of the stem cell population, this represents the most rigorous demonstration to date that ECM mechanics directly instructs a stem cell’s fate. (5) RhoA activity suppresses neurogenesis in the native hippocampal niche of NSCs in a manner strikingly similar to an ex vivo niche with similar stiffness properties. (6) NSC differentiation is mechanosensitive even in the absence of strong morphogenic factors that have been used in previous studies of stem cell mechanosensitivity.
This finding that RhoA and Cdc42 are important regulators of NSC fate expands and casts a new light on their relevance in neurobiology, beyond their previously known role in the morphological maturation of committed neurons. For example, RhoA and Cdc42 are weakly expressed within the granule cell layer of the hippocampal dentate gyrus (with slightly higher expression in the hilus) but are strongly expressed in the molecular layer and other surrounding regions [46]. This spatial pattern of expression, in particular the low RhoA and Cdc42 levels surrounding the subgranular zone neurogenic niche, is consistent with our finding that suppression of RhoA or Cdc42 activation promotes neuronal differentiation in vitro and in vivo. Our findings may also yield additional mechanistic insights into recent observations that administration of ROCK inhibitors (Y-27632 and Fasudil) into mammalian brains can offer neuroprotection against ischemia [47] and epileptic seizures [48], promote spatial learning and working memory in mice [49], and increase neurogenesis and generation of neurons in response to hypoxic conditions [50]. We may also place our findings in the context of recent studies that directly connect Rho GTPases to neural development. Consistent with our study, RNAi-mediated downregulation of the Rho GDP dissociation inhibitor γ (RhoGDIγ) in v-myc immortalized, multipotent C17.2 neural cells has been shown to decrease RhoA and Cdc42 activity, but increase Rac1 activity, as well as promote neuronal but not glial differentiation [51]. In contrast, activation of Cdc42 has been shown to enhance neuronal differentiation in C17.2 and P19 cells [52] and neuroblastoma [53], while siRNA knockdown of Cdc42 in P19 cells almost completely inhibited astrocytic differentiation while modestly inhibiting neuronal differentiation [54]. These different results may arise from distinct culture conditions and cell type, which originate from different species and/or subregions of the central nervous system and may thus exhibit different mechanobiological properties. Furthermore, it is entirely possible that Rho GTPases may affect lineage specification very differently from how they affect maturation of already lineage-committed cells. It would be interesting to revisit these studies to determine whether the observed effects of Rho GTPase signaling on neural differentiation have some mechanoregulatory component.
In principle, there are two distinct yet both physiologically relevant mechanisms by which ECM stiffness and Rho GTPases may impact lineage distributions: selection vs. instruction. In the hippocampus, some extracellular factors are known to instruct NSC commitment to specific lineages [55]. However, the majority of newborn cells in the hippocampus undergo apoptosis within 4 days [56], and ECM stiffness could alternatively affect lineage distributions through a selective mechanism, such as one that modulates cell survival. Our results show that Rho GTPase activity and ECM stiffness do not modulate NSC proliferation or apoptosis during differentiation in vitro, or NSC proliferation in vivo (Figures 4 and 6C), supporting an instructive mechanism in which ECM stiffness acts directly to perturb lineage commitment. While the mechanism of this instruction remains unclear, ECM mechanics may potentially function by modulating canonical signaling pathways including those downstream of Notch and Wnt/β-catenin, transcription factors like Sox2 and Tlx, or epigenetic regulators like RE-1 silencing transcription factor (REST) known to regulate NSC maintenance and differentiation [55, 57].
This study also adds new insight into the mechanobiology of stem cells. Previously with MSCs, pharmacological inhibition of myosin II reduced differentiation into all lineages on all ECM stiffnesses [24]. In NSCs, by contrast, we find that inhibition of contractility alters the distribution of differentiation trajectories (i.e. neuron vs. astrocyte) while actually modestly increasing differentiation. Two related innovations of our study are that we employed much lower effective dosages and included them only transiently during the period of lineage commitment, days before the appearance of lineage markers (e.g. 1 μM blebbistatin for 2 day pulse compared to 50 μM for full length of experiment). A similar early transient treatment of MSCs (12–24 hours) with Y-27632 in osteogenic differentiation media affected osteogenesis 7 days later, indicating the importance of early cytoskeletal contractility in stem cell differentiation [58]. By using transient exposures at a key early time window, we separated the regulatory contribution of early cellular mechanotransduction in multilineage stem cell differentiation from its longer-term contributions to maturation. Furthermore, the lower dosages of contractility inhibitors necessary for an observed phenotype, along with shifts in (rather than inhibition of) differentiation in NSCs compared to MSCs, suggests these two stem cell types may be sensitive to different ranges of ECM mechanical stiffnesses. This idea is further supported by the different ranges of cortical stiffnesses measured for the two cell types, with MSC stiffness generally exceeding 1 kPa [24] and NSC stiffness lying below 1 kPa even on the stiffest substrates (Figure 2). These findings are consistent with the notion that the differentiation of a specific stem cell population is most sensitive to ECM stiffness in a range that corresponds to its tissue(s) of residence, e.g., brain is softer than bone marrow and the connective tissues that MSCs chiefly populate.
Our study also reveals an interesting difference between NSCs and MSCs in the dynamic range of their stiffness-sensitive differentiation. The range of ECM stiffnesses required to alter differentiation is orders of magnitude smaller for NSCs compared to MSCs, here only 1 kPa (from 500–1500 Pa). This may reflect the fact that the NSCs used in this study arise from a single tissue with a well-defined, soft, anatomical niche, whereas MSCs arise from a broad range of tissues with niches that are comparatively poorly defined. This study also demonstrates that stem cells are capable of sensing much finer and subtler changes in microenvironmental stiffness than previously known. Future work should identify the mechanisms controlling the range of ECM stiffnesses in which specific stem cells are most sensitive and whether these ranges correlate with in vivo niche properties.
It is important to note that the interplay between biophysical and biochemical signaling may be more complicated than currently appreciated and suggests specific future avenues of study. For example, our finding that the inhibition of FAK, MLCK, and Src rescued neuronal differentiation on stiff but not soft ECMs (Figure 5), implies that distinct mechanotransductive pathways may be mobilized by specific microenvironmental contexts. Basal levels of cellular contractility or perhaps basal flux through particular biochemical signaling pathways may be significantly different on soft versus stiff ECMs, resulting in differential regulation based on the biophysical context of the microenvironment. Interestingly, an earlier study found that constitutive activation of ROCK, but not RhoA, can induce MSC osteogenesis even when these cells are forced to adopt a rounded morphology and are presumably limited in their ability to stiffen [59] suggesting biochemical and biophysical signaling may intersect at distinct places depending on the microenvironmental context. Similarly, we found that while DN RhoA/Cdc42 compromised the ability of NSCs to adapt their intrinsic mechanical properties to those of the ECM, CA RhoA/Cdc42 did not strongly enhance this behavior (Figure 2) yet still biased differentiation by reversing the enhanced neurogenesis observed on soft ECMs. The fact that CA RhoA/Cdc42 does not produce dramatic “hyperstiffening” is not entirely surprising given that ECM compliance places fundamental limits on how hard cells can pull on the matrix without rupturing adhesions and contracting [39]. Furthermore, cortical stiffness as measured by AFM is an integrated readout of cell-matrix tensional homeostasis and may not detect particularly subtle changes in tensile forces localized to individual adhesions that are believed to directly modulate adhesion-based signaling [60]. Future work in both MSCs and NSCs will be needed to further investigate the intracellular and extracellular components of mechanotransductive signaling networks, their connectivity and regulatory logic, and their relative importance as a function of microenviromental context [61–67].
Finally, in addition to adding to our understanding of the molecular and biophysical mechanisms regulating NSCs and their roles in health and disease, this study also indicates that modulation or even manipulation of the mechanical microenvironment may have implications for human health. For example, the elasticity of the brain changes significantly with age in both rats [21] and humans [68], raising the prospect that these changes may potentially contribute to age-related dysregulation of neuronal differentiation and cognitive decline. In addition, the mechanical changes observed during the progression of brain tumors and neurodegenerative scarring [69–71] may induce cancer growth and metastasis by increasing cancer cell proliferation and motility [18, 72]. Furthermore, our results in vivo strongly suggest that such stiffness increases in the native hippocampal NSC niche may, through upregulation of RhoA activity, suppress neurogenesis. Future developments of methods to modulate tissue stiffness without altering other niche properties such as niche biochemistry will be of significant interest given these findings. Investigating these hypotheses should contribute to our understanding of CNS diseases and may offer new and unexpected biomedical avenues.
Supplementary Material
Acknowledgments
We thank Rachel A. Segalman for access to an Asylum MFP3D AFM and G. Steven Martin for Rho GTPase cDNA (pcDNA3 myc CA RhoA Q63L, pcDNA3 myc DN RhoA T19N, pcDNA3 EGFP Cdc42 Q61L, pcDNA3 EGFP Cdc42 T17N, pcDNA3 EGFP Rac1 Q61L, pcDNA3 EGFP Rac1 T17N). We thank William Bretzlaff, Daniela Mehech, Meimei Dong and Mary West, for technical assistance. This work was supported by a National Defense Science and Engineering Graduate Fellowship and a National Science Foundation Graduate Research Fellowship to A. J. Keung. D. V. Schaffer wishes to acknowledge the support of NIH grants DE018044 and EB007295. S. Kumar wishes to acknowledge the support of a UC Berkeley Stem Cell Center Seed Grant, the Arnold and Mabel Beckman Young Investigator Award, a PECASE Award from the Army Research Office (W911NF-09-1-0507), and the NIH Director’s New Innovator Award (1DP2OD004213), a part of the NIH Roadmap for Medical Research.
Footnotes
Disclaimers: None.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
Author Contribution Summary:
Albert J. Keung: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing
Elena M. de Juan-Pardo: Conception and design, Collection and/or assembly of data, Data analysis and interpretation
David V. Schaffer: Financial Support, Conception and design, Data analysis and interpretation, Manuscript writing, Final approval of manuscript
Sanjay Kumar: Financial Support, Conception and design, Data analysis and interpretation, Manuscript writing, Final approval of manuscript
References
- 1.Palmer TD, Takahashi J, Gage FH. The Adult Rat Hippocampus Contains Primordial Neural Stem Cells. Molecular and Cellular Neuroscience. 1997;8(6):389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 2.Suh H, et al. In Vivo Fate Analysis Reveals the Multipotent and Self-Renewal Capacities of Sox2+ Neural Stem Cells in the Adult Hippocampus. Cell Stem Cell. 2007;1(5):515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balu DT, Lucki I. Adult hippocampal neurogenesis: Regulation, functional implications, and contribution to disease pathology. Neuroscience & Biobehavioral Reviews. 2009;33(3):232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blurton-Jones M, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proceedings of the National Academy of Sciences. 2009 doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011 doi: 10.1038/nature09817. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C-L, et al. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 7.Zhao C, et al. Distinct Morphological Stages of Dentate Granule Neuron Maturation in the Adult Mouse Hippocampus. J Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. Journal Of Neuroscience. 2002;22(3):629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barkho BZ, et al. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells and Development. 2006;15(3):407–421. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalani MYS, et al. Wnt-mediated self-renewal of neural stem/progenitor cells. Proceedings of the National Academy of Sciences. 2008;105(44):16970–16975. doi: 10.1073/pnas.0808616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohyama J, et al. Epigenetic regulation of neural cell differentiation plasticity in the adult mammalian brain. Proceedings of the National Academy of Sciences. 2008;105(46):18012–18017. doi: 10.1073/pnas.0808417105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma DK, et al. Neuronal Activity-Induced Gadd45b Promotes Epigenetic DNA Demethylation and Adult Neurogenesis. Science. 2009;323(5917):1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riquelme PA, Drapeau E, Doetsch F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1489):123–137. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt MHH, et al. Epidermal growth factor-like domain 7 (EGFL7) modulates Notch signalling and affects neural stem cell renewal. Nature Cell Biology. 2009;11(7):873–880. doi: 10.1038/ncb1896. [DOI] [PubMed] [Google Scholar]
- 15.Shen Q, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304(5675):1338–40. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 16.Thomas CH, et al. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proceedings Of The National Academy Of Sciences. 2002;99(4):1972–1977. doi: 10.1073/pnas.032668799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janmey PA. The Cytoskeleton and Cell Signaling: Component Localization and Mechanical Coupling. Physiological Reviews. 1998;78(3):763–781. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- 18.Ulrich TA, de Juan Pardo EM, Kumar S. The Mechanical Rigidity of the Extracellular Matrix Regulates the Structure, Motility, and Proliferation of Glioma Cells. Cancer Research. 2009;69(10):4167–4174. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelham RJ, Wang Y-l. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings Of The National Academy Of Sciences. 1997;94(25):13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elkin BS, et al. Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. Journal Of Neurotrauma. 2007;24(5):812–822. doi: 10.1089/neu.2006.0169. [DOI] [PubMed] [Google Scholar]
- 21.Gefen A, et al. Age-dependent changes in material properties of the brain and braincase of the rat. Journal of Neurotrauma. 2003;20(11):1163–1177. doi: 10.1089/089771503770802853. [DOI] [PubMed] [Google Scholar]
- 22.Uibo R, et al. Soft materials to treat central nervous system injuries: Evaluation of the suitability of non-mammalian fibrin gels. Biochimica et Biophysica Acta. 2009;1793(5):924–930. doi: 10.1016/j.bbamcr.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha K, et al. Substrate Modulus Directs Neural Stem Cell Behavior. Biophysical Journal. 2008;95(9):4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engler AJ, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Palmer TD, et al. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. Journal Of Neuroscience. 1999;19(19):8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domke J, Radmacher M. Measuring the elastic properties of thin polymer films with the atomic force microscope. Langmuir. 1998;14(12):3320–3325. [Google Scholar]
- 27.Rosenbluth MJ, Lam WA, Fletcher DA. Force microscopy of nonadherent cells: A comparison of leukemia cell deformability. Biophysical Journal. 2006;90(8):2994–3003. doi: 10.1529/biophysj.105.067496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teixeira AI, et al. The promotion of neuronal maturation on soft substrates. Biomaterials. 2009;30(27):4567–4572. doi: 10.1016/j.biomaterials.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30(36):6867–6878. doi: 10.1016/j.biomaterials.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee A, et al. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials. 2009;30(27):4695–4699. doi: 10.1016/j.biomaterials.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekblom P, Lonai P, Talts JF. Expression and biological role of laminin-1. Matrix Biology. 2003;22(1):35–47. doi: 10.1016/s0945-053x(03)00015-5. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki T, et al. Expression and Distribution of Laminin α1 and α2 Chains in Embryonic and Adult Mouse Tissues: An Immunochemical Approach. Experimental Cell Research. 2002;275(2):185–199. doi: 10.1006/excr.2002.5499. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh J, et al. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. Journal of Cell Biology. 2004;164(1):111–122. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abranches E, et al. Development of quantitative PCR methods to analyse neural progenitor cell culture state. Biotechnology And Applied Biochemistry. 2006;44:1–8. doi: 10.1042/BA20050218. [DOI] [PubMed] [Google Scholar]
- 35.Hsieh J, et al. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2004;101(47):16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuwabara T, et al. A Small Modulatory dsRNA Specifies the Fate of Adult Neural Stem Cells. Cell. 2004;116(6):779. doi: 10.1016/s0092-8674(04)00248-x. [DOI] [PubMed] [Google Scholar]
- 37.Lu YB, et al. Viscoelastic properties of individual glial cells and neurons in the CNS. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2006;103(47):17759–17764. doi: 10.1073/pnas.0606150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravin R, et al. Potency and Fate Specification in CNS Stem Cell Populations In Vitro. Cell Stem Cell. 2008;3(6):670–680. doi: 10.1016/j.stem.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Solon J, et al. Fibroblast Adaptation and Stiffness Matching to Soft Elastic Substrates. Biophysical Journal. 2007;93(12):4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maloney JM, et al. Influence of finite thickness and stiffness on cellular adhesion-induced deformation of compliant substrata. Physical Review E (Statistical, Nonlinear, and Soft Matter Physics) 2008;78(4):041923–15. doi: 10.1103/PhysRevE.78.041923. [DOI] [PubMed] [Google Scholar]
- 41.Allen WE, et al. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. Journal Of Cell Science. 1997;110:707–720. doi: 10.1242/jcs.110.6.707. [DOI] [PubMed] [Google Scholar]
- 42.Lange K, et al. Combined Lysophosphatidic Acid/Platelet-Derived Growth Factor Signaling Triggers Glioma Cell Migration in a Tenascin-C Microenvironment. Cancer Research. 2008;68(17):6942–6952. doi: 10.1158/0008-5472.CAN-08-0347. [DOI] [PubMed] [Google Scholar]
- 43.Inoue T, et al. An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nature Methods. 2005;2(6):415–418. doi: 10.1038/nmeth763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kempermann G, et al. Milestones of neuronal development in the adult hippocampus. Trends in Neurosciences. 2004;27(8):447. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Kempermann G, et al. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130(2):391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 46.O’Kane EM, Stone TW, Morris BJ. Distribution of Rho family GTPases in the adult rat hippocampus and cerebellum. Molecular Brain Research. 2003;114(1):1–8. doi: 10.1016/s0169-328x(03)00121-9. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, et al. Neuroprotective Potential of Fasudil Mesylate in Brain Ischemia-Reperfusion Injury of Rats. Cellular and Molecular Neurobiology. 2009;29(2):169–180. doi: 10.1007/s10571-008-9308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.İnan SY, Büyükafşar K. Antiepileptic effects of two Rho-kinase inhibitors, Y-27632 and fasudil, in mice. British Journal of Pharmacology. 2008;155(1):44–51. doi: 10.1038/bjp.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huentelman MJ, et al. Peripheral delivery of a ROCK inhibitor improves learning and working memory. Behavioral Neuroscience. 2009;123(1):218–223. doi: 10.1037/a0014260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding J, et al. Fasudil, a Rho kinase inhibitor, drives mobilization of adult neural stem cells after hypoxia/reoxygenation injury in mice. Molecular And Cellular Neuroscience. 2010;43(2):201–208. doi: 10.1016/j.mcn.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Lu W, Wang J, Wen TQ. Downregulation of Rho-GDI gamma promotes differentiation of neural stem cells. Molecular And Cellular Biochemistry. 2008;311(1–2):233–240. doi: 10.1007/s11010-008-9713-9. [DOI] [PubMed] [Google Scholar]
- 52.Oh JE, et al. Cdo promotes neuronal differentiation via activation of the p38 mitogen-activated protein kinase pathway. FASEB J. 2009;23(7):2088–2099. doi: 10.1096/fj.08-119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valentijn LJ, et al. Inhibition of a New Differentiation Pathway in Neuroblastoma by Copy Number Defects of N-myc, Cdc42, and nm23 Genes. Cancer Res. 2005;65(8):3136–3145. doi: 10.1158/0008-5472.CAN-04-2469. [DOI] [PubMed] [Google Scholar]
- 54.Endo M, Antonyak MA, Cerione RA. Cdc42-mTOR Signaling Pathway Controls Hes5 and Pax6 Expression in Retinoic Acid-dependent Neural Differentiation. J Biol Chem. 2009;284(8):5107–5118. doi: 10.1074/jbc.M807745200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suh H, Deng W, Gage FH. Signaling in Adult Neurogenesis. Annual Review Of Cell And Developmental Biology. 2009;25(1):253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 56.Sierra A, et al. Microglia Shape Adult Hippocampal Neurogenesis through Apoptosis-Coupled Phagocytosis. Cell Stem Cell. 2010;7(4):483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jessberger S, et al. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci. 2008 doi: 10.1038/nn.2148. advanced online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fu J, et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Meth. 2010;7(9):733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McBeath R, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 60.Klotzsch E, et al. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proceedings of the National Academy of Sciences. 2009;106(43):18267–18272. doi: 10.1073/pnas.0907518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chowdhury F, et al. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nature Materials. 2010;9:82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deisseroth K, et al. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42(4):535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 63.Kamal A, Goldstein LSB. Connecting vesicle transport to the cytoskeleton. Current Opinion in Cell Biology. 2000;12(4):503. doi: 10.1016/s0955-0674(00)00123-x. [DOI] [PubMed] [Google Scholar]
- 64.Mammoto A, et al. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457(7233):1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mammoto A, Huang S, Ingber DE. Filamin links cell shape and cytoskeletal structure to Rho regulation by controlling accumulation of p190RhoGAP in lipid rafts. Journal of Cell Science. 2007;120(3):456–467. doi: 10.1242/jcs.03353. [DOI] [PubMed] [Google Scholar]
- 66.Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. Journal of Cell Science. 2004;117(12):2449–2460. doi: 10.1242/jcs.01232. [DOI] [PubMed] [Google Scholar]
- 67.Wang J, Tolan DR, Pagliaro L. Metabolic Compartmentation in Living Cells: Structural Association of Aldolase. Experimental Cell Research. 1997;237(2):445–451. doi: 10.1006/excr.1997.3811. [DOI] [PubMed] [Google Scholar]
- 68.Sack I, et al. The impact of aging and gender on brain viscoelasticity. NeuroImage. 2009;46(3):652–657. doi: 10.1016/j.neuroimage.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 69.Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407(6807):963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- 70.Unsgaard G, et al. Intra-operative 3D ultrasound in neurosurgery. Acta Neurochirurgica. 2006;148(3):235–253. doi: 10.1007/s00701-005-0688-y. [DOI] [PubMed] [Google Scholar]
- 71.Woerly S, et al. Prevention of gliotic scar formation by NeuroGel™ allows partial endogenous repair of transected cat spinal cord. Journal of Neuroscience Research. 2004;75(2):262–272. doi: 10.1002/jnr.10774. [DOI] [PubMed] [Google Scholar]
- 72.Levental KR, et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.