Abstract
Vitamin D plays a significant role in musculoskeletal health by regulating calcium, phosphate and promoting new bone mineralization. The purpose of this study was to understand the effect of dietary vitamin D on general bone health during peri-operative bone healing via an in vivo dosing study of vitamin D in a rat posterolateral fusion model using autograft. Vitamin D Deficient (DD), vitamin D Insufficient (ID), Control vitamin D (CD), and Hyper-vitamin D (HD) groups were studied. Increasing dietary vitamin D improved quantitative measures of femoral geometry, including femoral strength, stiffness and density. Femoral biomechanics, cortical thickness, moment of inertia, cross sectional area, and measures from bone ashing were all greater in the HD group versus the CD. This suggests that additional dietary vitamin D above normal levels during spinal fusion may lead to improvement in bone health. Serum vitamin D levels were also observed to decrease during fusion healing. These results demonstrate that dietary vitamin D improves general bone health in the femur of a rat model during posterolateral spinal fusion. This suggests a role for further clinical evaluation of vitamin D dietary intake during the perioperative period, with the possibility of avoiding adverse consequences to general bone health.
Keywords: vitamin D, musculoskeletal health, bone healing, rat model, spinal fusion
Graphical Abstract
This study evaluated dietary vitamin D’s effect on bone health during peri-operative bone healing via an in-vivo dosing study of vitamin D in a rat posterolateral fusion model. Dietary vitamin D improved femoral biomechanics, cortical thickness, moment of inertia, cross sectional area, and bone ashing measures. Results indicate dietary vitamin D above normal levels during spinal fusion may lead to improvement in bone health. Study provides basis for a clinical study of vitamin D dietary intake during the perioperative period.
Introduction
The role of Vitamin D in musculoskeletal health has been appreciated even prior to its description as “vitamin D”. Dr. D. Scheutte, a German physician, prescribed cod liver oil as a treatment for rickets as early as 1824 (1). Vitamin D plays a significant role in musculoskeletal health by regulating calcium, phosphate and promoting new bone mineralization (2). Adequate levels are needed to support a variety of tissues and prevent sequelae of deficiency (3).
Vitamin D deficiency leads to osteomalacia, resulting in demineralized, weakened bone tissue with increased osteoclastic activity (4). In adults this presents as bone pain, proximal muscle weakness, and insufficiency fractures (5,6). It was the study of rickets that initially led to the discovery of Vitamin D (5,7,8). The work of authors such as McCollum and Mellanby demonstrated the existence of a “factor” in whole milk and cod liver oil that prevented the development of rickets in dogs (9,10). Chick et al. later demonstrated rickets in children could be cured by the same “factor” (11,12).
Vitamin D has been extensively studied for its role in overall bone health (13–18). Studies suggest supplementation may reduce fracture risk by 18–20%, although widespread use for the prevention of osteoporosis has not proven effective (14,19). A randomized clinical trial reported an increase in local bone mineral density in osteoporotic proximal humerus fractures with supplementation of both calcium and vitamin D(13). Animal studies have demonstrated accumulation of vitamin D within the fracture callus (20,21). Fu et al. reported an increase in bone volume with administration of vitamin D in a rat model. Likewise, Andreen et al. demonstrated an increase in callus turnover (22,23). However, these studies were confounded by either concomitant animal ovariectomies or manipulation of dietary calcium. No animal studies have isolated the effect of vitamin D on general bone health during a period of bone healing in metabolically unaltered rats.
The purpose of this study was to understand the effect of dietary vitamin D on general bone health during peri-operative bone healing via an in vivo dosing study of vitamin D in a rat posterolateral fusion model using autograft. The effect of vitamin D on healing in a spinal fusion model was previously investigated by our group (26). Briefly, we assessed fusion quality in a rat model using biomechanical testing, imaging, and palpation metrics, and found a significant increase in the rate of fusion and bone quality of the fusion mass with increased supplementation of vitamin D. Femurs were also collected at sacrifice and used to assess general bone health, which is the focus of this present study. General bone health during spinal fusion is of interest because it represents a greater burden of bone healing than a single long bone osteotomy (27). To date, studies analyzing the effect of vitamin D on bone healing during spinal fusion are limited to clinical case series (24,25).
We hypothesized that increasing dietary vitamin D would result in increased general bone health during spine fusion, through an increase in femoral bone density and stiffness as determined by biomechanical testing, micro CT analysis, and bone ashing. This study is unique as dietary calcium was not a confounding variable, no rats were ovariectomized, and spinal fusion, not long bone osteotomy, was used to model bone healing.
Methods
Experimental Design
Sprague Dawley rats (Charles River Laboratories, Inc., Reno, NV) were received at 12 weeks of age. After 4 weeks of dietary Vitamin D manipulation, rats underwent posterolateral inter-transverse process spinal fusion surgery with autogenous tail grafting; spinal fusion methods, results, and analysis were previously reported (26). 12 weeks after surgery (16 weeks of dietary manipulation) rats were sacrificed and both femora were harvested and assessed using micro CT, biomechanics, and bone ashing. For this investigation, femora data are presented and compared to the previously determined spinal fusion outcome measures (26). This study was approved by the Institutional Animal Care and Use Committee (IACUC, Protocol No. 003802).
Diet Manipulation & Serum Evaluation
Forty-eight male Sprague Dawley rats were randomly assigned into four rat chow regulated vitamin D groups: vitamin D Deficient (0 IU/g vitamin D rat chow, DD), vitamin D Insufficient (2.25 IU/g vitamin D rat chow, ID), Control vitamin D sufficient (5 IU/g vitamin D rat chow, CD), Hyper-vitamin D (40 IU/g vitamin D rat chow, HD). Water and food were given ad libitum. Dietary manipulations were given and maintained for 16 weeks until sacrifice. The rats were housed in a vivarium with no exposure to ultraviolet light. Plasma was collected at 4 weeks after the initiation of dietary manipulations and immediately after sacrifice. Serum 25(OH)D levels were determined via radioimmunoassay.
MicroCT Analysis
Ex vivo microcomputed tomography, microCT, (VivaCT 40, SCANCO Medical AG, Bruttisellen, Switzerland) was conducted on a trans-axial 3.5mm segment of the femoral mid-shaft. To maintain consistency in alignment, digital calipers with +/−.01mm accuracy were used to measure the center between the greater trochanter to the intercondylar notch of each femur. The scanner was set at 55 kVp and 145 mA with an integration time of 200 seconds, creating approximately 100 slices with a voxel size of 35 μm. Scanco evaluation software was used to quantify average architectural parameters including: cortical thickness, moment of inertia (anteroposterior), cross-sectional area, bone volume, total volume, bone volume fraction (BV/TV), and mean density across the mid-shaft.
Biomechanical Testing
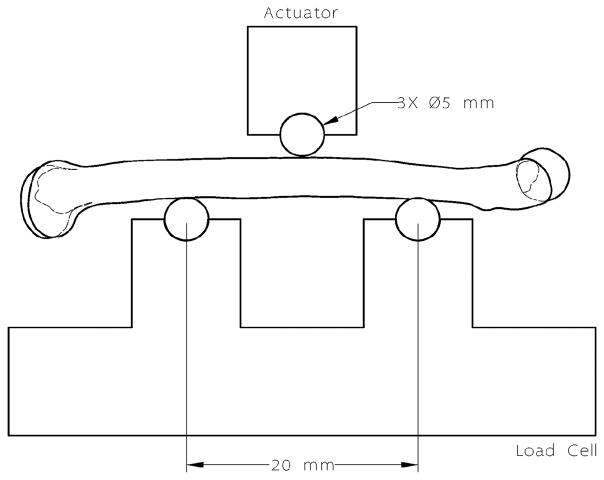
Three-point flexural bending was performed to determine strength and stiffness of the right femur for each specimen (Figure 1). Once cleaned of all soft-tissue, the femur was mounted onto a servo-hydraulic actuator (MTS Bionix 370.02, MTS Corp., Eden Prairie, MN) equipped with a mini load cell (MINI45 Transducer, API Corp., Apex, NC) and a three-point bending apparatus. The posterior side of the femur rested on two 5mm diameter steel roller contact points spanning 20mm. The actuator loaded the mid-shaft of the femur until failure using displacement control at a rate of 3mm/min to approximate quasi-static conditions. Stiffness of the femur was calculated as the slope within the elastic region of the measured load-displacement curves while the strength was measured as the maximum load applied before failure. Young’s Modulus (E) was calculated using the beam bending equation: E = (P/S)(L3/48xI), where P is the applied load, S is the deflection, L is the length of the femur, and I is the moment of inertia.
Figure 1.
Diagram of the 3-point bending test setup. Steel roller contact points with a diameter of 5 mm were set at 20 mm apart. The actuator applied a load on the top contact point at the mid-shaft until failure.
Bone Ashing
Bone ashing was performed to determine bone mineral content. Porcelain crucibles were initially cleaned and dried in a muffle furnace (Lindberg/Blue M 1100 °C Box Furnace, Thermo Fisher Scientific Inc., MA) at 700°C for 24 hours to eliminate any residue or moisture. Afterwards, bilateral rat femurs were dried in the muffle furnace at 105°C for 12 hours. After measuring dry weight, bone volume was measured using the water displacement technique. Each femur was then placed into a porcelain crucible and ashed in the muffle furnace at 650°C for 24 hours. Ash weight was determined and ash fraction was calculated as the ash weight divided by the dry weight. Ash density was calculated as the ash weight divided by the dry bone volume.
Statistical Analysis
ANOVA (SAS-GLM, SAS-Mixed Models, with Tukey tests, SAS Institute Inc., Cary, NC, USA) and Welch ANOVA when indicated were applied to evaluate the relationships of femoral dependent variables across vitamin D dietary groups. Mixed models (SAS-Proc Mixed) was used to test repeated measures of pre-surgical and sacrifice levels of serum vitamin D with dietary vitamin D group exploring different variance-covariance structures. Pearson correlation and regression were used to determine pairwise interrelationships among variables of femur stiffness and strength and spinal segment stiffness, dietary Vitamin D, serum vitamin D, and serum calcium. Multivariable regression, SAS-GLM SAS-Mixed procedures were applied to evaluate final relationships. A p<0.05 was used to determine significant relationships. For final models, where indicated, P was adjusted for multiple comparisons via Tukey.
Results
Vitamin D Status
Pre-surgical and sacrifice levels of serum plasma 25(OH)D were found to be positively related with levels of vitamin D adjusted chow (p<0.0001), validating the dietary manipulation yielded corresponding serum values, Table 1. Average serum vitamin D levels were significantly decreased by sacrifice compared to pre-surgery levels across all vitamin D groups (F= 22.08, p<0001) Table 1, Figure 2. No relationship existed between rat weight and vitamin D. Likewise, ex vivo femur length showed no relationship to serum or dietary vitamin D, Table 2. Femur length was correlated to rat weight both at initial observation prior to dietary intervention (r=0.29, p<0.05) and at day of surgery just prior to surgery (r= 0.33, p<0.05).
Table 1.
Descriptive Measures Across Vitamin D Adjusted Chow Groups
| Measurement | Deficient Vitamin D (DD) (0 IU/g) Ca = 0.95%* | Insufficient Vitamin D (ID) (2.25 IU/g) Ca = 0.95%* | Sufficient Vitamin D Control (CD) (5 IU/g) Ca = 0.95%* | Hyper-Vitamin D (HD) (40 IU/g) Ca = 0.94%* | P-Value |
|---|---|---|---|---|---|
| Serum Levels | |||||
| Serum Vitamin D (ng/mL) srg** | 9.26 ± 1.61 | 20.64 ± 3.27 | 28.30 ± 4.57 | 101.49 ± 17.27 | p<0.0001 |
| Serum Vitamin D (ng/mL) sac** | 6.25 ± 1.02 | 15.58 ±5.04 | 19.59 ± 5.64 | 88.44 ± 9.91 | p<0.0001 |
| Total Calcium (mg/dL) sac | 11.69 ± 1.20 | 11.78 ± 0.49 | 11.48 ± 0.89 | 12.86 ± 0.76 | p<0.001 |
| Free Calcium (mg/dL) sac | 6.59 ± 0.85 | 6.83 ± 0.81 | 6.84 ± 1.07 | 7.11 ± 1.09 | NS |
| Bound Calcium (mg/dL) sac | 5.10 ± 1.74 | 4.95 ± 0.72 | 4.65 ± 1.29 | 5.75 ± 1.15 | NS (p=0.06) |
Values given as mean ± SD, NS = Not Significant
% Calcium by weight of rat chow diet
Repeated measures SAS-Mixed model yielded significant differences within-subjects effect of pre-surgery (4 wks) vs. sac (16 wks) serum vitamin D (P<0.0001), a main grouping effect (P<0.0001), and interaction effect p<0.05. Serum Vitamin D was significantly decreased at sacrifice (16wks) compared to pre-surgery (4wks) – especially for HD: where serum vitamin D was significantly decreased to 88.44 (16wks) vs. 101.49 (4wks) (P<0.001), and for CD: 19.59 (16wks) vs. 28.30 (4wks) (p<0.05) (Tukey-Kramer/Bonferroni).
Total Calcium sac: R2 = 0.27 P<0.001, contrast HD > CD, ID, DD, (p<0.05)
NS, Bound Calcium sac: SAS-GLM R2 = 0.07, p<0.069; contrast HD > CD (p<0.05)
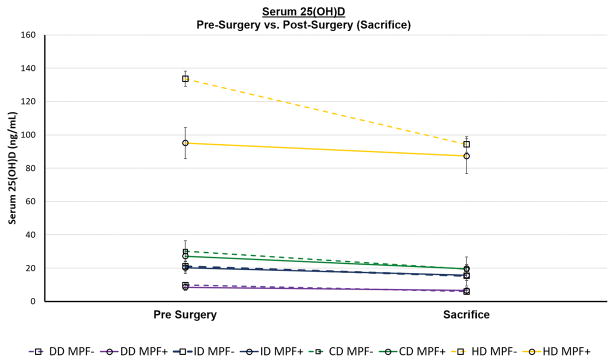
Figure 2.
Mean levels of serum plasma 25(OH)D at sacrifice were significantly lower than pre-surgical levels (F= 22.08, p<0.0001), decreasing lines indicate serum plasma vitamin D from pre-surgery to sacrifice. There was a significant grouping effect of levels of dietary vitamin D adjusted chow (F= 496.03, p<0.0001). Mean values are plotted as a function of spinal segment fused via manual palpation (MPF+, solid line) or not fused (MPF-, dashed line). SAS-Mixed model was used with a term for repeated measures of serum plasma vitamin D (pre-surgery vs. sacrifice) with dietary vitamin D as a grouping variable. The test for interaction between serum D and dietary was not significant (P=0.08) and the greatest contribution was from the HD, hyper-vitamin D dietary group.
Table 2.
Physical and Biomechanical Features of Femurs Across Vitamin D Adjusted Chow Groups
| Measurement | Deficient Vitamin D (DD) (0 IU/g) Ca = 0.95%* | Insufficient Vitamin D (ID) (2.25 IU/g) Ca = 0.95%* | Sufficient Vitamin D Control (CD) (5 IU/g) Ca = 0.95%* | Hyper-Vitamin D (HD) (40 IU/g) Ca = 0.94%* | P-Value |
|---|---|---|---|---|---|
| Characteristics of rats | |||||
| Intake Weight (g) (prior to dietary manipulation)** | 271.00 ± 4.80 | 272.50 ± 4.17 | 270.00 ± 7.04 | 270.50 ± 7.69 | NS |
| Pre-surgery Weight (g) | 421.64 ± 83.27 | 446.42 ±34.54 | 424.15 ± 70.61 | 431.42±70.99 | NS |
| Characteristics of femur | |||||
| Femur Length (mm) | 41.23 ± 1.85 | 41.71 ± 1.05 | 42.22 ± 1.47 | 41.43 ± 1.97 | NS |
| Femur MicroCT | |||||
| Cortical Thickness (mm) | 0.90 ± 0.07 | 0.86 ± 0.06 | 0.87 ± 0.06 | 0.93 ± 0.10 | NS (P=0.06)1 |
| Moment of Inertia (mm4) | 10.47 ± 1.66 | 10.14 ± 2.30 | 10.13 ± 1.94 | 12.05 ± 2.28 | p<0.012 |
| Cross Sectional Area (mm2) | 9.45 ± 0.57 | 9.21 ± 0.95 | 9.34 ± 0.86 | 10.16 ± 1.07 | p<0.013 |
| Bone Volume/Total Volume (fBV/TV) | 0.67 ± 0.03 | 0.66 ± 0.03 | 0.66 ± 0.03 | 0.67 ± 0.04 | NS |
| Bone Volume (fBV) | 34.26 ± 2.42 | 33.11 ± 3.39 | 33.59 ± 3.09 | 36.55 ± 3.81 | P<0.014 |
| Young’s Modulus (N/mm4) | 7202±736.92 | 7198 ± 950.57 | 7367±1500.00 | 7152±1029.00 | NS |
| Femur Biomechanics | |||||
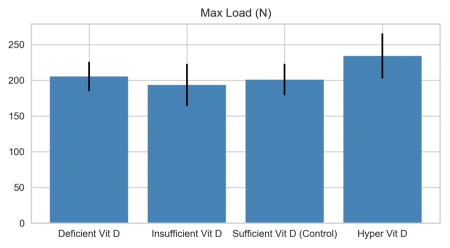
| Max Load (N) | 205.27 ± 20.37 | 193.48 ± 29.58 | 200.88 ± 21.99 | 233.84 ± 31.61 | p<0.015 |
| Stiffness (N/mm) | 445.91 ± 40.09 | 429.23 ± 74.91 | 433.75 ± 64.41 | 505.70 ± 58.50 | p<0.016 |
| Toughness (Nmm) | 116.45 ± 27.57 | 109.69 ± 25.27 | 109.89 ± 22.38 | 127.11 ± 40.79 | NS |
| Femur Bone Ashing | |||||
| Dry Weight | 0.92 ± 0.08 | 0.88 ± 0.08 | 0.93 ± 0.09 | 0.96 ± 0.02 | NS |
| Ash Weight | 0.59 ± 0.05 | 0.57 ± 0.06 | 0.60 ± 0.07 | 0.64 ± 0.08 | NS(P=0.06)7 |
| Ash Fraction (fAF) | 0.64 ± 0.02 | 0.64 ± 0.01 | 0.64 ± 0.02 | 0.67 ± 0.01 | p<0.0018 |
| Ash Density (fAD, g/mL) | 0.64 ± 0.05 | 0.67 ± 0.05 | 0.66 ± 0.07 | 0.74 ± 0.06 | p=0.0019 |
Mean ± SD measurement values of left and right femurs. Values given as mean ± SD, NS = Not Significant
% Calcium by weight of rat chow diet
SAS-Mixed, Main effect of weight gain across time for all rats (P<0.001), no Vitamin D group effect (NS). SAS-GLM:
Femur Cortical Thickness: with vitamin dietary group(NS); [w/pre surgery serum vitamin D (model R2=0.25, P<0.01)]
Femur Moment of Inertia: R2 = 0.13 P<0.01, contrast HD > CD, ID, (p<0.05)
Femur Cross Sectional Area: R2 = 0.15 P<0.01, contrast HD > CD, ID, (p<0.05); HD>DD, P=0.06
Femur Bone Volume (fBV): R2 = 0.13 P<0.01, contrast HD > CD, ID, (p<0.05)
Femur Max Load: R2 = 0.27 P<0.01, contrast HD > CD, ID, (p<0.05)
Femur Stiffness: R2 = 0.22 P<0.01, contrast HD > CD, ID, (p<0.05)
Femur Ash Weight: R2 = 0.16 P<0.06, contrast HD > ID, (p<0.05)
Femur Ash Fraction: R2 = 0.32 P<0.001, contrast HD > CD, ID, DD (p<0.05)
Femur Ash Density: R2 = 0.31 P<0.001, contrast HD > CD, ID, DD (p<0.05)
Femur Micro CT
Micro CT measures across dietary vitamin D groups are presented in Table 2 and Figure 2, with correlations presented in Table 3. Average femur cross sectional area (p<0.01) and moment of inertia (p<0.05) were significantly greater in the highest dietary vitamin D group. This pattern was also found for cortical thickness yet was not significant (P=0.06) alone, but was a significant function of the combined variable of dietary vitamin D with pre-surgery serum vitamin D levels (interaction term, R2=0.25, p<0.01). Strong positive correlations were found for measures of vitamin D with femur cross sectional area (dietary vitamin D, r= 0.38, p<0.01; serum vitamin D day of surgery, r=0.40, p<0.01; serum vitamin D level at sacrifice, r=0.35 p<0.01). Similarly, femur bone volume as determined by micro CT scanning showed significant correlation to vitamin D (serum srg, r=0.39 p< 0.01; serum sac r=0.33, p<0.05; dietary r=0.37, p<0.01), rat weight at initial observation prior to dietary intervention (r=0.35, p<0.05), weight on day of surgery (r= 0.42, p<0.01), and weight after sacrifice (r= 0.64, p<0.0001). Micro CT measures of femur bone volume, cortical thickness, cross sectional area, and moment of inertia were also intra-correlated (p<0.001), Table 3. Micro CT measures of femur bone mineral density (fBMD) was strongly related to bone volume fraction (BV/TV) (p<0.0001); yet, bone volume fraction and femur bone mineral density (fBMD) showed no significant relation with vitamin D (serum or dietary).
Table 3.
Heatmap of correlations indicating degree of association among femur variables (rows) and spine variables (columns).
| Vitamin D Status | Spine Micro CT Data | Spine Biomechanical Data | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dietary D | Serum D at surg | Serum D at sac | BMD | BV | BV/TV | Stiffness | Fusion MP | ||
| Vitamin D Status | Dietary D | 1.0000 | 0.9683 | 0.9818 | 0.4963 | 0.4106 | 0.5089 | 0.3328 | 0.1997 |
| Serum D (surg) | 0.9683 | 1.0000 | 0.9648 | 0.4721 | 0.3801 | 0.5055 | 0.3386 | 0.1233 | |
| Serum D (sac) | 0.9818 | 0.9648 | 1.0000 | 0.4985 | 0.3528 | 0.5187 | 0.3369 | 0.1841 | |
| Femur Micro CT Data | Cortical Thickness | 0.2703 | 0.3391 | 0.2505 | 0.1885 | 0.4239 | 0.3397 | 0.2963 | −0.1319 |
| X-sectional Area | 0.3831 | 0.4102 | 0.3524 | 0.0447 | 0.5533 | 0.1818 | 0.4160 | −0.0265 | |
| Moment of Inertia | 0.3590 | 0.3590 | 0.3334 | −0.0032 | 0.5093 | 0.1019 | 0.4020 | 0.0081 | |
| BMD | 0.0855 | 0.1454 | 0.0602 | 0.2226 | 0.2399 | 0.3165 | 0.0086 | −0.0620 | |
| BV | 0.3661 | 0.3895 | 0.3343 | 0.0468 | 0.5622 | 0.1941 | 0.3902 | −0.0123 | |
| BV/TV | 0.0845 | 0.1510 | 0.0611 | 0.1956 | 0.2525 | 0.3166 | −0.0345 | −0.0614 | |
| Femur Biomechanical Data | Stiffness | 0.4468 | 0.4496 | 0.4606 | 0.1633 | 0.5451 | 0.1954 | 0.2690 | −0.1075 |
| Max Load | 0.4927 | 0.4958 | 0.4828 | 0.1181 | 0.6061 | 0.2183 | 0.4942 | −0.0626 | |
| Young’s Modulus | −0.0373 | −0.0382 | 0.0049 | 0.1322 | −0.2006 | 0.0075 | −0.3014 | −0.1439 | |
| Femur Ashing Data | Dry Weight | 0.2425 | 0.2018 | 0.2458 | −0.0737 | 0.4378 | −0.0025 | 0.4452 | 0.0394 |
| Ash Weight | 0.3591 | 0.3248 | 0.3586 | 0.0316 | 0.5120 | 0.1092 | 0.4811 | 0.0781 | |
| Ash Fraction | 0.5687 | 0.5865 | 0.5528 | 0.4252 | 0.4230 | 0.4661 | 0.2328 | 0.1867 | |
| Ash Density | 0.5312 | 0.5886 | 0.5317 | 0.3738 | 0.2582 | 0.3878 | −0.0524 | −0.0792 | |
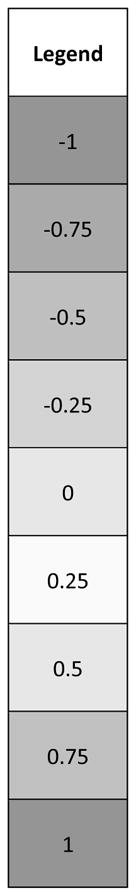
Cells correspond to correlation coefficients, r-values (range from −1 to +1), indicating degree of association. The more extreme the value, the more associated the variables. Corresponding p-values for these data are regarded as: r ≥ 0.6 | r ≤ −0.6, p<0.0001, ‘strong association’; r ≥ 0.48 | r ≤ −0.48, p<0.001, ‘moderate’; r ≥ 0.33 | r ≤ −0.33, p<0.01, ‘weak’; r ≥ 0.29 | r ≤ −0.29, p<0.05, ‘very weak’.
Femur Bone Ashing
Femur ash weight was significantly related to vitamin D (serum r=0.36, p<0.05; dietary r= 0.36, p<0.05), whereas femur dry weight was not related to vitamin D (serum D p= 0.096; dietary D p=0.10). Femur Ash Density (fAD) was positively related to Ash Fraction (fAF r= 0.66, p<0.0001) and both were significantly related to all measures of vitamin D (serum r= 0.57, p < 0.0001; dietary r= 0.53 p<0.0001).
Femur Biomechanical Testing
Vitamin D, dietary and serum, was significantly correlated to both femur stiffness (r=0.45, p <0.01) and femur strength (max load, r= 0.49, p<0.001) and were intra-correlated (max load, r= 0.75, p<0.0001), Table 3. Twenty-one percent of variability in femur stiffness was explained by vitamin D. In addition, femur stiffness and maximum load were both positively significantly related to femur dry weight as determined by bone ashing (both, r=0.58, p<0.0001).
Interrelationships among Femur and Spinal Fusion Characteristics
Significant correlations were found between micro CT measures of spine Bone Volume (sBV) and femur Bone Volume (fBV) (r=0.56, p<0.0001), cortical thickness (fCT r = 0.78, p<0.0001), cross sectional area (fCSA, r= 0.55, p<0.0001), and moment of inertia, (fMI, r= 0.51, p<0.001). Previously reported spine measures are reported in Table 4 (38). Spine Bone Mineral Density (sBMD) as determined by micro CT was not related to fBMD (p=0.16), although it was related to both femur ash fraction (r=0.43, p<0.01) and ash density (r=0.37, p<0.05).
Table 4.
Summary of previously published data (26) of physical and biomechanical features of spinal fusion segments across vitamin D adjusted chow groups.
| Measurement | Deficient Vitamin D (DD) (0 IU/g) Ca = 0.95%* | Insufficient Vitamin D (ID) (2.25 IU/g) Ca = 0.95%* | Sufficient Vitamin D Control (CD) (5 IU/g) Ca = 0.95%* | Hyper-Vitamin D (HD) (40 IU/g) Ca = 0.94%* | P-Value |
|---|---|---|---|---|---|
| Spine MicroCT | |||||
| BMD (sBMD) | 409.98 ± 58.40 | 422.36 ±37.12 | 441.00 ± 40.53 | 483.83 ± 56.50 | p<0.01** |
| Bone Volume (sBV) | 575.58 ± 81.00 | 488.27 ±87.27 | 512.97 ±110.14 | 651.61 ± 162.71 | p<0.01** |
| Bone Volume/Total Volume (sBV/TV) | 0.71 ± 0.09 | 0.74 ± 0.05 | 0.76 ± 0.05 | 0.81 ± 0.05 | p<0.01** |
| Spine Biomechanics | |||||
| Stiffness | 463.99 ± 76.67 | 385.67 ±75.78 | 494.04 ± 86.17 | 503.91 ± 67.44 | NS (p=0.08) |
| Fusion rate (manual palpation) | 45% (5/11) | 58% (7/12) | 61% (8/13) | 83% (10/12) | NS (p=0.08) |
Values given as mean ± SD, NS = Not Significant
% Calcium by weight of rat chow diet
SAS-GLM, ANOVA suggest main effect of dietary Vitamin D with significant group comparisons (Tukey’s HSD):
sBMD, significant effect of dietary Vitamin D (R2=0.25, F= 4.64, p <0.01). There was significantly higher sBMD in hyper-vitamin D (HD) group vs. ID (p<0.05) and HD vs. DD (p<0.05).
sBV, significant effect of dietary Vitamin D (R2=0.25, F= 4.20, p <0.01). There was significantly higher sBV in hyper-vitamin D (HD) group vs. CD (p<0.05) and HD vs. ID (p<0.05).
sBV/TV, significant effect of dietary Vitamin D (R2=0.29, F=5.10, P<0.01) with significantly higher sBV/TV in hyper-vitamin D (HD) group vs. ID (p<0.05) and HD vs. DD (p<0.05).
Dietary vitamin D was more highly related to femur stiffness (r=0.45, p<0.01) than to stiffness of fused spine segments (r=0.33, p=0.08) and fusion consolidation (fused vs. not fused) (r=0.25, p<0.08). Spinal stiffness did not correlate to femur stiffness but showed a significant positive relationship to femur max load (r= 0.49, p<0.01), which was best modeled by a linear equation, Figures 3 and 4. A common variability of 24% was determined between spinal stiffness and femur strength (r= 0.49, p <0.01), while only 7% common variability was determined between spinal stiffness with femur stiffness (r=0.27, NS).
Discussion
This study demonstrates that dietary vitamin D improves cortical bone health in the femur of a rat model during posterolateral spinal fusion. In particular, increasing dietary vitamin D in the perioperative period improved femoral strength, stiffness, and density. These results demonstrate an independent effect of dietary vitamin D on bone health despite a steady dietary amount of calcium in metabolically unaltered rats; including the ability of dietary vitamin D to increase serum total calcium. These findings complement the work of Nakamura et al., which demonstrated supra-physiologic vitamin D improves bone quality in rats not undergoing surgery (28). The results of this study are unique compared to prior studies which have focused on long bone osteotomies which have a smaller surface area of healing bone compared to a spinal fusion (13,22,29).
Biomechanical analysis showed an increase in both maximum load to failure and stiffness in 3-point bending with increased supplementation of vitamin D. Rats fed a hyper-D (HD) diet had an average 14% increase in maximum load to failure when compared to rats fed a deficient (DD) diet. This is likely due to changes in both the material properties and geometry of the bone. Such a conclusion is supported by studies in non-operative rats indicating that vitamin D depletion directly affects bone properties on a microscale (30–32). In addition, Martin et al. observed correlations between vitamin D and human femoral geometry, specifically increased femoral strength and cortical volume with increasing levels of vitamin D (33).
While increased vitamin D intake improved geometric parameters of femoral strength, some of the micro CT metrics of the femur, including BV/TV and BMD, did not increase. This may be due to an algorithmic error whereby the imaging density measurement software incorrectly subtracted the volume of the femoral canal when calculating density. This conclusion is supported by our bone ashing measurements where ash fraction, an adjusted measure of mineral content in the femur, was positively correlated to vitamin D. Several studies have reported trends of decreasing BV/TV and BMD with decreased intake of vitamin D, or alternatively, increasing bone density and strength as a function of reduced absorptive activity of osteoclasts with increased vitamin D (34–37). Hernandez et al. previously reported that ash fraction is a better predictor of bone strength than BV/TV.
Dietary vitamin D levels were maintained from 4 weeks prior to surgery until sacrifice. However, vitamin D serum levels dropped during fusion healing across all groups. Levels decreased by 33% (3.01 ng/mL) in DD, 25% in ID, and 31% in CD rats compared to a 13% (13.05 ng/mL) decrease in HD rats. Similar observations have been made in clinical studies (38, 39). Ettehad et al. observed a drop in serum vitamin D during healing of human tibial and femoral shaft fractures (38). Likewise, Alkalay et al. report increased intraosseus vitamin D in pertrochanteric fractures (39). These findings suggest bone healing from spinal fusion or fracture results in sequestration of vitamin D at the bone healing site. The impact of the associated drop in serum vitamin D on future fracture risk or long-term bone health has not been elucidated. However, this suggests that vitamin D deficient patients suffering a fracture may experience a further decrease in serum vitamin D levels during fracture healing. It is therefore important to clarify the activity of vitamin D during spinal fusion and fracture healing to determine the role of vitamin D and potential need for administration at the time of surgery or during fracture healing.
Femoral biomechanics, cortical thickness, moment of inertia, cross sectional area, and measures from bone ashing were all greater in the HD group versus the CD. This suggests that additional dietary vitamin D above normal levels during spinal fusion may lead to improvement in bone health. Our 3-point bending and micro CT results are based on analyses of the femoral mid-shaft and therefore represent changes to cortical bone. Previous studies looking at vitamin D and other nutritional factors that include outcome measures of trabecular bone demonstrate greater differences in trabecular bone compared to cortical bone (40–41). Extrapolating this information to the present study suggests vitamin D might play an even larger role in cancellous bone. Nonetheless, the role of vitamin D as a clinical therapeutic in those with otherwise normal vitamin D levels who are undergoing spinal fusion has not previously been described, but is a potential avenue for future research.
Limitations of the study design include the lack of a non-surgical control group of rats, exclusive use of male rats, limited dietary vitamin D dosages at the upper non-toxic levels, limited trabecular bone data, and limited sample size. Further, since there was a limited number of rats with successful fusion, and those that were fused were more likely to be in the hyper-vitamin D group comparisons between spinal stiffness and strength to femur measures had less statistical power. The relative proximity of dosing regimens among the three lower levels of dietary vitamin D (DD, ID, and CD groups) did not demonstrate a significant difference in measures of bone quality or quantity. The use of four manipulated dietary vitamin D groups with only one exceeding a ‘normal’ dose in rats limited our ability to characterize the relationship of bone quality and vitamin D. The use of a vitamin D dosage of 40 IU/g is well within safe limits for rat consumption. (42–46) However, our study was not designed to obtain safety data, and therefore future studies to demonstrate safety would need to collect biochemical, hematological and histopathological markers as previously described in studies of vitamin D toxicity (42). The highest calcium level recorded in the HD group was 13.62 mg/dL, above published normal limits, and therefore raises concern for sequelae of hypercalcemia (52). Human clinical evaluation would need to examine both safety and efficacy before any conclusions on clinical utility could be drawn. Further research is needed into the underlying biologic mechanisms responsible for the observed vitamin D drop at the time of spinal fusion, and dietary vitamin D’s ability to improve bone health in the perioperative spine fusion period.
When formulating the recommended daily allowance (RDA) for vitamin D the Institute of Medicine Committee reviews evidence regarding its impact on bone health, considered “desirable growth and maintenance of skeletal tissue” (47). However, the perioperative period for bone surgeries including spinal fusion has not been considered. Multiple studies report on the frequency of vitamin D deficiency in the orthopedic population (6,48–50). Our study suggests a role for further clinical evaluation of vitamin D dietary intake during the perioperative period, with the possibility of avoiding adverse consequences to general bone health.
Acknowledgments
Source of Funding: This study was partially funded by a grant from the Scoliosis Research Society, The National Center for Advancing Translational Sciences (NCATS, Grant UL1TR000124), and internal research funds from the department of Surgery at Cedars-Sinai Medical Center.
No author professional or financial affiliations are perceived to have biased this paper.
Footnotes
Author Contribution:
All authors contributed to trial design and data interpretation. Paper was drafted by authors Bhamb, Kanim, and Maldonado. Final paper edits were made by authors Bhamb, Kanim and Metzger. All authors read and approved the final manuscript.
Contributor Information
Neil Bhamb, Cedars-Sinai Medical Center, 444 S San Vicente Blvd, Suite 603, Los Angeles, CA 90048
Linda Kanim, Translational and Clinical Research, Spine Center, Cedars-Sinai Medical Center, 444 S San Vicente Blvd, Suite 901, Los Angeles, CA 90048
Ruben Maldonado, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Los Angeles, CA 90048
Mark Svet, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Los Angeles, CA 90048
Melodie Metzger, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Los Angeles, CA 90048
References
- 1.Wolf G. The Discovery of Vitamin D: The Contribution of Adolf Windaus. J Nutr. 2004 Jun 1;134(6):1299–302. doi: 10.1093/jn/134.6.1299. [DOI] [PubMed] [Google Scholar]
- 2.Costanzo LS. Physiology. 5. Philadelphia, Pa: Saunders Elsevier; 2014. p. 502. (Student consult) [Google Scholar]
- 3.Anderson PH, Atkins GJ, Turner AG, Kogawa M, Findlay DM, Morris HA. Vitamin D metabolism within bone cells: effects on bone structure and strength. Mol Cell Endocrinol. 2011 Dec 5;347(1–2):42–7. doi: 10.1016/j.mce.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet G, Dermauw V, Bouillon R. Vitamin D signaling in calcium and bone homeostasis: A delicate balance. Best Pract Res Clin Endocrinol Metab. 2015 Aug;29(4):621–31. doi: 10.1016/j.beem.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Vaishya R, Vijay V, Agarwal AK, Jahangir J. Resurgence of vitamin D: Old wine in new bottle. J Clin Orthop Trauma. 2015 Sep;6(3):173–83. doi: 10.1016/j.jcot.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogunovic L, Kim AD, Beamer BS, Nguyen J, Lane JM. Hypovitaminosis D in patients scheduled to undergo orthopaedic surgery: a single-center analysis. J Bone Joint Surg Am. 2010 Oct 6;92(13):2300–4. doi: 10.2106/JBJS.I.01231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elder CJ, Bishop NJ. Rickets. The Lancet. 2014 May 16;383(9929):1665–76. doi: 10.1016/S0140-6736(13)61650-5. [DOI] [PubMed] [Google Scholar]
- 8.Reid IR. What diseases are causally linked to vitamin D deficiency? Arch Dis Child. 2016 Feb;101(2):185–9. doi: 10.1136/archdischild-2014-307961. [DOI] [PubMed] [Google Scholar]
- 9.Mellanby E. Nutrition Classics. The Lancet 1:407-12, 1919. An experimental investigation of rickets. Edward Mellanby. Nutr Rev. 1976 Nov;34(11):338–40. doi: 10.1111/j.1753-4887.1976.tb05815.x. [DOI] [PubMed] [Google Scholar]
- 10.McCollum EV, Davis M. Observations on the isolation of the substance in butter fat which exerts a stimulating influence on growth. J Biol Chem. 1914;19(2):245–250. [Google Scholar]
- 11.Chick H, Dalyell E, Hume M, Mackay HH, Henderson S. The aetiology of rickets in infants: prophylactic and curative observations at the Vienna University Kinderklinik. The Lancet. 1922 Jul 1;ii:7–11. [Google Scholar]
- 12.Carpenter KJ. Harriette Chick and the Problem of Rickets. J Nutr. 2008 May 1;138(5):827–32. doi: 10.1093/jn/138.5.827. [DOI] [PubMed] [Google Scholar]
- 13.Doetsch AM, Faber J, Lynnerup N, Wätjen I, Bliddal H, Danneskiold-Samsøe B. The effect of calcium and vitamin D3 supplementation on the healing of the proximal humerus fracture: a randomized placebo-controlled study. Calcif Tissue Int. 2004 Sep;75(3):183–8. doi: 10.1007/s00223-004-0167-0. [DOI] [PubMed] [Google Scholar]
- 14.Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009 Mar 23;169(6):551–61. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- 15.Boonen S, Bischoff-Ferrari HA, Cooper C, Lips P, Ljunggren O, Meunier PJ, et al. Addressing the musculoskeletal components of fracture risk with calcium and vitamin D: a review of the evidence. Calcif Tissue Int. 2006 May;78(5):257–70. doi: 10.1007/s00223-005-0009-8. [DOI] [PubMed] [Google Scholar]
- 16.Brinker MR, O’Connor DP, Monla YT, Earthman TP. Metabolic and endocrine abnormalities in patients with nonunions. J Orthop Trauma. 2007 Sep;21(8):557–70. doi: 10.1097/BOT.0b013e31814d4dc6. [DOI] [PubMed] [Google Scholar]
- 17.Cranney A, Weiler HA, O’Donnell S, Puil L. Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. Am J Clin Nutr. 2008 Aug;88(2):513S–519S. doi: 10.1093/ajcn/88.2.513S. [DOI] [PubMed] [Google Scholar]
- 18.Harwood RH, Sahota O, Gaynor K, Masud T, Hosking DJ Nottingham Neck of Femur (NONOF) Study. A randomised, controlled comparison of different calcium and vitamin D supplementation regimens in elderly women after hip fracture: The Nottingham Neck of Femur (NONOF) Study. Age Ageing. 2004 Jan;33(1):45–51. doi: 10.1093/ageing/afh002. [DOI] [PubMed] [Google Scholar]
- 19.Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. The Lancet. 2014 Jan;383(9912):146–55. doi: 10.1016/S0140-6736(13)61647-5. [DOI] [PubMed] [Google Scholar]
- 20.Jingushi S, Iwaki A, Higuchi O, Azuma Y, Ohta T, Shida JI, et al. Serum 1alpha,25-dihydroxyvitamin D3 accumulates into the fracture callus during rat femoral fracture healing. Endocrinology. 1998 Apr;139(4):1467–73. doi: 10.1210/endo.139.4.5883. [DOI] [PubMed] [Google Scholar]
- 21.Lidor C, Atkin I, Ornoy A, Dekel S, Edelstein S. Healing of rachitic lesions in chicks by 24R,25-dihydroxycholecalciferol administered locally into bone. J Bone Miner Res Off J Am Soc Bone Miner Res. 1987 Apr;2(2):91–8. doi: 10.1002/jbmr.5650020203. [DOI] [PubMed] [Google Scholar]
- 22.Fu L, Tang T, Miao Y, Hao Y, Dai K. Effect of 1,25-dihydroxy vitamin D3 on fracture healing and bone remodeling in ovariectomized rat femora. Bone. 2009 May;44(5):893–8. doi: 10.1016/j.bone.2009.01.378. [DOI] [PubMed] [Google Scholar]
- 23.Andreen O, Larsson SE. Effects of parathyroidectomy and vitamin D on fracture healing. Fracture biomechanics in rats after parathyroidectomy and treatment with 1,25-dihydroxycholecalciferol. Acta Orthop Scand. 1983 Dec;54(6):805–9. doi: 10.3109/17453678308992913. [DOI] [PubMed] [Google Scholar]
- 24.Reid JJ, Johnson JS, Wang JC. Challenges to bone formation in spinal fusion. J Biomech. 2011 Jan 11;44(2):213–20. doi: 10.1016/j.jbiomech.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Plehwe WE, Carey RPL. Spinal surgery and severe vitamin D deficiency. Med J Aust. 2002 May 6;176(9):438–9. doi: 10.5694/j.1326-5377.2002.tb04487.x. [DOI] [PubMed] [Google Scholar]
- 26.Metzger MF, Kanim LEA, Zhao L, Robinson ST, Delamarter RB. The Relationship Between Serum Vitamin D Levels and Spinal Fusion Success: A Quantitative Analysis. Spine. 2015 Apr;40(8):E458–68. doi: 10.1097/BRS.0000000000000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson ST, Svet MT, Kanim LA, Metzger MF. Four-point bending as a method for quantitatively evaluating spinal arthrodesis in a rat model. Comp Med. 2015 Feb;65(1):46–50. [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura T, Kurokawa T, Orimo H. Increased mechanical strength of the vitamin D-replete rat femur by the treatment with a large dose of 24R,25(OH)2D3. Bone. 1989;10(2):117–23. doi: 10.1016/8756-3282(89)90009-4. [DOI] [PubMed] [Google Scholar]
- 29.Delgado-Martínez AD, Martínez ME, Carrascal MT, Rodríguez-Avial M, Munuera L. Effect of 25-OH-vitamin D on fracture healing in elderly rats. J Orthop Res Off Publ Orthop Res Soc. 1998 Nov;16(6):650–3. doi: 10.1002/jor.1100160604. [DOI] [PubMed] [Google Scholar]
- 30.Donnelly E, Chen DX, Boskey AL, Baker SP, van der Meulen MCH. Contribution of mineral to bone structural behavior and tissue mechanical properties. Calcif Tissue Int. 2010 Nov;87(5):450–60. doi: 10.1007/s00223-010-9404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donnelly E, Boskey AL, Baker SP, van der Meulen MCH. Effects of tissue age on bone tissue material composition and nanomechanical properties in the rat cortex. J Biomed Mater Res A. 2010 Mar 1;92(3):1048–56. doi: 10.1002/jbm.a.32442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, et al. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999 Nov;140(11):4982–7. doi: 10.1210/endo.140.11.7110. [DOI] [PubMed] [Google Scholar]
- 33.Martin E, Haney E, Shannon J, Cauley J, Ensrud K, Keaveny T, et al. Femoral Volumetric Bone Density, Geometry and Strength in Relation to 25-Hydroxy Vitamin D in Older Men. J Bone Miner Res Off J Am Soc Bone Miner Res. 2015 Mar;30(3):475–82. doi: 10.1002/jbmr.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez CJ, Beaupré GS, Keller TS, Carter DR. The influence of bone volume fraction and ash fraction on bone strength and modulus. Bone. 2001 Jul;29(1):74–8. doi: 10.1016/s8756-3282(01)00467-7. [DOI] [PubMed] [Google Scholar]
- 35.Braun JJ, Birkenhäger-Frenkel DH, Rietveld AH, Juttmann JR, Visser TJ, Birkenhäger JC. Influence of 1 alpha-(OH)D3 administration on bone and bone mineral metabolism in patients on chronic glucocorticoid treatment; a double blind controlled study. Clin Endocrinol (Oxf) 1983 Aug;19(2):265–73. doi: 10.1111/j.1365-2265.1983.tb02989.x. [DOI] [PubMed] [Google Scholar]
- 36.Gorter EA, Hamdy NAT, Appelman-Dijkstra NM, Schipper IB. The role of vitamin D in human fracture healing: a systematic review of the literature. Bone. 2014 Jul;64:288–97. doi: 10.1016/j.bone.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Kogawa M, Findlay DM, Anderson PH, Ormsby R, Vincent C, Morris HA, et al. Osteoclastic metabolism of 25(OH)-vitamin D3: a potential mechanism for optimization of bone resorption. Endocrinology. 2010 Oct;151(10):4613–25. doi: 10.1210/en.2010-0334. [DOI] [PubMed] [Google Scholar]
- 38.Ettehad H, Mirbolook A, Mohammadi F, Mousavi M, Ebrahimi H, Shirangi A. Changes in the Serum Level of Vitamin D During Healing of Tibial and Femoral Shaft Fractures. Trauma Mon [Internet] 2014 Jan 25;19(1) doi: 10.5812/traumamon.10946. [cited 2016 Apr 11] Available from: http://www.traumamon.com/?page=article&article_id=10946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alkalay D, Shany S, Dekel S. Serum and bone vitamin D metabolites in elective patients and patients after fracture. Bone Jt J. 1989 Jan 1;71–B(1):85–7. doi: 10.1302/0301-620X.71B1.2783695. [DOI] [PubMed] [Google Scholar]
- 40.Kaastad TS, Reikeras O, Madsen JE, Narum S, Stromme JH, Obrant KJ, Nordsletten L. Effects of clodronate on cortical and trabecular bone in ovariectomized rats on a low calcium diet. Calcif Tissue Int. 1997;61(2):158–164. doi: 10.1007/s002239900315. [DOI] [PubMed] [Google Scholar]
- 41.Kaastad TS, Reikeras O, Halvorsen V, Falch JA, Obrant KJ, Nordsletten L. Vitamin D deficiency and ovariectomy reduced the strength of the femoralneck in rats. Calcif Tissue Int. 2001;69(2):102–108. doi: 10.1007/s00223-001-0009-2. [DOI] [PubMed] [Google Scholar]
- 42.Chavhan SG, Brar RS, Banga HS, Sandhu HS, Sodhi S, Gadhave PD, et al. Clinicopathological Studies on Vitamin D3 Toxicity and Therapeutic Evaluation of Aloe vera in Rats. Toxicol Int. 2011;18(1):35–43. doi: 10.4103/0971-6580.75851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mortensen JT, Lichtenberg J, Binderup L. Toxicity of 1,25-dihydroxyvitamin D3, tacalcitol, and calcipotriol after topical treatment in rats. J Investig Dermatol Symp Proc Soc Investig Dermatol Inc Eur Soc Dermatol Res. 1996 Apr;1(1):60–3. [PubMed] [Google Scholar]
- 44.Martín-Lacave I, Ramos F, Utrilla JC, Conde E, Hevia A, Fernández R, et al. Chronic hypervitaminosis D3 determines a decrease in C-cell numbers and calcitonin levels in rats. J Endocrinol Invest. 1998 Feb;21(2):102–8. doi: 10.1007/BF03350323. [DOI] [PubMed] [Google Scholar]
- 45.Elshama SS, Osman H-EH, El-Kenawy AE-M, Youseef HM. Comparison between the protective effects of vitamin K and vitamin A on the modulation of hypervitaminosis D3 short-term toxicity in adult albino rats. Turk J Med Sci. 2016;46(2):524–38. doi: 10.3906/sag-1411-6. [DOI] [PubMed] [Google Scholar]
- 46.Marshall EF. CHOLECALCIFEROL: A UNIQUE TOXICANT FOR RODENT CONTROL. Proc Elev Vertebr Pest Conf; 1984; Mar 1, [Internet] Available from: http://digitalcommons.unl.edu/vpc11/22. [Google Scholar]
- 47.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D [Internet] Washington (DC): National Academies Press (US); The National Academies Collection: Reports funded by National Institutes of Health; 2011. [cited 2016 Oct 1] Available from: http://www.ncbi.nlm.nih.gov/books/NBK56070/ [PubMed] [Google Scholar]
- 48.Ravindra VM, Guan J, Holland CM, Dailey AT, Schmidt MH, Godzik J, et al. Vitamin D status in cervical spondylotic myelopathy: comparison of fusion rates and patient outcome measures. A preliminary experience. J Neurosurg Sci. 2016 Sep 2; doi: 10.23736/S0390-5616.16.03846-7. [DOI] [PubMed] [Google Scholar]
- 49.Parry J, Sullivan E, Scott AC. Vitamin D sufficiency screening in preoperative pediatric orthopaedic patients. J Pediatr Orthop. 2011 May;31(3):331–3. doi: 10.1097/BPO.0b013e3182104a94. [DOI] [PubMed] [Google Scholar]
- 50.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006 Mar;81(3):353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 51.Kerezoudis P, Rinaldo L, Drazin D, Kallmes D, Krauss W, Hassoon A, et al. Association Between Vitamin D Deficiency and Outcomes Following Spinal Fusion Surgery: A Systematic Review. World Neurosurg. 2016 Nov;95:71–6. doi: 10.1016/j.wneu.2016.07.074. [DOI] [PubMed] [Google Scholar]
- 52.Watchorn E. The normal serum-calcium and magnesium of the rat: their relation to sex and age. J Biochem. 1933;27(6):1875–1878. doi: 10.1042/bj0271875. [DOI] [PMC free article] [PubMed] [Google Scholar]