Abstract
Background
Investigation of exercise training in metastatic breast cancer has received minimal attention. We determined the feasibility and safety of aerobic training in metastatic breast cancer.
Methods
65 women (21 to 80 years) with metastatic (stage IV) breast cancer (57% receiving chemotherapy; >40% ≥ 2 lines of prior therapy) were allocated to an aerobic training (n=33) or stretching (n=32) group. Aerobic training consisted of 36 supervised treadmill walking sessions delivered thrice-weekly between 55% to 80% of peak oxygen consumption (VO2peak) for 12 consecutive weeks. Stretching was matched to aerobic training on location, frequency, duration, and intervention length. The primary end point was aerobic training feasibility a priori defined as rate of lost to follow up (LTF) (<20%) and attendance (≥70%). Secondary end points were safety, objective (VO2peak, functional capacity) and patient-reported (quality of life) outcomes.
Results
One of 33 (3%) patients receiving aerobic training was LTF whereas mean attendance was 63% ± 30%. Rates of permanent discontinuation and dose modification were 27% and 49%, respectively. Intention-to-treat analyses indicated improvements in patient-reported outcomes (PROs), favoring the attention control group (p’s>0.05). Per protocol analyses indicated 14 of 33 (42%) patients receiving aerobic training had acceptable tolerability (relative dose intensity ≥70%), which led to improvements in VO2peak and functional capacity (p’s <0.05).
Conclusion
Aerobic training at the dose and schedule tested is safe but not feasible in a significant proportion of patients with metastatic breast cancer. The acceptable feasibility and promising benefit in select patients warrants further evaluation in a dose-finding phase 1/2 study.
Keywords: Cardiorespiratory fitness, exercise, relative dose intensity, dose modification, patient reported outcome
INTRODUCTION
Meta-analyses conclude exercise training is a safe and feasible intervention associated with significant improvements across a broad array of symptom control outcomes (e.g., physical functioning, fatigue) in early-stage breast cancer.1, 2 Investigation of exercise in advanced or metastatic breast cancer has received minimal attention but may be of significant clinical interest to mitigate treatment and disease-related symptoms (e.g., exercise intolerance, quality of life, fatigue) and potentially to improve disease outcomes. Evaluation of exercise treatment in this setting presents a unique challenge given the high disease and treatment-related sequelae that collectively may alter exercise training tolerability, safety, and response.3 Thus, initial studies are an essential prerequisite prior to launching definitive trials in this population.4
We conducted a vanguard randomized clinical trial (RCT) to determine the feasibility and safety of aerobic training in patients with metastatic breast cancer. Secondary objectives were to explore the effects on symptom control outcomes as well as identify a subgroup of patients for which aerobic training was feasible. We hypothesized that aerobic training would be a feasible intervention associated with significant benefit compared to a non-exercise attention control group.
METHODS
Study design, participants, and procedures
Full study methods are described in the supplementary online content. We conducted a RCT among adult women with histologically-confirmed metastatic breast cancer regardless of menopausal status and concurrent or prior lines of therapy at Duke University Medical Center (DUMC) and Memorial Sloan Kettering Cancer Center (MSK). Other major eligibility criteria were: (1) Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1, (2) primary attending oncologist approval for a screening cardiopulmonary exercise test (CPET), (3) performing <150 mins of moderate-intensity exercise / wk,2 and (4) review and clearance of exercise electrocardiogram by cardiologist. All study procedures were reviewed and approved by the institutional review boards. Participation continued for a maximum of 12 weeks or until unacceptable toxicity, significant deterioration in performance status, or withdrawal of consent (Supplemental methods).
Randomization and masking
Patients were randomly allocated in a 1:1 ratio to receive aerobic training or attention control (stretching). The random allocation sequence was generated and implemented using REDCap with a random permuted block design. Randomization was stratified according to the prior lines of therapy (<1, ≥1) and menopausal status (post-, pre/peri-menopausal) at study entry. Neither patients nor exercise physiologists were blinded to group allocation.
Study treatments
Study treatments were matched in terms of setting (clinic-based), monitoring, frequency, duration/session, and length. Dedicated study personnel with Bachelor’s degrees in Exercise Science implemented the interventions and individually monitored all sessions in both groups. All sessions were by appointment only with patients contacted <24 h following an unscheduled missed session. Rescheduling of missed sessions was permitted.
Aerobic Training Treatment
Aerobic training consisted of 36 supervised treadmill walking sessions delivered thrice-weekly for 12 consecutive weeks (Supplemental methods). The intensity of each session alternated between four different dose intensities (i.e., 55%, 65%, 75%, and 80%) of maximal metabolic (MET) expenditure (i.e., VO2peak). Intensity was tailored to each patient on the basis of workload (i.e., treadmill speed / grade) corresponding to a specific percent of ventilatory thresholds measured during the pre-randomization or midpoint (week 6) CPET. The planned dose and scheduling of exercise treatment was continually altered and progressed in conjunction with appropriate rest / recovery sessions across the entire intervention period (i.e., non-linear periodized prescription; Figure 1), and standardized across all patients. This prescription approach was selected on the basis of a prior RCT among patients receiving neoadjuvant chemotherapy for operable breast cancer.5
Fig. 1. “Planned” Aerobic Training Dose Intensity and Schedule.
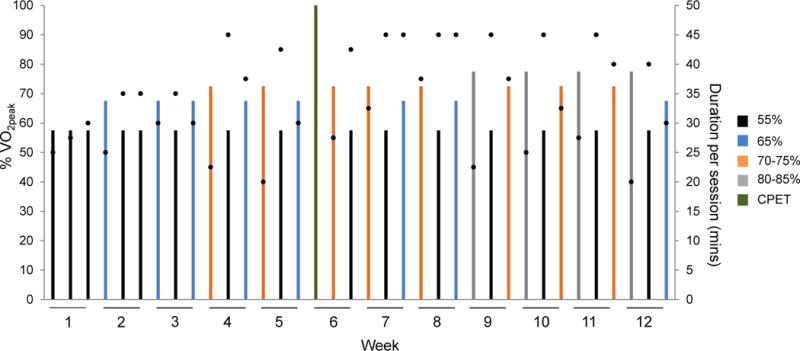
Schematic of the planned aerobic training prescription template implemented to all patients allocated to the aerobic training group. The intensity and duration of each individual session (i.e., dose) as well as the schedule of treatment dose across the study intervention period is presented. The intensity of each session was conducted at one of four different doses depicted by the colored bars as a percentage of VO2peak: (1) black – 55%, (2) blue – 65%, (3) orange – 70% to 75%, and (4) grey – 80%. All doses were individualized to each patient on the basis of the baseline cardiopulmonary exercise test (CPET). Black dots depict the planned duration of each session (mins), ranging from a minimum of 20 mins/session to a maximum of 45 mins/session. At the end of week 6, the CPET was repeated to re-prescribe exercise intensity (green bar). The prescription template depicts the planned intensity, duration, and scheduling of sessions as per protocol under the assumption that no sessions were missed.
Safety and verification of training intensity of each session was evaluated using a combination of heart rate (continuous), blood pressure (every 10 mins), and rate of perceived exertion (every 15 mins). Dose modification of any session was permitted and performed by the exercise physiologist monitoring each session using standardized criteria (Table S1).
Attention Control
Attention control consisted of three individualized stretching sessions per week, of 12 to 20 different positions, following a standardized progressive approach for 10 to 30 secs/stretch for a total of 20 – 45 min/session.7
Outcomes
The primary end point was feasibility evaluated by the composite end point of lost to follow up (LTF; completion of postintervention assessments) and attendance (ratio of total attended to planned treatments). Other secondary feasibility end points were permanent discontinuation (treatment discontinuation prior to week 12), treatment interruption (missing ≥3 consecutive sessions), dose modification [≥10% of sessions requiring modification (reduction / escalation) of intensity or duration], pre-treatment dose modification (reduction of pre-treatment session intensity), early session termination (termination of session prior to planned duration), and adherence (compliance with the planned dose/session). “Planned” and “completed” exercise dose in all sessions was quantified as METs/session, with relative dose intensity (RDI) defined as the ratio of total “completed” to total “planned” cumulative dose (Supplemental methods).6. Secondary end points were safety, VO2peak, functional capacity, and PROs. Safety was evaluated by the type and prevalence of serious (e.g., important medical events) and non-serious (e.g., knee, back pain) adverse events during aerobic training sessions. Hematological profile was evaluated via complete blood counts (CBCs). Cardiorespiratory fitness (VO2peak) was assessed by a symptom-limited CPET on an electronic motorized treadmill test with 12-lead ECG monitoring (Mac® 5000, GE Healthcare), according to standard procedures.8 All CPETs were conducted in a dedicated research laboratory at both institutions. Functional capacity was assessed by the six-minute walk test,9 30 second chair-stand test,10 and the timed up and go,11 while PROs included quality of life (Functional Assessment of Cancer Therapy – Breast (FACT-B),12 physical functioning (SF-36),13 fatigue, (FACIT-Fatigue),14 sleep quality (Pittsburgh Sleep Inventory (sleep quality),15 and pain (Brief Pain Inventory).16 Non-protocol exercise was assessed using a validated survey.17 Dedicated study personnel with degrees in Exercise Science conducted all baseline and postintervention physiological evaluations. All outcomes were evaluated at pre-randomization (study treatments were initiated ≤14 days) and were repeated ≤7 days of the final treatment session at postintervention (month 3).
Statistical Analysis
Feasibility was evaluated according to protocol-specified criteria for the intention to treat (ITT) population: (1) LTF rate <20%, and (2) mean attendance ≥70%. Standard definitions of feasibility with exercise training are not available for any clinical population, thus criteria selected here are based on LTF and attendance rates reported in prior exercise training studies in the oncology setting.18, 19 Allocation of 36 patients to the aerobic training group provides 80% power to differentiate between a 60% and 80% feasibility rate with a one-tailed one-sample binomial test with 0.05 level of significance. A protocol-specified stopping rule was a serious adverse event rate of ≥2 events/9 patients, ≥3/18, or ≥4/36 in the aerobic training group.
Baseline medical and demographic characteristics of each group are summarized using descriptive statistics (mean/SD and frequencies/%) and compared using the Wilcoxon Rank Sum test or Fisher’s Exact test, as appropriate. Aerobic training dose, feasibility, and safety are reported using descriptive statistics. We also explored differences in feasibility end points as a function of study site (DUMC, MSK). Wilcoxon Signed Rank tests were used to test for within-group and between-group changes in symptom control end points from baseline to postintervention. Analysis of covariance (ANCOVA) was used to estimate the difference in end points at postintervention between groups after adjustment for baseline values and study site. All analyses used the baseline carried forward imputation technique for patients LTF. Wilcoxon Signed Rank tests were applied to test for differences in study end points by tolerability (acceptable tolerability, RDI ≥ 70%) and to determine whether a RDI ≥ 70% was associated with superior benefit compared with a RDI <70%. A two-sided significance level of 0.05 was used for all statistical tests. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
A total of 65 patients were allocated to the aerobic training group (n = 33 patients) or the attention control group (n = 32 patients) (Figure 2). The study was conducted at DUMC between March, 2011 and October, 2013 and then continued at MSK from February 2015 to August, 2016 (for a total accrual period of three years), with final postintervention testing in November, 2016. Baseline characteristics were well balanced between study groups (Table 1). There were no between group differences in change in non-protocol exercise exposure during the intervention (p=0.44).
Fig. 2.

CONSORT Flow for Non-Pharmacological Trials
Table 1.
Characteristics of the Participants
| Characteristic | Overall (n=65) |
Attention Control Group (n=32) |
Aerobic Training Group (n=33) |
|---|---|---|---|
| Recruitment location – no. (%) | |||
| DUMC | 42 (65) | 19 (59) | 23 (70) |
| MSKCC | 23 (35) | 13 (41) | 10 (30) |
| Age (year) – mean (SD) | 54 ± 11 | 56 ± 12 | 52 ± 10 |
| Weight (kg) – mean (SD) | 71 ± 16 | 73 ± 17 | 69 ± 15 |
| BMI (kg/m2) – mean (SD) | 28 ± 16 | 31 ± 22 | 26 ± 5 |
| Hormone-receptor status – no. (%) | |||
| ER positive | 45 (69) | 21 (66) | 24 (73) |
| HER2/neu positive | 19 (29) | 11 (34) | 8 (24) |
| Visceral metastasis – no. (%) | 48 (74) | 21 (66) | 27 (82) |
| No. of disease sites – no. (%) | |||
| 1 | 23 (35) | 13 (41) | 10 (30) |
| 2 | 19 (29) | 8 (25) | 11 (33) |
| ≥3 | 23 (35) | 11 (34) | 12 (36) |
| Prior lines of therapy for metastatic disease – no. (%) | |||
| 0 | 8 (12) | 5 (16) | 3 (9) |
| 1 | 26 (40) | 11 (34) | 15 (46) |
| 2 | 20 (31) | 13 (41) | 7 (21) |
| ≥3 | 11 (17) | 3 (9) | 8 (24) |
| Current therapy for metastatic disease | |||
| Chemotherapy – no. (%) | 37 (57) | 15 (47) | 22 (67) |
| Endocrine therapy – no. (%) | 31 (48) | 16 (50) | 15 (46) |
| Radiotherapy – no. (%) | 3 (5) | 2 (6) | 1 (3) |
| Prior adjuvant therapy | |||
| Chemotherapy – no. (%) | 30 (46) | 14 (44) | 16 (49) |
| Endocrine therapy – no. (%) | 32 (49) | 15 (47) | 17 (52) |
| Radiotherapy – no. (%) | 24 (37) | 10 (31) | 14 (42) |
| Any comorbid condition – no. (%)* | 22 (34) | 11 (34) | 11 (33) |
| No comorbid conditions – no. (%) | 24 (37) | 9 (28) | 15 (46) |
| Exercise at baseline (min·wk−1) – mean (SD)† | 100 ± 151 | 97 ± 94 | 103 ± 192 |
| VO2peak (ml O2·kg−1.min−1) – mean (SD) | 22.5 ± 6.7 | 23.5 ± 6.6 | 22.5 ± 6.9 |
| Percent below age-sex sedentary norms – mean | −10 | −7 | −12 |
Continuous variables are reported as mean (SD) and categorical variables are reported as n (%).
All comparisons p>0.05
Exercise defined as total reported minutes of mild, moderate, and vigorous recreational physical activity over the past week.
Any comorbid condition defined patient presenting with any of the following: hypertension, hyperlipidemia, diabetes mellitus, coronary artery disease, pulmonary disease
Abbreviations: SD, standard deviation; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor; VO2peak, peak oxygen consumption.
For the primary end point, 1 of 33 (3%) patients receiving aerobic training was LTF whereas mean attendance was 63% ± 30% (range, 0% to 100%; 744 of 1,188 planned sessions). The most common reasons for missed sessions were health related (e.g., disease progression, pain) and non-health related (e.g., motivation, vacation) reasons (Table S2). For secondary feasibility end points, the mean cumulative “planned” and “completed” aerobic training dose was 81.2 ± 22.6 MET·hrs (range, 45.9 to 175.9 MET·hrs) and 45.7 ± 24.7 MET·hrs (range, 0.0 to 87.3 MET·hrs) (Figure 3A-B), equating to a mean RDI of 61% ± 30%. Aerobic training was permanently discontinued in 9 (27%) of 33 patients (Table S3). The reasons for discontinuation were disease progression (n=3, 9%), pain (n=2, 6%), and non-health related (motivational) reasons (n=4, 12%). The dose interruption rate was 46% (15 of 33 patients), with the most common reasons for interruption being time constraints (n=4, 12%) and vacation (n=3, 9%). The dose modification rate was 49% (16 of 33 patients); a total of 88 of 744 (12%) attended sessions required dose reduction. A total of 12 (36%) patients required at least one session to be terminated early due to a non-serious health related event. On the basis of study site, there were significant differences in attendance, dose modification, and pre-treatment dose modification with all being inferior at DUMC in comparison with MSK (Table S4). DUMC patients were more likely to have > 3 lines of prior treatment and lower VO2peak at study entry (Table S5). Two patients were transitioned from treadmill walking to stationary cycle ergometry due to pre-existing orthopedic comorbidities.
Fig. 3. Ratio of “planned” to “completed” aerobic training dose.
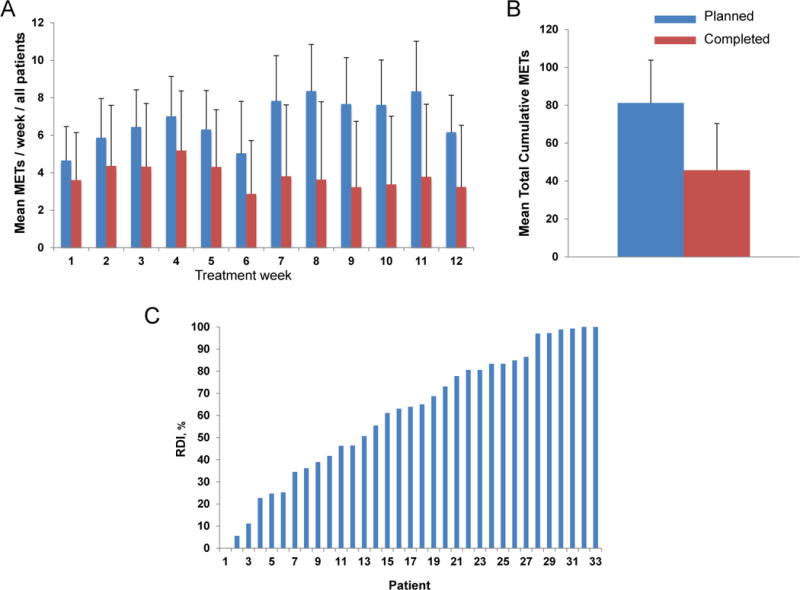
(A) Mean METs / week, (B) mean total cumulative dose, and (C) individual patient relative dose intensity. Data presented for the intention to treat population. “Planned” dose is depicted in the blue colored bars with “completed” dose depicted in the red colored bars. The average METs was assigned to sessions in which intensity was reduced (e.g., 75% reduced to 65%, imputed as 70%), whereas missed sessions were assigned zero METs.
Four of 69 (6%) consented patients were deemed ineligible owing to electrocardiogram abnormalities (n=3, 4%) or not achieving acceptable CPET test criteria (n=1, 2%). Electrocardiogram abnormalities were observed in one of 63 patients (<2%) at postintervention. No serious adverse events were observed during aerobic training. A total of 24 of 33 (73%) patients receiving aerobic training experienced at least one non-serious adverse event during aerobic training; a total of 76 independent non-serious events in 744 (10%) attended sessions were observed. The most common events were abnormal heart rate, pain in extremity, and fatigue (Table 2). No aerobic training-associated adverse events led to permanent discontinuation. There were no differences in hematological profile between patients receiving aerobic training or attention control (Table S6). A total of seven non-serious adverse events were observed in the attention control group.
Table 2.
Adverse Events During Aerobic Training Sessions*
| Variable | No. of Patients (n = 33) |
% |
|---|---|---|
| Any adverse events | 24 | 73 |
| Serious adverse events | ||
| Hospitalizations / life-threatening | 0 | 0 |
| All other adverse events† | ||
| Abnormal heart rate response‡ | 10 | 30 |
| Pain in extremity | 9 | 27 |
| Fatigue | 8 | 24 |
| Back pain | 6 | 18 |
| Dizziness | 2 | 6 |
| Diarrhea | 2 | 6 |
| Abnormal blood pressure response‡ | 1 | 3 |
| Acute polyneuropathy | 1 | 3 |
| Anemia | 1 | 3 |
| Dyspnea | 1 | 3 |
| Metastatic bone pain | 1 | 3 |
| Nausea | 1 | 3 |
Adverse events were coded according to the Medical Dictionary for Regulatory Activities (MedDRA).
Numbers may not add up to 100% due to rounding
Non-MedDRA coding terms
For ITT analyses, VO2peak and PROs were generally maintained in both groups (p’s>0.05; Table S7). The only between group differences were changes in FACT-General and FACT-SWB (Social Well-Being), favoring the attention control group (p=0.03 and p=0.04, respectively). All functional capacity end points significantly improved in both groups, with no significant differences between group (p’s>0.05; Table S8). Per protocol analyses indicated that aerobic training was feasible (RDI ≥ 70%) in 14 of 33 (42%) patients. These patients had higher VO2peak and had received < 3 lines of prior treatment at study entry, compared to patients with a RDI <70% (Table S9). Aerobic training feasibility on the basis of RDI is presented in Table S10. A RDI ≥70% was associated with significant improvements in functional capacity and cardiorespiratory fitness end points but in general, not PROs (Table S10).
DISCUSSION
On the basis of protocol-defined criteria supervised aerobic training at the dose and schedule tested is safe but not feasible for a significant subset of pretreated patients receiving concurrent cytotoxic treatment for metastatic breast cancer. Few prior exercise studies have been designed with this prespecified objective of feasibility and safety but rather focus on symptom control “efficacy” end points or feasibility of patient recruitment. Further, evaluation of exercise “feasibility” is limited to monitoring and reporting of adherence, typically limited to rates of LTF and attendance.20 In this context, the findings of this study are consistent with the only other randomized trial of exercise in metastatic breast cancer. The METT study evaluated the tolerability and efficacy of a 16-week exercise program in 101 patients receiving any prior line and any therapy for metastatic breast cancer (42% were receiving concurrent chemotherapy at study entry).21 Participation in regular exercise was not a study exclusion criterion (patients allocated to the exercise and control groups were moderately active at study entry). The exercise program consisted of a predominantly home-based prescription with the objective of achieving 150 minutes of moderate-intensity exercise/week. Details of the exercise prescription characteristics with regards to frequency, duration, and modality as well as how exercise was tailored to each patient were not provided. Over the 16-week study period, intervention participants increased exercise exposure by a mean of 62.4 (±102.8) minutes per week. The overall LTF rate was 22%, with patients assigned to exercise more likely to be LTF. Attendance rate was not reported.21
The findings of the present study are also not too dissimilar from randomized trials of supervised exercise training in patients receiving adjuvant chemotherapy for early-stage breast cancer. For example, in the START study, the LTF rate was 8%, with attendance rates of 72% and 68% in the aerobic training and resistance training groups, respectively.22 In the PACES study, LTF was 11%, with attendance rates of 71% in patients receiving supervised aerobic and resistance exercise training.23 On the basis of the predefined tolerability thresholds applied in the present study, exercise training would be considered of acceptable feasibility in the START and PACES studies. However, whether such thresholds are appropriate or should be different in the adjuvant versus metastatic setting is not clear since standardized definitions of feasibility and safety with exercise training are not available for any clinical population.
Metrics adapted from pharmacological trials indicated that despite a LTF rate of only 1%, approximately one-third of patients permanently discontinued aerobic training; a rate comparable to that observed in the METT study,21 the only other study to report exercise discontinuation rate in the oncology setting. Similarly, the dose modification and early session termination rates in the present study were 33% and 36%, respectively, yet the attendance rate for these sessions would be reported as 100%. Thus, use of standard exercise metrics such as LTF and attendance rates provide limited insight and could lead to erroneous conclusions regarding the actual feasibility or tolerability of exercise treatment for a given indication. To this end, our findings indicate that further analysis of feasibility, and potentially efficacy, as a function of site in multi-site trials is also likely to be important. Although not feasible for a significant subset of patients, the lack of serious adverse events indicates that aerobic training has an acceptable safety profile. Nevertheless, several non-serious events were observed that triggered dose modification and, more importantly, early session termination, highlighting the importance of close monitoring and supervision of exercise training interventions, at least in select patients, together with formalized monitoring and reporting of tolerability and safety.
In both pharmacological and non-pharmacological trials, several different terms including feasibility, tolerability, and adherence are used to describe the implementation or completion of study treatment. In oncology drug trials, the most commonly used term is “tolerability”, which appears appropriate given that non-compliance with the planned treatment schedule is mostly due to treatment-related dose-limiting toxicities (DLTs). In contrast however, non-compliance in exercise trials may be due to both health-related as well as non heath-related (e.g., motivational) reasons. Thus, the term “feasibility” may be more appropriate for reporting exercise treatment trials since this is a more all-encompassing term capturing the many unique elements for characterizing exercise treatment implementation / completion in a given clinical population and setting. The use of terminology is arguably a matter of investigator preference; the most important recommendation for future trials is that regardless of which term(s) are selected, all are clearly defined and operationalized to facilitate data interpretation and cross-trial comparisons.
Contrary to our hypotheses, aerobic training, in general, was not associated with improvements in cardiorespiratory fitness or PROs for the ITT population – these findings however are not unexpected in context of the high permanent discontinuation rate and moderate attendance rate. The lack of exercise benefit on both objective as well as PROs observed in the present study is consistent with that reported in patients with metastatic (advanced) cancer.19, 21 Specifically, in this study, VO2peak and PROs were generally maintained in both the aerobic training and attention-control groups over the 12-week study period although attention control was associated with superior improvements in quality of life end points in comparison with aerobic training. A closer inspection of the data indicate that these differences do not reflect a detrimental effect of aerobic training but rather the beneficial impact of the attention control stretching intervention. All prior randomized studies investigating the feasibility and efficacy of exercise in patients with metastatic cancer, as well as the majority of studies in the adjuvant setting, have compared efficacy of exercise to a non-intervention control group18 – groups that do not receive the same level of attention or social interaction as those allocated to exercise groups. It is therefore unclear whether the observed significant benefit of exercise on PROs reflects the actual psychosocial benefit of exercise or the social interaction aspects related to participation in an exercise intervention. Our data suggests that non-exercise interventions that match the degree of social interaction typically experienced in exercise groups are equally if not more efficacious at improving certain PROs, particularly social aspects of quality of life; on the other hand, exercise appears to be a more efficacious intervention for physical / functional aspects of quality of life.
In per-protocol analyses, patients with higher baseline VO2peak and received <3 lines of prior therapy were able to tolerate aerobic training (a RDI ≥70%) and derived significant physiological benefit. The significant ~11% VO2peak decline in patients with “unacceptable” feasibility of aerobic training (<70% RDI) is comparable to that observed in the adjuvant setting,5, 22, 23 although ours is the first to report such a decline in patients with metastatic breast cancer. Again, similar to the adjuvant setting,5, 22, 23 if feasible, aerobic training completely abrogates this decline. This may be of clinical importance since VO2peak and functional capacity measures24 are significant independent predictors of survival in numerous clinical populations, including metastatic breast cancer.25 Moreover, given the incurable nature of metastatic disease, treatment-related morbidity and PROs are of major clinical relevance.26 Collectively, our findings establish the platform to initiate phase 1/2 dose-finding studies in metastatic breast cancer patients with good performance status receiving either 1st or 2nd line therapy to determine the efficacy of exercise to both mitigate treatment-related toxicities and improve PRO.
Strengths of this trial include the rigorous conduct adhering to the principles of exercise training,27 adherence to reporting standards from the CONSORT guidelines for non-pharmacological trials28 and TIDieR29 statements, the novel adoption of pharmacological metrics to rigorously evaluate exercise training tolerability and safety, dual-site design, gold standard measurement of efficacy end points, and use of an attention-control comparison group as opposed to a non-intervention or wait-list control group.18 Limitations include the heterogeneous study cohort, the short intervention period, lack of generalizability to patients unable to attend supervised sessions (e.g., those with poor performance status or experiencing significant DLTs), and lack of clinical outcome data.
In conclusion, on the basis of predefined criteria supervised aerobic training at the dose and schedule tested is safe, but not feasible, among pretreated patients with metastatic breast cancer receiving concurrent therapy. The acceptable tolerability and promising benefit of aerobic training in a significant subset of patients warrants further evaluation in a phase 1/2 study.
Supplementary Material
Condensed Abstract.
We investigated the feasibility and safety of 36 supervised treadmill walking sessions delivered thrice-weekly between 55% to 80% of peak oxygen consumption (VO2peak) for 12 consecutive weeks in 65 patients with metastatic breast cancer. Aerobic training at the dose and schedule tested is safe but not tolerated in a significant proportion of patients with metastatic breast cancer; the acceptable tolerability and promising benefit in select patients warrants further evaluation in a dose-finding phase 1/2 study.
Acknowledgments
This study was supported by a research grant from the National Cancer Institute (R21-CA143254) awarded to LWJ. LWJ, JMS, TSN, CAC, DMC, and MM are supported by AKTIV Against Cancer, Kavli Trust, and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). The authors would like to thank Whitney Underwood for administrative support. We thank the patients who participated in this study and their families.
Footnotes
Trial Registration: Clinicaltrials.gov Identifier: NCT01725633
Declaration of interests: The authors declare no conflicts of interests.
Author Contributions
JMS, TSN, MM, JS, AY, SC, CTD, EAC, MND, JMP, and LWJ contributed to conception and design of the work, data acquisition and interpretation. ST and JH conducted statistical analysis. All authors reviewed and revised the manuscript, approved the final version, and agreed on all aspects of the work.
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Juvet LK, Thune I, Elvsaas IKO, Fors EA, Lundgren S, Bertheussen G, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: A meta-analysis. Breast. 2017;33:166–177. doi: 10.1016/j.breast.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 3.Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol. 2012;9:288–296. doi: 10.1038/nrclinonc.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones LW. Precision Oncology Framework for Investigation of Exercise As Treatment for Cancer. J Clin Oncol. 2015;33:4134–4137. doi: 10.1200/JCO.2015.62.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014;53:65–74. doi: 10.3109/0284186X.2013.781673. [DOI] [PubMed] [Google Scholar]
- 6.Nilsen TS, J M, Jones LW. Novel methods for reporting exercise training prescription dose and adherence in cancer. An exploratory analysis Medicine Science Sports and Exercise. doi: 10.1249/MSS.0000000000001545. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 8.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 9.Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 10.Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70:113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- 11.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 12.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 13.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 14.Lai JS, Cella D, Chang CH, Bode RK, Heinemann AW. Item banking to improve, shorten and computerize self-reported fatigue: an illustration of steps to create a core item bank from the FACIT-Fatigue Scale. Qual Life Res. 2003;12:485–501. doi: 10.1023/a:1025014509626. [DOI] [PubMed] [Google Scholar]
- 15.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 17.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 18.Steins Bisschop CN, Courneya KS, Velthuis MJ, Monninkhof EM, Jones LW, Friedenreich C, et al. Control group design, contamination and drop-out in exercise oncology trials: a systematic review. PLoS One. 2015;10:e0120996. doi: 10.1371/journal.pone.0120996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Temel JS, Greer JA, Goldberg S, Vogel PD, Sullivan M, Pirl WF, et al. A structured exercise program for patients with advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:595–601. doi: 10.1097/JTO.0b013e31819d18e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilsen TSSJ, Michalski M, Capaci C, Thomas ST, Herndon JE, Sasso J, Eves NE, Jones LW. Novel Methods for Reporting Exercise Training Prescription Dose and Adherence in Cancer: An Exploratory Analysis. Brit J Can. 2017 Submitted. [Google Scholar]
- 21.Ligibel JA, Giobbie-Hurder A, Shockro L, Campbell N, Partridge AH, Tolaney SM, et al. Randomized trial of a physical activity intervention in women with metastatic breast cancer. Cancer. 2016;122:1169–1177. doi: 10.1002/cncr.29899. [DOI] [PubMed] [Google Scholar]
- 22.Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 23.van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J Clin Oncol. 2015;33:1918–1927. doi: 10.1200/JCO.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- 24.Ross R, Blair SN, Arena R, Church TS, Despres JP, Franklin BA, et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 25.Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30:2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardoso F, Bedard PL, Winer EP, Pagani O, Senkus-Konefka E, Fallowfield LJ, et al. International guidelines for management of metastatic breast cancer: combination vs sequential single-agent chemotherapy. J Natl Cancer Inst. 2009;101:1174–1181. doi: 10.1093/jnci/djp235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neil-Sztramko SE, Winters-Stone KM, Bland KA, Campbell KL. Updated systematic review of exercise studies in breast cancer survivors: attention to the principles of exercise training. Br J Sports Med. 2017 doi: 10.1136/bjsports-2017-098389. [DOI] [PubMed] [Google Scholar]
- 28.Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P, Group CN CONSORT Statement for Randomized Trials of Nonpharmacologic Treatments: A 2017 Update and a CONSORT Extension for Nonpharmacologic Trial Abstracts. Ann Intern Med. 2017;167:40–47. doi: 10.7326/M17-0046. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann TC, Oxman AD, Ioannidis JP, Moher D, Lasserson TJ, Tovey DI, et al. Enhancing the usability of systematic reviews by improving the consideration and description of interventions. BMJ. 2017;358:j2998. doi: 10.1136/bmj.j2998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


