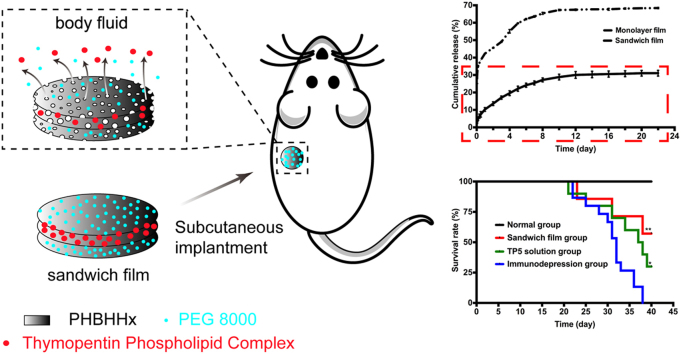
Abstract
Sustained release and non-parental formulations of peptides and protein drugs are highly desirable because of enhanced therapeutic effects as well as improved patient compliance. This is especially true for small peptides such as thymopentin (TP5). To this end, implantable sandwich poly (hydroxybutyrate-co-hydroxyhexanoate) (PHBHHx) films were designed to prolong release time and to inhibit burst release phenomenon of TP5 by a simple volatilization method. In vitro release studies revealed that sandwich films had nearly no burst release. In vivo release time of sandwich films was prolonged to 42 days. Pharmacodynamic evaluation demonstrated that TP5 sandwich films significantly increased survival rates in a rat immunosuppressive model and normalized CD4+/CD8+ values. These results suggest that TP5 released from sandwich films can attenuate cyclophosphamide's immunosuppressive activity, and possibly achieve results comparable to daily TP5 injection therapy. Thus, sandwich PHBHHx films show excellent potential as a sustained, burst-free release system for small molecular weight, hydrophilic peptide drugs.
KEY WORDS: Thymopentin, Sustained release, Burst-free release, Immune enhancement, PHBHHx
Graphical abstract
Subcutaneously implanted sandwich poly(hydroxybutyrate-co-hydroxyhexanoate) (PHBHHx) films were proven to prolong release time and to inhibit burst release phenomenon of thymopentin (TP5). Pharmacodynamics analysis revealed that implantable sandwich films could achieve comparative or even superior therapeutic outcomes to TP5 everyday injection therapy.
1. Introduction
The past decades have witnessed an incredible boom of biopharmaceuticals, mainly peptides and protein drugs. Among them, a synthetic pentapeptide, thymopentin or TP5 (Arg-Lys-Asp-Val-Tyr) has been widely used in clinic for immune enhancement and treatment of immune related diseases such as immune deficiency, cancers and various autoimmune diseases1, 2, 3. It has the ability to induce differentiation of T lymphocytes and to activate mature T lymphocytes in common with its parent molecule, thymic hormone thymopenin4, 5. As an effective immunomodulatory agent, however, TP5 has a very short plasma half-life (ca. 30 s) due to enzymatic degradation, extensive metabolism and poor membrane permeability6. Thus, daily intravenous infusions or injections are essential to ensure effective blood drug concentrations to stimulate CD8+ cells. Because this short half-life severely limits long-term TP5 therapy, a sustained TP5 release system would be highly desirable for improving patient compliance and therapeutic effects.
Biodegradable implantable films offer the potential for convenient and safe sustained release of medication which can improve patient compliance and therapy. In fact, subdermal contraceptive implants for sustained release are already on the market7. Generally, hydrophilic matrices may be unfit for prolonged release delivery system because of their fast dissolution rate in body fluid environment. Hydrophobic polymer poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx), is a member of bacteria-based natural polymer polyhydroxyalkanoates (PHA)8. It has been successfully applied in the field of tissue engineering scaffolds due to its advantageous biological characteristics, adjustable mechanical strength, reliable safety profile and strong hydrophobicity9, 10. In our previous study, we found that PHBHHx nanoparticles incorporating protein drugs were able to prolong their release11, 12. Besides, PHBHHx based 3D scaffolds have been proven to sustain the release of insulin13.
A common limitation of many sustained release systems is known as “burst release”, in which a large drug bolus can be initially released. One example was recently documented for the small molecular weight, hydrophilic peptide TP514, 15. Although the initial rapid release of drug may be appreciated in certain cases, it is usually viewed as a serious drawback because of unpredictable release and ineffective drug usage in terms of long-term controlled release systems. It was reported that the formation of cracks and pores may account for the phenomenon of burst release16. So, reduction in pore formation would largely avoid the phenomenon of burst release.
Thus far no reports have appeared on TP5-based subcutaneous implantable film systems. In the present study, for the first time a sandwich film entrapping TP5 phospholipid complexes was developed. The aim of sandwich film was to decrease burst release and to achieve a longer more stable release period. We investigated the release behavior from TP5 sandwich film systems both in vitro and in vivo, and assessed the therapeutic effects in rodent immunodepression models.
2. Materials and methods
2.1. Materials
Soybean phosphatidylcholines (Lipoid S-100, SPC) was purchased from Lipoid GmBH (Ludwigshafen, Germany). TP5 and C-terminally 5-carboxyfluorescein-labelled thymopentin (FAM-TP5) were gifts of Chengdu Kaijie Biotechnologies Co., Ltd. (Chengdu, China). PHBHHx (MW 534,000) containing 11% (mol/mol) of R-3-hydroxyhexanoate (HHx) was kindly donated by Guoqiang Chen (Tsinghua University, Beijing, China). Cyclophosphamide was product of Sigma (St. Louis, USA). Superoxide dismutase (SOD) kit was obtained from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China). Anti-rat CD3 APC, anti-rat CD4 FITC and anti-rat CD8b PE were supplied by eBioscience (San Diego, CA, USA). All other chemical reagents were of analytical grade.
2.2. Animals
Wistar rats (male, body weight 200–250 g) were obtained from the Experimental Animal Center of Sichuan University and were maintained on a light and dark cycle. All animals were allowed free access to standard rat chow and water. Temperature and relative humidity were kept at 25 °C and 50%, respectively. All animal care and experimental protocols were performed in compliance with the Animal Management Rules of the Ministry of Health of the People's Republic of China (No. 55, 2001) and the guidelines for the Care and Use of Laboratory Animals of Sichuan University (Chengdu, China).
2.3. Preparation of PHBHHx TP5 films
2.3.1. Monolayer films
The biodegradable PHBHHx films were fabricated by a simple solvent evaporation method. Briefly, TP5 and SPC (Lipoid S-100) (1:10, w/w) were prepared into TP5 phospholipid complexes (TP5-PLC) according to our previous study11. Monolayer films were obtained as follows: 30 mg TP5 PLC and 180 mg PHBHHx were solubilized in 1 mL chloroform in a 5 mL beaker. After complete dissolution, chloroform was removed by room temperature evaporation for 24 h, followed by room temperature vacuum evaporation for 48 h. Monolayer FAM-TP5 films were prepared in a similar fashion.
2.3.2. Sandwich films
Sandwich films were prepared as follows: first, 10 mg PEG 8000 and 40 mg PHBHHx were dissolved in 0.2 mL chloroform in a 5 mL beaker. The solvent was dried to form the first layer. Second, 30 mg TP5 PLC and 180 mg PHBHHx were solubilized in 1 mL chloroform. Once fully dissolved, they were dipped onto the first layer, and organic solvent removed again by evaporation and vacuum. Third, 10 mg PEG 8000 and 40 mg PHBHHx were mixed in 0.2 mL chloroform. This mixture was added onto the second layer, and then dried. FAM-TP5 sandwich films were obtained through the same method. All films were sterilized by ultraviolet (UV) for 30 min per side prior to use.
2.4. Solubility of FAM-TP5 in films and chloroform
Fluorescence photomicrographs were recorded in a fluorescence machine. Excitation wavelength set 470 nm, and emission wavelength set 520 nm. In chloroform, equal quantities of FAM-TP5 and SPC physical mixture, FAM-TP5 phospholipid complexes were added into equal volume chloroform, respectively. After 10 min of shaking, chloroform samples were photographed. Monolayer films with FAM-TP5 and SPC physical mixture and films with FAM-TP5 phospholipid complexes were separately fabricated in equal quantity, chloroform volume and same amount of PHBHHx. Film photomicrographs were recorded after samples were dried.
2.5. TP5 film in vitro release
TP5 sandwich films and monolayer films were transferred into dialysis bags (MWCO=3500 Da). Drug release experiments were performed by dynamic dialysis against 2 mL of phosphate buffered saline (PBS) at pH 7.4. The samples were thermostated at 37 °C with continuous agitation speed of 200 rpm for 22 days using a constant shaking incubator (THZ-C-1, Peiying, China). At each pre-established time point, aliquots were withdrawn and replaced with another 2 mL of fresh PBS to maintain sink condition. Drug content in the removed PBS was directly quantified by HPLC method.
2.6. Scanning electron micrographs (SEM) of films
The morphology of films was observed using a conventional SEM (JSM-5900LV, JEOL, Japan) at an accelerating voltage of 5 kV. Briefly, single sandwich film and monolayer film were observed separately after prepared and after being soaked into PBS (pH 7.4) for 5 days.
2.7. Pharmacokinetics of FAM-TP5 films
Male Wistar rats were randomly separated in two groups to receive FAM-TP5 monolayer film (3 mg TP5) or FAM-TP5 sandwich film (3 mg TP5) by subcutaneous implantation into the back, respectively (n=5 per group). Blood samples were collected at scheduled time points and centrifuged at 1469 × g for 10 min. Plasma (0.1 mL) was isolated and mixed with 0.4 mL of ethanol using vortex mixer for 1 min to extract FAM-TP5. The suspension mixture was centrifuged again at 5878 × g for 10 min in order to acquire FAM-TP5 solution. Plasma concentration of 5-FAM-TP5 was measured using an RF-5301PC spectrofluorophotometer (Shimadzu, Japan).
Pharmacokinetic parameters were calculated based on a noncompartmental model using the DAS version 2.0 program. The Cmax represented the maximum plasma concentration of 5-FAM-TP5, and the Tmax meant the time corresponding to Cmax. The AUC0‑t was calculated using the linear trapezoidal rule. The MRT expressed the mean retention time of 5-FAM-TP5.
2.8. Pharmacodynamics of TP5 films
Male Wistar rats were randomly divided into four groups (n=5), of which three were used for experiment and one for control. The rats in experiment groups received cyclophosphamide (CTX) solution intraperitoneally at a dose of 50 mg/kg per five days during the whole 40-day experiment process to implement a long-term chronic immunodepression rat model. Rats in the control group were administered PBS17. Immunodepression group received CTX but without further treatment. Sandwich film group were subcutaneously implanted a TP5 sandwich film (6 mg TP5) after the first time receiving CTX treatment. The TP5 solution group was given TP5 solution (0.6 mg/kg) by tail vein injection every day after the first time CTX treatment. On the 20th and 40th day, blood samples were collected from the rats and loaded into the anticoagulant tubes for the T-lymphocyte subsets analysis and the SOD activity assay.
2.8.1. Analysis of the lymphocyte population
Lymphocyte populations were subgrouped by flow cytometry with three-color analyses. 12.5 μL of antirat CD8b PE, 12.5 μL of antirat CD3 APC and 5 μL of antirat CD4 FITC were added in 100 μL blood sample, mixed well, and incubated in the dark at 25 °C for 30 min. Red blood cells were lysed with hemolysin completely. The samples were washed for 2 times with PBS and centrifuged at 400 × g for 5 min. The supernatant was discarded and the cell samples were resuspended in 0.4 mL PBS. T-lymphocyte subsets were measured within 4 h on a flow cytometer (Cytomices FC 500, Beckman coulter, USA). The number of CD4+ T cells and CD8+ T cells were calculated, as well as ratios of CD4+/CD8+ T cells in the blood samples.
2.8.2. Analysis of superoxide dismutase (SOD) activity
SOD values were measured by SOD kit instructions, got the read of 550 nm in a UV spectrophotometer (Varian, USA). Briefly, 20 μL of plasma samples were used to perform the assay and all the assay validation met the requirements of the SOD kit instruction.
2.9. Statistical analysis
All quantitative data were expressed as mean±standard deviation (SD) values from triplicate measurements, unless otherwise noted. Differences between groups were assessed for significance using single-factor ANOVA, followed by Student's t-test. The threshold for significance was P<0.05. Statistical analysis was performed using Prism 6 (GraphPad Software, USA).
3. Results
3.1. Formation of peptide phospholipid complexes
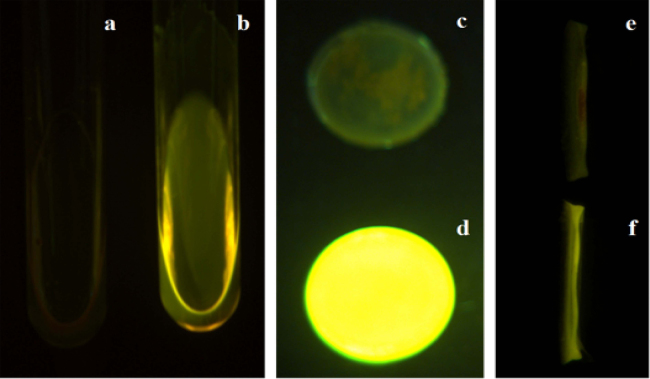
Fluorescence images in Fig. 1 illustrate the solubility of FAM-TP5 in chloroform. The physical mixture of FAM-TP5 was observed to float on the chloroform layer (Fig. 1a), indicating the very limited solubility of FAM-TP5 and phospholipids in chloroform. In contrast, FAM-TP5 can be solubilized uniformly in chloroform after being prepared into FAM-TP5 phospholipid complexes (Fig. 1b), confirming the successful preparation of the peptide phospholipid complexes. FAM-TP5 appeared only on the surface of film due to the physical mixture of FAM-TP5 and phospholipids (Fig. 1c and Fig. 1e). On the contrary, FAM-TP5 distributed homogeneously in the film when prepared with FAM-TP5 phospholipid complexes (Fig. 1d and f).
Figure 1.
Fluorescence pictures of FAM-TP5 in chloroform and films. (a) FAM-TP5 and SPC in chloroform; (b) FAM-TP5 phospholipid complex in chloroform; (c) front side of FAM-TP5 and SPC in PHBHHx film; (d) front side of FAM-TP5 phospholipid complex in PHBHHx film; (e) cross section of FAM-TP5 and SPC in PHBHHx film; (f) cross section of FAM-TP5 phospholipid complex in PHBHHx film.
3.2. In vitro release of TP5 in films
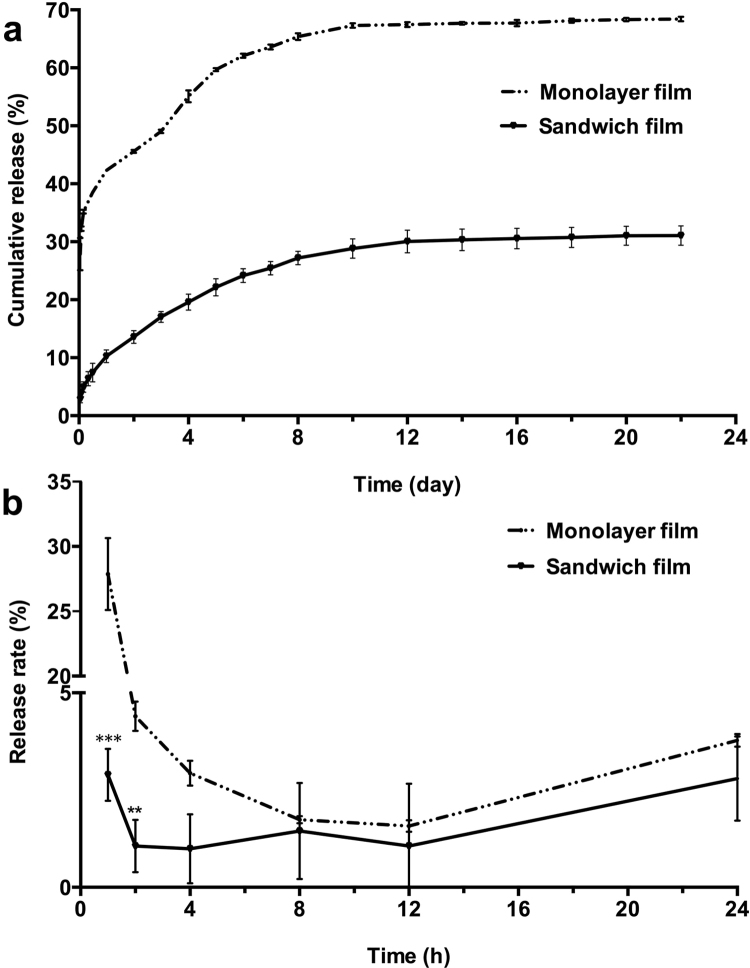
Fig. 2 demonstrates the slow and stable in vitro release of TP5 from sandwich films. Only about 10% of TP5 was released on the first day with nearly no burst release. However, a significant burst release was observed in monolayer films; approximately 42% of TP5 was released on the first day. On the 10th day, sandwich films released about 28% of drug vs. about 67% drug release in monolayer films. TP5 in monolayer films and sandwich films was slightly released in vitro after day 10. From the first day to the 20th day, monolayer films released around 26% of drug and sandwich films released around 21% of drug.
Figure 2.
In vitro TP5 film release profiles. (a) Cumulative release of sandwich films and monolayer films in 22 days. Data are mean±SD, n=3. (b) Release rate of sandwich films and monolayer films in 24 h. **P<0.01, compared with monolayer film group; ***P<0.001, compared with monolayer film group.
Thus, sandwich film decreased the burst release of drug significantly in the first two days (P<0.01). After the initial burst release, the release rate of drug did not significantly differ between the preparations (P>0.05).
3.3. Surface morphology of films
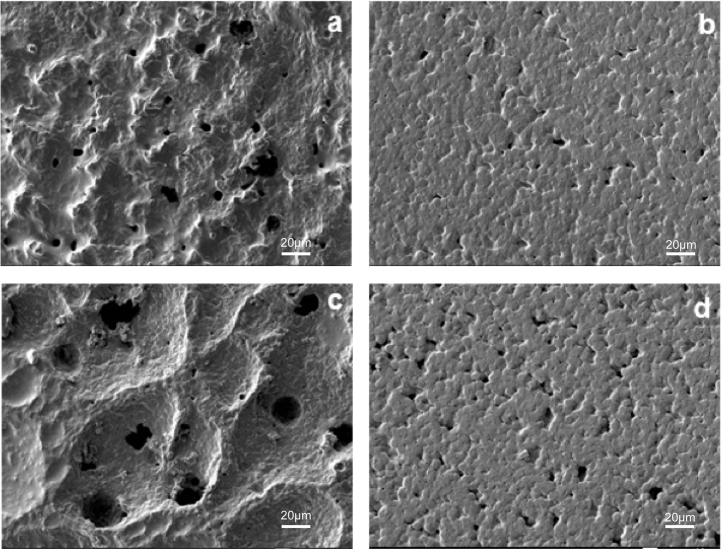
The morphology of the films was investigated using SEM (Fig. 3). The front side of the monolayer film which contained TP5, SPC and PHBHHx appeared lumpy and polyporous. However, that of the sandwich film which contained mainly PEG and PHBHHx appeared to be flatter with shallow holes. Soaking the films in PBS for five days produced distinct erosion in both. However, in monolayer films, variations in the integrity of the film and size of the holes were much more pronounced vs. the sandwich preparation. In the latter, the number and depth of holes were increased by soaking, but the surface remained flat. The enlarged holes produced by soaking the monolayer films is likely to explain the observed increases in burst release of drug as compared with the sandwich films.
Figure 3.
Surface morphology of TP5 films. (a) PHBHHx monolayer film after prepared; (b) PHBHHx sandwich film after prepared; (c) PHBHHx monolayer film in PBS after 5 days; (d) PHBHHx sandwich film in PBS after 5 days. Scale bars represent 20 μm.
3.4. Pharmacokinetics of films
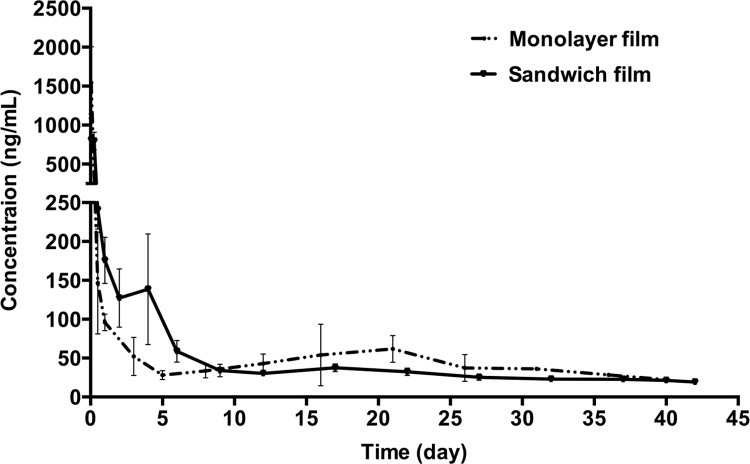
To assess whether subcutaneous films were able to achieve a long circulation lifetime in vivo, pharmacokinetic studies of FAM-TP5 monolayer films and sandwich films were carried out on male Wistar rats. As shown in Fig. 4, both monolayer films and sandwich films had the advantage of sustained release of drug over approximately 40 days. In the sandwich film group, FAM-TP5 remained detectable until day 42. Table 1 summarizes the pharmacokinetic parameters from the film data. Following subcutaneous implantation of films, plasma concentration increased quickly to peak values at 1 h in the monolayer group vs. 4h in the sandwich film group, a statistically significant (P<0.05) difference. Burst release was highly reduced when monolayer film was prepared into sandwich film.
Figure 4.
In vitro release profiles: mean plasma FAM-TP5 concentration-time curve of sandwich films and monolayer films. Data are mean±SD, n=5.
Table 1.
Pharmacokinetics parameters of monolayer films and sandwich films.
| Parameter | Monolayer filma | Sandwich filma |
|---|---|---|
| Cmax (μg/mL) | 1.546±0.462 | 0.981±0.185* |
| Tmax (h) | 1 | 4±2.739* |
| AUC0−t (μg/mL·h) | 49.941±2.757 | 49.724±2.568 |
| MRT0−t (h) | 343.902±37.413 | 257.563±65.14* |
| t1/2 (h) | 295.012±35.56 | 492.247±282.739 |
Data are mean±SD, n=5.
P<0.05, compared with monolayer films.
3.5. Pharmacodynamics of TP5 sandwich films
3.5.1. Change of body weight
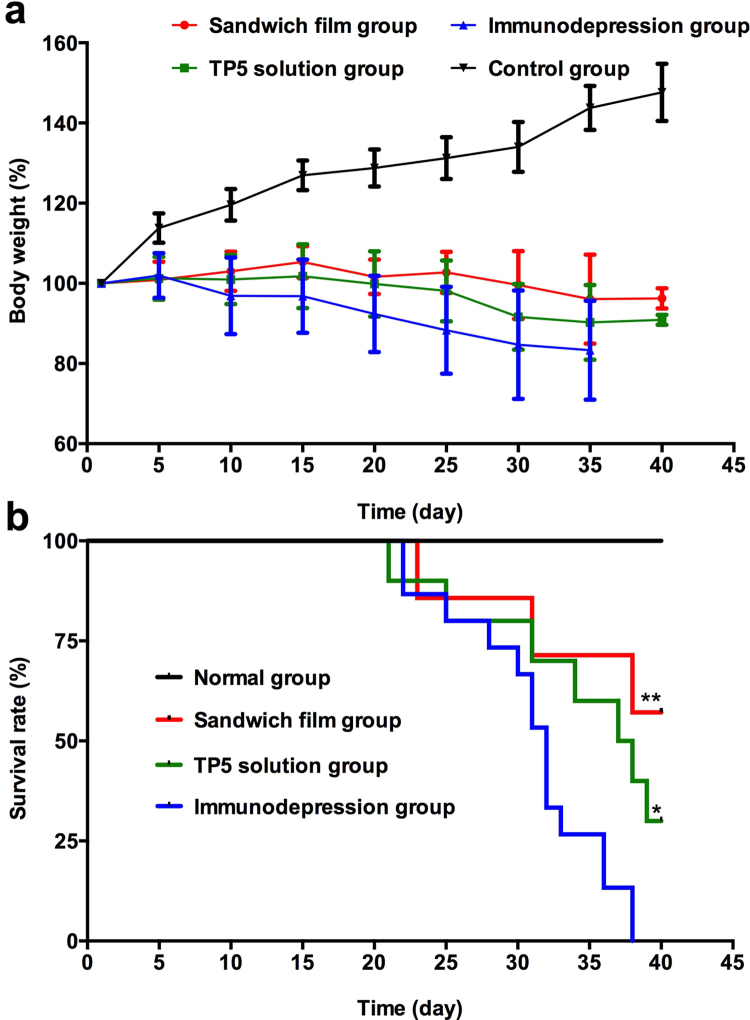
The body weight of control rats increased steadily throughout the experiment. Under the same conditions, rats in TP5 sandwich film group and TP5 solution group nearly maintained their body weight. Subjects in the immunodepression group continuously lost weight until death.
3.5.2. Survival rate
To investigate the immunomodulating effects of TP5 sandwich films, we compared it with the effects in the immunodepression group. As seen in Fig. 5b, there was a highly significant difference between TP5 sandwich film and immunodepression model groups (P<0.01), suggesting that the sandwich film increased the survival rate of immunodepressed rats. In addition, there was a significant difference between the TP5 solution group and immunodepression group (P<0.05), indicating that daily TP5 injections improved survival in immunosuppressed subjects.
Figure 5.
Protection of TP5 sandwich films against cyclophosphamide challenge. (a) Changes of body weight. (b) Survival rates. Data are mean±SD, n=15. *P<0.05,**P<0.01, compared with immunodepression group.
3.5.3. Change of CD4+/CD8+ value and SOD value
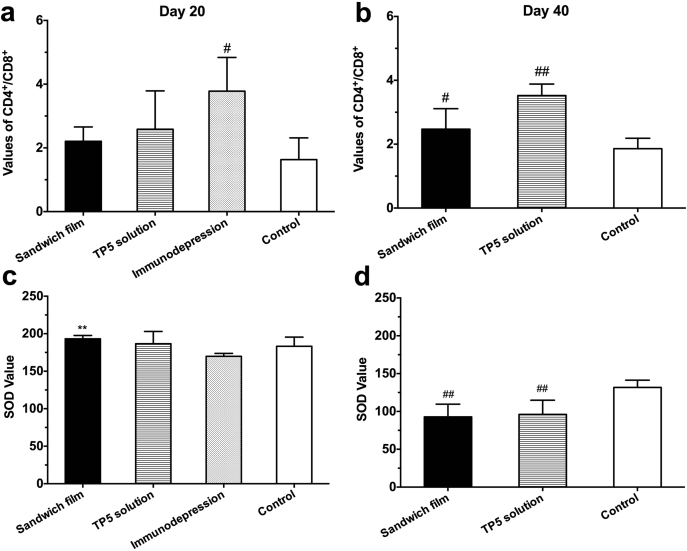
CD4+/CD8+ values indicate the level of immunological function. For example, immunologically deficient patients show an abnormally altered CD4+/CD8+ ratio18. The immunomodulatory effects of subcutaneous TP5 sandwich film and conditional TP5 solution were evaluated in a rat immunodepression model. Results from day 20 of the experiment (Fig. 6a) demonstrated a significant difference between control and immunodepression groups (P<0.05). The increase in the CD4+/CD8+ ratio to 3.78 in the immunodepression group showed that CTX had already modulated T-lymphocyte activity, confirming the adequacy of the model. Sandwich film and TP5 solution groups showed no significant differences vs. the control group (P>0.05), indicating TP5 released from sandwich films and solution did not attenuate the destruction of CTX to the body's immune system. Subjects did not survive beyond day 40 in the immunodepression group (Fig. 6b). Meanwhile, there was a significant difference (P<0.01) between TP5 solution group and control group indicating that daily injections of the TP5 solution can increase survival without normalizing the CD4+/CD8+ ratio.
Figure 6.
Pharmacodynamics of PHBHHx sandwich TP5 films. (a) Values of CD4+/CD8+ T-lymphocyte subsets in blood of rats on the 20th day. Data are mean±SD, n=6. (b) Values of CD4+/CD8+ T-lymphocyte subsets in blood of rats on the 40th day. Data are mean±SD, n=5. (c) SOD values on the 20th day. Data are mean±SD, n=6. (d) SOD values on the 40th day. Data are mean±SD, n=5. **P<0.01, compared with immunodepression group; #P<0.05, compared with control group; ##P<0.01, compared with control group.
Examination of data on day 20 (Fig. 6c) showed that CTX did not decrease the SOD level in either group. However, the SOD values of sandwich films increased significantly compared with those of immunodepression group (P<0.01). This may be due to immune-enhancing effects of the sustained release of TP5 from sandwich films. From Fig. 6d, both film group and solution group on the 40th day had significant differences from the control group suggesting the advanced nature of the immunodepression by this time. Thus, TP5 in either group could not increase SOD values to normal (P < 0.01).
4. Discussion
In this study, we designed a novel sandwich film to obtain a prolonged release profile of TP5 and to address the problem of burst release. This sandwich film contained TP5 PLC in the middle PHBHHx layer, which was entrapped between PEG and PHBHHx layers. Compared to PHBHHx monolayer films, sandwich films presented a more stable and prolonged release profile.
In order to obtain a sustained release profile for hydrophilic peptide drugs, a hydrophobic biodegradable polymer PHBHHx was chosen as the main film matrix because of its safety profile and slow dissolution rate in body fluid. However, the difference in solubility of these agents presents a challenge to entrap the small molecular weight, hydrophilic peptide TP5 into this matrix. Improving the lipophilicity of hydrophilic TP5 would remarkably promote its homogeneous dispersion through the PHBHHx films. This was achieved with TP5 and PHBHHx in chloroform. Our previous study found that the solubility of TP5 in chloroform increased 786 times by preparing TP5 phospholipid complex (TP5-PLC)11. This approach made it possible to entrap TP5 in the PHBHHx film by a simple volatilization method.
In vitro release studies confirmed a burst release phenomenon in monolayer films, but the release of TP5 from the sandwich films was much more stable even on the 1st day. TP5 was released from the films mainly through two ways. First, TP5 entrapped on the surface of the films was rapidly released because of its easy access to water medium. This may lead to monolayer films' burst release at the initial stage. Since the outer layers of PHBHHx/PEG8000 in sandwich films contain very little TP5, a much smaller burst release mechanism would be expected. Secondly, TP5 enclosed in the inner space of films is released slowly by diffusion through the film skeleton, or following degradation of the skeleton. Due to its strong hydrophobic property and high molecular weight (MW=53,400), the PHBHHx matrix is suggested to have impeded diffusion to body fluid or release media. Thus, TP5 is thought to have a much lower probability of burst release, rarely observed in literature19. Other findings revealed that PHBHHx films lost only 2%–6% of its weights in phosphate buffered saline solution containing lipase after 50 days20. The addition of PEG8000 played a vital role in TP5 released from PHBHHx sandwich films. On one hand, PEG8000 was more likely to dissolve in water, thus promoted water's infusion into films. On the other hand, its addition made the film surface more flat and compact, which was verified by SEM. Thus, the release rate from the sandwich film is determined by the dissolution rate of PEG 8000 and the degradation rate of PHBHHx.
Present results showed a greater cumulative release percentage from monolayer films than from sandwich films. It was reported that crystallinity and surface morphology of PHBHHx are two important factors affecting its biodegradation20. It was easy to see that monolayer films were more polyporous and lumpy than sandwich films, which granted easier access to water. Thus, hydrolysis of PHBHHx would be more rapid and complete in monolayer films than in sandwich films. In the present study, the strong hydrophobicity and relatively slow degradation of PHBHHx resulted in slower release of TP5 from sandwich films with nearly no burst release (Fig. 2a), which possibly met the requirement of long term in vivo release of TP5.
Previous studies showed that thymopentin was very susceptible to proteases in human plasma and was readily cleaved to the tetrapepetide (TP4, Lys-Asp-Val-Tyr) and the tripeptide (TP3, Asp-Val-Tyr)6, 21. While TP4 and TP3 still presented similar immunomodulatory properties to TP5, it was not considered to only measure the amount of TP5 in the plasma to characterize pharmacokinetics22, 23. In this study, FITC labeled TP5 was used for pharmacokinetic analysis to fully relate blood drug concentration to its effects. In vivo release curves showed that there was still FITC-TP5 detectable in sandwich film group in 42 days. The release duration time was much longer than that in vitro, which lasted for about 10 days. This may be ascribed to a higher hydrolysis rate of PHBHHx in vivo than in vitro.
TP5 exerts its immunomodulatory effects by promoting T lymphocyte differentiation and maturation. As important immunoregulatory cells, peripheral blood T lymphocytes with feature marker CD3 are comprised of at least three subsets, γδ+, CD4+, and CD8+. CD4+ and CD8+ represent maturation and activation of T lymphocytes24. T lymphocytes with CD4+ marker play an important role in regulating lymphocyte functions, while those with CD8+ marker take an active part in controlling acute viral infection. Many investigations of T lymphocyte populations have suggested that peripheral blood T lymphocytes for normal humans are validated to be stable in CD4+/CD8+ ratios (1.5–2.0). A biased ratio of CD4+ to CD8+ is a surrogate marker of impaired cell-medicated immunity in pathological disorders25. The biological significance of CD4+ and CD8+ molecules during activation of T lymphocytes make them an index of pharmacodynamic evaluation.
Superoxide dismutase (SOD) is one of the principal enzymes of the antioxidant system which is an index of the body's immune status. It catalyzed the dismutation of superoxide radical (O2) into hydrogen peroxide (H2O2) and elemental oxygen (O2). As such, SOD provides an important defense against toxicity of superoxide radical26. Our results showed that sandwich TP5 films could increase SOD activity in the first 20 days. However, by the 40th day, SOD values were significantly lower than normal. This suggests that CTX had caused irreversible destruction to immune system which could not be prevented or reversed by TP5. Our pharmacodynamic analysis demonstrates that TP5 solution therapy is not better than that of TP5 sandwich films. However, the obvious advantage of implanting a long-lasting TP5 delivery system vs daily solution injections suggests that sandwich films may provide a promising alternative for TP5 delivery.
5. Conclusions
In this paper, sandwich PHBHHx TP5 films were prepared using an ordinary volatilization method to provide an alternative delivery system for TP5. Physical mixtures of TP5 and phospholipids were theoretically very difficult to dissolve even in chloroform, but we prepared a TP5 phospholipid complex which could be distributed homogeneously in the same solvent, then prepare uniformity in a film. In vitro, monolayer films had a significant burst release phenomenon, which could be attributed to enlarged diameter of holes and increased extent of fluctuation. Holes in monolayer films led to a large burst release of drug. In contrast, sandwich films showed a lower increase in number and depth of holes, and a highly reduced burst release phenomenon. After monolayer films were prepared into sandwich films, release time was prolonged to 42 days, which meant sandwich films could decrease burst release phenomenon significantly and had a better chance to achieve sustained release. The survival rate results showed that sandwich film group and TP5 solution group could significantly increase survival rate compared to the immunodepression group, which meant that TP5 released from sandwich films and solution may attenuate the destruction of the body's immune system by CTX. Pharmacodynamic evaluation of the change in CD4+/CD8+ and SOD values showed that daily TP5 solution injections can only increase survival rate but not normalize CD4+/CD8+ values. Compared with control group, film group and solution group both formed immunodepression model and TP5 in both group could not increase SOD values to normal. The results of the present study show that it is feasible to design a controlled-release delivery system by building up sandwich PHBHHx TP5 films for treatments.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81673362).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Coppola S., Buccoliero G., Laddago V., Monno L., Perrone A., Guida G. Topical thymopentin therapy in HIV positive patients with recurrent oral candidiasis: a pilot study. New Microbiol. 1996;19:351–355. [PubMed] [Google Scholar]
- 2.Sundal E., Bertelletti D. Thymopentin treatment of rheumatoid arthritis. Arzneimittelforschung. 1994;44:1145–1149. [PubMed] [Google Scholar]
- 3.Xiaojing C., Yanfang L., Yanqing G., Fangfang C. Thymopentin improves cardiac function in older patients with chronic heart failure. Anatol J Cardiol. 2017;17:24–30. doi: 10.14744/AnatolJCardiol.2016.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein G., Scheid M.P., Boyse E.A., Schlesinger D.H., Van Wauwe J. A synthetic pentapeptide with biological activity characteristic of the thymic hormone thymopoietin. Science. 1979;204:1309–1310. doi: 10.1126/science.451537. [DOI] [PubMed] [Google Scholar]
- 5.Fan Y.Z., Chang H., Yu Y., Liu J., Zhao L., Yang D.J. Thymopentin (TP5), an immunomodulatory peptide, suppresses proliferation and induces differentiation in HL-60 cells. Biochim Biophys Acta. 2006;1763:1059–1066. doi: 10.1016/j.bbamcr.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Tischio J.P., Patrick J.E., Weintraub H.S., Chasin M., Goldstein G. Short in vitro half-life of thymopoietin 32–36 pentapeptide in human plasma. Int J Pept Protein Res. 1979;14:479–484. doi: 10.1111/j.1399-3011.1979.tb01959.x. [DOI] [PubMed] [Google Scholar]
- 7.Peralta O., Diaz S., Croxatto H. Subdermal contraceptive implants. J Steroid Biochem Mol Biol. 1995;53:223–226. doi: 10.1016/0960-0760(95)00051-z. [DOI] [PubMed] [Google Scholar]
- 8.Chen G.Q., Wu Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials. 2005;26:6565–6578. doi: 10.1016/j.biomaterials.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 9.Chen G.Q., Wu Q., Wang Y.W., Zheng Z. Application of microbial polyesters-polyhydroxyalkanoates as tissue engineering materials. Key Eng Mat. 2005;288-289:437–440. [Google Scholar]
- 10.Qu X.H., Wu Q., Zhang K.Y., Chen G.Q. In vivo studies of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) based polymers: biodegradation and tissue reactions. Biomaterials. 2006;27:3540–3548. doi: 10.1016/j.biomaterials.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Wu C., Zhang M., Zhang Z., Wan K.W., Ahmed W., Phoenix D.A. Thymopentin nanoparticles engineered with high loading efficiency, improved pharmacokinetic properties, and enhanced immunostimulating effect using soybean phospholipid and PHBHHx polymer. Mol Pharm. 2014;11:3371–3377. doi: 10.1021/mp400722r. [DOI] [PubMed] [Google Scholar]
- 12.Peng Q., Zhang Z.R., Gong T., Chen G.Q., Sun X. A rapid-acting, long-acting insulin formulation based on a phospholipid complex loaded PHBHHx nanoparticles. Biomaterials. 2012;33:1583–1588. doi: 10.1016/j.biomaterials.2011.10.072. [DOI] [PubMed] [Google Scholar]
- 13.Peng Q., Yang Y.J., Zhang T., Wu C.Y., Yang Q., Sun X. The implantable and biodegradable PHBHHx 3D scaffolds loaded with protein-phospholipid complex for sustained delivery of proteins. Pharm Res. 2013;30:1077–1085. doi: 10.1007/s11095-012-0944-9. [DOI] [PubMed] [Google Scholar]
- 14.Khang G., Kim S.W., Cho J.C., Rhee J.M., Yoon S.C., Lee H.B. Preparation and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) microspheres for the sustained release of 5-fluorouracil. Biomed Mater Eng. 2001;11:89–103. [PubMed] [Google Scholar]
- 15.Wei G., Jin L., Xu L., Liu Y., Lu W. Preparation, characterization and in vivo pharmacodynamic evaluation of thymopentin loaded poly(lactide acid)/poly(lactide-co-glycolide acid) implants. Int J Pharm. 2010;398:123–129. doi: 10.1016/j.ijpharm.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 16.Park T.G., Cohen S., Langer R. Controlled protein release from polyethyleneimine-coated poly(l-lactic acid)/pluronic blend matrices. Pharm Res. 1992;9:37–39. doi: 10.1023/a:1018971525301. [DOI] [PubMed] [Google Scholar]
- 17.Lallo M.A., Bondan E.F. Experimental meningoencephalomyelitis by Encephalitozoon cuniculi in cyclophosphamide-immunosuppressed mice. Arq Neuropsiquiatr. 2005;63:246–251. doi: 10.1590/s0004-282x2005000200010. [DOI] [PubMed] [Google Scholar]
- 18.McBride J.A., Striker R. Imbalance in the game of T cells: what can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 2017;13:e1006624. doi: 10.1371/journal.ppat.1006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin Y., Chen D., Qiao M., Lu Z., Hu H. Preparation and evaluation of lectin-conjugated PLGA nanoparticles for oral delivery of thymopentin. J Control Release. 2006;116:337–345. doi: 10.1016/j.jconrel.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y.-W., Mo W., Yao H., Wu Q., Chen J., Chen G.-Q. Biodegradation studies of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Polym Degrad Stab. 2004;85:815–821. [Google Scholar]
- 21.Amoscato A.A., Balasubramaniam A., Alexander J.W., Babcock G.F. Degradation of thymopentin by human lymphocytes: evidence for aminopeptidase activity. Biochim Biophys Acta. 1988;955:164–174. doi: 10.1016/0167-4838(88)90190-2. [DOI] [PubMed] [Google Scholar]
- 22.Denes L., Szende B., Hajos G., Szporny L., Lapis K. Therapeutic possibilities of thymopoietin fragments (TP3 and TP4) based on experimental animal models. Drugs Exp Clin Res. 1987;13:279–287. [PubMed] [Google Scholar]
- 23.Rajnavolgyi E., Kulics J., Szilagyvari M., Kisfaludy L., Nyeki O., Schon I. The influence of new thymopoietin derivatives on the immune response of inbred mice. Int J Immunopharmacol. 1986;8:167–177. doi: 10.1016/0192-0561(86)90056-1. [DOI] [PubMed] [Google Scholar]
- 24.Janeway C.A., Jr. The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol. 1992;10:645–674. doi: 10.1146/annurev.iy.10.040192.003241. [DOI] [PubMed] [Google Scholar]
- 25.Tositti G., Rassu M., Fabris P., Giordani M., Cazzavillan S., Reatto P. Chlamydia pneumoniae infection in HIV-positive patients: prevalence and relationship with lipid profile. HIV Med. 2005;6:27–32. doi: 10.1111/j.1468-1293.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 26.Treitinger A., Spada C., Verdi J.C., Miranda A.F., Oliveira O.V., Silveira M.V. Decreased antioxidant defence in individuals infected by the human immunodeficiency virus. Eur J Clin Invest. 2000;30:454–459. doi: 10.1046/j.1365-2362.2000.00642.x. [DOI] [PubMed] [Google Scholar]