Abstract
This nationwide, prospective cohort study evaluated pulmonary function and radiological sequelae according to infection severity in 73 survivors from the 2015 Middle East respiratory syndrome (MERS) outbreak in Korea. Patients with severe pneumonia in MERS-coronavirus infection had more impaired pulmonary function than those with no or mild pneumonia at the 1-year follow-up, which was compatible with the radiological sequelae. Severe pneumonia significantly impairs pulmonary function and makes long radiological sequelae in MERS.
Keywords: MERS, Complication, Coronavirus, Outbreak
Graphical Abstract
The 2015 outbreak of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in the Republic of Korea developed from a traveler returning from the Middle East,1 which is the largest outbreak outside of the Arabian Peninsula to date. This unprecedented nationwide outbreak resulted in 186 laboratory-confirmed cases with 38 fatalities and > 16,000 individuals being quarantined.2,3,4 During the outbreak, a comprehensive screening test including MERS-CoV real-time reverse transcription polymerase chain reaction (rRT-PCR) was performed in all possible contactors to prevent further spread of the disease. Positive MERS-CoV rRT-PCR findings were observed in patients with no or mild symptoms, who were also subjected to epidemiological investigation and follow-up.1
There have been many reports of long-term sequelae of severe acute respiratory syndrome (SARS),5,6,7,8,9,10 but to the best of our knowledge no report has addressed long-term sequelae in the follow-up of patients with MERS-CoV infection. The present study aimed to evaluate pulmonary function and radiological sequelae 1 year after MERS-CoV infection according to the severity of the infection.
In this nationwide cohort study the researchers tried to contact all survivors with laboratory-confirmed MERS-CoV infection during the outbreak by phone or mail. The patients who provided written informed consent were enrolled. The patients were followed up in five hospitals. The Institutional Review Board of each hospital approved the study protocol (National Medical Center, H-1510-059-007; Seoul National University Hospital, 1511-117-723; Seoul National University Boramae Medical Center, 26-2016-8; Seoul Medical Center, Seoul2015-12-102; Dankook University Hospital, DKUH2016-02-014; and Chungnam National University Hospital, CNUH2015-08-029).
The pulmonary function test, a standardized 6-minute walk test, and chest computed tomography (CT) were performed 1 year after MERS-CoV infection. The pulmonary function tests included total lung capacity (TLC), forced volume vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and diffusing capacity of the lung for carbon monoxide (DLCO). All pulmonary function values were presented as predicted percentage considering age, sex, height, body weight, and race. Radiological sequelae were scored as the number of involved lung segments (total score = 19) on chest CT that were suspected to be post-inflammation sequelae, including sub-segmental atelectasis, ground glass opacity, and consolidation by a radiologist.10 Emphysema, sequelae of tuberculosis, and bronchiectasis were excluded. Severe pneumonia was defined as the patient requiring oxygen therapy, mild pneumonia was defined as the patient presenting with infiltration on chest X-ray but not requiring oxygen therapy, and no pneumonia was defined as the patient without radiographic evidence of pneumonia.11
Linear regression or linear by linear association was used to evaluate the association between the severity of pneumonia and continuous or categorical variables, as appropriate. The correlation between pneumonia severity and pulmonary function or radiological sequelae was evaluated using a multivariable linear regression model including age, sex, underlying lung diseases, and smoking. P < 0.05 was considered significant. IBM SPSS Statistics (version 22; IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
Among a total of 146 survivors in the outbreak, 49 (34%) refused to participate in the study and 24 (16%) could not be contacted by any method. Therefore, 73 patients were enrolled in the study: 18 (25%) patients without pneumonia, 35 (48%) patients with mild pneumonia, and 20 (27%) patients with severe pneumonia. The mean patient age was 51 ± 13 years, 30 (41%) were female, and the severe pneumonia group tended to have more male patients (Table 1). Fourteen patients (19%) had a history of smoking and the patients with pneumonia were more likely to have a history of smoking. None of the underlying diseases were associated with the severity of pneumonia.
Table 1. Baseline characteristics of 73 patients with MERS-CoV infection.
| Variables | Total (n = 73) | No pneumonia (n = 18) | Mild pneumonia (n = 35) | Severe pneumonia (n = 20) | P value | |
|---|---|---|---|---|---|---|
| Age, yr | 51 (25–80) | 47 (25–80) | 56 (25–78) | 54 (28–69) | 0.872 | |
| Sex (female) | 30 (41) | 9 (50) | 17 (49) | 4 (20) | 0.056 | |
| Health care provider | 20 (27) | 8 (44) | 7 (20) | 5 (25) | 0.200 | |
| Smoker | 14 (19) | 0 (0) | 9 (26) | 5 (25) | 0.059 | |
| Underlying disease | ||||||
| Hypertension | 16 (22) | 5 (28) | 4 (11) | 7 (35) | 0.543 | |
| Diabetes | 9 (12) | 3 (17) | 3 (9) | 3 (15) | 0.904 | |
| Lung disease | 4 (6) | 1 (6) | 1 (3) | 2 (10) | 0.528 | |
| Liver disease | 3 (4) | 1 (6) | 2 (6) | 0 | 0.379 | |
| Solid tumor | 3 (4) | 0 | 3 (9) | 0 | 0.543 | |
| Heart disease | 2 (3) | 0 | 1 (3) | 1 (5) | 0.351 | |
| Kidney disease | 2 (3) | 1 (6) | 0 | 1 (5) | 0.957 | |
| Hematological malignancy | 1 (1) | 0 | 1 (3) | 0 | 0.970 | |
Data are presented as median (range) or No. (%).
MERS-CoV = Middle East respiratory syndrome coronavirus.
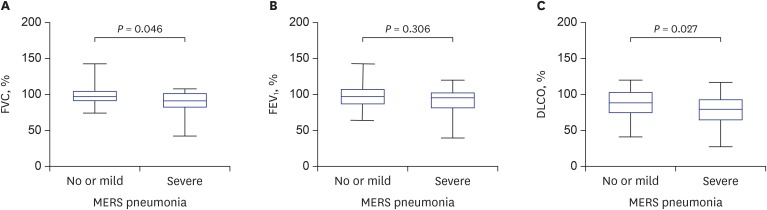
The frequency of patients with lung function parameters < 80% of predicted values was as follows: FVC (6/73, 8%), FEV1 (6/73, 8%), and DLCO (25/68, 37%). After adjusting for age, sex, underlying lung disease, and smoking, FVC and DLCO significantly correlated with the severity of pneumonia (P = 0.008 and P = 0.046; Table 2). The patients with severe pneumonia had lower FVC and DLCO than the patients with no or mild pneumonia (Fig. 1). TLC, FEV1, FEV1/FVC, and the walking distance in the 6-minute walk test were not significantly associated with the severity of pneumonia.
Table 2. Pulmonary function and radiological sequelae of 73 patients with MERS-CoV infection.
| Variables | Total | No pneumonia | Mild pneumonia | Severe pneumonia | P valuea |
|---|---|---|---|---|---|
| TLC, % | 97 (57–154) | 112 (87–154) | 95 (79–121) | 97 (57–148) | 0.843 |
| FVC, % | 93 (42–142) | 94 (81–142) | 94 (74–126) | 88 (42–108) | 0.008 |
| FEV1, % | 88 (40–154) | 91 (74–154) | 88 (64–143) | 84 (40–120) | 0.072 |
| FEV1/FVC | 79 (53–94) | 79 (67–94) | 78 (53–87) | 80 (74–85) | 0.291 |
| DLCO, % | 77 (28–117) | 75 (42–83) | 83 (70–117) | 68 (28–101) | 0.046 |
| 6 min walking test, m | 540 (50–738) | 563 (50–738) | 490 (317–600) | 540 (315–721) | 0.750 |
| Score of radiological sequelae | 1 (0–12) | 0 (0–5) | 1 (0–11) | 3 (0–12) | < 0.001 |
Data are presented as median (range) or No. (%).
MERS-CoV = Middle East respiratory syndrome coronavirus, TLC = total lung capacity, FVC = forced volume vital capacity, FEV1 = forced expiratory volume in 1 second, DLCO = diffusing capacity, CT = computed tomography.
aAdjusted for age, sex, underlying lung disease, and smoking.
Fig. 1. Comparison of pulmonary function in patients with no or mild pneumonia and patients with severe pneumonia. (A) FVC. (B) FEV1. (C) DLCO. P values were adjusted for age, sex, underlying lung disease, and smoking. Whiskers indicate minimum and maximum values.
FVC = forced volume vital capacity, FEV1 = forced expiratory volume in 1 second, DLCO = diffusing capacity, MERS = Middle East respiratory syndrome.
CT was performed 1 year after MERS-CoV infection in 65 (89%) patients. Radiological sequelae were revealed in 25% (4/16), 63% (19/30), and 95% (18/19) of patients in the no, mild, and severe pneumonia groups, respectively (P < 0.001). The median radiological sequelae score was 0, 1, and 3 in the no, mild, and severe pneumonia groups, respectively, and the radiological sequelae scores were significantly correlated with the severity of pneumonia (P < 0.001, Table 2).
This is the first cohort study showing long-term pulmonary complications of MERS-CoV infection. The findings suggest that more severe MERS pneumonia can result in more impaired lung function at least 1 year after MERS-CoV infection. These findings were compatible with radiological sequelae.
Several studies have examined the effect of SARS on pulmonary function 1 year after infection.5,10,12 A previous study showed that 24% of SARS survivors have impaired DLCO and 5% reduced lung volume at 12 months.5 Several studies on acute respiratory distress syndrome survivors showed that their pulmonary function usually returns to normal or near normal by 6–12 months,13,14 but a mild reduction of DLCO may persist in up to 80% of patients at 1 year after recovery.15 These findings were very similar to the results of the present study. We also showed that 37% of MERS survivors have impaired DLCO at 12 months, whereas only 8% of patients had a reduced FVC.
The Korean MERS outbreak in 2015 occurred in a hospital setting, and most patients with MERS had been admitted before the outbreak, though one-fourth of patients were healthcare providers.16 Thus, a comparison of lung function and exercise capacity between these MERS survivors and the general healthy population may mislead the results, as the underlying lung condition before MERS-CoV infection could impact lung function after illness. For this reason, we compared lung function according to the severity of pneumonia in order to evaluate the effect of MERS-CoV infection on pulmonary function. The finding that more severe MERS pneumonia resulted in more impaired lung function strongly suggests that pulmonary sequelae can remain at least 1 year after MERS-CoV pneumonia, which is also supported by the correlation of radiological sequela correlated with the severity of MERS pneumonia.
The previous study found that SARS survivors who required intensive care unit admission had lower predicted FVC and DLCO than those who did not, but there were no differences in the 6-minute walking test.5,12 These findings were also compatible with our results, which showed that severe pneumonia requiring oxygen therapy is associated with more impaired lung function, but there was no difference in exercise capacity.
The present study has several limitations. First, the patients with underlying lung diseases and impaired lung function may have more severe MERS pneumonia. The patients with severe pneumonia had more underlying lung diseases, though the difference was not significant. However, even after adjusting for underlying lung diseases and smoking, the correlation between the MERS pneumonia severity and lung function impairment was significant. Second, because we defined pneumonia as infiltration on chest X-ray, we may classify a patient with mild pneumonia in the group without pneumonia. In fact, radiological sequelae on chest CT was observed in approximately 25% of the patients without pneumonia. Third, only 50% of the eligible MERS-CoV infected survivors were enrolled, which may not represent all of the MERS-CoV survivors in Korea. Forth, no baseline pulmonary function or CT scans were not available. Lastly, our definition of severe pneumonia as requirement of oxygen therapy may be broad and subjective. Ventilator care or mortality may indicate the patients with more severe pneumonia, although small number of patients hampered further classification in this study.
In summary, patients with more severe MERS-CoV pneumonia may have more impaired pulmonary function at 1 year, which is compatible with the radiological sequelae.
Footnotes
Funding: This work was supported by a grant from the Korean Healthcare Technology R & D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C3227).
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Shin HS, Oh MD, Park WB. Dara curation: Park WB, Kim G, Choi JP, Rhee JY, Cheon S, Park JS, Kim Y, Joh JS, Chin BS, Choe PG, Bang JH, Park SW, Kim NJ, Kim YS. Formal analysis: Shin HS, Park WB, Jun KI, Lee CH, Lim DG. Writing - original draft: Shin HS, Park WB, Jun KI.
References
- 1.Korea Centers for Disease Control and Prevention. Middle East respiratory syndrome coronavirus outbreak in the Republic of Korea, 2015. Osong Public Health Res Perspect. 2015;6(4):269–278. doi: 10.1016/j.phrp.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang CK, Song KH, Choe PG, Park WB, Bang JH, Kim ES, et al. Clinical and epidemiologic characteristics of spreaders of Middle East respiratory syndrome coronavirus during the 2015 outbreak in Korea. J Korean Med Sci. 2017;32(5):744–749. doi: 10.3346/jkms.2017.32.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi WS, Kang CI, Kim Y, Choi JP, Joh JS, Shin HS, et al. Clinical presentation and outcomes of Middle East respiratory syndrome in the Republic of Korea. Infect Chemother. 2016;48(2):118–126. doi: 10.3947/ic.2016.48.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park WB, Kwon NJ, Choe PG, Choi SJ, Oh HS, Lee SM, et al. Isolation of Middle East respiratory syndrome coronavirus from a patient of the 2015 Korean outbreak. J Korean Med Sci. 2016;31(2):315–320. doi: 10.3346/jkms.2016.31.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui DS, Wong KT, Ko FW, Tam LS, Chan DP, Woo J, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128(4):2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonio GE, Wong KT, Hui DS, Wu A, Lee N, Yuen EH, et al. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience. Radiology. 2003;228(3):810–815. doi: 10.1148/radiol.2283030726. [DOI] [PubMed] [Google Scholar]
- 7.Hui DS, Joynt GM, Wong KT, Gomersall CD, Li TS, Antonio G, et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan KS, Zheng JP, Mok YW, Li YM, Liu YN, Chu CM, et al. SARS: prognosis, outcome and sequelae. Respirology. 2003;8(Suppl):S36–S40. doi: 10.1046/j.1440-1843.2003.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie L, Liu Y, Xiao Y, Tian Q, Fan B, Zhao H, et al. Follow-up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge. Chest. 2005;127(6):2119–2124. doi: 10.1378/chest.127.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X, Dong D, Ma D. Thin-section computed tomography manifestations during convalescence and long-term follow-up of patients with severe acute respiratory syndrome (SARS) Med Sci Monit. 2016;22:2793–2799. doi: 10.12659/MSM.896985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh MD, Park WB, Choe PG, Choi SJ, Kim JI, Chae J, et al. Viral load kinetics of MERS coronavirus infection. N Engl J Med. 2016;375(13):1303–1305. doi: 10.1056/NEJMc1511695. [DOI] [PubMed] [Google Scholar]
- 12.Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15(3):543–550. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson DL, Goodman M, Spector SL, Petty TL. Long-term follow-up and bronchial reactivity testing in survivors of the adult respiratory distress syndrome. Am Rev Respir Dis. 1978;117(3):449–454. doi: 10.1164/arrd.1978.117.3.449. [DOI] [PubMed] [Google Scholar]
- 14.Peters JI, Bell RC, Prihoda TJ, Harris G, Andrews C, Johanson WG. Clinical determinants of abnormalities in pulmonary functions in survivors of the adult respiratory distress syndrome. Am Rev Respir Dis. 1989;139(5):1163–1168. doi: 10.1164/ajrccm/139.5.1163. [DOI] [PubMed] [Google Scholar]
- 15.Orme J, Jr, Romney JS, Hopkins RO, Pope D, Chan KJ, Thomsen G, et al. Pulmonary function and health-related quality of life in survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2003;167(5):690–694. doi: 10.1164/rccm.200206-542OC. [DOI] [PubMed] [Google Scholar]
- 16.Oh MD, Choe PG, Oh HS, Park WB, Lee SM, Park J, et al. Middle East respiratory syndrome coronavirus superspreading event involving 81 persons, Korea 2015. J Korean Med Sci. 2015;30(11):1701–1705. doi: 10.3346/jkms.2015.30.11.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]