Abstract
Lentiviruses are highly efficient vehicles for delivering genes into cells. They readily transduce primary and immortalized cells in vivo and in vitro. Genes delivered by lentiviruses are incorporated and replicated as part of their host genome and therefore offer a powerful tool for creation of stable cell lines and transgenic animals. However, the zona pellucida surrounding the fertilized eggs acts as a barrier and hinders lentiviral transduction of embryos. Here, we utilize a laser, typically used to perforate the zona pellucida for in vitro fertilization, to permeabilize the zona for lentiviral gene delivery. A single hole in the zona is sufficient for the lentivirus to gain access to fertilized eggs without the need for microinjection for en masse gene delivery. Embryos generated by this method elicit no damage and can develop to term for creation of transgenic animals.
Keywords: gene delivery, lentivirus, XYclone laser, mouse fertilized eggs, mouse embryos
Introduction
Lentiviruses are members of the Retroviridae family of viruses that readily infect dividing and non-dividing mammalian cells delivering large amounts of genetic material. Their genome consists of two single stranded RNAs that shortly after infection are converted into DNA by the lentiviral reverse transcriptase protein (Stoye 2012). Upon entry into the cell nucleus, lentiviral DNA is inserted into the host chromosome by the integrase. Lentiviral transduction results in permanent incorporation of genetic material and delivered genes are replicated and passed on to daughter cells as part of the host genome. Pseudotyping lentiviruses with vesicular stomatitis virus glycoprotein (VSVG) broadens the range of lentiviral host cells and creates a robust research tool for gene delivery and creation of stable cell lines (Salmon and Trono 2007, Sakuma, Barry et al. 2012).
The rapid and highly-efficient stable integration of lentiviral genome into the host chromosome also offers a powerful mechanism for stable expression of genes in fertilized eggs, embryos, and creation of transgenic animals. Lentiviruses have been used to create transgenic mice, rats, chickens, sheep, quail, and pigs (Lois, Hong et al. 2002, Filipiak and Saunders 2006, McGrew, Sherman et al. 2010, Zhang, Xi et al. 2012, Zhang, Sun et al. 2012, Liu, Wang et al. 2013). For previous lentiviral transgenesis, viral particles were delivered via single cell embryo microinjection into the perivitelline cavity, the space between the zona pellucida and the fertilized egg, since the zona pellucida is a potent barrier against lentiviral transduction. In order to produce a transgenic embryo and subsequent genetically modified mice, single cell embryos were held in place by a holding pipet while a microinjection needle delivers the virus through the zona pellucida. While a skilled microinjectionist would find sub-zona microinjection trivial, the initial acquisition of a microinjection workstation and the subsequent steep learning curve for embryo manipulation and microinjections served as a strong entry barrier for new groups hoping to utilize lentiviruses to generate transgenic mouse lines. Therefore, while easier than pronuclear single cell embryo microinjection, the need to manipulate the embryo for sub-zona microinjection minimized the use of lentiviral gene delivery in transgenic research.
The zona pellucida is a multifunctional porous matrix of glycoproteins that envelopes mammalian oocytes and preimplantation-stage fertilized eggs to limit environmental interactions (Wassarman 1988, Clift and Schuh 2013). Moreover, sperm receptors on the zona secure species-specific fertilization and block polyspermy. Upon fertilization of oocytes, rapid chemical modifications initiate crosslinking of the zona matrix glycoproteins to form a barrier around the fertilized eggs. The zona hardens to establish a protective shell around the embryo during its journey through the female reproductive tract. The zona’s primary function of protecting the early embryo from environmental insults including viral infection is in direct conflict to viral based gene delivery.
Early attempts to chemically remove or weaken the zona for gene delivery have proven harmful and severely impede embryonic development and maturation (Nijs and Van Steirteghem 1987). Without the zona, fertilized eggs are vulnerable, adhesive, and are easily damaged. In conventional transgenic microinjection technique a “hatched” zona-less embryo is essentially deemed non-viable. The most commonly used methods for penetration of the zona for gene delivery is single cell embryo microinjection. Purified DNA for random host genome integration can be delivered directly to the nucleus via pronuclear microinjection (Gordon, Scangos et al. 1980) or viral elements can be microinjected beneath the zona for integrase-mediated genomic integration (Lois, Hong et al. 2002). Cultured rodent fertilized eggs can withstand microinjection with minimal damage and this method has been used to originate a number of transgenic animal models (Ikawa, Tanaka et al. 2003, Filipiak and Saunders 2006). Although highly effective, micromanipulation and microinjection of single cell embryos is a laborious practice that require highly skilled technologists and costly microinjection workstations.
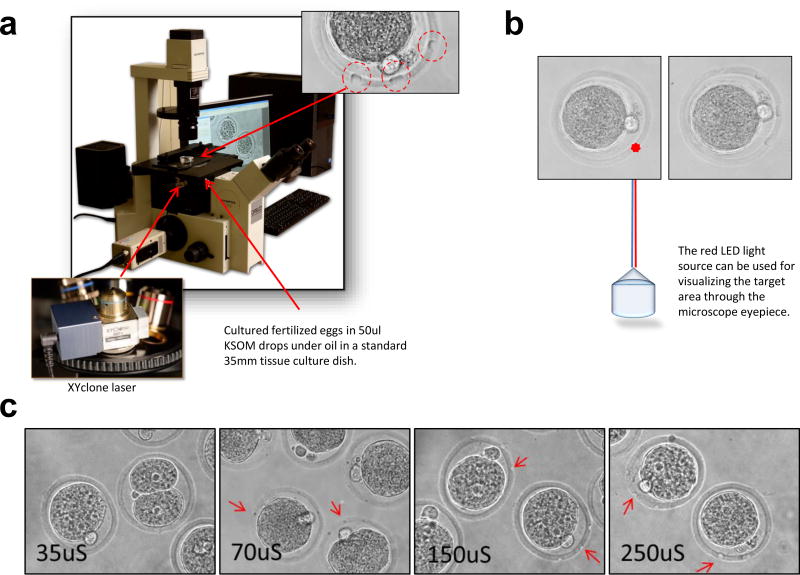
In order to facilitate gene delivery to mouse fertilized eggs, we report that perforating the zona using the XYclone laser (Hamilton Thorne Biosciences, Beverly, MA) renders fertilized eggs permeable for gene delivery using lentiviruses. The XYclone laser used in this study was initially designed for in vitro fertilization or thinning of the zona to facilitate sperm entry into unfertilized eggs and does not adversely affect the development of embryos to blastocysts (Woods, Qi et al. 2014). The XYclone laser is a small apparatus that is mounted in place of an objective lens on an inverted microscope (Fig. 1a). The controlling software allows for rapid laser fire while looking through the microscope eyepieces. A visible LED light serves as an indicator for the targeted area (Fig. 1b). The XYClone laser perforates targeted areas in the zona without micromanipulation. A novice user can utilize the system effectively to perforate the zona pellucida of free floating single cell embryos within minutes of first use. Conventional embryo microinjection requires more extensive training to develop expertise. By moving the LED light to the desired location on the zona while looking through the microscope eyepieces, the zona of large number of fertilized eggs can be perforated within minutes (Fig. 1b and 1c).
Fig. 1. An Infrared Laser Perforation System to Perforate the Zona.
(a) The laser is housed in a lens and can be attached to the turret of most inverted microscopes. The laser permits perforation of targeted areas in zona without micromanipulation of the embryos. Perforations in the zona are circled in red. Perforation size is controlled by the duration of laser firing, typically 250 microseconds. Photographs provided by Steven R. McCaw, NIEHS Multimedia Services. (b) The factory-aligned laser is locked in place and contains a red LED light source for visualizing the target area. The controlling software allows for rapid laser fire while looking through the microscope eyepieces. The fertilized eggs are laser treated while free floating in KSOM drops – before (left) and after (right) images of an embryo. (c) Size of the perforation by the XYclone laser directly affected the efficiency of gene delivery. Perforations generated by the laser treatments ranged from 35uS to 250uS. Larger perforations allowed for a more effective viral transduction. The results of seven independent experiments are shown in Table 2 summarizing the number of blastocysts and their fluorescence used in the experiments.
By combining the easy to use laser zona perforation method and gene delivery via lentiviruses, we were able to rapidly and efficiently transduce multiple single cell embryos in a drop of KSOM media. The lentiviral vectors were pipetted directly into the culture media containing the perforated embryos allowing for en masse infection and transduction. The embryos were cultured with the virus until they developed into blastocysts and non-surgically transferred into pseudopregnant mice, so viability and gene expression could be assessed. The laser-assisted lentiviral gene delivery can be used to transduce fertilized eggs in bulk, takes less time for a novice user, and removes many of the technical limitations imposed by conventional micromanipulation and microinjection. Lentiviral gene delivery also allows for spatiotemporal control of gene expression.
Results
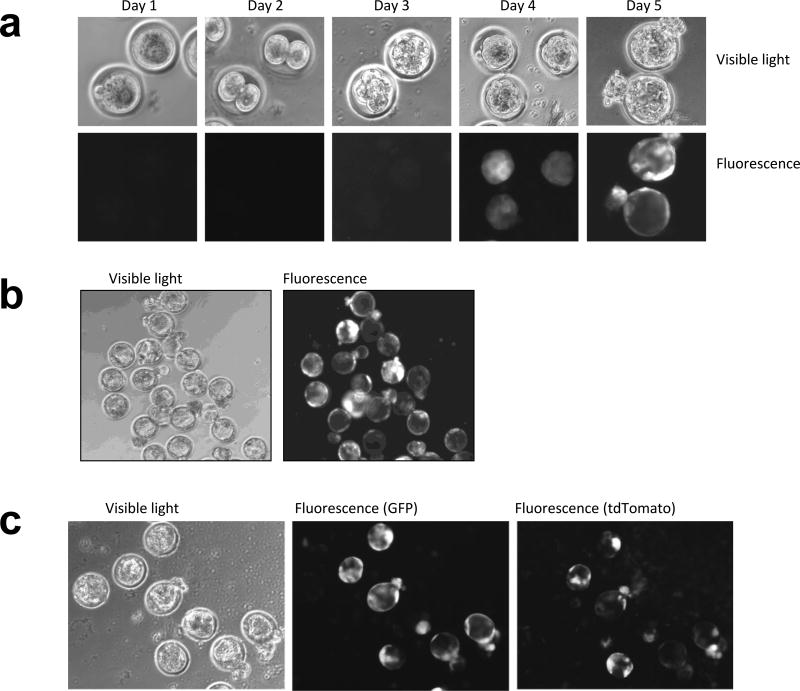
In the present study, either Crl:CD-1 (ICR) or C57BL/6J mouse single cell embryos were harvested and cultured according to standard protocols (see Material and Methods). The cumulus cells were removed by treating with 0.1% hyaluronidase in M2 media and the fertilized eggs were transferred to KSOM media under 5% CO2, 5% O2, and 90% N2, 37°C conditions for development. Single embryos were then incubated for two hours prior to laser zona pellucida perforation while free floating in KSOM media. For gene delivery, we used a lentiviral vector carrying a copepod green fluorescence protein (copGFP; abbreviated to GFP) gene driven by a constitutive elongation factor 1α (EF1α) promoter. 0.5–3 uL of concentrated GFP lentivirus (titer 108–109 transducing units per ml) was pipetted directly into the KSOM media containing the fertilized eggs with laser-perforated zona pellucida. In the following 3 days, embryos were cultured in KSOM media under 5% CO2, 5% O2, and 90% N2, 37°C conditions without further treatment to allow for the viral genome integration and the development into blastocysts. The expression of GFP was evident at the morula stage in cultured embryos of Crl:CD-1 (ICR) and the inbred strain C57BL/6J (Fig. 2a). Most laser perforated embryos were readily infected by the lentivirus and expressed GFP (Fig. 2b and Table 1). The laser-assisted lentiviral gene delivery can also be used to deliver multiple genes simultaneously. We exposed perforated fertilized eggs to two viruses carrying either GFP or tdTomato while cultured in KSOM. They expressed either both GFP and tdTomato fluorescence or no fluorescence (Fig. 2c). 80% of fertilized eggs expressing both GFP and tdTomato developed to blastocysts in culture. The number of fertilized eggs used for the three independent experiments and the expression of fluorescence in blastocysts are summarized in Table 1.
Fig. 2. En Masse Gene delivery to Fertilized Eggs.
(a) Development of C57BL/6J embryos from harvest (Day1) until the time of blastocyst implantation (Day 5). The embryos were perforated by the Xyclone laser (3 holes, 250uS) and treated with 2uL of high titer lentivirus (108–109 TU/ml) in 50uL KSOM drops. The presence of fluorescence was detected in days 3 and 4 and persisted until the time of implantation. The data is representative of five independent experiments - 49 blastocysts formed out of 82 treated fertilized eggs, 44 out of 49 blastocysts expressed GFP. (b) Most blastocysts infected with the lentivirus delivering GFP expressed fluoresce at the time of implementation. Cultured blastocysts from Crl:CD-1 (ICR) strain of mice are shown in the figure. (c) Co-infection of fertilized eggs with viruses expressing either GFP or tdTomato readily expressed both genes after 4 days. The pattern of expression for GFP and tdTomato in infected blastocysts are not identical although both genes are expressed from the EF1α promoter.
Table 1.
Number of resulting blastocyst following XYclone and/or lentiviral treatment
| Laser-Treatment Crl:CD-1 (ICR) |
Virus | # of Fertilized Eggs |
# Blastocyst | # of Fluorescent Blastocyst |
%Blastocyst | % Fluorescent Blastocyst |
|---|---|---|---|---|---|---|
| no treatment | - | 55 | 37 | 0 | 67% | 0 |
| 1 hole | - | 35 | 26 | 0 | 74% | 0 |
| 3 hole | - | 49 | 44 | 0 | 90% | 0 |
| 9 hole | - | 34 | 25 | 0 | 74% | 0 |
| 1 hole | 2ul GFP | 115 | 55 | 39 | 60% | 71% |
| 3 hole | 0.5ul GFP | 33 | 22 | 19 | 67% | 86% |
| 3 holes | 1ul GFP | 38 | 18 | 17 | 47% | 94% |
| 3 holes | 3ul GFP | 89 | 24 | 24 | 27% | 100% |
| 3 holes | 1ul GFP 1ul tdTomato | 64 | 53 | 51 | 80% | 96% |
| Laser-Treatment C57BL/6J |
Virus | # of Fertilized Eggs |
# Blastocyst | # of Fluorescent Blastocyst |
%Blastocyst | % Fluorescent Blastocyst |
|---|---|---|---|---|---|---|
| no treatment | - | 64 | 42 | 0 | 66% | 0 |
| 1 hole | - | 59 | 46 | 0 | 78% | 0 |
| 3 hole | - | 55 | 50 | 0 | 91% | 0 |
| 9 hole | - | 56 | 44 | 0 | 79% | 0 |
| 3 hole | 0.5ul GFP | 53 | 38 | 30 | 72% | 79% |
| 3 holes | 1ul GFP | 57 | 31 | 30 | 54% | 97% |
| 3 holes | 3ul GFP | 48 | 12 | 12 | 25% | 100% |
XYclone treatment of fertilized eggs had no adverse effect on the rate of embryonic development to blastocyst. Results for both strains of mice used in this study are listed. Laser-treatment resulting in 3 holes significantly improved blastocyst development (One-tailed T-test statistical analysis; 1 hole: P<0.5 for both strains; 3 holes: p=<0.005 for both strains; 9 holes: p<0.5 only for the Crl:CD-1 (ICR) strain). Addition of virus reduced the rate of blastocyst formation. The data is the sum of at least three independent experiments. Number of holes reflects the number of perforations by the XYclone laser on the surface of the zona. All holes were created using 250uS XYclone laser setting. The amount and type of virus added to each sample was shown in uL in Description.
Comparison between perforated and untreated fertilized eggs suggests that laser perforation was not harmful to the fertilized eggs. Earlier studies have evaluated laser-based microdrilling and use of lentiviruses for gene delivery to bovine oocyte and fertilized eggs (Ewerling, Hofmann et al. 2006). However, laser treatment resulted in reduced rate of bovine blastocyst formation in culture. The XYclone laser perforation of fertilized eggs had no adverse effect on the rate of embryonic development to blastocyst (66–67% for untreated fertilized eggs compared to 74–78%, 90–91%, and 74–79% containing 1, 3, and 9 holes on the perforated zona, respectively; Table 1). Interestingly, laser-perforation and perforation of the zona in mouse fertilized eggs improved the rate of development into blastocyst. This result echoes the reports that laser thinning of the zona pellucida has been shown to improve hatching of human embryos and enhance implantation (Blake, Forsberg et al. 2001, Kohli, Robles et al. 2007, Hiraoka, Fuchiwaki et al. 2008, Hammadeh, Fischer-Hammadeh et al. 2011). We hypothesize that laser-perforation improved access to nutrients in the cultured media and boosted development. Further studies are needed to determine the exact cause of this enhancement.
On the other hand, increasing the amount of lentivirus used for gene delivery adversely affected the development of fertilized egg into blastocysts similar to other reported studies (Lois, Hong et al. 2002). Although a single hole in the zona following laser perforation was sufficient for lentiviral gene delivery, the most effective means of laser-assisted lentiviral gene delivery was achieved by laser perforation of the zona in three places (250uS laser setting) and the use of 2ul of concentrated lentivirus (titer 108–109 transducing units per ml) in our experiments (Table 1). We chose to laser perforate the area close to the polar body of the fertilized eggs to take advantage of the distance between the zona and the cell body and to minimize damage. We also observed GFP expression in 5% of fertilized eggs without laser-perforation but treated with the lentivirus carrying GFP (Table 2). We hypothesize that the naturally occurring damage to the zona could also render the fertilized eggs permissive to lentiviral infections. Although, this is a rare occurrence.
Table 2.
Number of resulting fluorescent blastocyst following XYclone perforation
| Laser Treatment |
Virus | Laser Setting (uS) |
# of Fertilized Eggs |
# of Blastocyst |
# Fluor Blastocyst |
%Blastocyst | % Fluor Blast |
|---|---|---|---|---|---|---|---|
| no holes | 2ul | 0 | 102 | 63 | 3 | 62% | 5% |
| 3 holes | 2ul | 35 | 116 | 54 | 7 | 46% | 13% |
| 3 holes | 2ul | 70 | 120 | 54 | 17 | 45% | 31% |
| 3 holes | 2ul | 150 | 129 | 72 | 27 | 56% | 38% |
| 3 holes | 2ul | 250 | 114 | 54 | 46 | 48% | 85% |
The length of the laser exposure determines the size of the perforation. Longer laser treatments resulted in a more effective viral transduction. Number of holes reflects the number of perforations by the XYclone laser on the surface of the zona. The amount and the type of virus added to each sample was shown in uL in Description.
The thickness of the laser-perforated regions in the zona also impacts lentiviral gene delivery and embryo development (Li, Kinchen et al. 2013). Larger laser-perforated regions result in higher number of blastocyst expressing the virally delivered gene. However, the heat generated from the laser pulse could damage fertilized eggs. In our trials, we determined that minimum laser pulse of 250uS was necessary for greater than 80% of the cultured fertilized eggs to express GFP without incurring any damage to the embryo (Fig. 1c and Table 2). Varying the laser settings to remove larger portions of the zona had a direct effect on gene delivery.
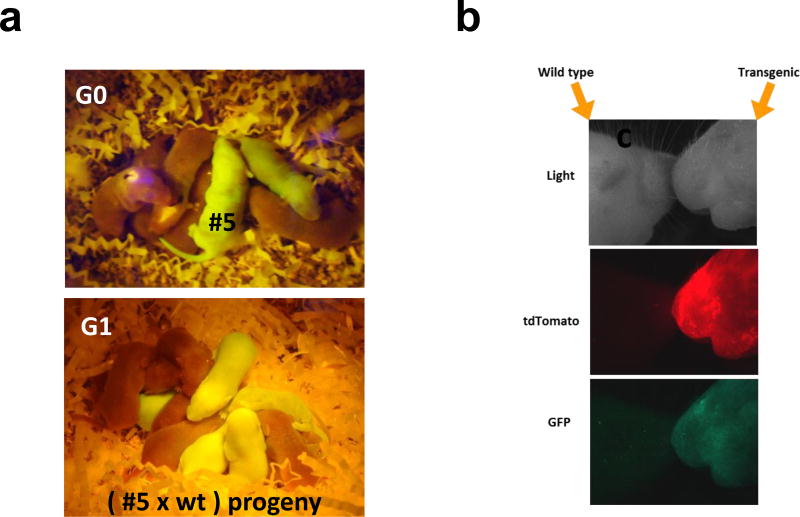
In order to prove the viability of the infected blastocyst, we utilized non-surgical embryo transfer (NSET) (Green, Bass et al. 2009) to pseudopregnant dams to create transgenic mice. 8–12 blastocysts were transferred to each pseudopregnant mouse yielding 1–5 pups (Generation 0, G0). Out of 68 implanted blastocysts (6 separate experiments), we recovered 12 pups or 18% birthrate. Out of 12 live pups, 9 expressed GFP to yield the transgenesis efficiency of 75%. We bred 2 of the transgenic GFP positive mice shown in Fig. 3a with wild type mice (3 rounds of breeding) to demonstrate germline transmission. The presence of the GFP gene in generated pups was determined by genotyping (Supplementary Table S1) and histological studies (data not shown). Generation 0 pups had incorporated 9 (Fig. 3a, #5) and 7 copies of the GFP gene into their genome. Germline transmission was also confirmed in G1 progeny (Fig. 3a and Supplementary Table S1). After exposing laser perforated fertilized eggs to two separate viruses carrying GFP and tdTomato genes, we observed the presence of both fluorescent genes in G0 pups (Fig. 3b). By employing this method, treatment with multiple lentiviruses can deliver genes simultaneously and independently.
Fig. 3. Laser-assisted Lentiviral Transgenesis.
(a) Images of GFP positive G0 founders and their progeny G1. Photographs provided by Charles Romeo and David Goulding, NIEHS (b) Lentiviral transduction of eGFP and tdTomato genes in the founder, G0.
Discussion
The laser-assisted lentiviral gene delivery is a rapid in vivo expression system. Current innovations in the field of lentiviral gene delivery has availed a wide range of vectors with tissue specific, recombinase activated, inducible promoters, and/or selectable markers to researchers. Any available lentivirus can be substituted for the purpose of transgenesis and used for laser-assisted lentiviral gene delivery. Genes delivered by lentiviruses can replicate silently as part of the host genome and turned-on in specific tissues or induced at various stages of development as dictated by their promoters; therefore, offering spatiotemporal control over gene expression.
Alternative methods such as electroporation and photoporation are also effective means of transient gene delivery to rodent single cell embryos (Hosokawa, Ochi et al. 2011). In a recent study, electroporation was used to successfully permeabilize the zona to deliver engineered endonucleases to rat fertilized eggs (Kaneko, Sakuma et al. 2014). This method is effective and ideal for short-term expression of genes such as recombinases and CRISPR/Cas9 components for creation of animal models. Electroporation of embryos is now becoming a standard method in CRISPR/Cas9 genome editing in single cell embryos in transgenic cores (Horii, Arai et al. 2014). CRISPR/Cas9 reagents delivered by electroporation, like microinjection, are transiently expressed and within days of electroporation, the delivered genes would either degrade or dilute away during cell division. For CRISPR/Cas9 genome editing, this transient expression is ideal for generating specific genomic manipulations at the early embryonic stage since stable or prolonged expression of CRISPR/Cas9 and guide RNA components would likely result in non-specific genetic modifications. Currently, the embryo electroporation system cannot be used to deliver a large stable genetic payload for host genome integration, or temporal control of CRISPR component expression via inducible or tissue specific promoters at the later developmental stage. Another efficient method for viral gene delivery and generation of transgenics is the lentiviral infection of spermatozoa which has been successfully used to create animal models for research (Hamra, Gatlin et al. 2002, Dann and Garbers 2008, Kanatsu-Shinohara, Kato et al. 2008, Chandrashekran, Sarkar et al. 2014). In this technique, mouse epididymis is isolated, punctured, and exposed to lentiviruses for stable gene delivery to mouse spermatozoa. The transduced spermatozoa are incubated with oocytes for in vitro fertilization (IVF) for an estimated 42% rate of transgenesis.
Laser perforation of the zona pellucida facilitates access to embryonic cells. It presents the cells for viral transduction without minimizing its protection of developing embryos. We anticipate that in laser-treated fertilized eggs, the zona permeability is not limited to retro/lentiviruses and may be exploited for gene delivery using other types of viral vectors or transfection reagents.
Lentiviral gene delivery has its limitations. The genetic load carried by each virus is limited to 10 kilobases. Multiple random insertion sites of viral payload into the genome of the mouse embryo creates mosaicism and chimeras. Therefore, it complicates the establishment of transgenic lines with a single transgenic locus and can only be attained by multiple rounds of breeding and careful genotyping of progeny, similar to other established methods used to create transgenic mice (Sauvain, Dorr et al. 2008).
The virus concentration and the length of embryo incubation with the lentivirus may also contribute to the number of gene integrations and chimerism in resulting transgenic mice. In this study, we used concentrated virus (105–106 transducing units) and incubated embryos with the virus until they developed into blastocysts to maximize gene delivery. The KSOM cultured media and DMEM were assayed daily for the presence of virus and titered (Supplementary Fig. 1). The virus was deactivated rapidly and number of transducing units dropped to 15–16% of the starting material within 24 hours of transduction. The viral titer in the embryonic KSOM media, 24 hours post infection, was close to a typical unconcentrated viral titer which is not potent enough to transduce embryonic cells. In our experiments, unconcentrated virus (103–104 transducing units) did not result in transduction of embryonic cells. Users should optimize viral concentration and time of incubation to reach custom gene delivery goals.
Laser-assisted lentiviral gene delivery offers an alternative method for creation of transgenic animals. This method can be used to validate in vivo consequences of protein variant expression, CRISPR or recombinase modifications to genome, or to explore the effects of dominant negative mutations, and to determine manifested phenotypic changes by genetic modulations. The laser-assisted gene delivery is an alternate and for most part complementary method to traditional transgenesis for robust gene delivery to single cell embryos. An investigator could use this method to deliver his/her gene of interest rapidly to mouse single cell embryos and observe the consequences prior to committing time and resources to creation of a transgenic animals using traditional methods. This method could also be used for in vivo protein purification.
Laser-perforated lentiviral gene delivery for embryo modification presents a simple and accessible option for gene delivery to fertilized eggs for many laboratories and specially for novice users. It is fast, effective, and less toxic to mouse fertilized eggs. The method employs the specialized XYclone laser which can be shared among a number of cores, research laboratories and departments due to the ease of use, its size, and the brief duration of its use per session, therefore, reducing cost while increasing efficiency. The observation that lentiviruses diffuse freely through thinned out/carved holes in the zona introduces an alternative method for gene delivery to fertilized eggs. This method should also be valuable in creating knock-downs and knock-outs by lentiviral delivery of shRNA or CRISPR components. This method is especially valuable for delivery and activation of CRISPR-Cas9 components for knocking out genes in specific tissues or at later stages of development. We expect this method to also have applications in gene delivery to fertilized eggs of other species with barriers similar to the mouse zona.
Materials and Methods
Reagents
The following reagents were purchased for use in our experiments: M2 and KSOM media (Millipore); Hyaluronidase (Sigma); HEK293T/17 cells (ATCC #Crl-11268); KSOM and M2 (Millipore), DMEM-10 (Gibco), FBS (Fisher), 4mM l-Glutamine (Gibco), 1mM NaPyruvate (Gibco); OPTI-MEM (Gibco); Pen/Strep (Pen/Strep 10,000 U Penicillin, 10 mg Streptomycin/ml in 0.9% NaC1, Sigma-Aldrich #P0781); Lipofectamine 2000 (Invitrogen); 0.25% Trypsin/EDTA (Gibco).
Plasmids
The following plasmids were used for packaging lentiviruses: lentiviral transfer vector pCDH511 expressing copGFP (System Biosciences); pWPXL-eGFP (Addgene#12257); pWPXL-tdTomato (tdTomato inserted in place of eGFP in pWPXL-eGFP); psPAX2 (encoding gag. Pol tat and rev proteins, Addgene#12260); pMD2.G (encoding VSV-G envelope protein, Addgene#12259).
Equipment
Non-surgical Embryo Transfer (NSET) device (Paratechs Corporation, Lexington, KY); XYclone Laser System (Hamilton Thorne Biosciences, Beverly, MA)
Production of Lentivirus
All lentiviruses were packaged and titered in HEK293T/17 cells (ATCC # CRL-11268) according to published Current Protocols in Neuroscience by P. Salmon and D. Trono (Salmon and Trono 2007). The sequences of primers used for titering are included in the aforementioned referenced paper. Briefly, 293T cells were transiently transfected with pMD2G, psPAX2 and transfer vector containing the desired gene using Lipofectamine 2000. Supernatant was collected 48 hours post transfection and concentrated by centrifugation at 50,000 g for 2 hours. Viral pellets were resuspended in PBS or KSOM media and used for infection. All titers were determined by performing quantitative PCR to measure the number of lentiviral particles that integrated into the transduced HEK293T genome. In addition to quantitative PCR, biological titration of viruses that expressed fluorescent moieties were determined by flow cytometry.
Animal Studies and Treatment
Animals used in this study were ordered from Charles River and Jackson Laboratories, USA. All animal procedures complied with NIH/NIEHS animal care guidelines and were approved by the Animal Care and Use Committee (ACUC) at NIH/NIEHS, animal Protocol # 2012-0004.
Isolation of fertilized eggs
Female mice from Crl:CD-1 (ICR) or C57BL/6J (The Jackson Laboratory Stock # 000664) strains were mated with intact male. The female was checked the following morning for the presence of a vaginal plug indicating successful mating. Females were humanely euthanized for ova/embryo collection via CO2 inhalation followed by cervical dislocation and vital organ transection. Fertilized eggs were isolated within 12–20 hours post mating. Upon euthanasia, the reproductive tract was removed. The collected fertilized eggs were stored in M2 sterile media prior to culturing in KSOM media at 37°C, 5% CO2, 5% O2 and 90% N2.
Embryo Transduction and Transfer
Pseudopregnancy was achieved by mating with vasectomized males. 3.5 days after mating, virally infected blastocysts were transferred into these females by non-surgical embryo transfer method. The Non-Surgical Embryo Transfer (NSET) Device was inserted approximately 5 mm into the cervix and fertilized eggs were deposited in a volume of approximately 2.0 ul (pictures and video available by the manufacturer at http://www.paratechs.com/nset). A sterile NSET device was used at the beginning of each transfer. All reagents used in the manipulation of the fertilized eggs were sterile. For transgenesis, each KSOM drop was seeded with 15–25 fertilized eggs and treated with 2uL of high titer lentivirus (109 TU/ml, final concentration of 4×107 TU/mL). 8–12 blastocysts from each drop were transferred to a pseudo pregnant females using NSET. 0–5 pups were obtained from each NSET transfer. Out of 68 implanted blastocysts (6 separate transfers), we recovered 12 pups or 18% birthrate. Out of 12 live pups, 9 expressed GFP. We bred 2 of the transgenic GFP positive mice to demonstrate germline transmission. The 68 implanted embryos were 85% GFP positive, therefore, 9 pups were recovered from 58 GFP positive blastocyst and 12 live births, resulting in 75% rate of transgenesis (9/12). The experiment was repeated six times. The transfer of blastocysts that expressed both GFP and tdTomato was performed once with 8 embryos transferred yielding 3 pups. All pups expressed both GFP and tdTomato. The presence of viruses in pups were confirmed by isolation of chromosomal DNA from pups’ tails and genotyping them.
Quality Assurance Testing
All weanlings were tested through the Comparative Medicine Branch’s Quality Assurance Laboratory (CMB/QAL) for the presence of adventitious rodent pathogens. Fecal samples from dams and weanlings were collected for PCR to detect pathogens including Helicobacter spp. and oropharyngeal swabs were collected to identify pathogens including Pasteurella pneumotropica. Blood was also collected when appropriate from euthanized foster dams at weaning for serology. Each set of weanlings was identified as a group and were not released by QAL until 3 negative tests were obtained. Groups of animals were separated by cubicles or racks in a rederivation holding room until released by the CMB/QAL. All reagents used in animals were tested by QAL and tested negative for the presence of contaminations prior to use in animals.
Perforating zona pellucida of fertilized eggs
The zona pellucida of fertilized eggs were laser perforated using XYclone Laser System (Hamilton Thorne Biosciences, Beverly, MA) in KSOM media. Zonas were carved with 1–9 holes of 0–12um (power 100%, pulse 35–250uS) according to recommended manufacturer’s protocols.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Health (NIH), National Institute of Environmental Health and Sciences (NIEHS). We are grateful to Dr. Franco Demayo, Dr. Ronald Cannon, Terry Blankenship, and Dr. Edward (Mitch) Eddy for critical reading of the manuscript and helpful advice. We would also like to acknowledge and thank Dr. Bernd Gloss, the Knockout core, the Flow Cytometry Facility, the Fluorescent Microscopy, and Imaging Center and the Comparative Medicine Branch facilities of the NIEHS for their technical contributions. We would like to thank Mr. David Goulding from the Comparative Medicine Branch; Mr. Steve McCaw, and Ms. Lois Wyrick of the Imaging Center at the NIEHS for providing us with photographs of our instruments and illustrations for the figures.
Footnotes
Competing Financial Interest
The authors declare no competing financial interests.
References
- Blake DA, Forsberg AS, Johansson BR, Wikland M. Laser zona pellucida thinning--an alternative approach to assisted hatching. Hum Reprod. 2001;16(9):1959–1964. doi: 10.1093/humrep/16.9.1959. [DOI] [PubMed] [Google Scholar]
- Chandrashekran A, Sarkar R, Thrasher A, Fraser SE, Dibb N, Casimir C, Winston R, Readhead C. Efficient generation of transgenic mice by lentivirus-mediated modification of spermatozoa. FASEB J. 2014;28(2):569–576. doi: 10.1096/fj.13-233999. [DOI] [PubMed] [Google Scholar]
- Clift D, Schuh M. Restarting life: fertilization and the transition from meiosis to mitosis. Nat Rev Mol Cell Biol. 2013;14(9):549–562. doi: 10.1038/nrm3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann CT, Garbers DL. Production of knockdown rats by lentiviral transduction of embryos with short hairpin RNA transgenes. Methods Mol Biol. 2008;450:193–209. doi: 10.1007/978-1-60327-214-8_14. [DOI] [PubMed] [Google Scholar]
- Ewerling S, Hofmann A, Klose R, Weppert M, Brem G, Rink K, Pfeifer A, Wolf E. Evaluation of laser-assisted lentiviral transgenesis in bovine. Transgenic Res. 2006;15(4):447–454. doi: 10.1007/s11248-006-0015-2. [DOI] [PubMed] [Google Scholar]
- Filipiak WE, Saunders TL. Advances in transgenic rat production. Transgenic Res. 2006;15(6):673–686. doi: 10.1007/s11248-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Gordon JW, Scangos GA, Plotkin DJ, Barbosa JA, Ruddle FH. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980;77(12):7380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M, Bass S, Spear B. A device for the simple and rapid transcervical transfer of mouse embryos eliminates the need for surgery and potential post-operative complications. Biotechniques. 2009;47(5):919–924. doi: 10.2144/000113257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadeh ME, Fischer-Hammadeh C, Ali KR. Assisted hatching in assisted reproduction: a state of the art. J Assist Reprod Genet. 2011;28(2):119–128. doi: 10.1007/s10815-010-9495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra FK, Gatlin J, Chapman KM, Grellhesl DM, Garcia JV, Hammer RE, Garbers DL. Production of transgenic rats by lentiviral transduction of male germ-line stem cells. Proc Natl Acad Sci U S A. 2002;99(23):14931–14936. doi: 10.1073/pnas.222561399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka K, Fuchiwaki M, Hiraoka K, Horiuchi T, Murakami T, Kinutani M, Kinutani K. Effect of the size of zona pellucida opening by laser assisted hatching on clinical outcome of frozen cleaved embryos that were cultured to blastocyst after thawing in women with multiple implantation failures of embryo transfer: a retrospective study. J Assist Reprod Genet. 2008;25(4):129–135. doi: 10.1007/s10815-008-9214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii T, Arai Y, Yamazaki M, Morita S, Kimura M, Itoh M, Abe Y, Hatada I. Validation of microinjection methods for generating knockout mice by CRISPR/Cas-mediated genome engineering. Sci Rep. 2014;4:4513. doi: 10.1038/srep04513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa Y, Ochi H, Iino T, Hiraoka A, Tanaka M. Photoporation of biomolecules into single cells in living vertebrate embryos induced by a femtosecond laser amplifier. PLoS One. 2011;6(11):e27677. doi: 10.1371/journal.pone.0027677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa M, Tanaka N, Kao WW, Verma IM. Generation of transgenic mice using lentiviral vectors: a novel preclinical assessment of lentiviral vectors for gene therapy. Mol Ther. 2003;8(4):666–673. doi: 10.1016/s1525-0016(03)00240-5. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Kato M, Takehashi M, Morimoto H, Takashima S, Chuma S, Nakatsuji N, Hirabayashi M, Shinohara T. Production of transgenic rats via lentiviral transduction and xenogeneic transplantation of spermatogonial stem cells. Biol Reprod. 2008;79(6):1121–1128. doi: 10.1095/biolreprod.108.071159. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Sakuma T, Yamamoto T, Mashimo T. Simple knockout by electroporation of engineered endonucleases into intact rat embryos. Sci Rep. 2014;4:6382. doi: 10.1038/srep06382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli V, Robles V, Cancela ML, Acker JP, Waskiewicz AJ, Elezzabi AY. An alternative method for delivering exogenous material into developing zebrafish embryos. Biotechnol Bioeng. 2007;98(6):1230–1241. doi: 10.1002/bit.21564. [DOI] [PubMed] [Google Scholar]
- Li MW, Kinchen KL, Vallelunga JM, Young DL, Wright KD, Gorano LN, Wasson K, Lloyd KC. Safety, efficacy and efficiency of laser-assisted IVF in subfertile mutant mouse strains. Reproduction. 2013;145(3):245–254. doi: 10.1530/REP-12-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Wang L, Li W, Zhang X, Tian Y, Zhang N, He S, Chen T, Huang J, Liu M. Highly efficient generation of transgenic sheep by lentivirus accompanying the alteration of methylation status. PLoS One. 2013;8(1):e54614. doi: 10.1371/journal.pone.0054614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295(5556):868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- McGrew MJ, Sherman A, Lillico SG, Taylor L, Sang H. Functional conservation between rodents and chicken of regulatory sequences driving skeletal muscle gene expression in transgenic chickens. BMC Dev Biol. 2010;10:26. doi: 10.1186/1471-213X-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijs M, Van Steirteghem AC. Assessment of different isolation procedures for blastomeres from two-cell mouse embryos. Hum Reprod. 1987;2(5):421–424. doi: 10.1093/oxfordjournals.humrep.a136561. [DOI] [PubMed] [Google Scholar]
- Sakuma T, Barry MA, Ikeda Y. Lentiviral vectors: basic to translational. Biochem J. 2012;443(3):603–618. doi: 10.1042/BJ20120146. [DOI] [PubMed] [Google Scholar]
- Salmon P, Trono D. Production and titration of lentiviral vectors. Curr Protoc Hum Genet. 2007 doi: 10.1002/0471142905.hg1210s54. Chapter 12: Unit 12 10. [DOI] [PubMed] [Google Scholar]
- Sauvain MO, Dorr AP, Stevenson B, Quazzola A, Naef F, Wiznerowicz M, Schutz F, Jongeneel V, Duboule D, Spitz F, Trono D. Genotypic features of lentivirus transgenic mice. J Virol. 2008;82(14):7111–7119. doi: 10.1128/JVI.00623-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye JP. Studies of endogenous retroviruses reveal a continuing evolutionary saga. Nat Rev Microbiol. 2012;10(6):395–406. doi: 10.1038/nrmicro2783. [DOI] [PubMed] [Google Scholar]
- Wassarman PM. Zona pellucida glycoproteins. Annu Rev Biochem. 1988;57:415–442. doi: 10.1146/annurev.bi.57.070188.002215. [DOI] [PubMed] [Google Scholar]
- Woods SE, Qi P, Rosalia E, Chavarria T, Discua A, Mkandawire J, Fox JG, Garcia A. Laser-assisted in vitro fertilization facilitates fertilization of vitrified-warmed C57BL/6 mouse oocytes with fresh and frozen-thawed spermatozoa, producing live pups. PLoS One. 2014;9(3):e91892. doi: 10.1371/journal.pone.0091892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xi Q, Ding J, Cai W, Meng F, Zhou J, Li H, Jiang Q, Shu G, Wang S, Zhu X, Gao P, Wu Z. Production of transgenic pigs mediated by pseudotyped lentivirus and sperm. PLoS One. 2012;7(4):e35335. doi: 10.1371/journal.pone.0035335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Sun P, Yu F, Yan L, Yuan F, Zhang W, Wang T, Wan Z, Shao Q, Li Z. Transgenic quail production by microinjection of lentiviral vector into the early embryo blood vessels. PLoS One. 2012;7(12):e50817. doi: 10.1371/journal.pone.0050817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.