Abstract
Renal sympathetic activity affects blood pressure in part by increasing renovascular resistance via release of norepinephrine (NE) from sympathetic nerves onto renal arteries. Here we test the idea that adipose tissue adjacent to renal blood vessels, i.e. renal perivascular adipose tissue (RPVAT), contains a pool of NE which can be released to alter renal vascular function. RPVAT was obtained from around the main renal artery/vein of the male Sprague Dawley rats. Thoracic aortic PVAT and mesenteric PVAT also were studied as brown-like and white fat comparators respectively. RPVAT was identified as a mix of white and brown adipocytes, because of expression of both brown-like (e.g. uncoupling protein 1) and white adipogenic genes. All PVATs contained NE (ng/g tissue, RPVAT:524±68, TAPVAT:740±16, MPVAT:96±24). NE was visualized specifically in RPVAT adipocytes by immunohistochemistry. The presence of RPVAT (+RPVAT) did not alter the response of isolated renal arteries to NE compared to responses of arteries without RPVAT (−RPVAT). By contrast, the maximum contraction to the sympathomimetic tyramine was ~2x greater in the renal artery +PVAT versus −PVAT. Tyramine-induced contraction in +RPVAT renal arteries was reduced by the α1-adrenoceptor antagonist prazosin and the NE transporter inhibitor nisoxetine. These results suggest that tyramine caused release of NE from RPVAT. Renal denervation significantly (>50%) reduced NE content of RPVAT but did not modify tyramine-induced contraction of +RPVAT renal arteries. Collectively, these data support the existence of a releasable pool of NE in RPVAT that is independent of renal sympathetic innervation and has the potential to change renal arterial function.
Keywords: catecholamines, PVAT, renal artery, hypertension
Graphical abstract
1.0 Introduction
Perivascular adipose tissue (PVAT) is fat that closely surrounds most blood vessels (except in the brain); it was originally considered to be connective tissue that provides mechanical support and cushioning to blood vessels. An entirely new function was suggested in a ground-breaking publication by Soltis and Cassis (50). They demonstrated that removal of the PVAT layer around the thoracic aorta shifted in vitro contraction to norepinephrine (NE) to the left, as did inhibition of NE uptake. These data suggested that PVAT can specifically modify responsiveness of underlying blood vessels to NE. Many subsequent studies confirmed that PVAT in healthy animals works through a variety of mechanisms to exert a largely anti-contractile function (44, 48, 51). Furthermore, it was discovered that PVATs at different locations along the vascular tree are not identical in either structure or function, including their relative amounts of white and brown fat (28).
In addition to the generally anti-contractile nature of PVAT described above, we have shown that PVAT contains measurable amounts of catecholamines (8–10). These include NE, epinephrine (EPI), the precursor L-3,4-dihydroxyphenylalanine (DOPA) and intermediate dopamine (DA). These findings are consistent with the work of Vargovic et al (52) who first showed that adipocytes from the mesentery contain catecholamines. Mesenteric PVAT appears to possess a functional adrenergic system. This includes the ability to release (9), take up (8) and metabolize NE (10). NE concentration – of all the amines listed above – is the highest of all the amines in PVAT (9). Most of our work on PVAT has focused on the mesenteric arteries and aorta, where we propose that NE released from PVAT could affect contractile function. In the present study, we investigate this concept in a vascular region currently receiving a great deal of attention in the field of hypertension—the renal circulation.
Reducing sympathetic nervous system activity to the kidneys, i.e. renal denervation (RDN), has been proposed to be an effective method for lowering blood pressure in patients with essential hypertension (25, 30, 49, 55), especially patients with drug resistant hypertension (21, 37), although this is being debated (14, 24). Renal sympathetic nerve activity can influence blood pressure by altering renal vascular tone, renin release and tubular sodium and water reabsorption, but the mechanisms by which RDN lowers blood pressure in humans with hypertension have not been identified. Nevertheless, there is substantial evidence that increased renal vascular resistance is a significant factor in the development and maintenance of essential hypertension in humans (23, 34) and that increased sympathetic nervous system activity is in part responsible (31). Furthermore, selective activation of renal adrenergic receptors by infusion of the sympathetic neurotransmitter norepinephrine (NE) can cause increased renovascular resistance and sustained hypertension in experimental animals (35, 43). Under normal circumstances, sympathetic influences on renovascular tone mainly derive from NE released from sympathetic nerve terminals in the adventitia of renal blood vessels. Circulating catecholamines play a much smaller role. Here we investigate the novel concept that another source of NE regulating renovascular function could be renal perivascular adipose tissue (RPVAT).
The initial part of this work was to characterize the type of adipocytes in RPVAT, because white and brown fat are known to differ in catecholamine concentration and innervation (11). Second, we compared the catecholamine content of RPVAT to that of other PVAT depots. Third, we determined if the presence of RPVAT affected the in vitro contractile response of the renal artery to NE and other agonists. Fourth, we used the sympathomimetic amine tyramine to determine if sufficient catecholamines could be released from RPVAT to cause arterial contraction in vitro. Last, we tested the hypothesis that part of the releasable NE in RPVAT exists independent of sympathetic innervation, such that NE from PVAT could control arterial function independent of the nerve. This work is the first to directly address RPVAT as a possible influence on the function of the renal artery.
2.0 Methods and Materials
2.1 Chemicals
Acetylcholine chloride, nisoxetine hydrochloride, norepinephrine hydrochloride, pargyline hydrochloride, phenol, phenylephrine hydrochloride and tyramine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2 Animals
Male Sprague-Dawley rats (225–275 gram or ~8–10 weeks of age, Charles River, Indianapolis, IN USA) were used. All protocols were approved by the MSU Institutional Animal Care and Use Committee and follow the “Guide for the Care and Use of Laboratory Animals”, 8th edition (2011) and the ARRIVE guidelines. Rats were anesthetized with sodium pentobarbital (60–80 mg/kg, IP). Anesthesia was verified by lack of paw pinch and eye blink reflexes. Death was assured by pneumothorax and exsanguination after which tissues were removed for one of the following protocols. Tissues removed included sections of the kidneys, main renal artery +PVAT, thoracic aorta +PVAT and mesenteric bed (including arteries +PVAT).
2.2.1 Tissue dissection
Tissues were placed in a Silastic® filled dish and kept under physiological salt solution [PSS in mM; NaCl 130; KCl 4.7; KH2PO4 1.18; MgSO4 • 7H2O 1.17; NaHCO3 14.8; dextrose 5.5; CaNa2EDTA 0.03, CaCl2 1.6 (pH 7.2)] during dissection (figure 1A and B, arrows point to RPVAT). Once removed from the kidney, vessels were cleaned of blood and of PVAT in the −PVAT groups. The endothelium was left intact in all renal arteries. The aorta was cannulated, and PVAT dissected off and frozen at −80 °C for later use. The small intestine was pinned out in the dish and PVAT around the arterial/vein dyads dissected off and frozen at −80 °C for later use.
Figure 1.
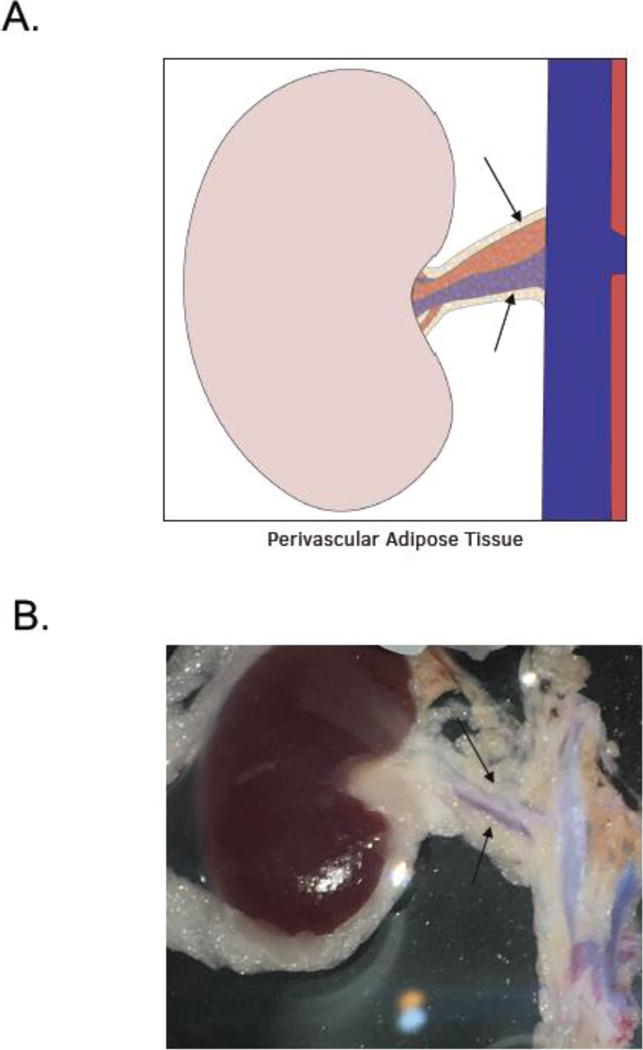
A. Illustration of the RPVAT (renal PVAT) around main renal artery, both used in experiments. B. Image showing the renal artery +PVAT used as the primary tissue of this study.
2.3 HPLC protocol
Catecholamine, 5-HT and tyramine measurements were made by homogenizing tissues (PVATs or tissues taken to validate renal denervation, below) in four times their weight of 0.1M percholoric acid, centrifugation and taking samples through a 30 kDa filtration tube. The filtrate was analyzed by HPLC. When measuring NE released from PVAT into buffer, only the buffer was used in HPLC. The HPLC system (ESA Biosciences, Chelmsford MA) consisted of a Coulochem III electrochemical detector set at −300mV with separation of the analytes on an HR-80 reverse-phase column (Thermo Scientific, Waltham MA). Cat-A-Phase II (Thermo) was the mobile phase with a flow rate of 1.1 mL min−1 and the separation column was maintained at 35 °C. Quantification of the analytes was accomplished by performing a standard curve periodically and the limit of detection was 0.1 ng/mL for catecholamines and 0.5 ng/mL for 5-HT. For release experiments, release was normalized to the amount of tissue from which it was released.
2.3.1 NE release experiments
PVAT was dissected away from both renal arteries and placed in a normal PSS. After blotting, PVATs were weighed and equally split into two tubes containing 250 μL fresh acidified (pH 6.0) PSS containing the metabolism inhibitors semicarbazide hydrochloride (to inhibit semicarbazide sensitive amine oxidase; 1 mM) and pargyline (to inhibit monoamine oxygenase; 10 μM). The tissues were minced at room temperature and then kept at 37°C for 30 min. Tyramine (100 μM) or vehicle (same volume) was added, and incubated at 37°C for 60 min. The supernatant was passed through a 30 kDa filter tube, centrifuged (12000 rpm, 20 min at 10°C) and stored in −80 °C for future analysis by HPLC as described above.
2.4 Immunohistochemical protocol
Paraffin-embedded rat adrenal (catalog no. RP-501; Zyagen, San Diego, CA, USA) and brown fat (interscapular; catalog no. RP-131; Zyagen) were used as positive controls for NE and UCP1, respectively. Slides were submerged twice in HistoChoice Clearing Agent (catalog no. H103; Amresco, Solon, OH, USA), four times in 100% isopropanol, and twice in dH2O, for 3 min each. Slides were submerged and microwaved in 1% antigen unmasking solution (catalog no. H-330; Vector Laboratories, Burlingame, CA, USA) for antigen retrieval and rinsed in dH2O. NE primary antibody [1:250 (Ab8887, Abcam, Cambridge, UK)] or UCP1 primary antibody [1:125 (MAB6158; R&D Systems, Minneapolis, MN, USA)] was added for 1 hour at 37 °C in a humidified chamber. Slides were developed using 3, 3-diaminobenzidine (catalog no. SK-4100; Vector) for 2 minutes. Hematoxylin QS (catalog no. H-3404; Vector) was added for 30 s as a counterstain. Coverslips were mounted using Vectamount (catalog no. H-5000; Vector). Imaging was performed on a Nikon TE2000 inverted microscope with MMI Cell Tools (Molecular Machines & Industries, Zurich, Switzerland).
2.5 RT-PCR and SuperArrays
2.5.1 PCR primers
Primers were obtained from Integrated DNA Technologies (Skokie, IL, USA) and Qiagen (Valencia, CA, USA; proprietary) and are listed as follows if nonproprietary. F = forward, R = reverse.
Tcf21-EXON 1–2 RN.PT.58.11686965; Ucp1-F CTT CTC AGC CGG CGT TTC TG, Ucp1-R GGT GAT GGT CCC TAA GAC ACC; Bact-F CCC ATC TAT GAG GGT TAC GC, Bact-R TTT AAT GTC ACG CAC GAT TTC; Cidea- Cat# PPR06597A, Ref Seq. NM_001170467.1; Cebpa-F TTT CGT AAC CGT CGC TCC TC, Cebpa-R TTC ACA TGT ACC TGC GCC TC; Adipoq- Exon 2b–3b, Rn.PT. 58.13973328, Pparg-F- GGT GTG ATC TTA ACT GTC GG; Pparg-R- TTC AGC TGG TCG ATA TCA CT. Bact was used as a calibrator gene and then mesenteric and aortic PVAT samples compared to the results from renal PVAT.
2.5.2 Tissue isolation and real time PCR
Tissue was homogenized using an Omni Bead Rupter (Omni, Kennesaw, GA). RNA was extracted with the Quick RNA MiniPrep kit (catalog no. R1053; Zymo Research, Irving CA, USA) and further purified with the RNA Clean & Concentrator (catalog no. R1017; Zymo Research). Purity (260/280 and 260/230 ratios ≥ 1.80) was assessed using a Nanodrop 2000C spectrophotometer (Thermoscientific, Wilmington, DE, USA). mRNA was reverse transcribed with a High Capacity cDNA Reverse Transcription kit (catalog no. 4368814; Thermoscientific). RT-PCR was performed using Power SYBR Green PCR Master Mix (catalog no. 4367659; Applied Biosystems, Foster City, CA, USA) on the ABI 7500 Fast Real-Time PCR system using the following parameters: 95 °C for 15 s, 60 °C for 1 min for 40 cycles.
2.6 Isometric contraction
Rat main renal artery (left or right) cleaned of fat (−PVAT) or with fat intact (+PVAT) were mounted into a Multi Wire Myograph System 620M (Danish Myo Technology, Denmark). Data were acquired using a PowerLab Data Acquisitions unit (ADInstruments, Colorado Springs, CO, USA). Baths contained warmed, oxygenated PSS (95% O2/5% CO2). In initial experiments, the optimum resting of renal rings +/− PVAT was determined by applying cumulative additions of passive tension and measuring the active response to the α1 adrenergic receptor agonist PE. In all subsequent experiments, rings were pulled to optimum resting tension (14 mN) with the aid of the DMT Normalization Module for LabChart software and PowerLab (Danish Myo Technology and ADInstruments; http://cdn.adinstruments.com/adi-web/brochures/DMT-Normalization-2011.pdf) and equilibrated for one hour with washes every 15 min. The arteries were exposed to an initial concentration of PE (10 μM) to test viability. Tissues were then washed and returned to baseline. The presence of the endothelium was confirmed by observing at least an 80% relaxation to the agonist acetylcholine (1 μM) after the pre-contraction with a half-maximal concentration of PE. After washing, cumulative concentration-effect curves for PE, NE or tyramine were performed. In some experiments, either vehicle or inhibitor was added for one hour incubation prior to generating a concentration response curve to agonist. In other experiments, the antagonist was added directly to a maximally-contracted tissue. At the end of each experiment, tissues were washed and a final PE addition was performed to test for tissue viability at the end of the experiment. Data are expressed as percent maximum contraction induced by PE (10 μM) at the beginning of the respective protocol.
2.7 Renal Denervation (RDN) in rats
Denervation of both afferent and efferent left renal nerves was performed as previously described by Jacob et al (33). Briefly, rats were anesthetized with 2% isoflurane, and the left renal nerves were then exposed through a midline abdominal incision. Using a dissecting microscope, the renal vein and artery were dissected out of the surrounding fascia and stripped of all visible renal nerve bundles. Following dissection, some of the rats had the renal artery painted with 10% phenol in ethanol to ensure the destruction of any remaining renal nerve fibers. The sham control was performed by externalizing the viscera, visualizing the renal artery and vein, replacing the organs, and then closing the wound.
2.7.1 Validation of RDN by HPLC
HPLC for NE was performed to quantify the extent of efferent renal nerve ablation. Kidneys were removed immediately after death and before the dissection of the left renal arteries (+ and − PVAT) for the contractility studies. Renal cortex and a small RPVAT sample were taken from left and right kidneys and flash frozen in liquid nitrogen. All frozen samples were stored at −80°C until being assayed as described above.
2.8 Data and statistical analysis
Data are reported as means±SEM for number of animals indicated by N or near each bar within the graphs. Statistical analyses were performed with GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA). All images taken were modified for brightness and contrast as a whole, never in part. Results within real time PCR are all reported relative to β-actin expression, and reported as 2−ddCT (39) using renal values as the comparator. In these experiments, normalized responses for renal artery were set to one for comparison to results from mesenteric and aortic PVAT. Contraction is reported as means±SEM as a percentage of the initial contraction to PE (10 μM) or as a percentage of tyramine-induced contraction remaining. Potency means (−log EC50, M) were calculated using GraphPad Prism 7.0. Where a maximum was not achieved, the values are estimated and true potencies are equal or greater than that reported. Data were analyzed by one-way ANOVA followed by the Newman-Keuls method for post hoc comparisons or unpaired Student-t test. P<0.05 was considered statistically significant. Statistical differences are shown only in the tables that report the pharmacological parameters so as to keep the figures clear.
3.0 Results
3.1 RPVAT contains catecholamines
PVAT isolated from directly outside of the renal vasculature (RPVAT) was processed for HPLC. All amines, with the exception of DOPA, were detected in the RPVAT (figure 2A). NE was the highest in concentration.
Figure 2.
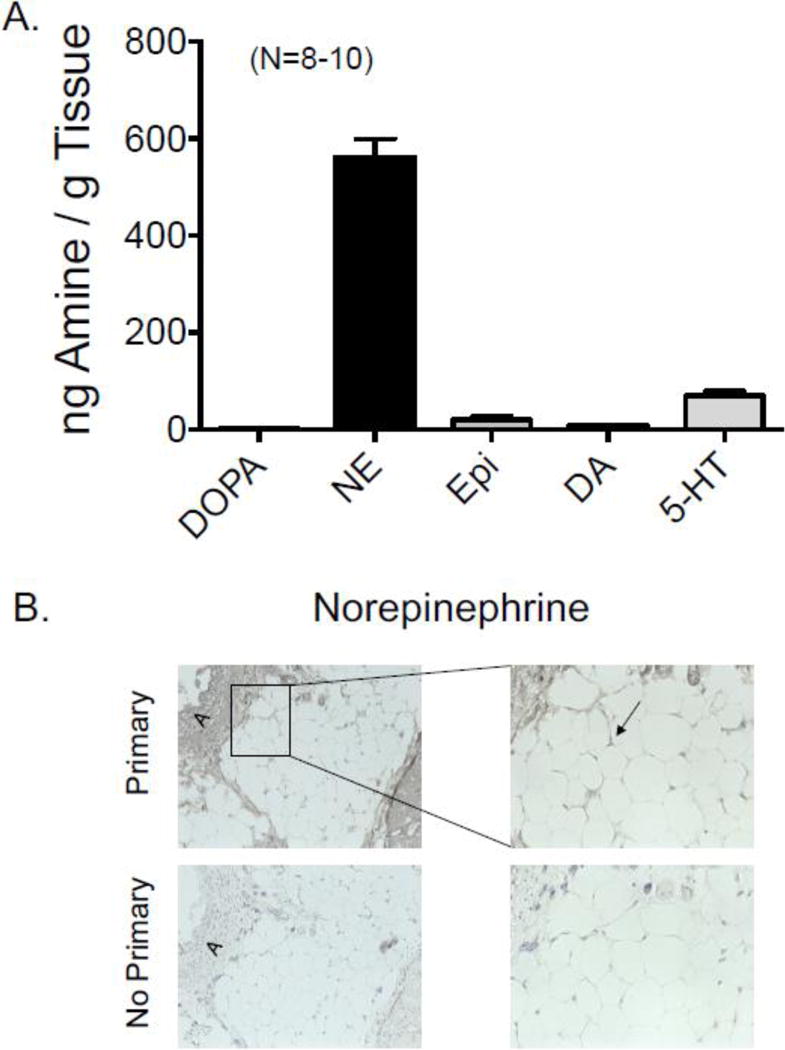
A. Content of amines in the PVAT around the main rat renal artery. DOPA = L-3, 4-dihydroxyphenylalanine, NE = norepinephrine, Epi = epinephrine, DA = dopamine, 5-HT = 5-hydroxytryptamine. Bars are means±SEM for number of animals in parentheses. B. Immunohistochemical staining of NE around the rat main renal artery. primary = sections in which primary antibody against NE was used, no primary = sections in which primary antibody was left out of reaction. “A” marks the vessel and PVAT is immediately adjacent. Representative of five (5) different male rats.
3.2 NE is located in RPVAT adipocytes
Immunohistochemical sections of the renal artery +PVAT were incubated with an antibody directed against NE. Figure 2B shows the results of these experiments which support the HPLC finding that NE is present in RPVAT, given the clear loss of signal in sequential sections incubated without the primary antibody. NE was observed in the cytoplasm around the lipid droplet of the adipocytes in RPVAT.
3.3 RPVAT is a mix of brown and white fat
To determine the type of fat that constitutes RPVAT, both immunohistochemistry (figure 3A) and RT-PCR was used (figure 3B). UCP-1 was used as a marker for brown fat, and an antibody against UCP-1 stained islands of fat within RPVAT, but not all RPVAT. The distinctive phenotype of small, multilocular brown adipocytes was clear and contrasted with white adipocytes (unilocular) that resided immediately adjacent. Consistent with this observation, genes expressed by brown fat were detected in renal PVAT (ucp1, cidea), using the rat thoracic aorta PVAT (TAPVAT) as positive control and white mesenteric PVAT (MPVAT) as a negative control, with all tissues being from the same animal. Importantly, RPVAT expressed Tcf1, a gene largely possessed by white adipocytes, as is shown by the significant expression in MPVAT but not TAPVAT (figure 3B, top line). All three PVATs tested expressed genes that are possessed by adipocytes, and these include genes for adiponectin (adipoq), CCAAT/enhancer-binding protein alpha (CEBPA) and peroxisome proliferator-activated receptor-γ (pparg) (figure 3B, bottom line).
Figure 3.
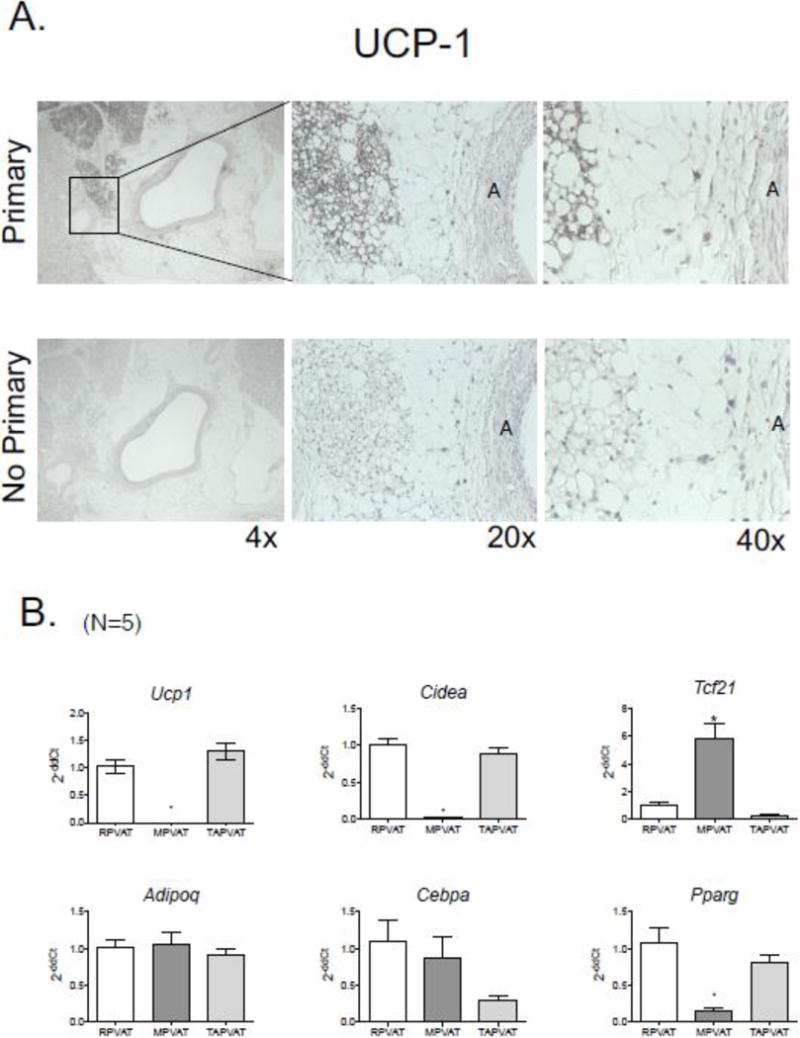
A. Immunohistochemical staining of the brown fat marker uncoupling protein 1 (UCP1) in the PVAT around the rat main renal artery, shown at three different magnifications. Primary = sections in which primary antibody against NE was used, no primary = sections in which primary antibody was left out of reaction. “A” marks the artery and PVAT is immediately adjacent. Representative of five (5) different male rats. B. Expression of fat type genes (top panel) and adipocyte genes (bottom row) as expressed in and normalized to the main renal artery PVAT (RPVAT) in the mesenteric (MPVAT) and thoracic aorta PVAT (TAPVAT) from the same animals. Bars are means±SEM for number of animals in parentheses. * mark statistical differences (p< 0.05).
3.4 Renal artery contractility +/− PVAT
3.4.1 RPVAT mass is consistent
Renal arterial sections, 3 mm in length, were used for all the following experiments. The renal arteries +PVAT were considerably greater in mass than those without PVAT, both when measured wet and dry (figure 4A). These weights were taken so as to determine the variability in the PVAT mass associated with vessels because this could modify the outcome of these experiments. With the high sampling as is shown in figure 4A, the variability was small in renal arteries +PVAT (<10%) and thus we have confidence that differences observed in the contractile studies to be presented is not because of differences within the burden of PVAT carried by tissues.
Figure 4.
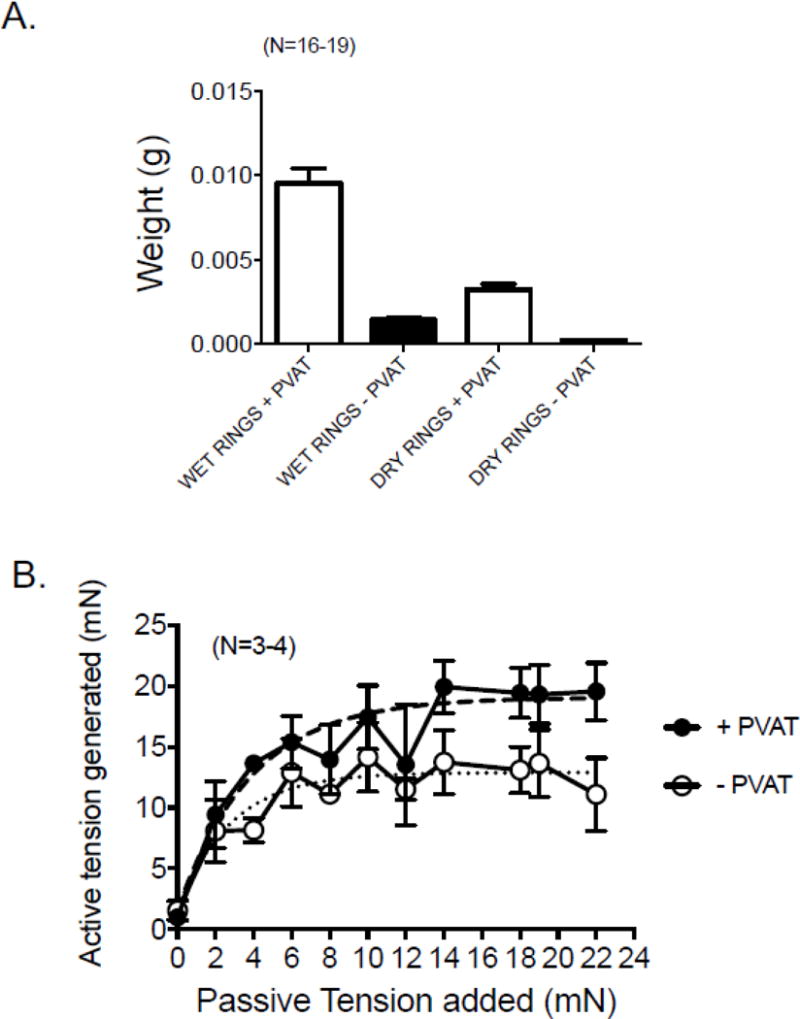
A. Masses of renal artery sections (3 mm in length) with PVAT (+) and without PVAT (−) directly after experimentation (wet) and 24 hours after drying (dry). Bars are means±SEM for number of animals in parentheses. B. Length tension curves for renal arterial rings + and − PVAT. Dashed/dotted lines indicate fitted curves for both groups. Points are means±SEM for number of animals in parentheses.
3.4.2 PVAT does not influence the optimum passive tension in renal artery
We performed step wise, cumulative length tension response curves in the renal artery + and − PVAT using a maximal concentration of PE to assess active response. Figure 4B demonstrates that past 14 mN of applied passive tension, both +PVAT and −PVAT arteries performed maximally. Thus, 14 mN is the amount of passive tension applied to all tissues in the following experiments.
3.4.3 RPVAT does not reduce arterial contraction to NE or PE
Cumulative concentration response curves to NE (figure 5A) and PE (figure 5B) were performed to determine if RPVAT was anti-contractile, an observation made previously in multiple isolated arteries. Neither the potency of these adrenergic agonists nor the maximums achieved were reduced by the presence of PVAT (table 1).
Figure 5.
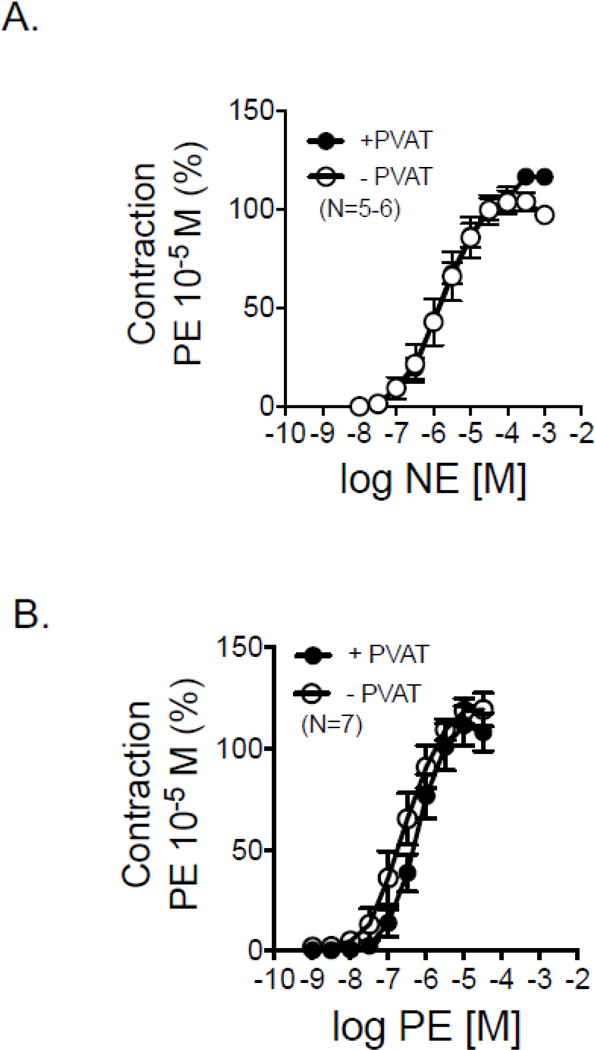
Cumulative concentration response curves to NE (A) and PE (B) in isolated rat renal rings with (+) and without (−) PVAT. Points are means±SEM for number of animals in parentheses.
Table 1.
Potency and efficacy of NE, PE and Tyramine in renal arteries + and − PVAT. Points are means±SEM for number of animals indicated on figures 5 and 6.
| +PVAT | −PVAT | |||
|---|---|---|---|---|
| −log EC50 [M] | Max | −log EC50 [M] | Max | |
| NE | 5.64±0.16 | 122.4±6.0 | 5.86±0.25 | 106.6±4.5 |
| PE | 6.47±0.13 | 111.4±9.5 | 6.73±0.22 | 119.5±8.1 |
| Tyramine | 4.67±0.15 | 68.36±6.20 | 4.43±0.20 | 44.46±6.20* |
= statistically significant differences (p<0.05) between + and − PVAT variable.
3.4.4 Tyramine causes a PVAT−, alpha-1 adrenergic receptor-dependent contraction
By contrast to that observed with adrenergic agonists, the indirect sympathomimetic tyramine caused a PVAT-dependent contraction in the renal artery (figure 6A). Specifically, the maximum contraction caused by tyramine was greater in the presence of PVAT vs the absence (table 1). The potency of tyramine in stimulating contraction was not different between tissues + and − PVAT. This finding suggests tyramine caused contraction in part by releasing NE from the rervoir known to be present in RPVAT (figures 2 and 3). Supporting this idea is that the α1 adrenergic antagonist prazosin (1 μM) significantly reduced tyramine-induced contraction to ~ 25% of control (figure 6B).
Figure 6.
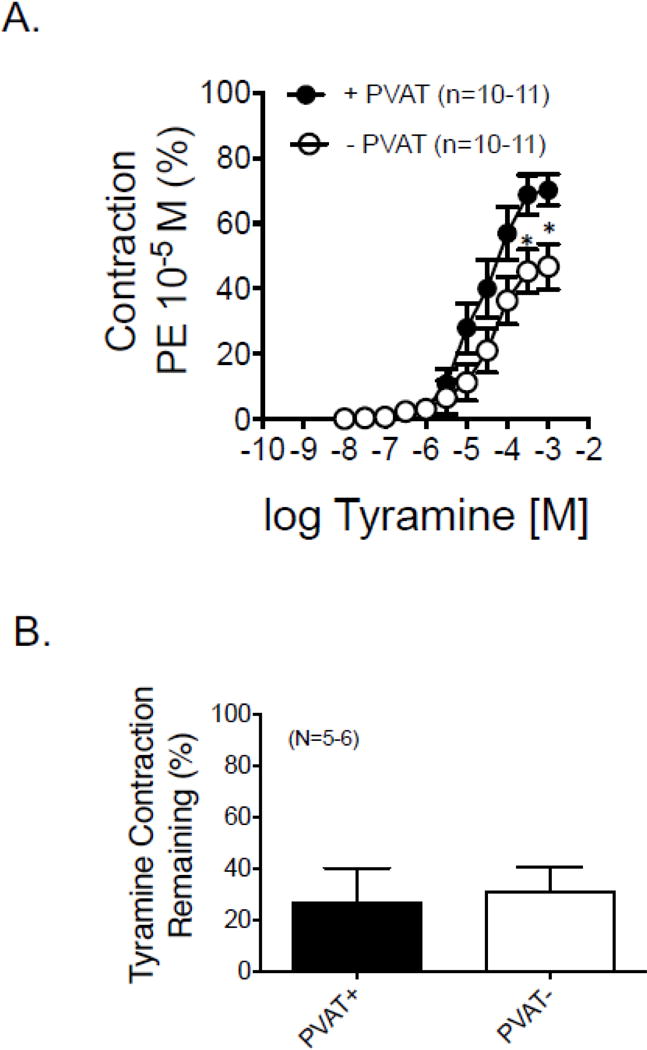
A. Concentration response curves to the indirect sympathomimetic tyramine in isolated rat renal rings with (+) and without (−) PVAT. Points are means±SEM for number of animals in parentheses. B. Effect of the α1 adrenergic receptor antagonist prazosin (1 μM) on the maximum tyramine-induced contraction in isolated rat renal arteries with (+) and without (−) PVAT. Bars are averages ± SEM for the number of animals shown in parentheses. Statistics are marked on Table 1, which reports the pharmacological values for the curves shown in this figure.
3.4.5 Tyramine releases NE from RPVAT; dependence on NET
Consistent with the idea that RPVAT contains releasable amines, the indirect sympathomimetic tyramine (100 μM, near maximal in contraction) caused a quantifiable and detectable release of NE from RPVAT (figure 7A). Because NE release is typically dependent on actions of the NE transporter (NET), we tested the ability of the NET inhibitor nisoxetine to alter tyramine-induced contraction in the renal artery + and − PVAT. Figure 7B and table 2 shares these data. In these specific experiments, the differences between the tyramine-induced contraction in vehicle incubated tissues + and − PVAT was greater than shown in figure 6A. We attribute this to the fact that these tissues were in the tissue bath for a longer time because tissues were incubated with vehicle or nisoxetine for one hour prior to performing a cumulative concentration response curve to tyramine. There appears to be a loss of the tyramine-induced contraction in −PVAT tissues. Nisoxetine was effective in shifting contraction to tissues +PVAT but not −PVAT (table 2). These data support that the NE release from RPVAT, as stimulated by tyramine, is NET-dependent.
Figure 7.
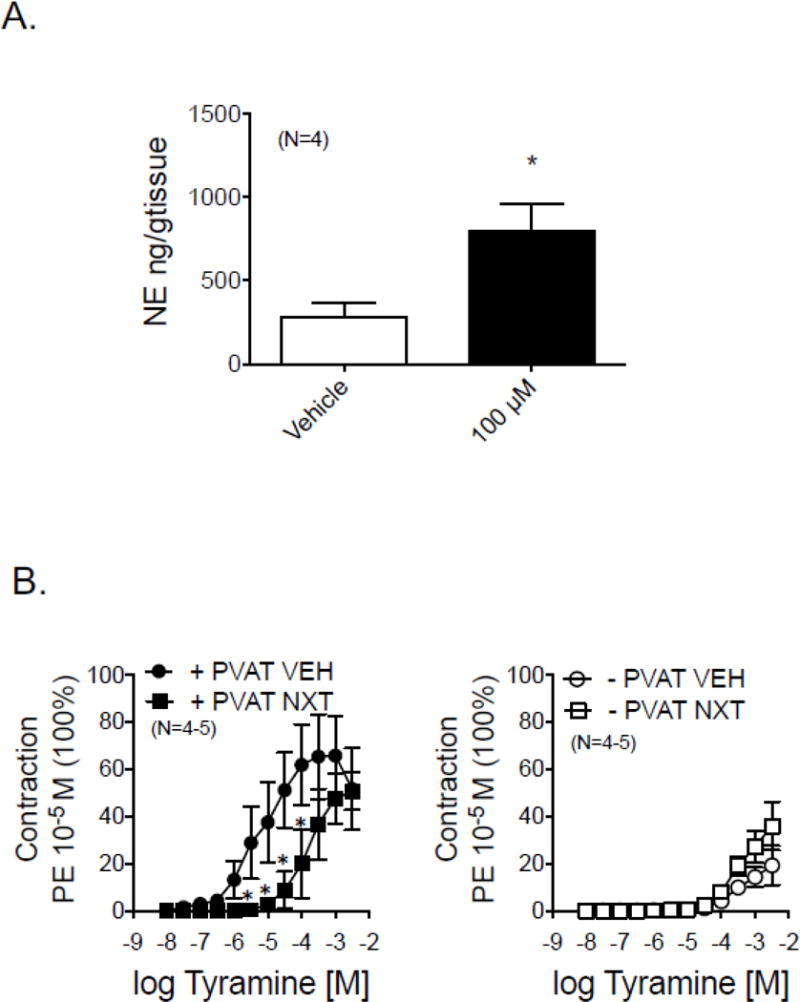
A. Release of the catecholamine NE as stimulated by the indirect sympathomimetic tyramine (100 μM) in isolated male RPVAT. Bars represent means±SEM for number of animals in parentheses. B. Efficacy of vehicle or the norepinephrine transporter inhibitor nisoxetine (NXT; 1 μM) on tyramine-induced contraction in isolated rat renal rings with (+) and without (−) PVAT. Statistics are marked on Table 2, which reports the pharmacological values for the curves shown in this figure
Table 2.
Potency and efficacy of tyramine in renal arteries + and − PVAT in the absence and presence of the NET inhibitor nisoxetine. Points are means±SEM for number of animals indicated on figure 7.
| +PVAT | −PVAT | |||
|---|---|---|---|---|
| −log EC50 [M] | Max | −log EC50 [M] | Max | |
| Vehicle | 4.95±0.28 | 51.6±17.4 | 2.80±0.30 | 19.2±8.2 |
| Nisoxetine | 3.61±0.23* | 50.7±7.8 | 3.08±0.32 | 36.0±10.2 |
= statistically significant differences (p<0.05) between + and − PVAT variable.
3.4.6 NE content is partially nerve dependent while tyramine-induced contraction is not nerve dependent
Renal arteries are significantly innervated by the sympathetic nervous system, and thus it is possible that part of the reason tyramine contracts the renal artery is because it is stimulating neuronal release of NE, a release independent of the content in adipocytes. Renal denervation (RDN) was performed in five pairs of animals, with one receiving a sham renal denervation while the other rat was formally denervated. Arteries were taken from these animals 5 days after surgery. The effectiveness of renal denervation was validated through HPLC experiments in which the amount of NE in the cortex of the kidney that was denervated (figure 8A; left) was significantly reduced when compared to the right kidney from the same animal or the left kidney of sham denervated animals. NE content in RPVAT was reduced but not abolished in tissues from the RDN rats compared to sham (figure 8A right; RDN = 233.0±73 ng/g tissue; Sham RDN = 773.3±78.5 ng/g tissue; p<0.05). Figure 8B left demonstrates that renal denervation did not change the contraction of renal arteries to NE. In these same tissues, renal denervation did not reduce the maximum contraction to tyramine and significantly increased the sensitivity of tyramine in causing contraction when compared to sham controls (figure 8B; table 3). Similar to what was reported in figure 6, prazosin (1 μM) reduced tyramine-induced contraction in all tissues to ~25% of control tyramine contraction in all groups. These data support that the PVAT-dependent contraction to tyramine is not nerve dependent but that NE content is at least partly nerve dependent in the isolated male rat main renal artery.
Figure 8.
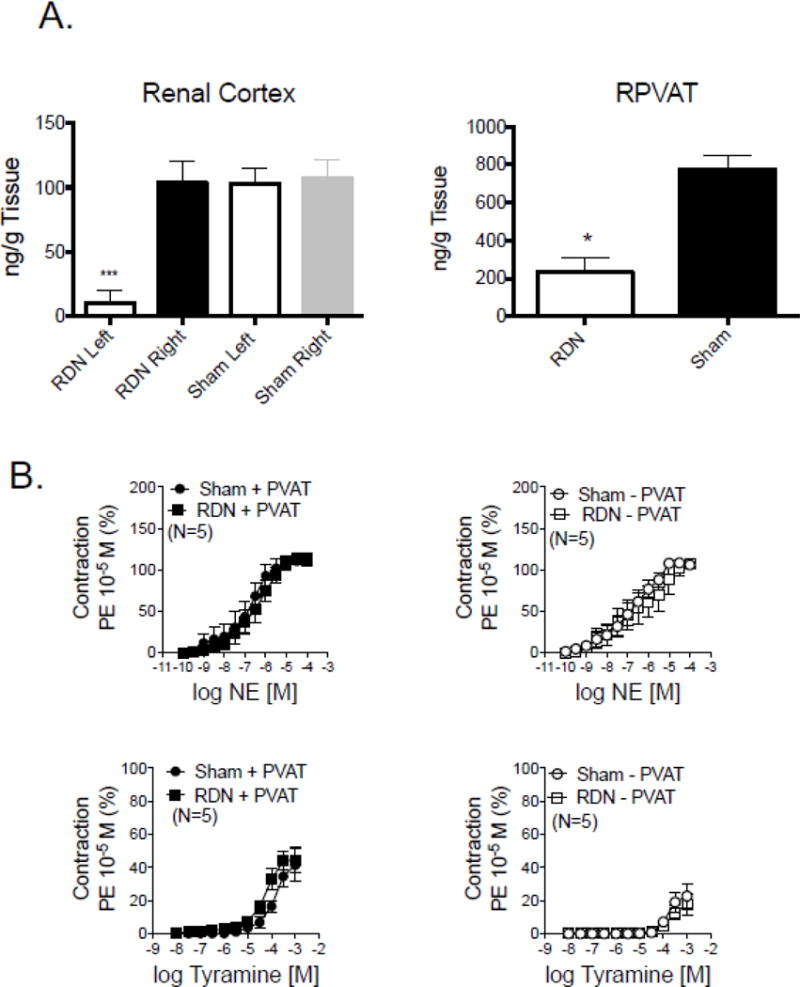
A. left: Validation of the effectiveness of renal denervation in the left kidney as evidenced by a significantly reduced NE content when compared to its body control (right; black bar) and kidneys from sham denervated rats (open, gray bars). Right: RPVAT NE content with denervation and sham procedure. Bars represent means±SEM for number of animals indicated in parentheses. * = statistical reduction using unpaired t tests. B. Contraction of isolated renal arteries taken from sham denervated (sham) or denervated (RDN) rats to NE (left) and tyramine (right). Points represent means±SEM for number of animals indicated in parentheses. Statistics are marked on Table 3, which reports the pharmacological values for the curves shown in this figure.
Table 3.
Potency and efficacy of tyramine in renal arteries + and − PVAT from the sham renal denervated (Sham RDN) and denervated (RDN) rats. Points are means±SEM for number of animals indicated on figure 8.
| +PVAT | −PVAT | |||
|---|---|---|---|---|
| −log EC50 [M] | Max | −log EC50 [M] | Max | |
| Sham RDN | 4.05±0.17 | 56.40±11.61 | 3.81±0.06 | 20.60±5.85* |
| RDN | 4.24±0.10 | 47.62±5.48 | 3.95±0.15 | 15.51±5.50* |
= statistically significant differences (p<0.05) between + and − PVAT variable.
4.0 Discussion
4.1 RPVAT contains brown and white fat
In the rat, the renal artery is different from many other arteries in that the fat type that makes up RPVAT is mixed. Both immunohistochemical, histological and PCR results support that brown and white fat make up renal PVAT in the artery. We compared the renal artery to the thoracic aorta and mesenteric resistance artery, vessels with relatively homogenous PVATs made of brown and white fat, respectively. They served as excellent controls for demonstrating that the RPVAT is ‘middle of the road’ between these two. This, again, underscores the importance of studying each regional PVAT in its own right, and not making generalizations about all PVATs (45, 46). The presence of brown fat may be part of the reason NE content in the renal artery PVAT was the magnitude observed. TAPVAT (brown fat) contains significant amounts of NE (~700–800 ng/g tissue), mesenteric resistance artery PVAT (white fat) does not (~80–100 ng/g tissue; 9, 10). RPVAT was ~580 ng/g tissue, lower than mesenteric resistance PVAT but not as great as that of TAPVAT. These findings are internally consistent.
4.2 RPVAT does not afford anti-contractile protection
The original studies done investigating the effect of PVAT on vascular contraction used the rat thoracic aorta as the model tissue (41, 50). In these studies, the presence of PVAT shifted NE-induced contraction rightward. Such protection, or anti-contractile action, has also been observed against multiple agonists in the porcine coronary of the female (5), rat mesenteric arteries (18, 27, 54), rat aorta (6, 12, 13, 36, 50), mouse aorta (2, 36), mouse mesenteric artery (3) and human (4). In hypertension and obesity, this anticontractile facet is lost, at least in vessels like the aorta and mesenteric arteries (2, 3, 16, 19, 32, 38, 47).
The renal artery appears to be different. Neither agonist potency nor maximum were different in arteries with and without PVAT. This argues that the renal artery is not protected by PVAT against the contractile actions of agonists. This protection could be provided by, in part, adiponectin which is expressed, at least at the level of mRNA, in RPVAT (figure 3). PVAT can provide protection to vessels by means other than by reducing contraction. Though adiponectin reduces vessel constriction, it also appears to reduce the inflammation that can accompany hypertension (47). We have only addressed one aspect of how PVAT may be protective of the renal artery. It is unknown if the lack of protection afforded by RPVAT relative to contractility extends to other aspects of vessel health and function. It will be of interest to determine if adiponectin protein is expressed in RPVAT, if the renal artery expresses the adiponectin receptors and whether these receptors are prone to the adiponectin resistance in ways other arteries might not be (53). A limitation to making this conclusion about the anti-contractile nature of RPVAT is that we used only adrenergic agonists. It is possible that RPVAT could be protective against other agonists. It is clear, though, that other PVATs are anticontractile against NE itself, specifically in the aorta of the same species, the rat (13), in mouse mesenteric artery (2, 42, 50) and in human gluteal biopsy arteries (4). Similarly, PVAT was anticontractile to a different adrenergic agonist, PE, in the rat aorta (12, 41). These studies suggest that our findings in the renal artery are specific to RPVAT.
4.3 RPVAT possesses a releasable pool of catecholamines independent of renal nerve
We approached this study unsure of the role that PVAT, independent of the nerve, would play in the underlying renal artery in contributing to NE content/release as indicated by tyramine-induced contraction. An independence of tyramine-induced contraction from direct innervation in and near PVAT would support that constituents of PVAT outside of the nerve contribute the NE that mediates tyramine-induced contraction. In our hands, renal denervation did not modify the PVAT-dependent contraction to tyramine. This finding is important because it validates that renal PVAT should be considered a tissue that, on its own, has the ability to affect renal arterial tone (and thus function) through paracrine measures. What was somewhat surprising is that the NE content of PVAT from RDN-rats was reduced by 70%, possibly reflecting loss of sympathetic nerve fibers within RPVAT after RDN (see below and figure 9B), yet tyramine-induced contraction remained normal. The NE measured in RPVAT could be taken up from exogenous sources (circulating catecholamines or renal sympathetic nerves) or made within PVAT. We do not yet know whether cells within RPVAT, independent of sympathetic nerves, express tyrosine hydroxylase and thus have the potential to make NE. This differs significantly from the mesenteric PVAT in which removal of sympathetic innervation through celiac ganglionectomy reduced NE content by only 15–20% and tyramine-induced contraction in superior mesenteric arteries +PVAT was not modified. Similarly, adipocytes within the mesenteric fat express tyrosine hydroxylase (52).
Figure 9.
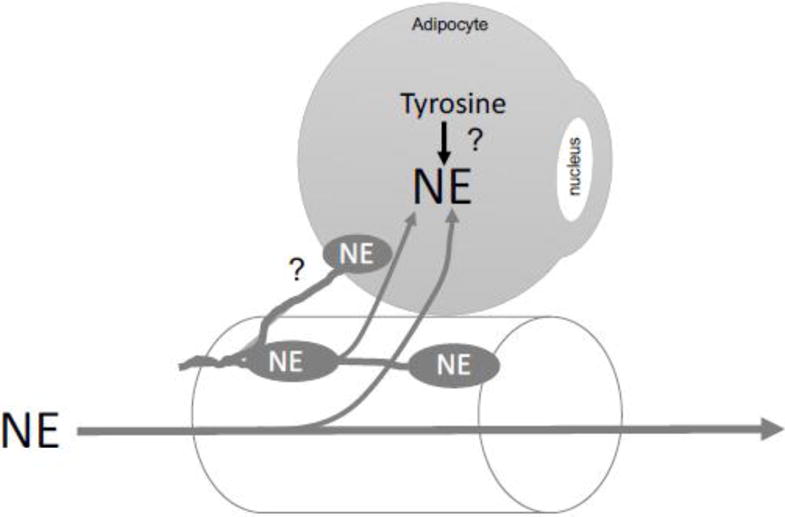
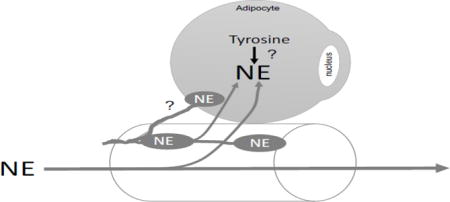
Diagram of conclusions supported by the work within this manuscript and speculation (?) as to whether RPVAT is innervated by the sympathetic nervous system as indicated by the oval nerve boutons containing NE. The lipid droplet of the adipocyte depicted has been removed so as to have more space within the cell to draw. NE = norepinephrine.
Several possibilities exist for this seeming inconsistency. First, the NE that remained in the RPVAT after denervation may be sufficient to mediate tyramine-induced contraction. Second, tyramine could be eliciting contraction independent of NE release. This, however, is unlikely. Tyramine does not have affinity for α adrenergic receptors (7, 9, 15) but tyramine-induced contraction was significantly dependent on α adrenergic receptor stimulation. There is a a percentage of tyramine-induced contraction - ~20%-that was not reduced by the α1 receptor antagonist prazosin; we do not know the mediators of that contraction. It is possible that trace amine receptors mediate this remaining contraction to tyramine (15).
A remaining question is whether the adipocytes/constituents of PVAT — all PVATs but especially that of the renal vasculature — are formally innervated by the sympathetic, parasympathetic or sensory nerves. This has been suggested (1. 17, 29) and reports from the 1970s suggest that mesenteric fat adipocytes may be sparsely innervated (22). A recent review by Loesch and Dashwood (40) brings together our current knowledge and questions with respect to innervation of PVAT. To our knowledge, no formal, systematic study of at least the sympathetic nervous system innervation of any PVAT has been done. Figure 9B portrays our current thoughts on the relationship between the sympathetic nervous system, and renal artery and RPVAT. We do not know if NE is synthesized in the RPVAT adipocyte and/or if all the NE found in RPVAT depends on external sources (e.g. circulating NE, NE from sympathetic nerve boutons). Our findings that RPVAT contains catecholamines that are at least partly dependent on innervation must also be weighed as a factor for why denervation trials may have had such varied outcomes; was the RPVAT and contributions made by it a factor in that variability? Could NE remaining in RPVAT after denervation influence renal arterial function? It has only been in the last 15 years or so that PVAT has been formally studied, and renal PVAT has not been amongst the PVATs studied. However, it is notable that two previous studies in patients with essential hypertension (20, 26) revealed a significant link between renal sinus fat mass and hypertension development. Specifically, Foster et al (26) concluded the following: “Fatty kidney is a common condition that is associated with an increased risk of hypertension and chronic kidney disease. Renal sinus fat may play a role in blood pressure regulation and chronic kidney disease.” These findings collectively highlight the importance of considering RPVAT as a tissue with physiological function.
5.0 Conclusions
These studies support the conclusions that RPVAT in the rat is a mix of brown and white fat, the sum of which contain significant concentrations of catecholamines, making RPVAT similar to other PVATs (aortic, mesenteric). Additionally, tyramine-induced contraction was also PVAT-dependent, as has been found with other arteries. However, RPVAT differs from other PVATs in the lack of protection afforded to α adrenergic agonist contraction and significant loss of NE content with denervation. However, NE in RPVAT was not abolished with renal denervation. These studies provide the first formal investigation of the possible function of renal PVAT.
Acknowledgments
This work was funded by the National Institutes of Health P01 HL70687 and by the State of Sao Paulo Research Foundation to CBAR (Fundacao de Amparo a Pesquisa do Estado de Sao Paulo: Fapesp:2015/25822-9).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abu Bakar H, Dunn WRT, Daly C, Ralevic V. Sensory innervation of perivascular adipose tissue: a crucial role in artery vasodilatation and leptin release. Cardiovasc Res. 2017 doi: 10.1093/cvr/cvx062. [DOI] [PubMed] [Google Scholar]
- 2.Agabiti-Rosei C, De Ciuceis C, Rossini C, Porteri E, Rodella LF, Withers SB, Heagerty AM, Favero G, Agabiti-Rosei E, Rizzoni Rezzani R. Anticontractile activity of perivascular fat in obese mice and the effect of long-term treatment with melatonin. J Hypertens. 2014;32:1264–1274. doi: 10.1097/HJH.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 3.Agabiti-Rosei C, Favero G, De Ciuceis C, Rossini C, Porteri E, Rodella LF, Franceschetti L, Maria Sarkar A, Agabiti-Rosei E, Rizoni D, Rezzani R. Effect of long-term treatment with melatonin on vascular markers of oxidative stress/inflammation and on the anticontractile activity of perivascular fat in aging mice. Hypertens Res. 2017;40:41–50. doi: 10.1038/hr.2016.103. [DOI] [PubMed] [Google Scholar]
- 4.Aghamohammadzadeh R, Greenstein AS, Yadav R, Jeziorska M, Hama S, Soltani F, Pemberton PW, Ammori B, Malik RA, Soran H, Heagerty AM. Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J Am Coll Cardiol. 2013;62:128–135. doi: 10.1016/j.jacc.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad AA, Randal MD, Robert RE. Sex differences in the regulation of porcine coronary artery tone by perivascular adipose tissue: a role of adiponectin. Br J Pharmacol. 2017 doi: 10.1111/bph.13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Jarallah A, Oriowo MA. Loss of anticontractile effect of perivascular adipose tissue on pregnant rats: a potential role of tumor necrosis factor-α. J Cardiovasc Pharmacol. 2016;67:145–151. doi: 10.1097/FJC.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 7.Anwar MA, Ford WR, Herbert AA, Broadley KJ. Signal transduction and modulating pathways in tryptamine-evoked vasopressor responses of the rat isolated perfused mesenteric bed. Vasc Pharmacol. 2013;58:140–149. doi: 10.1016/j.vph.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayala-Lopez N, Jackson WF, Burnett R, Wilson JN, Thompson JM, Watts SW. Organic cation transporter 3 contributes to norepinephrine uptake into perivascular adipose tissue. Am J Physiol Heart Circ Physiol. 2015;309:H1904–1914. doi: 10.1152/ajpheart.00308.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayala-Lopez N, Martini M, Jackson WF, Darios E, Burnett R, Seitz B, Fink GD, Watts SW. Perivascular adipose tissue contains functional catecholamines. Pharmacol Res Perspect. 2014;2(3):e00041. doi: 10.1002/prp2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayala-Lopez N, Thompson JM, Watts SW. Perivascular adipose tissue’s impact on norepinephrine-induced contraction of mesenteric resitance arteries. Front Physiol. 2017;8(8):37. doi: 10.3389/fphys.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartness TJ, Ryu V. Neural control of white, beige and brown adipocytes. Int J Obes Suppl. 2015;5(S1):S35–S39. doi: 10.1038/ijosup.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beltowski J, Guranowski A, Jamroz-Wisniewska A, Wolski A, Halas K. Hydrogen-sulfide mediated vasodilatory effect of nucleoside 5′-monophosphorothioates in perivascular adipose tissue. Can J Physiol Pharmacol. 2015;93:585–595. doi: 10.1139/cjpp-2014-0543. [DOI] [PubMed] [Google Scholar]
- 13.Boydens C, Pauwels B, Van de Voorde J. Effect of resveratrol and orchidectomy on the vasorelaxing influence of perivascular adipose tissue. Heart Vessels. 2016;31:508–515. doi: 10.1007/s00380-015-0664-2. [DOI] [PubMed] [Google Scholar]
- 14.Briasoulis A, Bakris G. Renal denervation after SYMPLICITY HTN-3: where do we go? Can J Cardiol. 2015;31:642–648. doi: 10.1016/j.cjca.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Broadley KJ. The vascular effects of trace amines and amphetamines. Pharmacol Therap. 2010;125:363–375. doi: 10.1016/j.pharmthera.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Brown NK, Zhour Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol. 2014;34:1621–1630. doi: 10.1161/ATVBAHA.114.303029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulloch JM, Daly CJ. Autonomic nerves and perivascular fat: interactive mechanisms. Pharmacol Ther. 2014;143:61–73. doi: 10.1016/j.pharmthera.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Bussey CE, Withers SB, Aldous RG, Edwards G, Heagerty AM. Obesity related perivascular adipose tissue damage is reversed by sustained weight loss in the rat. Arterioscler Thromb Vasc Biol. 2016;36:1377–1385. doi: 10.1161/ATVBAHA.116.307210. [DOI] [PubMed] [Google Scholar]
- 19.Chaldakov GN, Tonchev AB, Stankulov IS, Ghenev PI, Fiore M, Aloe L, Rancic G, Panayotov P, Kostov DD. Periadventitial adipose tissue (tunica adipose): enemy or friend around? Arch Pathol Lab Med. 2007;131:1766. doi: 10.5858/2007-131-1766a-PATTAE. [DOI] [PubMed] [Google Scholar]
- 20.Chugtai HL, Morgan TM, Rocco M, Stacey B, Brinkley TE, Ding J, Nicklas B, Hamilton C, Hundley WG. Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular event. Hypertension. 2010;56:901–906. doi: 10.1161/HYPERTENSIONAHA.110.157370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coppolino G, Pisano A, Rivoli L, Bolignano D. Renal denervation for resistant hypertension. Cochrane Database Syst Rev. 2017;2:CD011499. doi: 10.1002/14651858.CD011499.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cushman SW. Structure Function relationships in the adipose cell. I. Ultrastructure of the isolated adipose cell. Cell Biol. 1970;46:326–341. doi: 10.1083/jcb.46.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damkjaer M, Jensen PH, Schwämmle V, Sprenger RR, Jacobsen IA, Jensen ON, Bie P. Selective renal vasoconstriction, exaggerated natriuresis and excretion rates of exosomic proteins in essential hypertension. Acta Physiol (Oxf. 2014;212(1):106–18. doi: 10.1111/apha.12345. [DOI] [PubMed] [Google Scholar]
- 24.Esler M, Guo L. The future of renal denervation. Auton Neurosci. 2017;204:131–138. doi: 10.1016/j.autneu.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Fink GD, Phelps JT. Can we predict the blood pressure response to renal denervation? Auton Neurosci. 2017;204:112–118. doi: 10.1016/j.autneu.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster MC, Hwang S-J, Porter SA, Massaro JM, Hoffmann U, Fox CS. Fatty kidney, hypertension, and chronic kidney disease: the Framingham Heart Study. Hypertension. 2011;58:784–790. doi: 10.1161/HYPERTENSIONAHA.111.175315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galvez-Prieto B, Dubrovska G, Cano MV, Delgado M, Aranguez I, Gonzalez MC, Ruiz-Gayo M, Gollach M, Fernandez-Alfonos0 MS. A reduction in the amount and anti-contractile effect of periadventitial mesenteric adipose tissue precedes hypertension development in spontaneously hypertensive rats. Hypertens Res. 2008;31:1415–1423. doi: 10.1291/hypres.31.1415. [DOI] [PubMed] [Google Scholar]
- 28.Gil-Ortega M, Somoza B, Huang Y, Gollasch M, Fernandez-Alfonso MS. Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends Endocrinol Metabol. 2015;26:367–375. doi: 10.1016/j.tem.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Gollasch M. Adipose-vascular coupling and potential therapeutics. Annu Rev Pharmacol Toxicol. 2017;57:417–436. doi: 10.1146/annurev-pharmtox-010716-104542. [DOI] [PubMed] [Google Scholar]
- 30.Henegar JR, Zhang Y, De Rama R, Hata C, Hall ME, Hall JE. Catheter-based radiofrequency renal denervation lowers blood pressure in obese hypertensive dogs. Am J Hypertens. 2014;27:1285–1292. doi: 10.1093/ajh/hpu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollenberg NK, Adams DF. The renal circulation in hypertensive disease. Am J Med. 1976;60(6):773–84. doi: 10.1016/0002-9343(76)90891-3. [DOI] [PubMed] [Google Scholar]
- 32.Huang F, Lezama MAR, Ontiveros JAP, Bravo G, Villafana S, del-Rio-Navarro BE, Hong E. Effect of losartan on vascular function in fructose-fed rats: the role of perivascular adipose tissue. Clin Exp Hypertens. 2010;32:98–104. doi: 10.3109/10641960902993129. [DOI] [PubMed] [Google Scholar]
- 33.Jacob F, Clark LA, Guzman PA, Osborn JW. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. Am J Physiol Heart Circ Physiol. 2005;289:H1519–H1529. doi: 10.1152/ajpheart.00206.2005. [DOI] [PubMed] [Google Scholar]
- 34.Johnson RJ, Feig DI, Nakagawa T, Sanchez-Lozada LG, Rodriguez-Iturbe B. Pathogenesis of essential hypertension: historical paradigms and modern insights. J Hypertens. 2008;26:381–391. doi: 10.1097/HJH.0b013e3282f29876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katholi RE, Carey RM, Ayers CR, Vaughan ED, Jr, Yancey MR, Morton CL. Production of sustained hypertension by chronic intrarenal norepinephrine infusion in conscious dogs. Circ Res. 1977;40(5 Suppl 1):I118–26. [PubMed] [Google Scholar]
- 36.Kohn C, Schleifenbaum J, Szijarto IA, Marko L, Dubrovska G, Huang Y, Gollasch M. Differential effects of cystathionine-γ-lyase-dependent vasodilatory H2S in periadventitial vasoregulation of rat and mouse aorta. PLoS One. 2012;7:e41951. doi: 10.1371/journal.pone.0041951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laffin LJ, Bakris CL. Catheter-based renal denervation for resistant hypertension: will it ever by ready for “prime time”? Am J Hypertens. 2016;30:841–846. doi: 10.1093/ajh/hpw123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee RMKW, Ding L, Lu C, Su L-Y, Gao Y-J. Alteration of perivascular adipose tissue function in angiotensin II-induced hypertension. Can J Physiol Pharmacol. 2009;87:944–953. doi: 10.1139/y09-088. [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using RealTime Quantitative PCR and the 22DDCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.126. [DOI] [PubMed] [Google Scholar]
- 40.Loesch A, Dashwood MR. Nerve-perivascular fat communication as a potential influence on the performance of blood vessels used as coronary artery bypass grafts. J Cell Commun Signal. 2017 doi: 10.1007/s.12079-017-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lohn M, Dubrovska G, Lauterbackh B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 42.Lynch FM, Withers SB, Yao Z, Werner ME, Edwards G, Weston AH, Heagerty AM. Perivascular adipose tissue-derived adiponectin activates BK(Ca) channels to induced anticontractile responses. Am J Physiol Heart Circ Physiol. 2013;304:H786–H795. doi: 10.1152/ajpheart.00697.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osborn JL, Plato CF, Gordin E, He XR. Long-term increases in renal sympathetic nerve activity and hypertension. Clin Exp Pharmacol Physiol. 1997;24(1):72–6. doi: 10.1111/j.1440-1681.1997.tb01786.x. [DOI] [PubMed] [Google Scholar]
- 44.Ozen G, Topal G, Gomez I, Ghorreshi A, Boukais K, Benyahia C, Kanyinda L, Longrois D, Teskin O, Uydes-Dogan BS, Norel X. Control of human vascular tone by prostanoids derived from perivascular adipose tissue. Prostaglandins Other Lipid Mediat. 2013;107:13–17. doi: 10.1016/j.prostaglandins.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Padilla J, Jenkins NT, Vieira-Potter VJ, Laughlin MH. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul Integr Comp Physiol. 2013;304:R543–R552. doi: 10.1152/ajpregu.00567.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rittig K, Dolderer JH, Balletschofer B, Machann J, Schick F, Meile T, Kuper M, Stock USA, Staiger H, Machicao F, Schaller HE, Konigsrainer A, Haring HU, Siegel-Axel DI. The secretion pattern of perivascular fat cells is different from that of subcutaneous and visceral fat cells. Diabetologia. 2012;55:1514–1525. doi: 10.1007/s00125-012-2481-9. [DOI] [PubMed] [Google Scholar]
- 47.Ruan CC, Ma Y, Ge Q, Li Y, Zhu LM, Zhang Y, Kong LR, Wu QH, Li F, Cheng L, Zhao AZ, Zhu DL, Gao PJ. Complement-mediated inhibition of adiponectin regulates perivascular inflammation and vascular injury in hypertension. FASEB J. 2017;31:1120–1129. doi: 10.1096/fj.201600780R. [DOI] [PubMed] [Google Scholar]
- 48.Schinzari F, Tesauro M, Cardillo C. Endothelial and perivascular adipose tissue abnormalities in obesity-related vascular dysfunction: novel targets for treatment. J Cardiovasc Pharmacol. 2017;69:360–368. doi: 10.1097/FJC.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 49.Schlaich MP. What we need to know about renal nerve ablation for treatment of hypertension and other states of sympathetic overactivity. Am J Physiol Renal Physiol. 2016;311:F1267–F1270. doi: 10.1152/ajprenal.00058.2016. [DOI] [PubMed] [Google Scholar]
- 50.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens. 1991;13:277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 51.Van de Voorde J, Boydens C, Pauwels B, Decaluwe K. Perivascular adipose tissue, inflammation and vascular dysfunction in obesity. Curr Vasc Pharmacol. 2014;12:403–411. doi: 10.2174/1570161112666140423220628. [DOI] [PubMed] [Google Scholar]
- 52.Vargovic P, Ukropec J, Laukova M, Cleary S, Manz B, Pacak K, Kvetnansky R. Adipocytes as a new source of catecholamine production. FEBS Lett. 2011;585:2279–2284. doi: 10.1016/j.febslet.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Ma XL, Lau WB. Cardiovascular adiponectin resistance: the critical role of adiponectin receptor modification. Trends Endocrinol Metab. 2017;28:519–530. doi: 10.1016/j.tem.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaborska KE, Edwards G, Austin C, Wareing M. The role of O-GlcNAcylation in perivascular adipose tissue dysfunction of offspring of high-fat diet fed rats. J Vasc Res. 2017;54:79–91. doi: 10.1159/000458422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Z, Yang K, Zeng L, Wang X, Jiang F, Tu S, Liang Q, Shen Z. Renal simplicity denervation reduces blood pressure and renal injuries in an obesity-induced hypertension dog model. Clin Exp Pharmacol Physiol. 2016 doi: 10.1111/1440-1681.12661. [DOI] [PubMed] [Google Scholar]


