Abstract
Background context
Open laminectomy has been regarded as the standard surgical method up to date in degenerative spinal stenosis or herniation of intervertebral disc. The conventional method may lead to instability and provoke chronic lower back pain by scarifying facet joint, posterior ligamentous complex as well as paraspinal muscle. For this reason, the new technique using an endoscope, which could protect soft tissue and facet joint, recently got spotlight.
Purpose
The aim of this study is to introduce a new spinal surgical technique using a 30-degreed endoscopy through bi- or tri- portals and to report the preliminary result of this technique.
Study design
retrospective study
Methods
One hundred five patients who were suffering from neurologic symptoms by degenerative lumbar spine disease were included even after preoperative conservative treatment. Two or three portals were used for each level. One portal was used for viewing, the others, for working of a certain instrument. Unilateral laminotomy was followed by bilateral decompression under 30° endoscopy. Clinical outcomes were analyzed in view of modified-Macnab criteria, Oswestry Disability Index (ODI), Visual analog scale (VAS), and postoperative complications were analyzed.
Results
The ODI improved from 67.4 ± 11.5 preoperatively to 22.9 ± 12.4 postoperatively. VAS for leg decreased from 7.7 ± 1.5 to 2.4 ± 1.3 at final follow up. Eighty-eight percent of the patients were improved over a level of good based on the Macnab criteria. There were not infection case.
Conclusions
The 30-degreed endoscopy had the advantages of obtaining a wider view. Full endoscopic decompression using 30-degreed endoscopy allowed satisfactory result clinically and reduction of surgical infection. It could be alternative method of microscopic laminectomy.
1. Introduction
Spinal stenosis is an encroachment on the neural structure by surrounding soft tissue and bone.1 It is the most common reason for spine surgery in adults over 60 years.1 The treatment of lumbar spinal stenosis has been increasing in old age. Posterior spinal fusion is regarded as the current standard surgical treatment of various lumbar spinal stenosis.2 However, these operations lead to several kind of problems. The traditional operation of this disease involves wide dissection of paraspinal muscle for the enough space for the proper procedure, followed by excessive removal of bone structure and ligament structure for decompression.3 Inevitably, most surgeons would operate posterior lumbar fusion to prevent due to iatrogenic instability. Although posterior lumbar fusion with screw fixation leads to a satisfactory outcome. A firm fusion is able to accelerate degeneration of the adjacent unfused segment.2 In addition, it could be vulnerable to adjacent segmental degeneration by wide posterior dissection that injures paraspinal muscle due to operating posterior lumbar fusion. Multifidus muscle injury and atrophy are common after posterior lumbar spine surgery and are associated with lower back pain and functional disability.4 The posterior splitting approach is an important cause of multifidus muscle injury and atrophy in posterior lumbar spinal surgery.4 Denervation and disuse may be important factors in muscle atrophy in the splitting approach. Infection and significant blood loss could also be problems in case of lumbar fusion and wide laminectomy. The prevalence of spinal surgical site infection was reported 3.5% in validation cohort study. The predisposing factors for surgical site infection were old age, higher BMI and, presence of certain comorbidities.5 Reduction of infection rate is regarded as important due to characteristic of patients who have the spinal stenosis in old age as well as having certain morbidity. According to Huang YH et al., the incidence of significant blood loss (over 500 ml) during lumbar fusion was reported over 53% and substantial bleeding in lumbar fusion is associated with a greater incidence of morbidities and prolonged length of hospital stay.6 In case of open spinal surgery, since the above problem may occur, various instruments and techniques were invented in order to prevent these problems. Recently, biportal endoscopic spinal surgery were reported by several authors7, 8, 9, 10,and have started to get spotlighted. So far, there are few reports that present clinical outcomes, specifically technical reports about biportal endoscopic surgery for spinal stenosis. The purpose of this study evaluates the unilateral biportal endoscopic spinal surgery for in degenerative spinal stenosis and presents a technical note.
2. Material and methods
The study was carried out after the approval of the institutional review board. All patients who received unilateral biportal endoscopic decompression consented and signed prior to operation. The clinical outcomes including Oswestry Disability Index, Modified Macnab criteria, VAS, operation time, and complication rate were analyzed from patients who were treated by unilateral biportal decompression using 30° endoscopy. The number of patients was one hundred five. These patients were diagnosed with spinal stenosis and treated by our institution via unilateral biportal endoscopic decompression. Other patients with other diagnoses such as lumbar disc herniation or re stenosis due to adjustment segmental degeneration were excluded from the patient pool. Unilateral biportal endoscopic decompression was operated by a single surgeon. Instruments including basic spine instruments and 30° 4 mm arthroscopy (Linvatec®) which were commonly used in joint arthroscopy, and Arthrocare (Smith & Nephew®), 4.2 mm arthroscopic burr, shaver were used in operation. The procedures were operated under spinal or general anesthesia. The patients were positioned prone along with the abdomen free, the spine was flexed to widen inter laminar space.
2.1. Operation technique
Level confirmation was conducted under C-arm prior to operation. A proximal portal was made just below the pedicle of lamina that had to be laminotomized, and a distal portal was made in the same manner just below the pedicle of the lower lamina (Fig. 1). The two portals were 0.5 cm in diameter, enough to insert instruments and endoscope. The distance of the two portal was different depending on the height of patient and level. In general, the distal portal was located 2–3 cm caudal to proximal portal. The scopic trocha was introduced to the proximal portal and the round-shape, smooth periosteal elevator was inserted to the distal portal through the paraspinal muscle without any dissection until it was located at the end of the lamina. (Fig. 2) Sweep the muscle overlying lamina and inter lamina space with periosteal elevator to make enough space for vision. In case of failure to acquire the proper portal, another portal also can be added for smooth progression. Lower pressure inflow water that is under 30 mmHg should be initiated through the proximal portal. Outflow should be made through the distal portal to achieve continuous irrigation to prevent congestion of soft tissue. If there is no water flow with water congestion in the operation field, there are some problem that can occur. First, when the flow of water was congested without a definite water current, the visual field of operation was not clear, consequentially. Second, water could infiltrate to the multifidus muscle so that could undergo significant trouble when working due to limited space by soft tissue swelling of the muscle. Third, the camera lens can be destroyed due to overheating by radiofrequency catheter. The water current should be maintained not to experience these problems. Problem of the water congestion can be solved by inserting root retractor or 5.5 mm transparent plastic cannula through the portal (Fig. 3). Identification of the facet joint, a significant landmark in the surgery is very important after obtaining working space. The facet joint looks bright by the joint capsule on endoscope. The lamina should be identified along the facet joint. A shaver function or RF catheter can be used to clean soft tissue and debris over the lamina. The arthroscopic 4.2 mm burr or osteotome was used to make thin ipsilateral lamina and a part of the spinous process followed by the Kerrison roungeur to achieve a laminotomy (Fig. 4). The ligamentum flavum should be preserved because it can play a role as a protective barrier until the completion of the bony work at the contralateral lamina. If 30° endoscopy is at a 12 O’clock angle, it can visualize the base of the contralateral lamina and the spinous process. In the case of a 6 O’clock angle, it can visualize inferior articular process and inferior lamina. When partial laminotomy of superior part at inferior lamina is necessary, switching the portals for convenient working could be helpful during operation. Ipsilateral decompression should be performed with arthroscopic burr and Kerrison rongeur until the superior part of the ligamentum flavum is exposed, (Fig. 5A), followed by the undercut of contralateral lamina and the base of spinous process in order to make enough space for handling in contralateral space. The inferior portion of ligamentum flavum should be ablased in order to expose the superior end of inferior lamina using RF catheter. After switching the two portals again, the superior portion of the inferior lamina was thinning with burr. A blunt dissector such as a curret or dura elevator verifies the plane between the ligament flavum and the dura. After confirming no adhesion between the ligamentum flavum and dura, a flavectomy could be performed using the Kerrison punch and pituitary forcep for ipsilateral decompression of the neural structure. For contralateral decompression, both the scope and working instrument should exceed the top of the lamina in order to approach the contralateral ligamentum flavum. Blind usage of the Kerrison punch at contralateral ligamentum flavum could lead to a high possibility of dura tear due to the tight ligamentum flavum and folding of dura in severe spinal stenosis. The Over the top technique is very useful that contralateral side should be decompressed. For Over the top technique, arrow of scope should be rotated to 11 O’clock to obtain an adequate view of the contralateral side in case of left decompression case. (Fig. 5B).When the scope is rotated to 1 O’clock, the operator can obtain a better view in right side case. The smooth curret could be used to detach the ligamentum flavum from the contralateral lamina wall and then make space between the dura and ligamentum flavum for more safety while working (Fig. 5C). The ligamentum flavum should be removed using a Kerrison punch and pituitary forcep on the condition that it is detached from both the dura and lamina wall. When tight adhesion is present between ligamentum flavum and dura it is recommended to use several tips
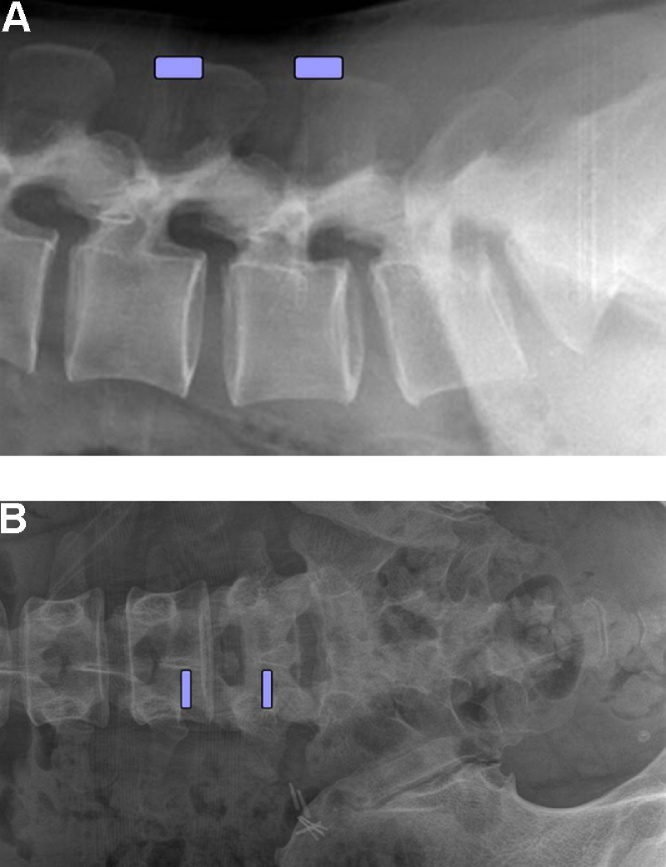
Fig. 1.
A lateral and anteroposterior view of this lumbar spine exhibits the locations of the two portals.
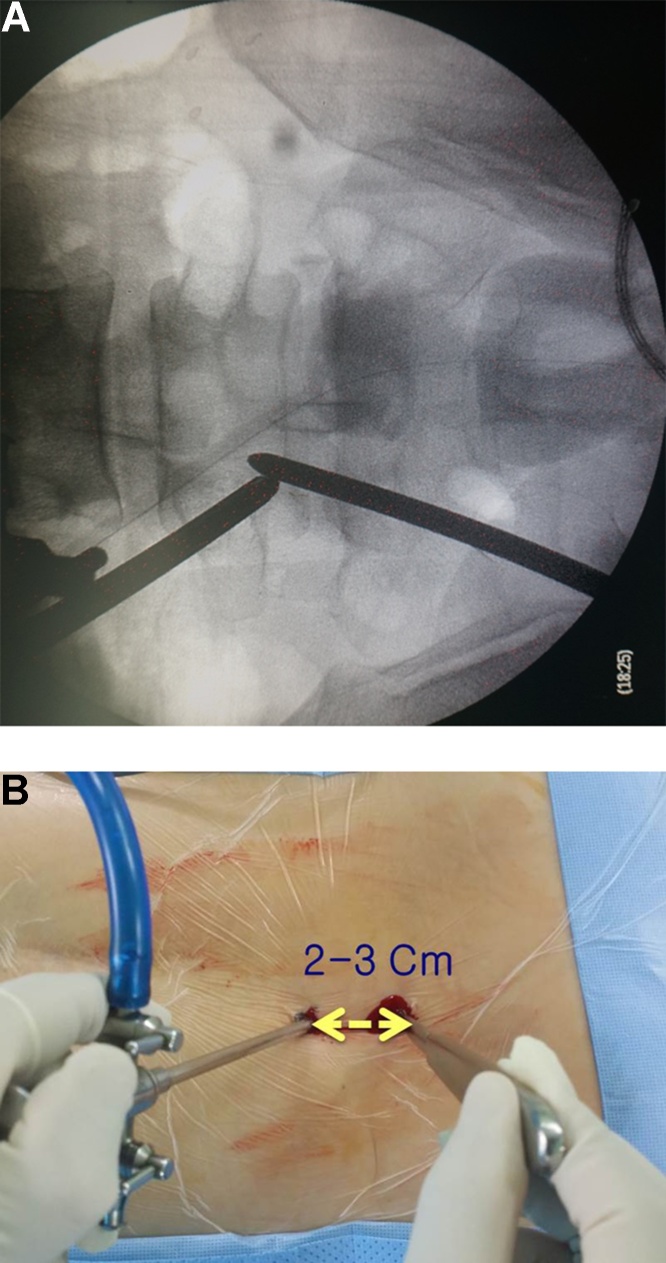
Fig. 2.
Intraoperative fluoroscopy image of the lumbar spine and a gross photo of both the working and viewing portals. The two portals meet at the end of the lamina.
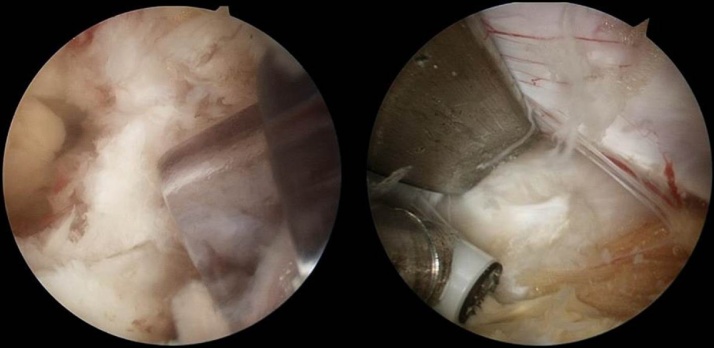
Fig. 3.
Endoscopic images of (Right) 5.5 mm transparent plastic cannula and (Left) root retractor which can solve water congestion.
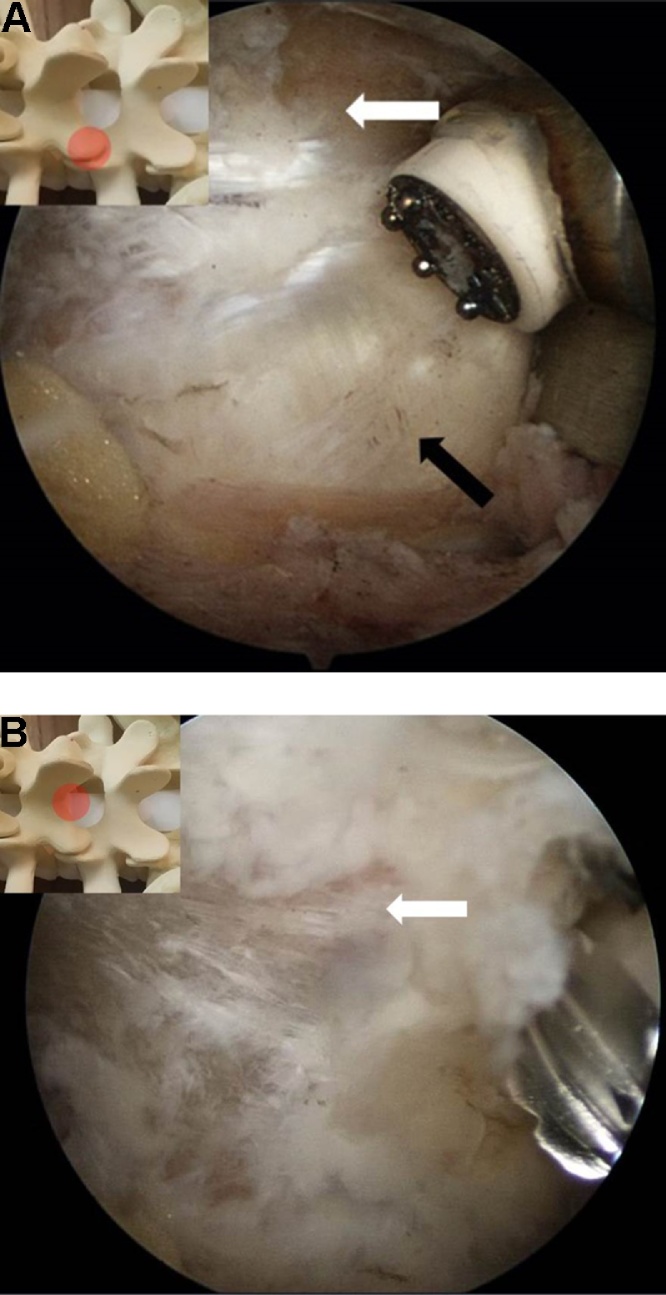
Fig. 4.
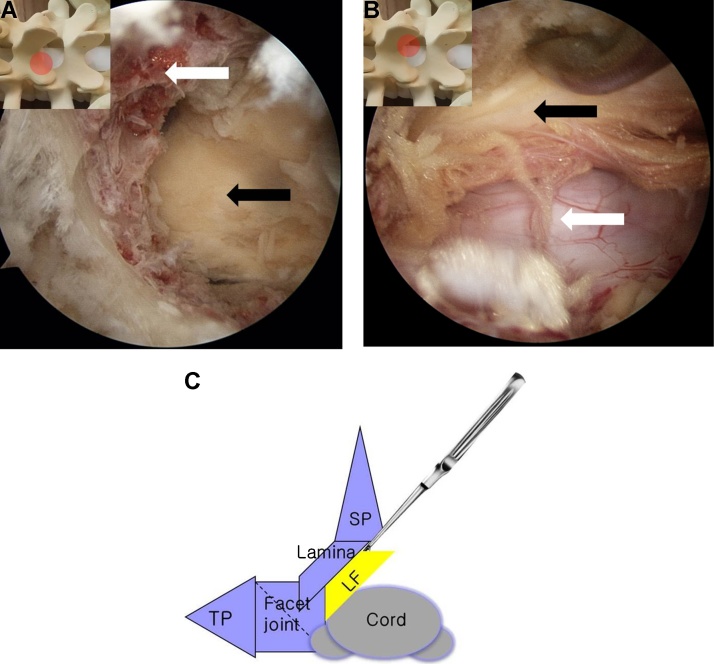
(A)Endoscopic views at the facet joint and (B) laminotomy with burr. The round figure indicates the location of the endoscopic view. (White arrow) lamina, (Black arrow) facet.
Fig. 5.
(A) An endoscopic view of ipsilateral laminectomy. spinous process (white arrow), ligamentum flavum (black arrow). (B) Contralateral decompression with Over the top technique. ligamentum flavum (black arrow), dura (white arrow). (C)The schematic view of ‘Over the top technique'., TP transverse process, SP, spinous process, LF, ligamentum flavum.
First, the magnification of arthroscopy can help surgeons do more sophisticated work and prevent accidental dura tear due to kerrison punch. Second, in cases of severe adhesions such as epidural fat loss, gentle adheolysis using continuous irrigation and blunt dura elevator can provide space between dura and ligamentum flavum. Unlike open surgery, adheolysis is easier because of the penetration of water into tissues.
Additional annuloplasty is performed with a RF catheter in the case of disc protrusion which provokes a canal compromise. The operation is finished after confirmation of bilateral decompression use of blunt probe (Fig. 6). After the instruments and endoscope were removed, the skin incision were closed. After drain removal, postoperative MRI was checked. (Fig. 7).
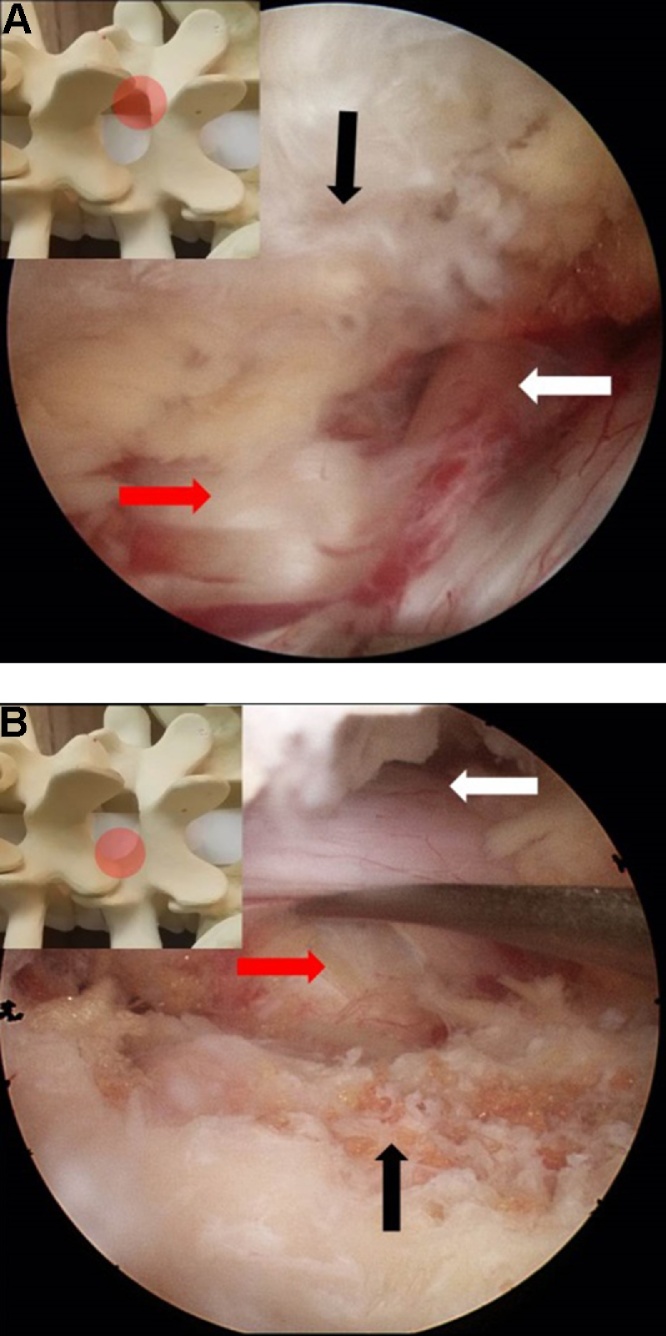
Fig. 6.
(A) An endoscopic view of the contralateral side after decompression. annulus (red arrow), contralateral facet joint (black arrow), traversing root (white arrow). (B) The complete decompression of the spinal canal. annulus (red arrow), ipsilateral facet (black arrow), ipsilateral traversing root (white arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
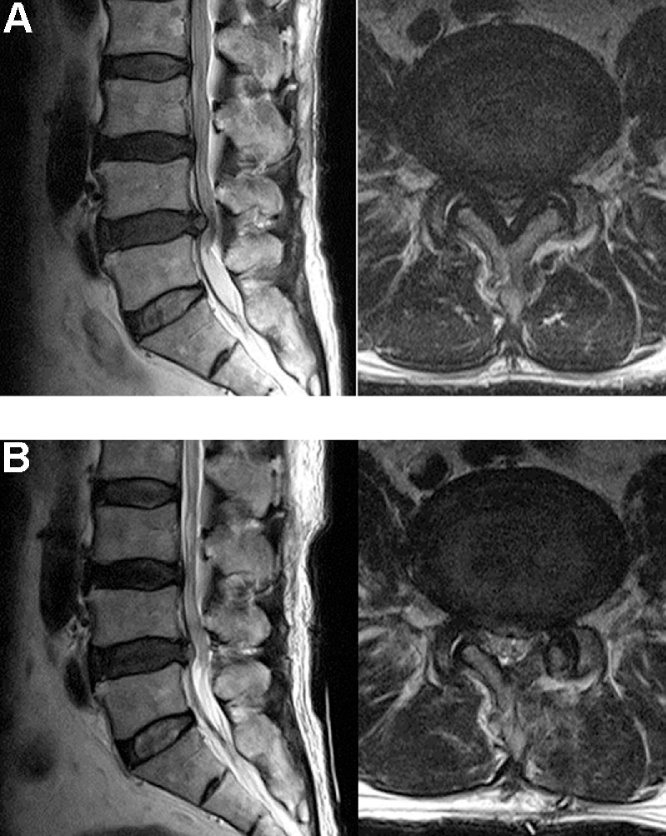
Fig. 7.
(A) Central spinal stenosis of L4-5 is shown in this preoperative MRI. (B) Postoperative MRI showing that the spinal canal was decompressed completely by unilateral biportal decompression with 30°endoscopy.
2.2. Statistatical analysis
All stastistical analyses were performed using Statistical Package for Social Sciences (version 18; SPSS, Chicago, Illinois). Values are presented as means ± standard deviation. Patient data was analyzed using the paired t-test. P < 0.05 was regarded as statistically significant.
2.3. Surgical indication
Radiating leg pain or neurogenic claudication dominant spinal stenosis, multilevel spinal stenosis Grade I spondylolithesis,
3. Result
The one hundred five patients were treated in our institution. There were forty six male patients and fifty nine female patients. The ages of the patients ranged from 52 to 86 years old, with a mean age of 71.2 ± 8.9. The operation was performed by unilateral biportal endoscopic decompression. A record of blood loss was not acquired due to the character of the operation which uses continuous watery irrigation (Table 1). The mean follow up period was 14 months. The mean operative time for 1 level was 53 ± 13.5 min. The postoperative mean VAS for leg decreased from 7.7 ± 1.5 to 2.4 ± 1.3 (P < 0.01) at final follow up. The postoperative mean ODI significantly improved from 67.4 ± 11.5 to 22.9 ± 12.4 (P < 0.01). 88% of patients improved over a good base on the Macnab criteria. The recorded outcomes, according to the modified Macnab criteria, were excellent in 81 patients (77%), good in 12 (11%), fair in 10 patient (10%), and poor in 2 patient (2%) (Table 2). Complications with unilateral biportal endoscopic decompression by 30° endoscopy were limited to 3 cases. Although dura tear occurred in 2 cases, symptoms improved after conservative treatment. One patient who had neurologic symptoms got revision surgery due to postoperative epidural hematoma. The patient was treated again by unilateral biportal decompression by 30° endoscopy through the same portals which were used previously.
Table 1.
Record of blood loss.
| Preoperative | Postoperative (Final f/u) | |
|---|---|---|
| VAS for Leg | 7.7 ± 1.5 | 2.4± 1.3 |
| ODI. | 67.4 ± 11.5 | 22.9 ± 12.4 |
| Macnab criteria | Above good – 88% |
Table 2.
Recorded outcomes.
| Characteristic | Value |
|---|---|
| Age | 52 to 86 (71.2 ± 8.9) |
| Male/Female | 46/59 |
| Mean operation time for 1 level. | 53 ± 13.5 |
| Complication (dura tear) | 2 |
| (epidural hematoma) | 1 |
| (infection) | 0 |
4. Discussion
Open decompressive laminectomy is known as the conventional treatment of spinal stenosis. However, back pain and muscle atrophy can occur due to excessive dissection of the paraspinal muscle, especially postoperative multifidus.4 The number of posterior lumbar fusions also increases due to inevitable iatrogenic instability, caused by wide decompression including facetectomy.11 Adjacent segmental degeneration can occur after posterior lumbar fusion. On this account, spinal stenosis can recur and develop lower back pain over time.12 Minimal invasive spinal surgical techniques were developed and spotlighted to reduce surgical trauma and decrease the rate of iatrogenic lumbar fusion surgery due to these problem. Problems also include inevitable muscle dissection and difficulty of decompression at the contralateral side in the case of ULBD (unilateral laminotomy with bilateral decompression), which is most similar to the unilateral biportal endoscopic decompression technique. When tubular spinal surgery is operated, the diameter of tube is not wide enough to work properly in a 16 mm tube. In addition, it has not been verified with regard to spinal instability due to the damage of the supraspinous ligament by tubular spinal surgery up to date.13 Although, microscopic decompression technique is anatomically familiar to spine surgeons, more muscle dissection is required compared to the unilateral biportal endoscopic technique. It is relatively not easy to obtain good contralateral view depending on the patient. In contrast, the advantage of the unilateral biportal endoscopic technique is that it provides a good view of the contralateral, sublaminar foraminal area without additional incision. In addition, there is less muscle dissection. The risk of infection is low and clear visualization and less intraoperative bleeding can be obtained due to continuous saline irrigation. However, the surgeon should understand the concept of triangulation and overcome a steep learning curve in order to perform this operation. Meningeal irritation can be caused by excessive saline irrigation after surgery.
Although Komp et al. recently reported a retrospective study related to decompression surgery using single portal endoscopy in spinal stenosis. There are disadvantages to viewing and working in a single portal compared to two portals.14 The single portal technique is narrow in vision because viewing and working are done by one portal. The single portal has also a limited working space. In the biportal technique, working is done freely in the space between the muscle and the lamina, not the tube. In addition, the triangulation technique is used as a basic concept for arthroscopy and biporal endoscopic surgery has a substantial advantage over the other technique as it provides unconstrained working space through two independent portals. Therefore, while decompression of only the central stenosis is possible by the single portal technique, the biportal technique can decompress both central stenosis and lateral stenosis.
Unilateral biportal endoscopic decompression technique allows the decompression of the contralateral side and the identification of the contralateral facet as well as the traversing root without excessive resectioning of the bony structure. The portals made in the intermuscular plane of the multifidus provide space between the multifidus and the lamina in order to prevent failed back syndrome, which is caused by muscle atrophy and denervation of the posterior ligament complex and the paraspinal muscle. Also, tissue debris did not congest due to continuous water flow through an in and out point. Although the prevalence of spinal surgical site infection was reported 3.5% in validation cohort study.5 There are not any infection cases up to date because of the continuous irrigation. It can be considered that the improvement of the inflammatory environment was achieved by continuous irrigation. There were two cases of dura tear. The dura tear of both cases occurred early in the learning curve, and fortunately it was a small tear within about 5 mm. The symptoms of patients improved after conservative treatment 3 days after operation. There have not been any revision surgeries due to dura tear so far. There were not transfusion cases due to postoperative bleeding.
Unilateral biportal endoscopic decompression is a novel technique that fully utilized advantages and features of both endoscopy and microscopic ULBD. It allows to obtain high quality of vision by using continuous irrigation through inflow and outflow, to provide better verification of sublaminar, contralateral facet, foramen comparing microscope. Biportal endoscopic surgery was introduced, and its results were reported by several authors in previous studies. However, valuable technical tips such as the over the top technique for safe contralateral decompression and the method of controlling water flow for a better visual field of operation has not been introduced in other up to date literature. There has not been literature that introduces, in detail, the 30° scopic view according to location and direction of the scopic arrow.
This study had relatively short follow up periods and few populations. Another limitation is the study of a single group without a comparison group.
5. Conclusion
The 30-degreed endoscopy had the advantages of obtaining a wider view. Full endoscopic decompression using 30-degreed endoscopy allowed satisfactory result clinically. It could become the alternative method for microscopic laminectomy on the condition that surgeons overcome the necessary learning curve. This novel technique may be a good method to reduce infection and inevitable lumbar fusion due to iatrogenic instability by wide decompression in even severe spinal stenosis.
Conflict of interest
None.
References
- 1.Weinstein J.N., Tosteson T.D., Lurie J.D. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358(February (8)):794–810. doi: 10.1056/NEJMoa0707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J.Y., Ryu D.S., Paik H.K. Paraspinal muscle, facet joint, and disc problems: risk factors for adjacent segment degeneration after lumbar fusion. Spine. 2016;16(July (7)):867–875. doi: 10.1016/j.spinee.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Iguchi T., Kurihara A., Nakayama J. Minimum 10-year outcome of decompressive laminectomy for degenerative lumbar spinal stenosis. Spine. 2000;25(July (14)):1754–1759. doi: 10.1097/00007632-200007150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Hu Z.J., Fang X.Q., Zhou Z.J. Effect and possible mechanism of muscle-splitting approach on multifidus muscle injury and atrophy after posterior lumbar spine surgery. J Bone Joint Surg Am. 2013;95(December (24)) doi: 10.2106/JBJS.L.01607. [e192(1–9)] [DOI] [PubMed] [Google Scholar]
- 5.Klemencsics I., Lazary A., Szoverfi Z. Risk factors for surgical site infection in elective routine degenerative lumbar surgeries. Spine. 2016;9(August) doi: 10.1016/j.spinee.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y.H., Ou C.Y. Significant Blood Loss in Lumbar Fusion Surgery for Degenerative Spine. World Neurosurgery. 2015;84(September (3)):780–785. doi: 10.1016/j.wneu.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Choi C.M., Chung J.T., Lee S.J. How I do it? Biportal endoscopic spinal surgery (BESS) for treatment of lumbar spinal stenosis. Acta neurochirurgica. 2016;158(March (3)):459–463. doi: 10.1007/s00701-015-2670-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwa Eum J., Hwa Heo D., Son S.K. Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: a technical note and preliminary clinical results. J Neurosurg Spine. 2016;24(April (4)):602–607. doi: 10.3171/2015.7.SPINE15304. [DOI] [PubMed] [Google Scholar]
- 9.Soliman H.M. Irrigation endoscopic assisted percutaneous pars repair: technical note. Spine Journal. 2016;(June (23)) doi: 10.1016/j.spinee.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Soliman H.M. Irrigation endoscopic decompressive laminotomy. A new endoscopic approach for spinal stenosis decompression. The Spine Journal. 2015;15(October (10)):2282–2289. doi: 10.1016/j.spinee.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Lipson S.J. Spinal-fusion surgery – advances and concerns. N Engl J Med. 2004;350(February (7)):643–644. doi: 10.1056/NEJMp038162. [DOI] [PubMed] [Google Scholar]
- 12.Imagama S., Kawakami N., Kanemura T. Radiographic adjacent segment degeneration at five years after L4/5 posterior lumbar interbody fusion with pedicle screw instrumentation: evaluation by computed tomography and annual screening with magnetic resonance imaging. Clinical spine surgery. 2016;(May (2)) doi: 10.1097/BSD.0b013e31828aec78. [DOI] [PubMed] [Google Scholar]
- 13.Mikami Y., Nagae M., Ikeda T. Tubular surgery with the assistance of endoscopic surgery via midline approach for lumbar spinal canal stenosis: a technical note. Eur Spine J. 2013;22(September (9)):2105–2112. doi: 10.1007/s00586-013-2806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komp M., Hahn P., Oezdemir S. Bilateral spinal decompression of lumbar central stenosis with the full-endoscopic interlaminar versus microsurgical laminotomy technique: a prospective, randomized, controlled study. Pain Physician. 2015;18(January-February (1)):61–70. [PubMed] [Google Scholar]