Abstract
Non-Hodgkin lymphoma (NHL) is the most common hematologic malignancy. More than 20,000 people in United States, more than 37,000 people in Europe and more than 199,000 people worldwide die of NHL every year. Recent advances in immunotherapeutic approaches for cancer have resulted in development of new classes of very effective immunotherapeutic approaches including chimeric antigen receptor T (CAR-T) cell therapy that are designed to bypass cancer immune evasion. Here we review recent advances in CAR-T cell therapy for NHL. US food and drug administration (FDA) recently approved axicabtagene ciloleucel (Yescarta) a CD19 CAR T cell therapy for treatment of relapsed refractory diffuse large B cell lymphoma (DLBCL), high grade lymphoma, and primary mediastinal B cell lymphoma (PMBCL). Approval of Yescarta and rapid development of other CAR T cell therapies at various stages of development are opening up the door for a new wave of CAR T cell therapies that will dramatically change the way we treat NHL and hopefully other malignancies in the near future.
Keywords: CAR (Chimeric Antigen Receptor) T Cells, NHL (non-Hodgkin lymphoma)
Introduction
NHL is the most common hematologic malignancy with the annual incidence of more than 70,000 in the US, more than 93000 cases in the Europe, and more than 385,000 cases worldwide [1–3]. Approximately more than 600,000 people live with NHL in the US. Estimated number of annual death due to NHL in the US, Europe and worldwide are more than 20,000, more than 37,000, and more than 199000 respectively [1–3]. Diffuse large B cell lymphoma (DLBCL) is the most common NHL (30%) followed by follicular lymphoma (FL), mantel cell lymphoma (MCL), chronic lymphocytic leukemia/small lymphocytic leukemia (CLL/SLL), and T cell lymphoma. Annual incidences of DLBCL in the US, Europe, and the world are 7, 4–5, and 5–6 among 100,000 people respectively [1–4]. Mortality rate of DLBCL is one case per 100,000 per year with approximately 75000 people die of DLBCL worldwide every year [1–5]. Although majority of patients with DLBCL are cured with current chemo-immunotherapy regimens, approximately 20–30% of patients with DLBCL will be refractory to chemotherapy or relapse after salvage chemotherapy and autologous stem cell transplantation [4]. These patients have a very poor prognosis with overall response rate (ORR) and complete remission (CR) rate of 26% and 7% respectively with median survival of 6.3 months with standard salvage chemotherapy [6].
CAR T cell therapy is a cellular therapy that redirects T cells against tumor associated antigens bypassing tumor evasion mechanisms. Based on the results of ZUMA-1, FDA approved axicabtagene ciloleucel (Yescarta) first CAR-T cell therapy for relapsed refractory DLBCL, high risk lymphoma and PMBCL [7]. Here we review ZUMA-1 and other advances in CAR T cell therapies for NHL. At the end a brief review of recent preclinical advances in CAR T for T cell NHL will be presented.
CAR T overview
Biology
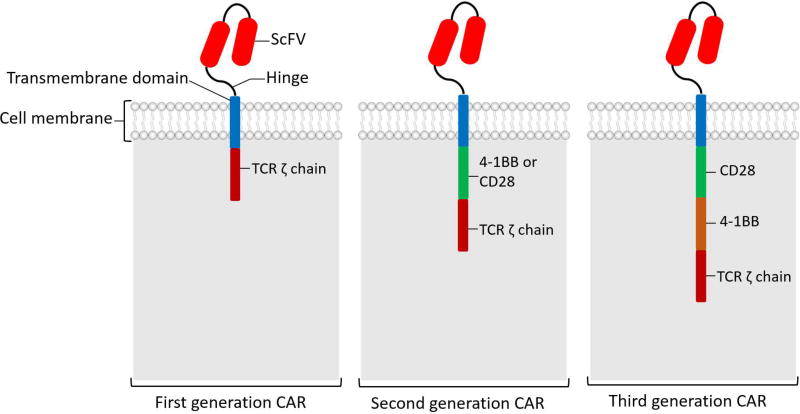
CAR T is a T cell transduced with chimeric antigen receptor that is specific to a tumor associated antigen (TAA). CAR is called “chimeric” because it is a combination of antigen binding site of B cell receptor (BCR) and activating domain of T cell receptor (TCR) in a single chain molecule. CAR has three main components, extracellular domain, transmembrane domain, and intracellular domain (Figure 1) [8, 9]. Extracellular domain is comprised of a single chain variable fragment (ScFv) that binds to tumor associated antigen (TAA), and a hinge that connect ScFV to transmembrane domain. Based on number of activating intracellular domain, CARs are divided to first generation with single CD3ζ chain as the only activating domain, second generation CARs with 2 activating domains (usually CD3ζ chain plus CD28 or 4-1BB) and third generations CARs with three intracellular activating domains. Almost all CAR T cells used in clinical trials are second generation with CD3ζ chain plus CD28 or 4-1BB activating domains. Review of differences between CD28 and 4-1BB CAR T cells is out of the scope of this review; in general CD 28 CAR T cells expands faster but are less persistent comparted with 4-1BB CAR T cells [4, 8, 9].
Figure 1.
Chimeric antigen receptor (CAR) structure. This diagram shows various components of CAR and different CAR generations. ScFV (single-chain variable region) of an antibody, that provides target specificity against tumor associated antigens.
Production
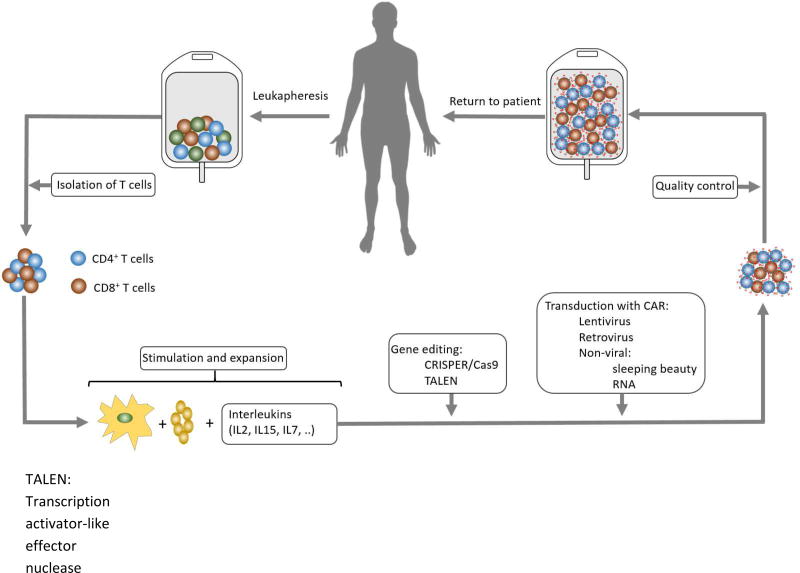
Figure 2 presents overall schema of CAR T therapy. The first step in CAR T production is collection of peripheral blood mononuclear cells (PBMC) that are collected in steady state by leukapheresis (12-15L). Collected PBMCs then transferred to good manufacturing practice (GMP) facility and T cells (CD3+ cells) are isolated as next step. Isolated T cells are then activated, expanded, and transduced with CAR gene by using lentiviral vectors, retroviral vectors, or non-viral methods like electroporation and sleeping beauty system. Transduced T cells with CAR gene expressing CAR receptor on their surface are called “CAR T cells”. These CAR T cells will be expanded more, and after quality control usually frozen and shipped to treating centers if they are produced outside of the treating center or just transferred from GMP facility to cellular therapy labs for storage. Patients first receive lymphodepleting chemotherapy that is usually 3 days of cyclophosphamide only or cyclophosphamide plus fludarabine. A few days later (usually after 1–2 days of rest), frozen CAR T cells are thawed and transfused intravenously using a central line [8, 9]. The interval from leukapheresis to infusion of CAR T cells is called “vein to vein interval”. This is an important factor especially in patients with rapidly progressing disease who have a short therapeutic window and need to get CAR T cells as soon as possible.
Figure 2.
CAR T cell therapy overall process.
CAR T for Lymphoma
Diffuse Large B Cell Lymphoma (DLBCL)
Patients with relapsed refractory DLBCL have a very poor prognosis. SCHOLAR-1 study was a retrospective study that analyzed response rate and survival outcome of salvage chemotherapy in 636 patients with relapsed refractory DLBCL defined as no response to 4 cycles of initial chemotherapy or 2 cycles of salvage chemotherapy or relapse within 12 months after autologous stem cell transplant (auto-HCT). Based on SCHOLAr-1, ORR, CR and median OS in these group of relapsed and refractory patients were 26%, 7% and 6.3 months respectively [6].
ZUMA-1 (NCT02348216) was a phase 1 and 2 single arm trial evaluated safety and efficacy of axicabtagene ciloleucel (axi-cel, Yescarta™) in patients with relapsed refractory high grade lymphoma [10, 11]. Axi-cel is a second generation CD19 CAR T cell with CD28 costimulatory domain. Retroviral vector was used as the mode of transduction for production of axi-cell. One hundred and eighteen patients with relapse refractory DLBCL, transformed FL and PMBCL were enrolled. Relapsed refractory was defined as no response to last line of chemotherapy or relapse within 12 months of auto-HCT. Seven patients enrolled in phase one and 111 patients enrolled in phase 2. Patients were older than 18 years old (median 58 years, range 23–76 years). Seventy percent were refractory to 3 or more lines of therapies, and 21% had relapsed disease within 12 months after auto-HCT. Patients with relapsed post allogeneic stem cell transplant (allo-HCT) and patients with CNS disease were excluded. Among 111 patients enrolled in phase 2, axi-cel was successfully manufactured in 110 patients (99%) and 101 patients received axi-cel (91%) with median time from leukapheresis to delivery of axi-cell to the treating facility of 17 days. No bridging chemotherapy (chemotherapy after leukapheresis and before lymphodepleting chemotherapy while waiting for manufacturing of CAR T cells) was allowed. Lymphodepleting chemotherapy included fludarabine 30 mg/m2/day plus cyclophosphamide 500 mg/m2/day for 3 days on days -5, -4, and -3. After 2 days of rest (on day 0) patients received axi-cel with the target dose of 2 × 106 CAR T cells/kg based on recipient body weight. Response was evaluated one month after infusion of axi-cel. Overall response rate (ORR) and complete response (CR) rates were 82% and 54% respectively. Interestingly 23/61 patients with either partial response (PR) (11/35) or stable disease (SD) (12/25) at one month post infusion evaluation, achieved CR in subsequent restaging without additional treatment with median time from PR to CR of 64 days (range, 49 – 424 days). With median follow up of 15.4 months 40% of patients continued to be in CR with overall survival (OS) and disease free survival (DFS) of 52% and 41% at 18 months respectively. In phase 2 portion of the study, 3 patients died, 2 related to axi-cel (one hemophagocytic lymphohistiocytosis and one cytokine release syndrome) and one unrelated to axi-cel (pulmonary embolism). Grade 3 and higher CRS and neurotoxicity happened in 13% and 28% respectively. Median time from axi-cel infusion to CRS onset was 2 days (range, 1–12 days) and median duration of CRS was 7 days (range, 2–58 days). Median time from axi-cell infusion to the onset of neurotoxicity was 4 days (range, 1–43 days) with median neurotoxicity duration of 17 days [10–12]. Based on the results of this trial, on October 18, 2017 US FDA approved axicabtagene ciloleucel (axi-cel, Yescarta™) for treatment of adults with relapsed refractory high grade lymphoma after two or more lines of systemic therapy including DLBCL not otherwise specified, PMBCL, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma [7, 12].
JULIET (NCT02445248) is a phase 2 single arm pivotal multicenter trial of tisagenlecleucel (kymriah™) formerly known as CTL019 in adults patients with relapsed refractory DLBCL [13, 14]. Kymriah is a second generation CD19 CAR with 4-1BB activating domain. Lentiviral vector is used for transduction of autologous T cells in order to manufacture Kymriah. Kymriah was approved by US FDA on 8/30/2017 for pediatric and young adult patients (up to 25 years of age) with relapsed refractory B cell acute lymphocytic leukemia (B cell ALL). The same product was used in JULIET trial. Patients enrolled in JULIET trial were 18 years or older with relapsed refractory DLBCL defined as relapsed or refractory after 2 or more lines of therapies or relapse after auto-HCT. Primary end point was ORR and secondary endpoints included OS and duration of response (DOR) and safety. The median age was 56 years (rage, 22–76 years). Fifty one percent of patients were status post 3 or more lines of therapies and 51% had relapsed disease post auto-HCT. Ninety nine out of 147 patients enrolled received single infusion of Kymriah. Ninety percent of patients received bridging chemotherapy and 92% of patients received lymphodepleting chemotherapy including fludarabine 25 mg/m2/day plus cyclophosphamide 250 mg/m2/day × 3 days in 73% of patients and bendamustine 90 mg/m2/day × 2 days in 19% of the patients. Median (range) of CTL019 transduced cells was 3.1 × 108 (0.1–6.0 × 108) cells. Data regarding 81 patients with at least 3 months follow up post Kymriah was reported. ORR and CR rates were 53.1% (95% CI, 42% to 64%; P <.0001) and 39.5% respectively. CR rates at 3 months and 6 months were 32% and 30% respectively. Ninety five percent of patients in CR at 3 months remained in CR at 6 months. Median duration of response and OS was not reached. The probability of overall survival at 6 months was 64.5%. Eighty six percent of patients developed grade 3 or higher toxicities. Grade 3 and higher CRS (based on University of Pennsylvania grading) happened in 23% of patients with no grade 5 CRS. Grade 3 and higher neurotoxicity occurred in 12% of patients. No grade 5 neurotoxicity was reported. Three patients died within one month of infusion due to disease progression. No death related to CTL019 was reported [13, 14]. On January 17 2018 US FDA granted priory review of supplemental Biological License Application (sBLA) for Kymriah for treatment of patients with relapsed refractory DLBCL [15].
CTL019 was used in another trial at University of Pennsylvania (NCT02030834) that enrolled patients with CD19+ relapsed refractory lymphomas [16]. A cohort of 14 patients with relapsed refractory DLBCL were treated. Median age was 58 (range, 25–77). Eighty six percent of patients had chemorefractory disease and 50% had relapsed disease post auto-HCT. Various lymphodepleting chemotherapies were used. CTL019 dose was 5.79×106 (range, 3.08×106 to 8.87×106) transduced T cells/kg of recipient body weight. The median number of days from apheresis to infusion of CTL019 was 39 (range, 27–145). ORR and CR rates were 50% and 43% respectively. With median follow up of 28.6 months, 43% of patients were progression free. Among all enrolled patients (including a cohort of patients with FL), grade 3 and higher CRS and neurotoxicity happened in 18% and 11% of patients respectively. No grade 5 CRS or neurotoxicity was reported in the DLBCL cohort [16].
TRANSCEND NHL001 (NCT02631044) is an open-label, multicenter Phase 1 trial of lisocabtagene maraleucel (liso-cel) formerly known as JCAR 017 in patients with relapsed refractory DLBCL and MCL [17]. This study was designed to determine the recommended regimen for the planned pivotal cohort and is currently recruiting patients with the estimated primary completion date of October 2018. Liso-cel is a second generation CD19 directed CAR T cell product with 4-1BB costimulatory domain and a defined composition of CD4+ and CD8+ cells. A lentiviral vector is used for transduction of autologous T cells for manufacturing of liso-cel. This trial has two cohorts, a full DLBCL cohort (including patients with DLBCL NOS, DLBCL transformed from FL or MZL, and FL grade 3B) and a mantel cell lymphoma (MCL) cohort. A subset of full DLBCL cohort including patients with DLBCL NOS, and DLBCL transformed from FL is defined as pivotal (core) cohort. Relapsed refractory disease was defined as refractory to 2 lines of therapy or relapse post auto-HCT or allo-HCT. Interim analysis of the full DLBCL cohort and pivotal (core) cohort was reported in December 2017. Median age was 61 years (range, 26–82 years), median number of prior therapies was 3 (range, 1–12), 67% were chemorefractory, and 46% had relapsed disease post-transplant. Bridging chemotherapy is allowed. Lymphodepleting chemotherapy includes fludarabine and cyclophosphamide daily for 3 days. There was 3 dose levels of JCAR017: DL1 single infusion of 5 × 107 CAR T cells, DL1 double infusion of 5 × 107 CAR T cells, and DL2 single infusion of 1 × 108 CAR T cells. In full DLBCL cohort, 69 patients were evaluable for safety and 68 patients were evaluable for efficacy analysis. In full DLBCL cohort best ORR, 3 month response rate and 6 month response rate were 75%, 49%, and 40% respectively. The best overall CR rate, 3 month and 6 month CR rates were 56%, 40%, and 37% respectively. In subset analysis of pivotal (core) group of patients with DLBCL NOS or DLBCL transformed from FL (n=49), ORR, 3 month and 6 month response rates were 84%, 65%, and 57% respectively. The best overall CR rates, 3 month and 6 month CR rates in core group were 61%, 53%, and 52% respectively. Median DOR and OS for the full DLBCL cohort was 5 months and 13.7 months respectively. Median DOR for the pivotal (core) DLBCL cohort was 9.2 months and median OS for the pivotal (core) cohort was not reached. Grade 3 and higher CRS and neurotoxicity happened in 1% and 14% of patients respectively with no death related to CRS or neurotoxicity. Median time from infusion of liso-cel to onset of CRS and neurotoxicity was 5 days (range, 2–12) and 10 days (range, 5–23) respectively. There were 2 deaths on study: one due to disease progression and one related to chemotherapy and JCAR017 [17, 18].
Table 1 is a summary of these 3 trials side by side. The summary of other smaller published trials was recently reviewed [19, 20].
Table 1.
Summary of ZUMA-1, JULIET, and TRANSCEND trials.
| ZUMA-1 Yescarta |
JULIET Kymriah |
TRANSCEND JCAR-017 |
|
|---|---|---|---|
|
| |||
| Sponsor | Kite/Gilead | Novartis | Juno/Celgene |
|
| |||
| Source | Phase 1/2 | Phase2 | Phase 1 |
| N Engl J Med 2017;377:2531-44 (NCT02348216) [10, 11] | ASH 2017 # 577: Blood 2017 130:577 (NCT02445248) [13] | ASH 2017 # 581: Blood 2017 130:581 (NCT02631044) [17] | |
|
| |||
| Population |
|
|
(CORE; N = 49)
|
|
| |||
| Enrollment |
|
|
|
|
| |||
| CAR |
|
Second generation, 41BBLentiviral vector |
|
|
| |||
| Dose | 2.0 × 106 CAR T cells/kg | Median, 3.1 × 108 transduced cells | DL1 5.0 × 107 CAR T cells |
| >100 kg: 2.0 × 108 fixed dose | (range, 0.1–6.0 × 108 transduced cells) | DL2 1.0 × 108 CAR T cells | |
|
| |||
| Lymphodepleting chemotherapy | Flu 30 mg/m2 and Cy 500 mg/m2 on days −5, − 4, and − 3 |
|
Flu 30 mg/m2 and Cy 300 mg/m2 for 3 days |
|
| |||
| Efficacy | mITT = 108
|
Median follow up: 5.6 m
|
(CORE; N = 49)
|
|
| |||
| Safety |
|
|
|
Abbreviations: Auto-HCT: autologous stem cell transplant; DLBCL NOS: diffuse large B cell lymphoma not otherwise specified; TFL: transformed follicular lymphoma; DL: dose level; Flu: fludarabine; Cy: cyclophosphamide; Benda: bendamustine; mITT: modified intention to treat; ORR: overall response rate; CR: complete response; m: month; DOR:duration of response; OS: overall survival; NR: not reached; CRS: cytokine release syndrome; NT: neurotoxicity; Gr: grade.
Follicular Lymphoma (FL)
FL is the second most common NHL (10–20% of all NHL) in the US and Europe with annual incidence of 10,000 cases in the US [1, 21]. Although there has been significant advances in treatment of FL with median OS more than 10 years [22, 23], approximately 20% of patients with FL relapse within 2 years of their CR. Patients with elapsed refractory FL have a poor prognosis with median progression free survival of close to one year [21, 24–28]. For patients with chemo refractory FL disease or FL with rapid relapse within 2 years of CR, development of new therapies is warranted.
CTL019 was used in a phase IIa study of 14 patients with FL with disease progression within 2 years of 2 or more lines of therapy (NCT02030834) [16, 29]. Nine patients had grade 1–2 FL and 5 patients had grade 3a FL. The median age was 59 years (range, 43–72) with median number of prior therapies of 5. Two patients had disease relapse post auto-HCT and one patient had disease relapse post allo-HCT. Lymphodepleting chemotherapy included bendamustine (6 patients), cyclophosphamide (2 patients), cyclophosphamide-fludarabine (1 patient), radiation-cyclophosphamide (3 patients), EPOCH (1 patient), and carboplatin-gemcitabine (1 patient). CTL019 dose was 6.08 × 106 (range, 3.08–8.87 × 106) transduced cells. ORR and CR rates at 3 months was 79% (11/14) and 50% (7/14) respectively. Three patients who were in PR at 3 months converted to CR at 6 months. CR rates at 6 months was 71% (10/14). With median follow up of 28.6 months, 70% were progression free with median DOR not reached. While median time to next treatment (TTNT) was 7.2 months for antecedent therapy before CTL019 infusion, median TTNT after CLTL019 was not reached at the time of the report. Two patients (14%) developed grade 3 or higher CRS with no death related to CRS. One patient died of encephalitis possibly related to CTL019 [16, 29].
ZUMA-5 (NCT03105336) is a phase 2 multicenter study of Axicabtagene Ciloleucel in subjects with relapsed/refractory indolent NHL including FL. This study is currently enrolling patients with the estimated primary completion date of December of 2018.
Patients with FL have been enrolled in other CAR T cell trials as small subgroup of patients among other NHL patients. Table 2 summarizes published data of CAR T for FL [30–32].
Table 2.
Selected publications of CAR T cell therapy for follicular lymphoma
| Study |
ClinicalTrials.gov identifier |
Phase | Number of subjects with FL |
CAR | Lymphodepleting chemotherapy |
ORR and CR in subjects with FL |
|---|---|---|---|---|---|---|
|
| ||||||
| Schuster et al. (2017) [16, 29] | NCT02030834 | IIa | 14 |
|
Various regimens | ORR: 79% |
| CR: 50% at 3 m, 70% at 6 m | ||||||
|
| ||||||
| Kochenderfer et al. (2017) [30] | NCT00924326 | I | 2 |
|
Flu 30 mg/m2 daily plus Cy 300 mg/m2 or 500 mg/m2 daily for 3 days | ORR: 100% |
| CR: 100% | ||||||
|
| ||||||
| Turtle et al. (2016) [31] | NCT01865617 | I/II | 6 |
|
|
ORR: 80% |
| CR: 40% | ||||||
|
| ||||||
| Kochenderfer et al. (2012) [32] | NCT00924326 | I | 4 |
|
Cy 60 mg/kg daily for 2 days, followed by Flu 25 mg/m2 daily for 5 days | ORR: 100% |
| CR: 0% | ||||||
Abbreviations: FL: follicular lymphoma; Cy: cyclophosphamide; Flu: fludarabine; ORR: overall response rate; CR: complete response.
Mantle Cell Lymphoma (MCL)
MCL accounts for 7% of all NHL with incidence of 0.55 cases/100,000 people per year in US and 0.45 cases/100,000 people in Europe. It usually affects older people with a median age at diagnosis of 68 years [1, 2]. Standard treatment is upfront chemoimmunotherapy followed by auto-HCT in first CR. These treatments are not curative for MCL with median overall survival of 5–7 years. Relapsed refractory MCL has a poor prognosis [33]. Only a fraction of patients with MCL undergoing allogeneic transplant can be cured suggesting effective cellular therapy can be curative for MCL [33]. CAR T therapy can be an effective treatment for MCL and has a potential to change MCL to a curable disease. ZUMA-2 (NCT02601313) is a phase 2, multicenter, open-label study evaluating the efficacy of KTE-C19 (autologous second generation CD19 CAR T with CD28 activating domain) in subjects with relapsed/refractory MCL with estimated primary completion date of July 2018. Table 3 summarized the outcome of small number of patients with MCL enrolled as subgroup of NHL patients enrolled in CAR T trials [30, 31, 34, 35].
Table 3.
Selected publications of CAR T cell therapy for mantle cell lymphoma
| Study |
ClinicalTrials.gov identifier |
Phase | Number of subjects with MCL |
CAR | Lymphodepleting chemotherapy |
ORR and CR in subjects with MCL |
|---|---|---|---|---|---|---|
|
| ||||||
| Turtle et al. (2016) [31] | NCT01865617 | I/II | 4 |
|
|
ORR: 25% |
| CR: 0% | ||||||
|
| ||||||
| Brudno et al. (2016) [34] | NCT01087294 | I | 5 |
|
No lymphodepleting chemotherapy | ORR: 25% |
| CR: 0% | ||||||
|
| ||||||
| Kochenderfer et al. (2017) [30] | NCT00924326 | I | 1 |
|
Flu 30 mg/m2 daily plus Cy 300 mg/m2 or 500 mg/m2 daily for 3 days | ORR: 100% |
| CR: 100% | ||||||
|
| ||||||
| Schuster et al. (2015) [35] | NCT02030834 | IIa | 2 |
|
Various regimens | ORR: 50% |
| CR: ? | ||||||
Abbreviations: MCL: mantle cell lymphoma; Cy:
Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL)
SLL accounts for 6% of all NHL cases [1]. Although median survival of patients with CLL/SLL is high; CLL/SLL is not curable with standard treatments and patients with relapsed refractory CLL/SLL especially patients with poor cytogenetic features have a poor prognosis with short median survival [36, 37]. Although dramatic responses of first few patients with CLL/SLL receiving CD19 CAR T started a widespread interest in CAR T cells for hematologic malignancies [38] subsequent studies showed lower ORR and CR rates in CLL/SLL compared with ALL. Porter et al., reported the results of phase 2 study of CTL019 (autologous CR19 CAR T with 4-1BB costimulatory domain) in 49 patients with relapsed refractory CLL/SLL defined as refractory or relapse within 2 years after 2 or more prior lines of therapy (NCT01747486) [39]. Median age was 62 years. Forty percent had P53 deletion and 23% failed ibrutinib. ORR and CR rate were 53% and 35% respectively. Turtle et al., recently reported trial of a second generation CD19 CAR in 24 patients with relapsed refractory CLL. ORR and CR rates were 74% and 21% respectively [40]. T cells in patients with CLL/SLL have intrinsic functional defect impairing their function [41]. Fraietta et al. showed that impaired function of T cells in patients with CLL/SLL can be reversed by using Ibrutinib [41]. They also showed that T cells exposed to ibrutinib are better sources of T cells for CAR T cell production and concurrent use of CAR T cells and ibrutinib in mice model resulted in better efficacy and engraftment of CAR T cells [41]. Indeed combination of Ibrutinib with CAR T cells resulted in significant improvement in response to CAR T cells in the subsequent trial (NCT02640209) [42]. This trial included patients with CLL/SLL who failed at least 6 months of ibrutinib therapy and at least 1 regimen before ibrutinib, unless they had del 17p or a TP53 mutation. CTL119 (humanized autologous second generation CD19 CAR T with 4-1BB activating domain) was used. Patients continued ibrutinib throughout the trial (during leukapheresis and CAR T infusion and after CAR T infusion). Preliminary results showed 8 out of 9 (89%) evaluable patients achieved MRD negative CR confirming preclinical data [42].
T cell NHL
In western countries, T cell lymphomas account for 5% to 10% of NHL cases. Although ALK+ anaplastic large cell lymphoma (6.6% of T cell NHL cases) has a very good prognosis with 5 year survival of more than 70%; Other subtypes of T cell lymphomas have a poor prognosis with a 5 year overall survival ranging from 10% to 50% suggesting that new therapeutic approaches are warranted in treatment of T cell lymphomas [43]. Development of CAR T for T cell malignancies is very challenging because tumor associated targets are also expressed on the surface of normal T cells resulting in fratricide (self-killing of CAR T cells by CAR T cells). Recently our group developed a third generation off the shelf universal CD7 CAR T (UCART7) with both CD28 and 4-1BB costimulatory domains [44]. CRISPER/Case 9 gene editing was used for simultaneous bi-allelic deletion of both CD7 to prevent fratricide and T cell receptor alpha chain (TRAC) to prevent graft vs. host disease. In vitro and mouse studies showed rapid expansion of UCART cells without fratricide with robust efficacy of UCART7 against CD7 positive tumors without GVHD in mice. UCART7 is a fratricide-resistant allo-tolerant off-the-shelf CAR T that represent the first clinically feasible CAR T for T cell lymphomas [44].
Conclusion
Recent advances in CAR T cell therapy has provided us with very effective options for treatment of patients with NHL. Recent approval of Yescarta for relapsed refractory DLBCL, high grade lymphomas and PMBCL has paved the way for new CAR T cell therapies, not only for NHL but other hematologic malignancies in the near future. Although CAR T cells are very effective, there is still room for improving efficacy and duration of response. Additionally, while CRS and neurotoxicity can almost always be treated and reversed, these complications can cause significant morbidity in patients receiving CAR T. Therefore new strategies to block or prevent these side effects without affecting the efficacy of CAR T cells are warranted. In conclusion, advances in CAR T cell therapy, in conjunction with advances in targeted molecular therapies, checkpoint inhibitors, antibody drug conjugates, and T cell engagers are dramatically changing the way we are treating NHL and other hematologic malignancies.
Acknowledgments
This work is supported by NIH UL1TR002345.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure: AG reports advisory board fee and grant support from Kite Pharma.
References
- 1.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107:265–76. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116:3724–34. doi: 10.1182/blood-2010-05-282632. [DOI] [PubMed] [Google Scholar]
- 3.http://gco.iarc.fr/today/online-analysis-multi-bars?mode=population&mode_population=continents&population=900&sex=0&cancer=26&type=0&statistic=0&prevalence=0&color_palette=default.
- 4.Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology American Society of Hematology Education Program. 2011;2011:498–505. doi: 10.1182/asheducation-2011.1.498. [DOI] [PubMed] [Google Scholar]
- 5.www.lymphomacoalition.org/docman2/leip/853-dlbcl-subtype-report/file.
- 6.Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–8. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm581216.htm.
- 8.Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nature reviews Clinical oncology. 2016;13:370–83. doi: 10.1038/nrclinonc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nature reviews Cancer. 2016;16:566–81. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neelapu SS. An interim analysis of the ZUMA-1 study of KTE-C19 in refractory, aggressive non-Hodgkin lymphoma. Clinical advances in hematology & oncology : H&O. 2017;15:117–20. [PubMed] [Google Scholar]
- 11.Locke FL, Neelapu SS, Bartlett NL, Siddiqi T, Chavez JC, Hosing CM, et al. Phase 1 Results of ZUMA-1: A Multicenter Study of KTE-C19 Anti-CD19 CAR T Cell Therapy in Refractory Aggressive Lymphoma. Molecular therapy : the journal of the American Society of Gene Therapy. 2017;25:285–95. doi: 10.1016/j.ymthe.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.https://www.fda.gov/downloads/UCM581226.pdf.
- 13.Schuster SBM, Tam C, et al. Primary Analysis of Juliet: A Global, Pivotal, Phase 2 Trial of CTL019 in Adult Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Blood. 2017;130:577. [Google Scholar]
- 14.Schuster SJ, Bishop MR, Tam C, Waller EK, Borchmann P, McGuirk J, et al. Global Pivotal Phase 2 Trial of the Cd19-Targeted Therapy Ctl019 in Adult Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma - an Interim Analysis. Haematologica. 2017;102 [Google Scholar]
- 15.https://www.novartis.com/news/media-releases/novartis-granted-us-fda-priority-review-kymriahtm-tisagenlecleucel-formerly-ctl019-adults-rr-dlbcl.
- 16.Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak O, et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. New Engl J Med. 2017;377:2545–54. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abramson J, Palomba, Gordon L, et al. High Durable CR Rates in Relapsed/Refractory (R/R) Aggressive B-NHL Treated with the CD19-Directed CAR T Cell Product JCAR017 (TRANSCEND NHL 001): Defined Composition Allows for Dose-Finding and Definition of Pivotal Cohort. Blood. 2017;130:581. [Google Scholar]
- 18.http://www.onclive.com/conference-coverage/icml-2017/jcar017-impresses-in-updated-dlbcl-data.
- 19.Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nature Reviews Clinical Oncology. 2018;15:31–46. doi: 10.1038/nrclinonc.2017.128. [DOI] [PubMed] [Google Scholar]
- 20.Gauthier J, Yakoub-Agha I. Chimeric antigen-receptor T-cell therapy for hematological malignancies and solid tumors: Clinical data to date, current limitations and perspectives. Curr Res Transl Med. 2017;65:93–102. doi: 10.1016/j.retram.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. Ca-Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 22.Fisher RI, LeBlanc M, Press OW, Maloney DG, Unger JM, Miller TP. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005;23:8447–52. doi: 10.1200/JCO.2005.03.1674. [DOI] [PubMed] [Google Scholar]
- 23.Nooka AK, Nabhan C, Zhou X, Taylor MD, Byrtek M, Miller TP, et al. Examination of the follicular lymphoma international prognostic index (FLIPI) in the National LymphoCare study (NLCS): a prospective US patient cohort treated predominantly in community practices. Ann Oncol. 2013;24:441–8. doi: 10.1093/annonc/mds429. [DOI] [PubMed] [Google Scholar]
- 24.Groves FD, Linet MS, Travis LB, Devesa SS. Cancer surveillance series: Non-Hodgkin's lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J Natl Cancer I. 2000;92:1240–51. doi: 10.1093/jnci/92.15.1240. [DOI] [PubMed] [Google Scholar]
- 25.Coiffier B, Osmanov EA, Hong XN, Scheliga A, Mayer J, Offner F, et al. Bortezomib plus rituximab versus rituximab alone in patients with relapsed, rituximab-naive or rituximab-sensitive, follicular lymphoma: a randomised phase 3 trial. Lancet Oncol. 2011;12:773–84. doi: 10.1016/S1470-2045(11)70150-4. [DOI] [PubMed] [Google Scholar]
- 26.Kahl BS, Bartlett NL, Leonard JP, Chen L, Ganjoo K, Williams ME, et al. Bendamustine Is Effective Therapy in Patients With Rituximab-Refractory, Indolent B-cell Non-Hodgkin Lymphoma Results From a Multicenter Study. Cancer. 2010;116:106–14. doi: 10.1002/cncr.24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher RI, Kaminski MS, Wahl RL, Knox SJ, Zelenetz AD, Vose JM, et al. Tositumomab and iodine-131 tositumomab produces durable complete remissions in a subset of heavily pretreated patients with low-grade and transformed non-Hodgkin's lymphomas. J Clin Oncol. 2005;23:7565–73. doi: 10.1200/JCO.2004.00.9217. [DOI] [PubMed] [Google Scholar]
- 28.Leahy MF, Turner JH. Radioimmunotherapy of relapsed indolent non-Hodgkin lymphoma with 131I-rituximab in routine clinical practice: 10-year single-institution experience of 142 consecutive patients. Blood. 2011;117:45–52. doi: 10.1182/blood-2010-02-269753. [DOI] [PubMed] [Google Scholar]
- 29.Chong E, Svoboda J, Dwivedy Nasta S, et al. Chimeric Antigen Receptor Modified T Cells Directed Against CD19 (CTL019) in Patients with Poor Prognosis, Relapsed or Refractory CD19+ Follicular Lymphoma: Prolonged Remissions Relative to Antecedent Therapy. Blood. 2016;128:1100. [Google Scholar]
- 30.Kochenderfer JN, Somerville RPT, Lu T, Shi V, Bot A, Rossi J, et al. Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels. J Clin Oncol. 2017;35:1803–13. doi: 10.1200/JCO.2016.71.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, et al. Immunotherapy of non-Hodgkin's lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Science translational medicine. 2016;8:355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghielmini M, Zucca E. How I treat mantle cell lymphoma. Blood. 2009;114:1469–76. doi: 10.1182/blood-2009-02-179739. [DOI] [PubMed] [Google Scholar]
- 34.Brudno JN, Somerville RPT, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J Clin Oncol. 2016;34:1112-+. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuster S, Svoboda J, Nasta SW, et al. Sustained Remissions Following Chimeric Antigen Receptor Modified T Cells Directed Against CD19 (CTL019) in Patients with Relapsed or Refractory CD19+ Lymphomas. Blood. 2015;126:183. [Google Scholar]
- 36.Dreger P, Schetelig J, Andersen N, Corradini P, van Gelder M, Gribben J, et al. Managing high-risk CLL during transition to a new treatment era: stem cell transplantation or novel agents? Blood. 2014;124:3841–9. doi: 10.1182/blood-2014-07-586826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown JR. The Treatment of Relapsed Refractory Chronic Lymphocytic Leukemia. Hematol-Am Soc Hemat. 2011:110–8. doi: 10.1182/asheducation-2011.1.110. [DOI] [PubMed] [Google Scholar]
- 38.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric Antigen Receptor-Modified T Cells in Chronic Lymphoid Leukemia. New Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porter D, Frey N, Melenhorst J, et al. Randomized, phase II dose optimization study of chimeric antigen receptor (CAR) modified T cells directed against CD19 in patients (pts) with relapsed, refractory (R/R) CLL. Journal of Clinical Oncology. 2016 May;34(15_suppl):3009–3009. [Google Scholar]
- 40.Turtle CJHK, Hanafi L-A, Li D, Cherian S, Chen X, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chi-meric antigen receptor – modified T-cells after failure of ibrutinib. J Clin Oncol. 2017;10(35):3010–20. doi: 10.1200/JCO.2017.72.8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng ZH, Barrett DM, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127:1117–27. doi: 10.1182/blood-2015-11-679134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saar Gill SFN, Hexner E, et al. CD19 CAR-T cells combined with ibrutinib to induce complete remission in CLL. 7509 Journal of Clinical Oncology. 2017 May;35(15_suppl):7509–7509. [Google Scholar]
- 43.Vose JM, Neumann M, Harris ME Project IT-CL. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–30. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 44.Cooper M, Choi J, Staser K, et al. An Off-the-Shelf™ Fratricide-Resistant CAR-T for the Treatment of T Cell Hematologic Malignancies. Blood. 2017;130:844. doi: 10.1038/s41375-018-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]