SUMMARY
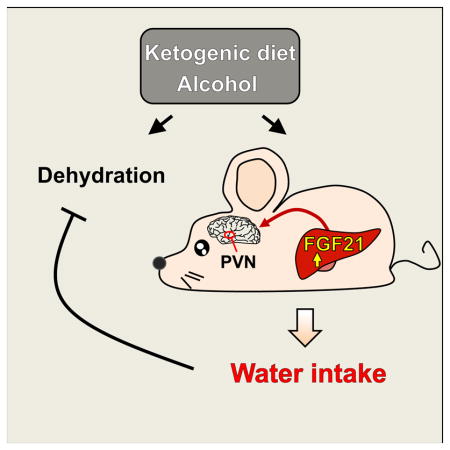
Alcohol and ketogenic diets increase water consumption. Here, we show that the hormone FGF21 is required for this drinking response in mice. Circulating levels of FGF21 are increased by alcohol consumption in humans, and by both alcohol and ketogenic diets in mice. Pharmacologic administration of FGF21 stimulates water drinking behavior in mice within 2 hours. Concordantly, mice lacking FGF21 fail to increase water intake in response to either alcohol or a ketogenic diet. The effect of FGF21 on drinking is mediated in part by SIM1-positive neurons of the hypothalamus and is inhibited by β-adrenergic receptor antagonists. Given that FGF21 also is known to suppress alcohol intake in favor of pure water, this work identifies FGF21 as a fundamental neurotropic hormone that governs water balance in response to specific nutrient stresses that can cause dehydration.
Keywords: FGF21, water drinking, β-klotho, β-adrenergic signaling, hypothalamus, neurons, alcohol, ketogenic diet
eTOC Blurb
XXX et al show that the metabolic hormone, FGF21, is involved in water drinking in response to metabolic stresses such as alcohol-intake and ketogenic diets, which can cause dehydration. The effect of FGF21 on water balance is mediated by a hypothalamic circuit that is dependent on beta-adrenergic signaling.
INTRODUCTION
The hormone FGF21 is synthesized and secreted into the blood from liver in response to a wide variety of nutritional stresses, including starvation and low protein diets, high fat/low carbohydrate ketogenic diets, simple sugars such as sucrose and fructose, and alcohol (Fisher and Maratos-Flier, 2016; Owen et al., 2015). Among its myriad downstream actions, FGF21 regulates carbohydrate and lipid metabolism and energy expenditure. Pharmacologically, FGF21 causes weight loss and improves insulin sensitivity and lipid parameters in rodent and primate models of obesity (Kharitonenkov and DiMarchi, 2017; Owen et al., 2015). In two recent clinical trials, long-acting FGF21 derivatives lowered body weight and insulin levels in obese subjects with type 2 diabetes (Gaich et al., 2013; Talukdar et al., 2016b). FGF21 acts through a cell-surface receptor composed of a conventional FGF receptor, FGFR1c, in complex with a single pass-transmembrane protein, β-Klotho (Kuro-o, 2012). In mice, FGFR1c and β-Klotho are co-expressed in only a few tissues, including white and brown adipose tissue and the hypothalamus and hindbrain (Bookout et al., 2013; Fon Tacer et al., 2010).
FGF21 crosses the blood-brain barrier (Hsuchou et al., 2007) and mediates its chronic pharmacologic effects on body weight and insulin sensitivity by acting on its receptor complex in neurons (Owen et al., 2014). This in turn activates sympathetic nervous system outflow to both brown and white adipose tissue depots (Douris et al., 2015; Owen et al., 2014), resulting in thermogenesis and weight loss. In humans, a long-acting FGF21 analog increased blood pressure and heart rate (Kim et al., 2017), which may be a consequence of FGF21 inducing sympathetic nerve activity.
In human GWAS studies, SNPs in and around the FGF21 and β-Klotho genes were linked to carbohydrate and alcohol consumption (Chu et al., 2013; Schumann et al., 2016; Tanaka et al., 2013). In two bottle preference experiments performed with mice, pharmacologic administration of FGF21 suppressed intake of both alcohol and sweetened water by acting on the nervous system (Schumann et al., 2016; Talukdar et al., 2016a; von Holstein-Rathlou et al., 2016). These results provided evidence for a feedback liver-brain endocrine pathway that limits alcohol and carbohydrate consumption. Interestingly, pharmacologic FGF21 administration also increased water consumption in mice (Camporez et al., 2013; Talukdar et al., 2016a). However, these studies did not address whether endogenous FGF21 regulates water intake and, if so, its underlying mechanism of action.
In this report, we show that FGF21 acts directly on the nervous system to markedly stimulate drinking in both pharmacologic and physiologic contexts, including in response to alcohol exposure and a ketogenic diet. These findings, together with the previous results from two bottle preference studies (Schumann et al., 2016; Talukdar et al., 2016a; von Holstein-Rathlou et al., 2016), show that FGF21 plays an important role in regulating water balance in response to specific nutritional stresses.
RESULTS
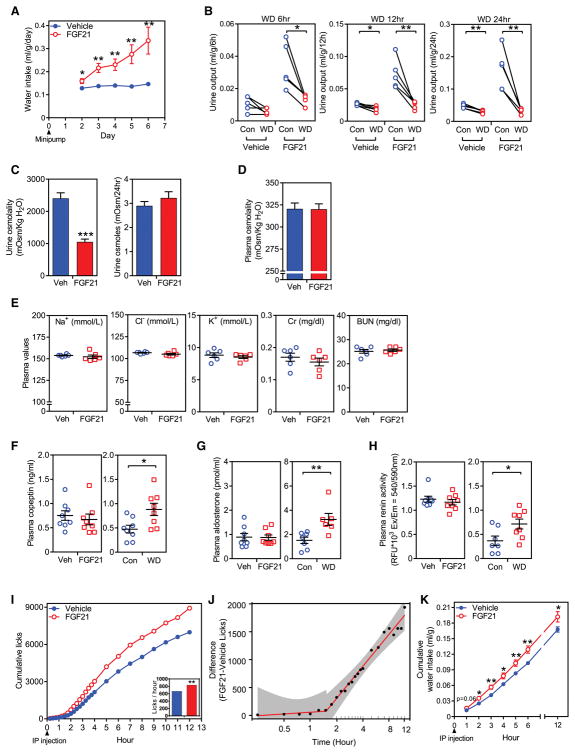
In agreement with previous reports (Camporez et al., 2013; Talukdar et al., 2016a), we found that FGF21 administration by osmotic minipump, which resulted in ~50 ng/ml FGF21 in plasma, increased water consumption approximately 3-fold in mice (Figure 1A) with no change in food intake (Figure S1A). FGF21 caused a corresponding increase in urine output (Figure 1B) and decrease in urine osmolality (Figure 1C, left panel), with no change in urine osmole excretion rate (Figure 1C, right panel) or plasma osmolality (Figure 1D). Plasma sodium, chloride, potassium, and creatinine concentrations, and blood urea nitrogen were also unchanged (Figure 1E). When subjected to water deprivation, vehicle and FGF21-treated mice decreased urine output to the same absolute level at 6, 12 and 24 hour time points (Figure 1B), demonstrating that FGF21-induced polydipsia is not secondary to polyuria. Accordingly, FGF21 administration did not change the plasma concentration of copeptin, a stable surrogate for the antidiuretic hormone, arginine vasopressin (AVP; Figure 1F, left panel), nor did it change plasma levels of aldosterone (Figure 1G, left panel) or renin activity (Figure 1H, left panel). In contrast, 24 hour water deprivation increased plasma copeptin and aldosterone concentrations and plasma renin activity as expected (Figures 1F–H, right panels). Thus, FGF21 stimulates drinking through a mechanism that does not appear to involve AVP or the renin-angiotensin-aldosterone pathways.
Figure 1. FGF21 induces water drinking.
(A) Daily water intake in C57BL/6J mice administered either vehicle or FGF21 (1 mg/kg/day) by osmotic minipump (n = 5/group). This study was repeated independently five times with similar results.
(B) Urine output over a 24 hour period in groups of mice administered FGF21 or vehicle by osmotic minipump with either ad libitum access to water (Control) or water deprivation (WD) (n = 5/group).
(C–E) Urine osmolality and urine osmoles (C), plasma osmolality (n = 7/group) (D), and electrolytes, creatinine (Cr) and blood urea nitrogen (BUN) (E) in C57BL/6J mice administered FGF21 or vehicle by osmotic minipump (n = 6/group).
(F–H) Plasma copeptin (F) and aldosterone (G) levels, and renin activity (H) in C57BL/6J mice administered either vehicle or FGF21 by osmotic minipump (n = 8/group). Data from groups of 24-hour water-deprived (WD) mice are included as positive controls. This study was repeated independently twice with similar results.
For (A–H), values are means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s t-test. (I, J) Licking behavior in C57BL/6J mice after a single i.p. injection of FGF21 (1 mg/kg) or vehicle (n = 18/group). Plotted results represent the mean of 6 independent experiments using 3 mice per group (shown in Fig. S1B). The mean number of cumulative licks in the FGF21 group (838.7 licks/hour) was significantly higher than in the control group (670.0 licks/hour) (p = 0.0026) (I). In (J), differences in cumulative licks between the FGF21 and vehicle group (dots) were fitted to a two-piece linear regression model (red line) of the data in (I). The break point for the statistical difference between FGF21 and vehicle treatment was detected at 1.52 hour with 95% confidence interval [0.82–2.81] shown in grey. (K) Cumulative water consumption in C57BL/6J mice after a single i.p. injection of FGF21 (1 mg/kg) or vehicle (n = 9/group).
See also Figure S1.
To determine the kinetics with which FGF21 induces drinking, we performed experiments with a gustometer, which detects licks on a drinking tube. A significant increase in mouse licking behavior was seen 1.5 hours after a single i.p. injection of FGF21 compared to vehicle, and this increased licking continued to rise for the entire 12 h recording period of the experiment (Figures 1I, 1J and S1B). A corresponding increase in cumulative water intake was detected within 2 hours after FGF21 administration (Figure 1K). Metabolic cage experiments performed in parallel showed that the FGF21-induced increase in water intake was not secondary to changes in energy expenditure (Figure S1C).
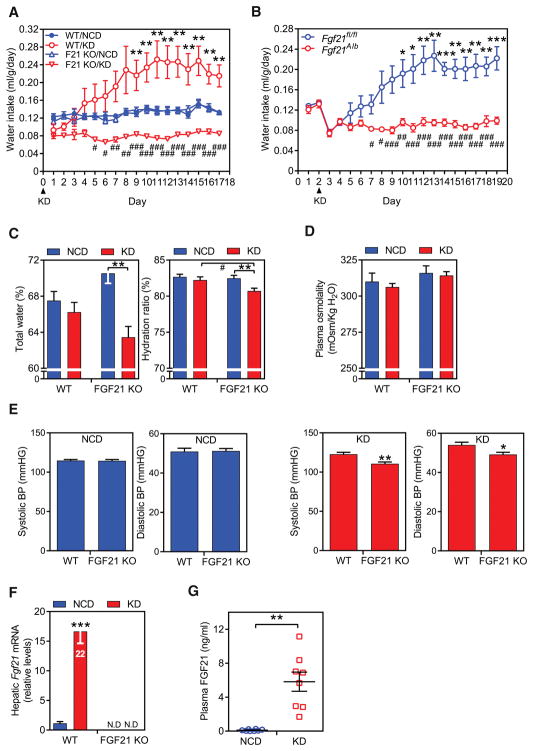
To examine whether endogenous FGF21 induces drinking, we compared water consumption in groups of wild-type (WT) and whole-body FGF21-knockout (KO) mice fed either normal chow or a ketogenic diet, which strongly induces FGF21 levels (Badman et al., 2007). There was no difference in water intake between WT and FGF21-KO mice on the chow diet (Figure 2A). WT mice fed the ketogenic diet gradually increased water consumption until it plateaued approximately 2.5-fold higher after 8 days (Figure 2A). In marked contrast, FGF21-KO mice on the ketogenic diet significantly decreased their water intake (Figure 2A). While there was a trend toward increased food consumption in mice fed the ketogenic diet, there was no difference in food intake between the WT and FGF21-KO mice on either diet (Figure S2A). Mice selectively lacking FGF21 in hepatocytes showed this same decrease in water intake in response to the ketogenic diet as in mice with global FGF21 deletion (Figure 2B), demonstrating that FGF21 derived from liver is responsible for increased drinking. While the basis for decreased water consumption in KO mice fed ketogenic diet is not entirely clear, approximately 30% of it can be accounted for by increased water content in the ketogenic diet compared to normal chow diet. Consistent with their decreased water intake, whole-body FGF21-KO mice on the ketogenic diet had a decreased total body water level and hydration ratio compared to WT mice (Figure 2C) but no detectable change in plasma osmolality (Figure 2D), suggesting changes in total body water in proportion to solutes. We also measured blood pressure, since it can be affected by dehydration. While there was no difference between WT and FGF21-KO on the normal chow diet, FGF21-KO mice on the ketogenic diet mice had lower systolic and diastolic pressures (Figure 2E). As expected, FGF21 mRNA in liver and protein concentrations in blood were increased by the ketogenic diet (Figures 2F and 2G). Thus, endogenous FGF21 released from liver stimulates drinking.
Figure 2. Liver-derived FGF21 is required for ketogenic diet-induced drinking.
(A) Daily water intake (ml/g·BW) in wild type (WT) and FGF21-knockout (F21 KO) mice fed either a normal chow diet (NCD) or ketogenic diet (KD) (n = 8/group). The study was repeated independently three times with similar results. Values are means ± SEM. **p < 0.01 for WT NCD vs. WT KD groups; #p < 0.05, ##p < 0.01, ###p < 0.001 for WT + KD vs. KO + KD by two-way ANOVA with Tukey's post hoc test.
(B) Daily water intake in groups of WT and hepatocyte-specific FGF21-KO (Fgf21Alb) mice fed either a NCD (days 1 and 2) or a KD (days 3–19) (n = 6/group). Values are means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 for Fgf21fl/fl NCD (on days 1 and 2) vs. Fgf21fl/fl KD groups; #p < 0.05, ##p < 0.01, ###p < 0.001 for Fgf21fl/fl vs. Fgf21alb by two-way ANOVA with Tukey's post hoc test.
(C) Total body water (left panel) and hydration ratios (right panel) were measured using an Echo MRI in WT and FGF21-KO mice after 3 weeks on either a NCD or KD (n = 7–8/group). Values are means ± SEM. **p < 0.01, for NCD vs. KD groups with the same genotype; #p < 0.05, for WT + KD vs. KO + KD by two-way ANOVA with Tukey's post hoc test.
(D) Plasma osmolality in wild type (WT) and FGF21-knockout (KO) mice fed either a normal chow diet (NCD, n = 6/group) or ketogenic diet (KD, n = 7/group). Values are means ± SEM.
(E) Systolic and diastolic blood pressure measurements in groups of WT and FGF21-KO mice fed either NCD or KD (n = 8/group). Measurements were done using a tail-cuff device over a 7 day period. Values are means ± SEM. *p < 0.05, **p < 0.01 for WT vs. FGF21-KO groups by Student’s t-test.
(F, G) Hepatic Fgf21 mRNA (F) and plasma FGF21 concentrations (G) in WT and FGF21-KO mice fed a NCD or KD. In (F), the Fgf21 QPCR Ct value for the WT/KD group is shown. Values are means ± SEM. **p < 0.01, ***p < 0.001 for NCD vs. KD groups by Student’s t-test. N.D., not detected.
See also Figure S2.
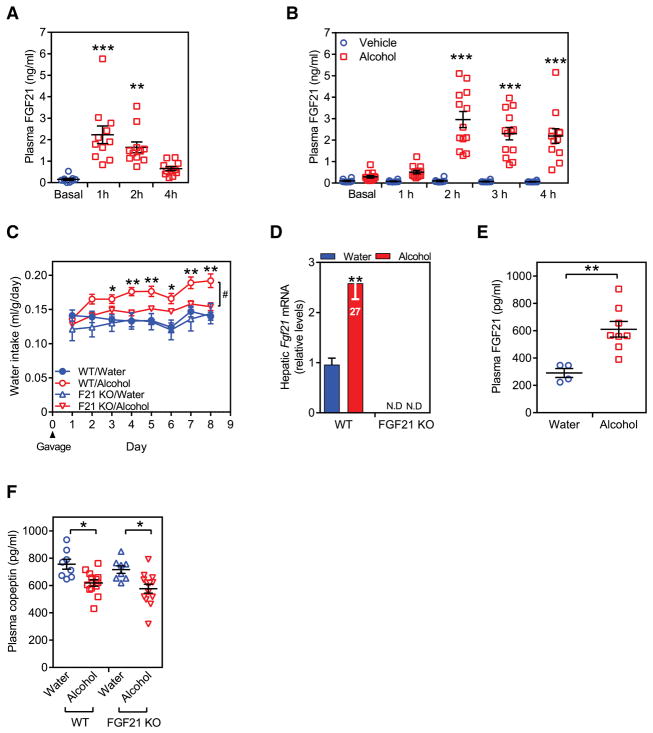
Chronic alcohol consumption also induces circulating FGF21 concentrations in mice (Zhao et al., 2015). To determine if FGF21 is induced by acute alcohol exposure, mice were administered a single dose of ethanol (31.5% v/v, 3.5 g/kg) by oral gavage and plasma FGF21 concentrations were measured at 1, 2 and 4 hours. Plasma FGF21 concentrations were significantly elevated at 1 hour post treatment and declined after that (Figure 3A). To determine whether acute alcohol exposure likewise induces FGF21 in humans, healthy volunteers were administered either alcohol (40.0% v/v, 2 ml/kg; n = 13) or the juice vehicle alone (n = 8) over a 30 minute period and serum FGF21 concentrations measured hourly after the first drink. Alcohol—but not the juice vehicle—increased circulating FGF21 concentrations at the 2–4 hour time points to levels comparable to those achieved by ketogenic diet in mice (Figure 3B). These findings are consistent with a recent independent report in mice and humans (Desai et al., 2017). Thus, acute alcohol exposure strongly induces FGF21 levels in both mice and humans.
Figure 3. FGF21 is required for alcohol-induced drinking.
(A) Plasma FGF21 levels in C57BL/6 mice given a single bolus of water or alcohol (3.5 g/kg) by oral gavage. Values are means ± SEM. **p < 0.01, ***p < 0.001 for basal (n = 9) vs. alcohol gavage groups (n = 11) at each time point by one-way ANOVA with Tukey's post hoc test.
(B) Plasma FGF21 levels in healthy volunteers who drank either alcohol in juice (2 ml/kg; n = 13) or the juice vehicle alone (n = 8) over a 30-minute period. FGF21 concentrations were measured in plasma taken at the indicated times after the first drink. Values are means ± SEM. ***p < 0.001 for vehicle vs. alcohol groups at each time point by two-way ANOVA with Tukey's post hoc test.
(C) Daily water intake (ml/g·BW) in wild type (WT) and FGF21-knockout (KO) mice administered water or alcohol (3.5 g/kg/day) by oral gavage (n = 4 for water and 7–8 for alcohol groups). Values are means ± SEM. *p < 0.05, **p < 0.01 for WT + water vs. WT + alcohol groups; #p < 0.05 for WT + alcohol vs. KO + alcohol groups by two-way ANOVA with Tukey's post hoc test. This study was repeated independently three times with similar results.
(D, E) Hepatic Fgf21 mRNA (D), and plasma FGF21 levels (E) after 8 days of treatment. ND, not detected. Values are means ± SEM. **p < 0.01 by Student’s t-test. In (D), the Fgf21 QPCR Ct value for the WT/alcohol group is shown.
(F) Plasma copeptin levels in WT and FGF21-KO mice after 8 days of treatment. Values are means ± SEM. *p < 0.05 for water vs. alcohol groups by two-way ANOVA with Tukey's post hoc test.
See also Figure S2.
We next examined whether endogenous FGF21 contributes to alcohol-induced increases in water consumption. Groups of WT and FGF21-KO mice were administered either alcohol (3.5 g/kg/day) or an equal volume of water by oral gavage once daily for 8 days with free access to water. From Day 3, alcohol administration significantly increased water intake in WT mice (Figure 3C). Importantly, this effect was absent in FGF21-KO mice (Figure 3C). As expected, alcohol administration increased hepatic Fgf21 mRNA and plasma FGF21 levels in WT mice (Figures 3D and 3E). Moreover, alcohol significantly decreased plasma copeptin concentrations in WT mice (Figure 3F), which is consistent with the well-established inhibitory effect of alcohol on AVP release from the pituitary gland (Cicero, 1981). Alcohol also decreased circulating copeptin levels in FGF21-KO mice (Figure 3F). Taken together, these data show that alcohol stimulates drinking through an FGF21-dependent mechanism that appears to be independent of AVP levels.
We also examined whether FGF21 is regulated by high salt intake or water deprivation. Mice fed a high salt diet had a striking increase in water consumption (Figure S2B) without changes in FGF21 mRNA levels in liver (Figure S2C) or protein levels in blood (Figure S2D). Thus, FGF21 does not contribute to salt-induced drinking. FGF21 mRNA (Figure S2E) and protein levels (Figure S2F) were also unchanged by 24 hour water deprivation, indicating that FGF21 is not induced by dehydration per se, at least in this time frame.
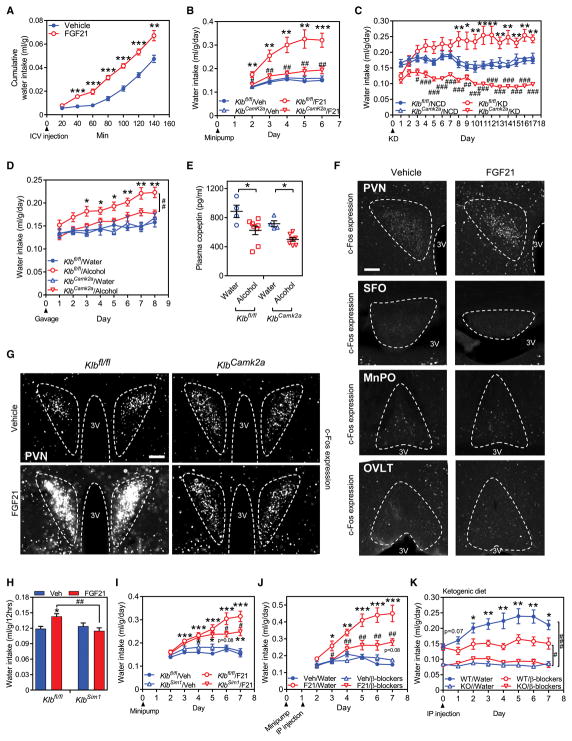
FGF21 mediates many of its effects on metabolism and behavior by acting directly on its receptor in the nervous system (Owen et al., 2015). In time course studies, direct i.c.v. injection of FGF21 into the brain increased water intake within 40 minutes (Figure 4A), which was faster than i.p. injection (Figure 1K), providing evidence that FGF21 can act centrally. To test whether peripheral FGF21 also stimulates drinking by acting on the nervous system, we used mice in which the β-Klotho gene (Klb), which encodes the obligate co-receptor for FGF21 (Kuro-o, 2012), was selectively eliminated in neurons (KlbCamk2a mice) (Bookout et al., 2013). In pharmacologic studies, administration of recombinant FGF21 by osmotic minipump increased water consumption in control Klbfl/fl but not KlbCamk2a mice (Figure 4B). There was no difference in food intake between the Klbfl/fl and KlbCamk2a mice (Figure S3A). Notably, the effect of both ketogenic diet (Figure 4C) and alcohol (Figures 4D) on water intake was also eliminated in the KlbCamk2a mice. Alcohol exposure decreased plasma copeptin concentrations in both Klbfl/fl and KlbCamk2a mice (Figure 4E), providing additional evidence that the effect of FGF21 on drinking is independent of AVP. Thus, endogenous FGF21 stimulates water consumption by acting directly on the nervous system.
Figure 4. FGF21 acts on neurons to induce drinking.
(A) Cumulative water consumption in C57BL/6J mice after a single i.c.v. injection of FGF21 (1 μg) or vehicle (n = 8/group). Values are means ± SEM. **p < 0.01, ***p < 0.001 for vehicle vs. FGF21.
(B) Daily water intake (ml/g·BW) measured in control mice (Klbfl/fl) and neuron-specific β-Klotho-knockout mice (KlbCamk2a) administered either vehicle (n = 4/group) or FGF21 (1 mg/kg/day, n = 5/group) by osmotic minipump. Values are means ± SEM. **p < 0.01, ***p < 0.001 for Klbfl/fl + vehicle vs. Klbfl/fl + FGF21 groups; #p < 0.05, ##p < 0.01 for Klbfl/fl + FGF21 vs. KlbCamk2a + FGF21 groups by two-way ANOVA with Tukey's post hoc test. This study was repeated independently three times with similar results.
(C) Daily water intake (ml/g·BW) in Klbfl/fl and KlbCamk2a mice fed a normal chow diet (NCD, n = 5/group) or ketogenic diet (KD, n = 7/group). Values are means ± SEM. *p < 0.05, **p < 0.01 for NCD vs. KD groups for Klbfl/fl group; #p < 0.05, ##p < 0.01, ###p < 0.001 for Klbfl/fl + KD vs. KlbCamk2a + KD by two-way ANOVA with Tukey's post hoc test. This study was repeated independently three times with similar results.
(D) Daily water intake (ml/g·BW) in Klbfl/fl and KlbCamk2a mice administered either water or alcohol (3.5 g/kg/day) by gavage (n = 4 for water and 8 for alcohol group). Values are means ± SEM. *p < 0.05, **p < 0.01 for Klbfl/fl + water vs. Klbfl/fl + alcohol gavage groups, ##p < 0.01 for Klbfl/fl + alcohol vs. KlbCamk2a + alcohol treated group by two-way ANOVA with Tukey's post hoc test.
(E) Plasma copeptin levels in Klbfl/fl and KlbCamk2a mice after 8 days of water or alcohol administration. Values are means ± SEM. *p < 0.05 for water vs. alcohol groups by two-way ANOVA with Tukey's post hoc test.
(F, G) c-Fos immunofluorescence staining in the indicated brain regions of C57BL/6J mice (F) or Klbfl/fl and KlbCamk2a mice (G) 2 hours after vehicle or FGF21 (1 mg/kg) administration by i.p. injection. c-Fos fluorescence is white. 3V, third ventricle. Scale bar = 50 μm in (F) and 100 μm in (G).
(H) Cumulative water consumption in Klbfl/fl and KlbSim1 mice after a single i.p. injection of FGF21 (1 mg/kg, n = 7/group) or vehicle (n = 5/group).
(I) Daily water intake (ml/g·BW) measured in Klbfl/fl and KlbSim1 mice administered either vehicle or FGF21 (1 mg/kg/day) by osmotic minipump (n=10 ~ 12/group). Values are means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 for vehicle vs FGF21 groups, #p < 0.05 for Klbfl/fl + FGF21 vs. KlbSim1 + FGF21 groups by two-way ANOVA with Tukey's post hoc test.
(J) Daily water intake (ml/g·BW) in groups of mice administered FGF21 or vehicle by osmotic minipump and either β-blockers or water vehicle by i.p. injection (n = 5 for water groups and 6 for β-blocker groups). Values are means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 for vehicle vs. FGF21-treated groups; #p < 0.05, ##p < 0.01 for FGF21 + water vs. FGF21 + β blockers by two-way ANOVA with Tukey's post hoc test.
(K) Daily water intake (ml/g·BW) in KD-fed mice administered water or β-blockers by i.p. injection (n = 6/group for water and β-blocker treatment). Injection was started after 7 days on the KD. *p < 0.05, **p < 0.01 for water vs. β-blocker-treated groups with the same genotype; #p < 0.05, ###p < 0.001 for indicated groups by two-way ANOVA with Tukey's post hoc test.
See also Figure S3.
Within the brain, the drinking response is controlled by a number of different nuclei including the subfornical organ (SFO), the organum vasculosum of the lamina terminalis (OVLT) and the median preoptic nucleus (MnPO), which together comprise the lamina terminalis in the forebrain; the area postrema (AP) and nucleus tractus solitarii (NTS), which are components of the dorsal-vagal complex in the hindbrain (McKinley and Johnson, 2004; Zimmerman et al., 2017); and the paraventricular nucleus (PVN), which mediates increased water consumption in response to direct injection by norepinephrine (Leibowitz, 1978). We previously showed that β-Klotho is expressed in the AP and NTS (Bookout et al., 2013). Using KlbPhox2b mice, in which β-Klotho expression is disrupted in the dorsal-vagal complex (Bookout et al., 2013), we tested whether these hindbrain nuclei are involved in the FGF21-induced drinking response. In osmotic minipump experiments, FGF21 stimulated water intake equally well in control Klbfl/fl and KlbPhox2b mice (Figure S3B). Thus, FGF21 does not appear to act through the dorsal-vagal complex to induce drinking.
To assess whether FGF21 stimulates drinking by acting on the PVN, SFO, OVLT or MnPO, we first examined β-Klotho mRNA expression by in situ hybridization using brain sections from control Klbfl/fl and KlbCamk2a mice. In the PVN, where FGF21 is known to have other effects on metabolism and behavior (Douris et al., 2015; Liang et al., 2014; von Holstein-Rathlou et al., 2016), β-Klotho mRNA was detected in Klbfl/fl mice, and the levels were significantly lower in KlbCamk2a PVN (Figure S3C). Likewise, β-Klotho expression was detected in SFO from Klbfl/fl mice, with a trend towards decreased expression in KlbCamk2a SFO (Figure S3C). β-Klotho mRNA was not detected in OVLT or MnPO (Figure S3C). We next examined whether FGF21 could signal in these brain regions using c-Fos induction studies to measure neuron activation. Whereas i.p. FGF21 injection caused a robust increase in c-Fos levels in the PVN that was β-Klotho dependent (Figures 4F, G and S3D), FGF21 injection had no effect on c-Fos expression in the SFO, OVLT or MnPO (Figure 4F). These data suggest that FGF21 is acting on the PVN, but not on the lamina terminalis to regulate drinking.
To further assess the contribution of the PVN to FGF21’s effect on drinking, we crossed Klbfl/fl mice with Sim1-Cre mice, in which Cre expression is enriched in the PVN (Balthasar et al., 2005). β-Klotho mRNA was reduced by ~40 % in the PVN of KlbSim1 mice, based on in situ hybridization analysis (Figure S3E). The remaining β-Klotho expression may reflect either incomplete knockout of β-Klotho or its expression in cells that are not Sim1+. Notably, the effect of FGF21 on water intake during the first 12 hours after injection was lost in these KlbSim1 knockout mice (Figure 4H). In longer-term osmotic minipump experiments, the effect of FGF21 on water intake was significantly blunted but not absent in KlbSim1 mice (Figure 4I). This remaining FGF21 activity could be due to either incomplete knockout of β-Klotho in the PVN or effects of FGF21 on other brain regions. We conclude that FGF21 mediates its effect on drinking in part by acting on Sim1+ neurons most likely in the PVN.
To examine whether FGF21 stimulates drinking through the β-adrenergic signaling pathway suggested by previous norepinephrine/PVN injection studies (Leibowitz, 1978), we combined propranolol, a β-1 and β-2 adrenergic receptor antagonist, with SR59230A, a β-3 adrenergic receptor antagonist, to inhibit all three β-adrenoreceptor subtypes. Injection of these β-blockers inhibited the dipsogenic activity of FGF21 delivered by osmotic minipump without having any effect on water intake in vehicle-treated mice (Figure 4J). Similarly, β-blocker administration inhibited the increase in water drinking caused by ketogenic diet in WT mice without affecting water intake in FGF21-KO mice (Figure 4K). Thus, the effect of FGF21 on drinking involves signaling through the β-adrenergic receptor pathway.
DISCUSSION
Drinking is induced by various stimuli including hypertonicity acting on osmoreceptors in the hypothalamus, and hypovolemia and hypotension acting via baroreceptors in the heart, kidney and vasculature (McKinley and Johnson, 2004). Drinking can also be stimulated by the consumption of food. Here, we describe a distinct pathway mediated by the endocrine hormone FGF21 that stimulates water consumption in response to alcohol or a ketogenic diet. It is not induced by 24 hour water deprivation or a high salt diet nor is it secondary to increased food intake. Since this pathway is activated by nutrients that have the potential to disrupt water balance, it likely serves as a pre-emptive mechanism for maintaining hydration.
Our findings are particularly noteworthy with respect to alcohol, which is generally believed to mediate much of its effect on water balance and drinking by reducing circulating concentrations of AVP (Cicero, 1981). While this is likely true during acute alcohol exposure, we show here that most of the effect of chronic alcohol exposure on drinking is lost in both FGF21-KO and the neuron-specific β-Klotho-KO mice. Moreover, in agreement with a recent study (Desai et al., 2017), we show that FGF21 is strongly induced in humans by acute exposure to alcohol, suggesting a similar pathway exists in humans. Notably, FGF21 not only causes drinking but also reduces alcohol and sweet preference (Schumann et al., 2016; Talukdar et al., 2016a; von Holstein-Rathlou et al., 2016). Thus, FGF21 stimulates both water consumption and a behavioral drive towards drinking pure water, both of which can negate the anticipated water loss from suppression of AVP by alcohol. These data suggest a broad role for FGF21 in regulating water balance in response to specific nutrients that can cause dehydration.
We show that FGF21’s effect on drinking is accelerated by direct injection into the brain and, conversely, eliminated by removing β-Klotho from neurons. Thus, FGF21 induces drinking by acting directly on the nervous system. A unique aspect of FGF21’s action is that there is a relatively long delay (>30 min) between the time of FGF21 administration and the initiation of licking/drinking, suggesting that changes in gene expression or other longer-term signaling processes are required for FGF21 to elicit its effect on drinking. We further show that FGF21’s dipsogenic effect is partially blunted by either selectively disrupting β-Klotho expression in Sim1+ neurons, which are highly enriched in the PVN, or by systemic administration of β-blockers. Previous studies showed that central injection of β-adrenergic receptor agonists or norepinephrine increased water intake in rats (Lehr et al., 1967; Leibowitz, 1971, 1975) whereas β-adrenergic receptor antagonists inhibited drinking (Leibowitz, 1971). A follow-up study showed that injection of norepinephrine directly into the PVN stimulated drinking (Leibowitz, 1978). Interestingly, a more recent study showed that disrupting β-Klotho expression in the PVN impaired FGF21-mediated suppression of sucrose intake in two-bottle preference tests (von Holstein-Rathlou et al., 2016). Thus, the PVN appears to be an important target for FGF21’s effects on both water intake and preference, although we cannot rule out that other brain regions are involved. Additional studies will be required to determine whether FGF21 impacts water consumption and preference through a common neural pathway.
In summary, we show that FGF21 is a potent dipsogenic hormone, thereby defining a new physiologic pathway that regulates drinking. Since ketogenic diets, alcohol and simple sugars in excess can all cause dehydration, this provides a unifying basis for the induction of FGF21 in liver by these diverse stimuli. In addition to its beneficial pharmacologic effects on body weight, insulin sensitivity and lipid levels, endogenous FGF21 protects mice against steatosis and liver injury caused by alcohol or ketogenic diet (Badman et al., 2009; Desai et al.; Liu et al., 2016). Since hydration can affect thermogenesis, body weight and other metabolic parameters (Boschmann et al., 2007; Boschmann et al., 2003; Gerich et al., 1973; Thornton, 2016), we speculate that some of these salutary actions may be secondary to FGF21’s effects on water balance.
Limitations of the Study
As noted above, our studies implicate Sim1+ neurons in the PVN as important targets of FGF21’s effects on water drinking. However, other neuronal populations in the PVN or other brain regions are likely to be required. We also do not yet know if these neuronal sites overlap with FGF21’s effects on sweet and alcohol preference. Future work is being directed toward dissecting the neuronal pathways involved in FGF21’s diverse effects on thirst, behavior, and metabolism. In addition, it is not yet clear whether FGF21 stimulates water drinking in humans, a question that will also be addressed by future studies.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contacts, Steven Kliewer (steven.kliewer@utsouthwestern.edu) and David Mangelsdorf (davo.mango@utsouthwestern.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mouse studies
All procedures and use of mice were approved by the Institutional Animal Care and Use Committees of University of Texas Southwestern Medical Center or Columbia University.
FGF21-KO (Potthoff et al., 2009) and KlbCamk2a mice (Bookout et al., 2013) were generated as described. Hepatocyte-specific FGF21-KO mice were generated by crossing Fgf21fl/fl mice (Potthoff et al., 2009) with albumin (Alb)-Cre mice (Jackson Laboratory, Stock #003574) to generate Fgf21Alb mice. FGF21-KO mice were on a C57BL/6J background. All other transgenic mouse lines were on mixed C57BL/6J;129/Sv background.
C57BL/6J mice were generated by the animal breeding core at University of Texas Southwestern Medical Center. Mice with cannulae in the third ventricle were purchased from Jackson Laboratory (C57BL/6, USA stock# 000664). For i.c.v injections, an injector attached to PE-50 tubing was inserted into the cannula, and vehicle or FGF21 was delivered in a 2 μl volume over 2 minutes.
Mice were maintained on a 12 hour light-dark cycle with lights on at 7 am. Housing rooms were maintained at 22–23°C. Mice were maintained on a rodent chow diet (Harlan Teklad, TD.2916), and fed a ketogenic diet (BioServ F3666) or 8% high salt diet (Harlan Teklad, TD.92012) as indicated. Male littermates were used for all experiments, except for the control group in the hepatocyte-specific FGF21-KO experiment, in which three of the control Fgf21fl/fl mice were from a separate litter born on the same day. Mice were randomly assigned to experimental groups.
Human studies
The alcohol drinking study was approved by the Institutional Review board at the Medical University of Graz (28–255 ex 15/16). Informed consent was obtained before the study in accordance with the declaration of Helsinki and with local laws and the study was performed according to good clinicap practice principles. The study was registered at clinicaltrials.gov (NCT02568904). Healthy volunteers received either alcohol in juice (n=13, including 10 men and 3 women, ages 21–38) or juice alone (n=8, including 2 men and 6 women, ages 21–42). Volunteers were randomly assigned to experimental groups.
METHOD DETAILS
Mouse Studies
Recombinant human FGF21 protein was provided by Novo Nordisk and administered by subcutaneous osmotic mini-pumps (Alzet) at a dose of 1 mg/kg/day. Mice were single caged after minipump surgery, which was conducted under isoflurane anesthesia followed by 24 hours of buprenorphine analgesia. Water intake was measured at the same time every day (9:00 a.m., 2 hours after lights on) and normalized to body weight for each mouse. Body composition was measured using an EchoMRI-100 Body Composition Analyzer. The hydration ratio was calculated using the formula [(total water-free water)/lean mass]. For alcohol experiments, mice were administered 3.5 g/kg/day of 31.5 % (v/v, tap water) ethanol (200 proof ethyl alcohol, Pharmco-Aaper) or water as a control by oral gavage using 20-gauge (1.5 inches) sterile gavage needles. For β-blocker experiments, propranolol (10 mg/kg/day, TOCRIS Bioscience-0624) and SR59230A (2.5 mg/kg/day, TOCRIS Bioscience-1511) were administered by i.p. injection daily at 6 pm.
Immunofluorescence
For c-Fos analyses, mice were treated with 1 mg/kg FGF21 or vehicle for 2 hours. The mice were anesthetized with isofluorane and transcardially perfused first with phosphate buffered saline (PBS) followed by 10% neutral buffered formalin (NBF). The brains were then harvested from the skull and incubated in 10% NBF overnight at 4°C. Next, the brains were embedded in 4% low-melting agarose and sectioned in a Leica VT1000 S vibratome at a section thickness of 50 μm. The brain sections were washed 3 times in PBS, 5 minutes/wash, and then incubated in blocking buffer (5% normal goat serum, 1% BSA, 0.4% Triton X-100 in PBS) for 1 hour. All primary and secondary antibodies were diluted in blocking buffer and incubations were done in an orbital shaker at 200 rpm. The sections were incubated overnight at 4°C in primary antibody against c-Fos (Santa Cruz SC-52; 1:1000). The next day the sections were washed 3 times in PBS, 5 minutes/wash, and then incubated in goat-anti rabbit Alexa Fluor 647 secondary antibody (Thermo Fisher; 1:500) and DAPI (Thermo, Fisher; 1:3000) for 1 hour at room temperature. Sections were washed 3 times in PBS, 5 minutes/wash, mounted onto Superfrost Plus slides (Fisher) using Aqua-Poly/Mount medium (Polysciences, Inc.). The slides were imaged using a Zeiss Axioscan Z1 with a 20X objective. All image analyses were done using ImageJ software. For RNAscope, brains were dissected from control, KlbCamk2a and KlbSim1 mice and fixed for 24 hours at 4°C with 10% neutral buffered formalin. The brains were then submitted to the UT Southwestern Pathology core where they were embedded in paraffin and sectioned coronally at a thickness of 5 μm. In situ hybridization was performed using the RNAscope 2.5 HD detection kits (Advanced Cell Diagnostics). The Klb probe (#415221) was purchased from Advanced Cell Diagnostics. Hybridized sections were counterstained with hematoxylin, dehydrated, cleared, and mounted with Ecomount (Biocare Medical). Images were taken using a Zeiss Axioscan Z1 with a 20X objective.
Licking Behavior
Immediate licking responses were assayed using a custom-made gustometer as described previously (Chandrashekar et al., 2010). Seven-week-old mice were housed in behavioral cages in a temperature and humidity-controlled vivarium on a 12:12 hour light-dark cycle. Water bottles were removed from the cages of mice approximately 36 hour prior to lickometer training. Water-restricted mice were subsequently given 30 minute training sessions with ab libitum access to water for 3 days before testing. After training, mice administered either FGF21 (1 mg/kg, i.p. injection) or vehicle (3 mice/group) were placed inside the behavioral box at 7:30 PM and licking was measured every 15 minutes for 4 hours and then every hour for a total of 12 hours. The results from 6 independent experiments were used in the analysis (n=18/group).
Energy expenditure measurements
Indirect calorimetry using LabMaster metabolic cages (TSE Systems) was used to determine energy expenditure per gram of body weight. Energy expenditure was measured and calculated by UT Southwestern phenotyping core as a function of O2 consumption and CO2 production according to the following formula: EE (kcal/h) = (3.941 x vO2 (ml/h) + 1.106 x vCO2 (ml/h))/1,000.
Blood Pressure Measurements
Blood pressure was measured by the tail-cuff method using a Visitech Systems BP-2000 Series II Blood Pressure Analysis System. Mice were acclimated for at least four days prior to initiating measurements by placement in the restrainer on the warmed platform (37°C). For measurements, 15–20 consecutive readings were averaged after blood pressure values stabilized.
Urine Analysis
Mice were acclimated to metabolic cages (1 mouse/cage, Hatteras Instruments) for 3 days. For urine output and water intake measurements, data were collected for an additional 4 days. For water deprivation experiments, water was removed for 24 hours starting on day 7. Control mice had free access to water. Urine osmolality was measured using the freezing point depression method (Osmometer 3D3, Advanced Instruments) and total osmoles were calculated by multiplying urine osmolality by 24 hour urine volumes.
Blood Analysis
Mice were killed by decapitation and plasma was collected using EDTA or heparin (Sarstedt) after centrifugation. Plasma electrolytes were measured by UT Southwestern’s Metabolic Phenotyping Core using a Vitros 250 chemistry analyzer. Plasma FGF21 and copeptin were measured by ELISA (Biovendor for FGF21, NeoScientific for copeptin) according to the manufacturers' instructions. Plasma renin activity was measured by fluorescence resonance energy transfer using a peptide containing renin cleavage sequence and a fluorescence quencher (Sigma). Fluorescence signal induced by proteolytic cleavage was measured using a Multilabel Plate Reader (Perkin Elmer Victor 3V). Plasma aldosterone levels were measured by ultra-high performance liquid chromatography (Nexera X2: Shimadzu) coupled to a triple quadrupole mass spectrometer (Qtrap 6500: Sciex) (Van Der Gugten et al., 2012). Briefly, a 100 μl aliquot of plasma containing 10 nM of deuterated aldosterone internal standard (Sigma-Aldrich) was extracted with 500 μl of methyl tert-butyl ether and the organic phase dried under nitrogen. Samples were reconstituted with water/methanol (200 μl 50:50, v:v) and chromatography performed on a Poroshell 120, SB-C18, 2.1 x 150mm, 2.7μm (Agilent) column using water + 2 mM ammonium acetate as mobile phase A and methanol + 2mM ammonium acetate as mobile phase B, delivered in a gradient mode. Eluate from the analytical column was directed into the source of the mass spectrometer using electrospray ionization (ESI) in negative ionization mode. Two MRM transitions were used to measure aldosterone (359.2 → 189.0, quantifier; 359.2 → 331.1, qualifier) and one MRM transition used to quantify the internal standard (367.1 → 194). MS data was analyzed and quantification performed using Analyst software (Sciex).
QPCR Analysis
Total RNA was isolated from tissue using RNA-STAT60 reagent, and RNA was reverse-transcribed into cDNA (Invitrogen). Gene expression (Mouse Fgf21 - forward: ctctaggtttctttgccaacag; reverse: aagctgcaggcctcaggat) was measured with an Applied Biosystems 7900HT Sequence Detection System using the CT assay and normalized to Gapdh as described (Bookout et al., 2006).
Human Alcohol Drinking Study
Healthy volunteers received either 2 ml/kg·BW vodka (40% v/v alcohol) in a total volume of 300 ml orange or strawberry juice (n=13, including 10 men and 3 women) or juice alone (n=8, including 2 men and 6 women). The alcohol or juice alone was consumed within 30 minutes and blood samples were taken at baseline and every hour for 4 hours after the first drink. FGF21 was measured in lithium heparin plasma by ELISA (BioVendor, Czech Republic) according to the manufacturers' instructions.
QUANTIFICATION AND STATISTICAL ANALYSIS
All data are expressed as means ± SEM. Statistical analysis between the two groups was performed by unpaired two-tailed Student’s t-test using Excel or GraphPad Prism (GraphPad Software, Inc.). For multiple comparisons, two-way ANOVA with post hoc Tukey was performed using SPSS. For licking behavior, a mixed-effects model (Pinheiro and Bates, 2000) was used to compare the number of licks in the FGF21 and vehicle groups, and a piecewise linear model was used to estimate the change point of mean differences between the two groups over time (Muggeo, 2016).
Supplementary Material
Highlights.
FGF21 stimulates water drinking behavior in response to alcohol and a ketogenic diet.
SIM1-neurons in the hypothalamus contribute to FGF21’s effect on drinking behavior.
The FGF21-induced thirst response depends on beta-adrenergic signaling.
Footnotes
Author Contributions
P.S., G.H., J.C., V.Sondhi, V.Stadlbauer, A.H., and B.L. performed experiments. P.S., C.Z., G.H., J.C., M.W., M.C.H., O.W.M., D.J.M., and S.A.K. designed experiments and analyzed data. Y.X. analyzed data. D.J.M. and S.A.K. supervised the project. P.S., C.Z., G.H., M.C.H., O.W.M., D.J.M., and S.A.K. wrote the paper.
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150:4931–4940. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Bookout AL, Cummins CL, Mangelsdorf DJ, Pesola JM, Kramer MF. High-throughput real-time quantitative reverse transcription PCR. Curr Protoc Mol Biol. 2006;Chapter 15(Unit 15.18) doi: 10.1002/0471142727.mb1508s73. [DOI] [PubMed] [Google Scholar]
- Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, Kliewer SA. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nature Medicine. 2013;19:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschmann M, Steiniger J, Franke G, Birkenfeld AL, Luft FC, Jordan J. Water drinking induces thermogenesis through osmosensitive mechanisms. J Clin Endocrinol Metab. 2007;92:3334–3337. doi: 10.1210/jc.2006-1438. [DOI] [PubMed] [Google Scholar]
- Boschmann M, Steiniger J, Hille U, Tank J, Adams F, Sharma AM, Klaus S, Luft FC, Jordan J. Water-induced thermogenesis. J Clin Endocrinol Metab. 2003;88:6015–6019. doi: 10.1210/jc.2003-030780. [DOI] [PubMed] [Google Scholar]
- Camporez JP, Jornayvaz FR, Petersen MC, Pesta D, Guigni BA, Serr J, Zhang D, Kahn M, Samuel VT, Jurczak MJ, Shulman GI. Cellular mechanisms by which FGF21 improves insulin sensitivity in male mice. Endocrinology. 2013;154:3099–3109. doi: 10.1210/en.2013-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu AY, Workalemahu T, Paynter NP, Rose LM, Giulianini F, Tanaka T, Ngwa JS, Qi Q, Curhan GC, Rimm EB, Hunter DJ, Pasquale LR, Ridker PM, Hu FB, Chasman DI, Qi L. Novel locus including FGF21 is associated with dietary macronutrient intake. Human molecular genetics. 2013;22:1895–1902. doi: 10.1093/hmg/ddt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ. Neuroendocrinological effects of alcohol. Annu Rev Med. 1981;32:123–142. doi: 10.1146/annurev.me.32.020181.001011. [DOI] [PubMed] [Google Scholar]
- Desai BN, Singhal G, Watanabe M, Stevanovic D, Lundasen T, Fisher fM, Mather ML, Vardeh HG, Douris N, Adams AC, Nasser IA, FitzGerald GA, Flier JS, Skarke C, Maratos-Flier E. Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Molecular Metabolism. doi: 10.1016/j.molmet.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai BN, Singhal G, Watanabe M, Stevanovic D, Lundasen T, Fisher FM, Mather ML, Vardeh HG, Douris N, Adams AC, Nasser IA, FitzGerald GA, Flier JS, Skarke C, Maratos-Flier E. Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Mol Metab. 2017;6:1395–1406. doi: 10.1016/j.molmet.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douris N, Stevanovic D, Fisher FM, Cisu TI, Chee MJ, Ly Nguyen N, Zarebidaki E, Adams AC, Kharitonenkov A, Flier JS, Bartness TJ, Maratos-Flier E. Central Fibroblast Growth Factor 21 Browns White Fat via Sympathetic Action in Male Mice. Endocrinology. 2015 doi: 10.1210/en.2014-2001. en20142001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Maratos-Flier E. Understanding the Physiology of FGF21. Annu Rev Physiol. 2016;78:223–241. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, Goetz R, Mohammadi M, Kuro-o M, Mangelsdorf DJ, Kliewer SA. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24:2050–2064. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell metabolism. 2013;18:333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Gerich J, Penhos JC, Gutman RA, Recant L. Effect of dehydration and hyperosmolarity on glucose, free fatty acid and ketone body metabolism in the rat. Diabetes. 1973;22:264–271. doi: 10.2337/diab.22.4.264. [DOI] [PubMed] [Google Scholar]
- Hsuchou H, Pan W, Kastin AJ. The fasting polypeptide FGF21 can enter brain from blood. Peptides. 2007;28:2382–2386. doi: 10.1016/j.peptides.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, DiMarchi R. Fibroblast growth factor 21 night watch: advances and uncertainties in the field. J Intern Med. 2017;281:233–246. doi: 10.1111/joim.12580. [DOI] [PubMed] [Google Scholar]
- Kim AM, Somayaji VR, Dong JQ, Rolph TP, Weng Y, Chabot JR, Gropp KE, Talukdar S, Calle RA. Once-weekly administration of a long-acting FGF21 analogue modulates lipids, bone turnover markers, blood pressure, and body weight differently in obese hypertriglyceridemic subjects and in non-human primates. Diabetes Obes Metab. 2017 doi: 10.1111/dom.13023. [DOI] [PubMed] [Google Scholar]
- Kuro-o M. Klotho and betaKlotho. Advances in experimental medicine and biology. 2012;728:25–40. doi: 10.1007/978-1-4614-0887-1_2. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF. Hypothalamic alpha- and beta-adrenergic systems regulate both thirst and hunger in the rat. Proc Natl Acad Sci U S A. 1971;68:332–334. doi: 10.1073/pnas.68.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF. Paraventricular nucleus: a primary site mediating adrenergic stimulation of feeding and drinking. Pharmacol Biochem Behav. 1978;8:163–175. doi: 10.1016/0091-3057(78)90333-7. [DOI] [PubMed] [Google Scholar]
- Liang Q, Zhong L, Zhang J, Wang Y, Bornstein SR, Triggle CR, Ding H, Lam KS, Xu A. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes. 2014;63:4064–4075. doi: 10.2337/db14-0541. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhao C, Xiao J, Liu L, Zhang M, Wang C, Wu G, Zheng MH, Xu LM, Chen YP, Mohammadi M, Chen SY, Cave M, McClain C, Li X, Feng W. Fibroblast growth factor 21 deficiency exacerbates chronic alcohol-induced hepatic steatosis and injury. Sci Rep. 2016;6:31026. doi: 10.1038/srep31026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley MJ, Johnson AK. The physiological regulation of thirst and fluid intake. News Physiol Sci. 2004;19:1–6. doi: 10.1152/nips.01470.2003. [DOI] [PubMed] [Google Scholar]
- Muggeo VMR. Testing with a nuisance parameter present only under the alternative: a score-based approach with application to segmented modelling. Journal of Statistical Computation and Simulation. 2016;86:3059–3067. [Google Scholar]
- Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA, Mangelsdorf DJ. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metabolism. 2014;20:670–677. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen BM, Mangelsdorf DJ, Kliewer SA. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends in endocrinology and metabolism: TEM. 2015;26:22–29. doi: 10.1016/j.tem.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D. Mixed-Effects Models in S and S-PLUS. Springer; New York: 2000. [Google Scholar]
- Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, Liu C, O'Reilly P, Gao H, Song P, Xu B, Ruggeri B, Amin N, Jia T, Preis S, Segura Lepe M, Akira S, Barbieri C, Baumeister S, Cauchi S, Clarke TK, Enroth S, Fischer K, Hallfors J, Harris SE, Hieber S, Hofer E, Hottenga JJ, Johansson A, Joshi PK, Kaartinen N, Laitinen J, Lemaitre R, Loukola A, Luan J, Lyytikainen LP, Mangino M, Manichaikul A, Mbarek H, Milaneschi Y, Moayyeri A, Mukamal K, Nelson C, Nettleton J, Partinen E, Rawal R, Robino A, Rose L, Sala C, Satoh T, Schmidt R, Schraut K, Scott R, Smith AV, Starr JM, Teumer A, Trompet S, Uitterlinden AG, Venturini C, Vergnaud AC, Verweij N, Vitart V, Vuckovic D, Wedenoja J, Yengo L, Yu B, Zhang W, Zhao JH, Boomsma DI, Chambers J, Chasman DI, Daniela T, de Geus E, Deary I, Eriksson JG, Esko T, Eulenburg V, Franco OH, Froguel P, Gieger C, Grabe HJ, Gudnason V, Gyllensten U, Harris TB, Hartikainen AL, Heath AC, Hocking L, Hofman A, Huth C, Jarvelin MR, Jukema JW, Kaprio J, Kooner JS, Kutalik Z, Lahti J, Langenberg C, Lehtimaki T, Liu Y, Madden PA, Martin N, Morrison A, Penninx B, Pirastu N, Psaty B, Raitakari O, Ridker P, Rose R, Rotter JI, Samani NJ, Schmidt H, Spector TD, Stott D, Strachan D, Tzoulaki I, van der Harst P, van Duijn CM, Marques-Vidal P, Vollenweider P, Wareham NJ, Whitfield JB, Wilson J, Wolffenbuttel B, Bakalkin G, Evangelou E, Liu Y, Rice KM, Desrivieres S, Kliewer SA, Mangelsdorf DJ, Muller CP, Levy D, Elliott P. KLB is associated with alcohol drinking, and its gene product beta-Klotho is necessary for FGF21 regulation of alcohol preference. Proc Natl Acad Sci U S A. 2016;113:14372–14377. doi: 10.1073/pnas.1611243113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Owen BM, Song P, Hernandez G, Zhang Y, Zhou Y, Scott WT, Paratala B, Turner T, Smith A, Bernardo B, Muller CP, Tang H, Mangelsdorf DJ, Goodwin B, Kliewer SA. FGF21 Regulates Sweet and Alcohol Preference. Cell Metab. 2016a;23:344–349. doi: 10.1016/j.cmet.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, Somayaji V, Weng Y, Clark R, Lanba A, Owen BM, Brenner MB, Trimmer JK, Gropp KE, Chabot JR, Erion DM, Rolph TP, Goodwin B, Calle RA. A Long-Acting FGF21 Molecule, PF-05231023, Decreases Body Weight and Improves Lipid Profile in Non-human Primates and Type 2 Diabetic Subjects. Cell Metab. 2016b;23:427–440. doi: 10.1016/j.cmet.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Ngwa JS, van Rooij FJ, Zillikens MC, Wojczynski MK, Frazier-Wood AC, Houston DK, Kanoni S, Lemaitre RN, Luan J, Mikkila V, Renstrom F, Sonestedt E, Zhao JH, Chu AY, Qi L, Chasman DI, de Oliveira Otto MC, Dhurandhar EJ, Feitosa MF, Johansson I, Khaw KT, Lohman KK, Manichaikul A, McKeown NM, Mozaffarian D, Singleton A, Stirrups K, Viikari J, Ye Z, Bandinelli S, Barroso I, Deloukas P, Forouhi NG, Hofman A, Liu Y, Lyytikainen LP, North KE, Dimitriou M, Hallmans G, Kahonen M, Langenberg C, Ordovas JM, Uitterlinden AG, Hu FB, Kalafati IP, Raitakari O, Franco OH, Johnson A, Emilsson V, Schrack JA, Semba RD, Siscovick DS, Arnett DK, Borecki IB, Franks PW, Kritchevsky SB, Lehtimaki T, Loos RJ, Orho-Melander M, Rotter JI, Wareham NJ, Witteman JC, Ferrucci L, Dedoussis G, Cupples LA, Nettleton JA. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. The American journal of clinical nutrition. 2013;97:1395–1402. doi: 10.3945/ajcn.112.052183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton SN. Increased Hydration Can Be Associated with Weight Loss. Front Nutr. 2016;3:18. doi: 10.3389/fnut.2016.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Gugten JG, Dubland J, Liu HF, Wang A, Joseph C, Holmes DT. Determination of serum aldosterone by liquid chromatography and tandem mass spectrometry: a liquid-liquid extraction method for the ABSCIEX API-5000 mass spectrometry system. J Clin Pathol. 2012;65:457–462. doi: 10.1136/jclinpath-2011-200564. [DOI] [PubMed] [Google Scholar]
- von Holstein-Rathlou S, BonDurant LD, Peltekian L, Naber MC, Yin TC, Claflin KE, Urizar AI, Madsen AN, Ratner C, Holst B, Karstoft K, Vandenbeuch A, Anderson CB, Cassell MD, Thompson AP, Solomon TP, Rahmouni K, Kinnamon SC, Pieper AA, Gillum MP, Potthoff MJ. FGF21 Mediates Endocrine Control of Simple Sugar Intake and Sweet Taste Preference by the Liver. Cell Metab. 2016;23:335–343. doi: 10.1016/j.cmet.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Liu Y, Xiao J, Liu L, Chen S, Mohammadi M, McClain CJ, Li X, Feng W. FGF21 mediates alcohol-induced adipose tissue lipolysis by activation of systemic release of catecholamine in mice. Journal of lipid research. 2015;56:1481–1491. doi: 10.1194/jlr.M058610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman CA, Leib DE, Knight ZA. Neural circuits underlying thirst and fluid homeostasis. Nat Rev Neurosci. 2017;18:459–469. doi: 10.1038/nrn.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.