SUMMARY
Intermittent fasting (IF) improves cardiometabolic health; however, it is unknown whether these effects are due solely to weight loss. We conducted the first supervised controlled feeding trial to test whether IF has benefits independent of weight loss by feeding participants enough food to maintain their weight. Our proof-of-concept study also constitutes the first trial of early time-restricted feeding (eTRF), a form of IF that involves eating early in the day to be in alignment with circadian rhythms in metabolism. Men with prediabetes were randomized to eTRF (6-hour feeding period, with dinner before 3 pm) or a control schedule (12-hour feeding period) for five weeks and later crossed over to the other schedule. eTRF improved insulin sensitivity, β cell responsiveness, blood pressure, oxidative stress, and appetite. We demonstrate for the first time in humans that eTRF improves some aspects of cardiometabolic health and IF’s effects are not solely due to weight loss.
Keywords: intermittent fasting, early time-restricted feeding, eTRF, meal timing, circadian system, circadian rhythms, prediabetes
eTOC
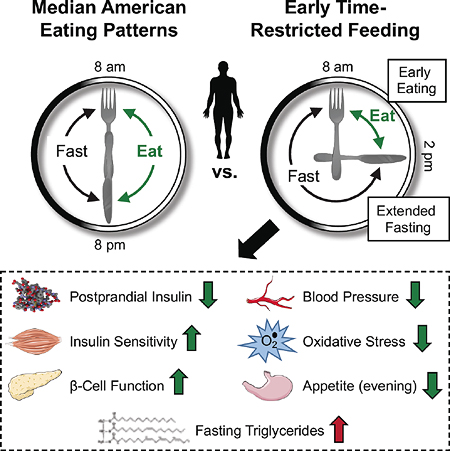
Sutton et al. conduct the first supervised controlled feeding trial to test whether intermittent fasting has benefits in humans in the absence of weight loss. Prediabetic men following a form of intermittent fasting called early time-restricted feeding improved their insulin sensitivity, blood pressure, and oxidative stress levels, without losing weight.
INTRODUCTION
Intermittent fasting (IF)—the practice of alternating periods of eating and fasting—has emerged as an effective therapeutic strategy for improving multiple cardiometabolic endpoints in rodent models of disease, ranging from insulin sensitivity and ectopic fat accumulation to hard endpoints such as stroke and diabetes incidence (Antoni et al., 2017; Harvie and Howell, 2017; Mattson et al., 2016; Patterson and Sears, 2017). The first clinical trials of IF in humans began about a decade ago, including trials on alternate-day fasting (Catenacci et al., 2016; Heilbronn et al., 2005a; Heilbronn et al., 2005b), alternate-day modified fasting (ADMF) (Bhutani et al., 2013; Eshghinia and Mohammadzadeh, 2013; Halberg et al., 2005; Hoddy et al., 2016; Hoddy et al., 2014; Johnson et al., 2007; Klempel et al., 2013; Kroeger et al., 2018; Soeters et al., 2009; Trepanowski et al., 2017a; Trepanowski et al., 2017b; Varady et al., 2009; Varady et al., 2013; Wegman et al., 2015), the 5:2 diet (Carter et al., 2016; Harvie et al., 2013; Harvie et al., 2011; Harvie et al., 2016), and the fasting-mimicking diet (Brandhorst et al., 2015; Choi et al., 2016; Wei et al., 2017; Williams et al., 1998). Data from these trials suggests that IF has similar benefits in humans: IF can reduce body weight or body fat, improve insulin sensitivity, reduce glucose and/or insulin levels, lower blood pressure, improve lipid profiles, and reduce markers of inflammation and oxidative stress (Bhutani et al., 2013; Brandhorst et al., 2015; Carter et al., 2016; Catenacci et al., 2016; Eshghinia and Mohammadzadeh, 2013; Halberg et al., 2005; Harvie et al., 2013; Harvie et al., 2011; Harvie et al., 2016; Heilbronn et al., 2005a; Heilbronn et al., 2005b; Hoddy et al., 2016; Hoddy et al., 2014; Johnson et al., 2007; Klempel et al., 2013; Trepanowski et al., 2017b; Varady et al., 2009; Varady et al., 2013; Wegman et al., 2015; Wei et al., 2017; Williams et al., 1998).
However, it was unknown whether these benefits are solely due to weight loss. Many have speculated that IF improves cardiometabolic health more than conventional dieting, even when matched for weight loss. Indeed, data in rodents suggest that IF improves cardiometabolic endpoints even when food intake and/or body weight is matched to the control group (Anson et al., 2003; Belkacemi et al., 2012; Hatori et al., 2012; Olsen et al., 2017; Sherman et al., 2012; Woodie et al., 2017; Wu et al., 2011; Zarrinpar et al., 2014). However, preliminary evidence in humans suggests that the benefits of IF are due mostly or only to weight loss (Halberg et al., 2005; Harvie et al., 2011; Soeters et al., 2009; Trepanowski et al., 2017b). Initially, a single-arm, two-week trial reported that IF improves insulin sensitivity even when participants are approximately weight-stable (Halberg et al., 2005), but the study was uncontrolled. Later, two better controlled, randomized crossover trials reported that IF did not improve glucose or lipid metabolism (Carlson et al., 2007; Soeters et al., 2009; Stote et al., 2007). More recently, the longest IF study in humans reported that adults who practiced ADMF for one year were not any healthier than conventional dieters who lost a similar amount of weight, yet they had a higher attrition rate (Trepanowski et al., 2017b). However, none of these studies matched food intake and meal frequency nor supervised participants to ensure that they were following the prescribed dietary intervention. Drawing a parallel to metabolic (bariatric) surgery—which is widely believed to be more effective than conventional caloric restriction—four studies now show that the most popular form of metabolic surgery, called Roux-en-Y gastric bypass surgery, is no better or may be even worse at improving glycemic control than calorie-matched weight loss (Campos et al., 2010; Isbell et al., 2010; Jackness et al., 2013; Lingvay et al., 2013). Such findings underscore the critical need to determine whether the benefits of interventions such as IF are mediated only through weight loss or through mechanisms that are independent of weight loss.
To test whether IF can have benefits independent of weight loss, we therefore decided to perform a proof-of-concept trial using a relatively new form of IF called time-restricted feeding (TRF). TRF is a type of IF that extends the daily fasting period between dinner and breakfast the following morning, and, unlike most forms of IF, it can be practiced either with or without reducing calorie intake and losing weight. Since the median American eats over a 12-hour period (Kant and Graubard, 2014), we define TRF as a form of IF that involves limiting daily food intake to a period of 10 hours or less, followed by a daily fast of at least 14 hours. Studies in rodents using feeding windows of 3–10 hours report that TRF reduces body weight, increases energy expenditure, improves glycemic control, lowers insulin levels, reduces hepatic fat, prevents hyperlipidemia, reduces infarct volume after stroke, and improves inflammatory markers, relative to grazing on food throughout the day (Belkacemi et al., 2011; Belkacemi et al., 2012; Belkacemi et al., 2010; Chung et al., 2016; Duncan et al., 2016; Garcia-Luna et al., 2017; Hatori et al., 2012; Kudo et al., 2004; Manzanero et al., 2014; Olsen et al., 2017; Park et al., 2017; Philippens et al., 1977; Sherman et al., 2011; Sherman et al., 2012; Sundaram and Yan, 2016; Woodie et al., 2017; Wu et al., 2011; Zarrinpar et al., 2014). We chose to test TRF over other forms of IF in part because TRF consistently improves health endpoints in rodents, even when food intake and/or body weight is matched to the control group (Belkacemi et al., 2012; Hatori et al., 2012; Olsen et al., 2017; Sherman et al., 2012; Woodie et al., 2017; Wu et al., 2011; Zarrinpar et al., 2014).
In humans, four pilot trials of TRF (4–10 hour feeding periods) have been conducted to date. Surprisingly, the results of TRF in humans appear to depend on the time of day of the eating window (Carlson et al., 2007; Gill and Panda, 2015; Moro et al., 2016; Stote et al., 2007; Tinsley et al., 2017). Restricting food intake to the middle of the day (mid-day TRF [mTRF]) reduced body weight or body fat, fasting glucose and insulin levels, insulin resistance, hyperlipidemia, and inflammation (Gill and Panda, 2015; Moro et al., 2016). However, restricting food intake to the late afternoon or evening (after 16:00 h; late TRF [lTRF]) either produced mostly null results or worsened postprandial glucose levels, β cell responsiveness, blood pressure, and lipid levels (Carlson et al., 2007; Stote et al., 2007; Tinsley et al., 2017).
The circadian system, or internal biological clock, may explain why the effects of TRF appear to depend on the time of day. Glucose, lipid, and energy metabolism are all regulated by the circadian system, which upregulates them at some times of day and downregulates them at others (Poggiogalle et al., 2018a; Scheer et al., 2009). For instance, in humans, insulin sensitivity, β cell responsiveness, and the thermic effect of food are all higher in the morning than in the afternoon or evening, suggesting that human metabolism is optimized for food intake in the morning (Morris et al., 2015a; Morris et al., 2015b; Poggiogalle et al., 2018a; Scheer et al., 2009). Indeed, studies in humans show that eating in alignment with circadian rhythms in metabolism by increasing food intake at breakfast time and by reducing it at dinnertime improves glycemic control, weight loss, and lipid levels and also reduces hunger (Garaulet et al., 2013; Gill and Panda, 2015; Jakubowicz et al., 2013a, b; Jakubowicz et al., 2015; Keim et al., 1997; Ruiz-Lozano et al., 2016). This suggests that the efficacy of IF interventions may depend not only on weight loss but also on the time of day of food intake. Moreover, these data from circadian studies suggest that combining two different meal timing strategies—IF and eating in alignment with circadian rhythms—may be a particularly beneficial form of IF. We call such a combined intervention early time-restricted feeding (early TRF; eTRF), and we define it as a subtype of TRF where dinner is eaten in the mid-afternoon. To date, however, there had been no trials of eTRF in humans.
We therefore decided to test eTRF in our proof-of-concept trial. Our goals were two-fold: (1) to determine whether eTRF can improve cardiometabolic health and (2) to determine whether IF can have benefits independent of weight loss and food intake. Our objective was not to examine the effectiveness or feasibility of eTRF but rather to determine the efficacy of eTRF when participants strictly adhere to their assigned meal times, food intake is precisely matched and monitored, and no weight loss occurs—that is, to measure the pure physiologic effects of eTRF uncontaminated by non-adherence. As such, our study is both the first clinical trial of eTRF and the most rigorously controlled trial of any form of IF in humans. We hypothesized that eTRF would improve glycemic control, improve vascular function, and reduce markers of inflammation and oxidative stress, even when food intake is matched and no weight loss occurs.
RESULTS AND DISCUSSION
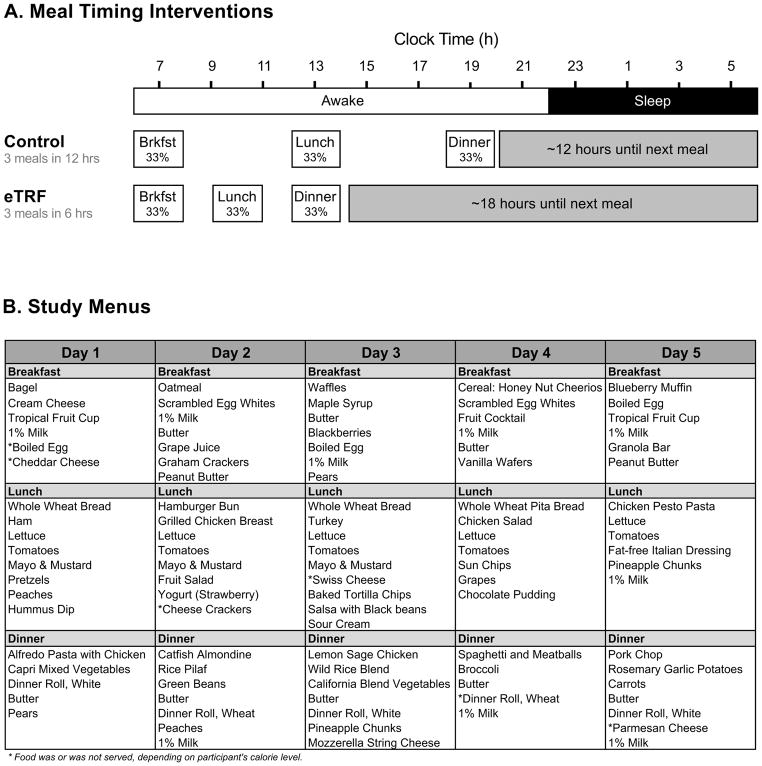
We performed a five-week, randomized, crossover, isocaloric and eucaloric controlled feeding trial testing eTRF in men with prediabetes. In brief, participants adopted an eTRF schedule (6-hour daily eating period, with dinner before 15:00 h) and a control schedule (12-hour eating period) for five weeks each, separated by a washout period of approximately seven weeks. Participants chose a habitual time between 06:30 – 08:30 h to start eating breakfast every day, and lunch and dinner were timed accordingly. For example, participants who ate breakfast at 07:00 h then ate lunch and dinner at 10:00 h and 13:00 h in the eTRF arm and at 13:00 h and 19:00 h in the control arm (Figure 1). During the intervention phases, participants were required to eat only food provided by study staff, were fed enough food to maintain their weight, and ate all meals while being monitored by study staff. Furthermore, food intake was matched on a meal-by-meal basis across the two arms to eliminate any confounding effects from differences in food intake or meal frequency. As a result, our trial is the most rigorously controlled trial of IF in humans to date, achieving a level of rigor intermediate between metabolic ward conditions and a standard outpatient feeding trial (in which food is provided, but food intake is not measured, monitored, or enforced). The primary endpoints were glucose tolerance, postprandial insulin, and insulin sensitivity as measured using a 3-hour Oral Glucose Tolerance Test (OGTT), while the secondary endpoints were cardiovascular risk factors and markers of inflammation and oxidative stress. Metabolic hormones were added later as an exploratory outcome. Differences between meal timing schedules were assessed by comparing the two within-arm changes against each other; these treatment effects are denoted by Δ.
Figure 1. Dietary Interventions.
(A) Meal Timing Interventions. An example schedule for a person who eats breakfast at 07:00 h. (B) Study Menus. Food was prepared according to a five-day sequence of menus. Each menu provided three meals/day and was composed of 35% fat, 50% carbohydrate, and 15% protein. Caloric intake was tailored to each participant’s unique energy requirements, and each meal provided about 33% of daily caloric needs. See also Figure S1 and Table S1.
Participants
Controlled feeding trials are very demanding because they require participants to eat all meals under supervision for weeks. This, in turn, makes recruitment challenging since most people cannot take time off work daily to eat their meals while being monitored. As shown in Figure S1, 934 individuals expressed interest in trying eTRF and applied to participate in the trial. Of these, most were excluded for being unable to eat all meals under supervision. Ultimately, 130 men were screened in the clinic, and, of those, 18 had both elevated HbA1c levels and impaired glucose tolerance indicative of prediabetes, and 15 met all eligibility requirements. Twelve men were enrolled in order to have the requisite eight completers. Of the four who did not complete the intervention, two withdrew for unrelated medical reasons (severe neck pain necessitating surgery, abnormally low potassium levels at baseline that did not improve over time), and another two withdrew because of unexpected changes to their work schedule. The eight overweight men with prediabetes who completed the trial (aged 56 ± 9 years; 6 Caucasian, 1 African-American, 1 South Asian) had a mean BMI of 32.2 ± 4.4 kg/m2, fasting glucose of 102 ± 9 mg/dl, fasting insulin of 25.1 ± 14.5 mU/l, and 2-hour glucose tolerance of 154 ± 17 mg/dl (Table S1). At screening, their mean blood pressure was at the lower end of the prehypertensive range (systolic: 123 ± 8 mm Hg; diastolic: 82 ± 7 mm Hg), while their mean lipid levels were in the normal ranges.
Compliance
Compliance was outstanding: participants who completed the trial were 100.0 ± 0.0% and 98.9 ± 1.8% compliant to eating the provided meals when following the eTRF and control schedules, respectively. Every exception to eating the provided food (aside from a single meal by one participant and three sick days by another participant) was approved ahead of time by study staff, and food intake was then re-calculated and matched in the second arm of the trial. Furthermore, participants were 98.2 ± 2.9% and 99.0 ± 1.9% compliant to adhering to the required meal times when following the eTRF and control schedules, respectively. In addition, body weight was approximately stable, and changes in body weight were similar between arms (−1.4 ± 1.3 kg vs. −1.0 ± 1.1 kg; Δ=−0.5 ± 0.3 kg; p=0.12). Importantly, since food intake was matched across arms, the lack of a treatment effect for body weight suggests that TRF does not impact energy expenditure in humans. We suspect that the non-significant difference in the within-arm change in body weight was due to a reduction in glycogen levels and the accompanying loss of water weight, which arises from the longer fasting duration on the eTRF schedule.
Adverse Events
There were no serious adverse events. There were about one dozen adverse events identified as possibly related to the study intervention. These included vomiting (1 participant in the eTRF arm); frequent urination and drowsiness (1 participant in the control arm); and headaches, increased thirst, and diarrhea (each of which afflicted 2 participants in the eTRF arm and 1 participant in the control arm). Unrelated to the study intervention, one participant reported a worsening of neck pain requiring surgery during the washout period, which precipitated his withdrawal from the study.
eTRF Reduces Insulin Levels and Improves Insulin Sensitivity and β cell Responsiveness
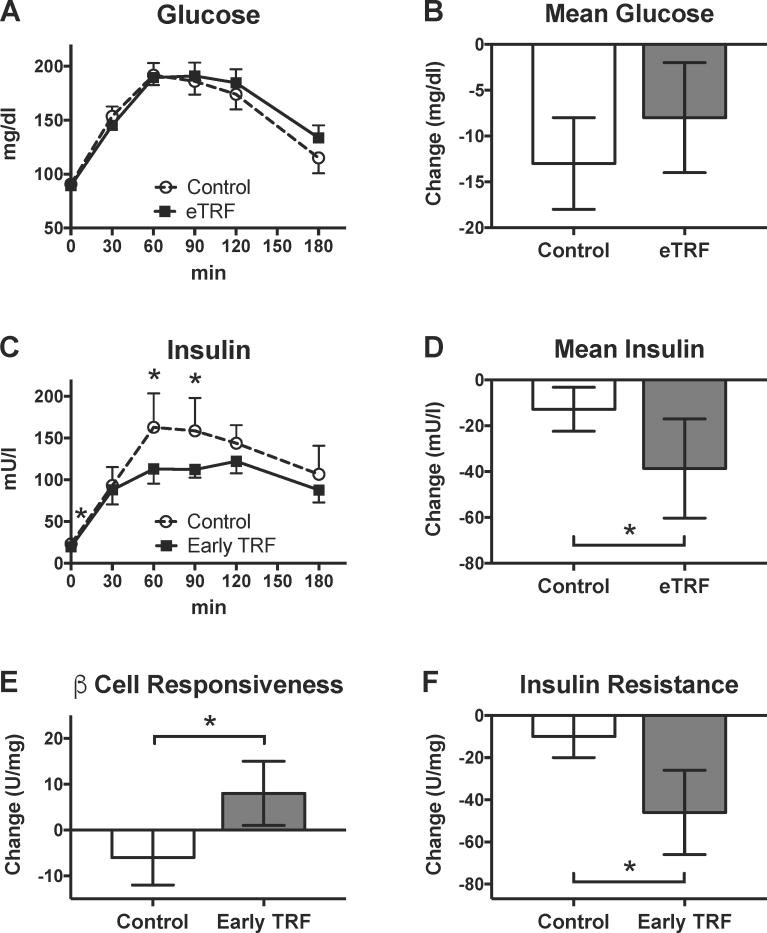
Participants underwent 3-hour OGTTs in the morning at baseline and post-intervention for each study arm. As shown in Figures 2 and S2, five weeks of eTRF did not affect fasting glucose (Δ=−2 ± 2 mg/dl; p=0.49) or glucose levels at any time point during the 3-hour OGTT (p≥0.13). Consequently, mean glucose levels were unchanged (Δ=5 ± 5 mg/dl; p=0.40). However, eTRF did affect insulin levels. eTRF decreased fasting insulin by 3.4 ± 1.6 mU/l (p=0.05) and decreased insulin levels at t=60 min and 90 min post-load (p≤0.01). In aggregate, eTRF reduced mean and peak insulin values by 26 ± 9 mU/l (p=0.01) and 35 ± 13 mU/l (p=0.01), respectively. We also investigated the impact of eTRF on OGTT-derived indices of β cell responsiveness and insulin resistance. eTRF increased the insulinogenic index, a marker of β cell responsiveness, by 14 ± 7 U/mg (p=0.05) and decreased insulin resistance, as measured by the 3-hour incremental AUC ratio, by 36 ± 10 U/mg (p=0.005).
Figure 2. Glycemic Control.
eTRF did not affect (A) individual or (B) mean values for glucose during a 3-hour OGTT. However, eTRF did lower (C) insulin levels at multiple time points and (D) mean insulin levels. Overall, eTRF improved (E) β cell responsiveness and (F) insulin resistance, as measured by the insulinogenic index and the incremental AUC ratio, respectively. (Post-intervention values shown above for (A) glucose and (C) insulin were adjusted for differences at baseline.) All data are paired, with N=8 completers in each arm. Data are presented as least squares mean ± SEM, with the exceptions of panels A and C, which display the data as raw mean ± SEM. * p ≤ 0.05. See also Figure S2 and Table S2.
Although five weeks of eTRF did not improve glucose levels, it dramatically lowered insulin levels and improved insulin sensitivity and β cell responsiveness. This is consistent with several other trials in humans that suggest that IF may be more effective at reducing insulin levels and improving insulin sensitivity than at lowering glucose levels (Bhutani et al., 2013; Harvie et al., 2013; Harvie et al., 2011; Heilbronn et al., 2005a; Heilbronn et al., 2005b; Trepanowski et al., 2017b; Wegman et al., 2015; Williams et al., 1998).
In our trial, the reductions in insulin levels were largest in participants with worse hyperinsulinemia at baseline, and these improvements were driven more (but not exclusively) by differences at baseline (see Figure S2). Much to our surprise, even after the seven-week washout period, all but one participant who first completed the eTRF intervention entered the second arm of the trial with substantially lower (≥25%) mean postprandial insulin levels. (The only exception was one participant who traveled multiple time zones away during his washout period.) Although these within-subject differences at baseline were not statistically significant for the four men who followed eTRF first (−46 ± 14 mU/l; p=0.55) or for all eight completers (−20 ± 14 mU/l; p=0.13), our linear mixed models did uncover statistically significant sequence and period effects. (By comparison, the insulin sensitivity endpoint was not affected by either sequence or period effects, while β cell responsiveness was affected only by period effects). This suggests that eTRF may have longer-lasting benefits even after being discontinued. As a result, the true effect size for improvements in mean postprandial insulin could be smaller or larger than the 26 ± 9 mU/l decrease that we observed, and our study therefore merits replication to confirm the effect size in men with prediabetes. Nonetheless, all but one of our participants experienced improvements of 5 mU/l or greater in mean postprandial insulin levels on eTRF relative to the control schedule, suggesting that such effects are real. Interestingly, the one participant whose insulin levels worsened on eTRF had reported a long history of overnight shift work prior to enrolling in the trial. Given that circadian rhythms are altered in adults who perform overnight shift work, it will be important to determine whether some subpopulations have altered circadian rhythms and would benefit more from alternative meal timing interventions.
An important consideration in interpreting our results is that our data may underestimate the glycemic benefits of eTRF for two reasons. First, we did not match the fasting duration prior to testing: participants fasted for about 18 hours prior to testing in the eTRF arm but for only 12 hours in the control arm. Acute fasting induces insulin resistance and worsens β cell responsiveness, even after only 24 hours, and this is mediated at least partially through elevation of triglycerides and/or free fatty acids from lipolysis (Antoni et al., 2016; Browning et al., 2012; Halberg et al., 2005; Salgin et al., 2009). In one trial, 24 hours of fasting decreased insulin sensitivity the following morning by 54% and the acute response of insulin, a marker of β cell responsiveness, by 22% (Salgin et al., 2009). In retrospect, given that the eTRF arm involved fasting for 18 hours prior to testing and that we observed a large 57 ± 13 mg/dl increase in triglyceride levels at the start of the OGTT (described below), it is quite remarkable that we found an improvement in both β cell responsiveness and insulin sensitivity. This limitation can be resolved in future studies by matching the fasting duration in both arms on the day prior to testing.
The second reason why our data may underestimate the glycemic benefits of eTRF is that we measured glucose levels only in the morning. Although we observed no difference at habitual breakfast time, eTRF may still lower mean 24-hour glucose levels simply by shifting the timing of lunch and dinner to earlier in the day, when the circadian system promotes better glucose tolerance (Poggiogalle et al., 2018a). In future studies, it will be important to measure glucose metabolism over a 24-hour period to determine whether improvements in insulin levels and insulin sensitivity—without an accompanying reduction in glucose levels—is indeed a hallmark of IF or is an artifact of not measuring glucose levels over the 24-hour day.
In sum, our results show that eTRF can be used to treat insulin resistance and to improve pancreatic β cell function; however, its effects on 24-hour glucose levels remain to be determined.
eTRF Lowers Blood Pressure but Does Not Affect Arterial Stiffness, LDL Cholesterol, or HDL Cholesterol
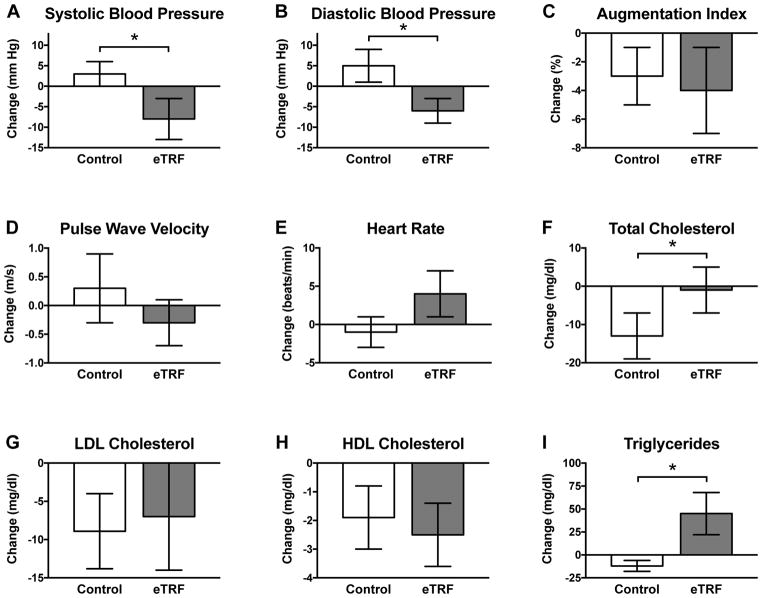
As shown in Figure 3, five weeks of eTRF lowered morning levels of systolic and diastolic blood pressure by 11 ± 4 mm Hg (p=0.03) and 10 ± 4 mm Hg (p=0.03), respectively, relative to the control schedule. This is a surprisingly and dramatically large improvement for a dietary intervention of only five weeks that did not induce weight loss; it is on par with the effectiveness of anti-hypertensive medications such as angiotensin-converting enzyme (ACE) inhibitors (Heran et al., 2008). Although other IF trials have observed improvements in blood pressure (Bhutani et al., 2013; Eshghinia and Mohammadzadeh, 2013; Varady et al., 2009; Wei et al., 2017), none have reported effects this large. Given some evidence that elevated insulin levels may directly increase blood pressure (Bhanot and McNeill, 1996; Biston et al., 1996; Persson, 2007), one possibility is that the improvements in blood pressure were driven by the reduction in insulin levels. Another possibility is that eTRF promotes natriuresis by shifting salt intake to earlier in the daytime when sodium excretion is upregulated by the circadian system (Johnston et al., 2016).
Figure 3. Cardiovascular Disease Risk Factors.
eTRF dramatically lowered (A) systolic blood pressure and (B) diastolic blood pressure in the morning. However, it increased or tended to increase morning values for (E) resting heart rate, (I) triglycerides, and, in turn, (F) total cholesterol. The (C) augmentation index, (D) pulse wave velocity, (G) LDL cholesterol, and (H) HDL cholesterol were unaffected.
All data are paired, with N=8 completers in each arm. Data are presented as least squares mean ± SEM. * p ≤ 0.05. See also Table S2.
However, five weeks of eTRF did not affect the augmentation index (Δ=−1.4 ± 2.1%; p=0.53) or pulse wave velocity (Δ=−0.5 ± 0.4 m/s; p=0.23), which are measures of arterial stiffness. Similarly, eTRF did not affect HDL cholesterol (Δ=−0.6 ± 0.9 mg/dl; p=0.48) or LDL cholesterol (Δ=2 ± 6 mg/dl; p=0.75). eTRF did increase morning fasting levels of triglycerides by 57 ± 13 mg/dl (p=0.0007), which translated into a 13 ± 5 mg/dl relative increase in morning fasting levels of total cholesterol (p=0.02). (The relative increase in total cholesterol was driven by an improvement in the control arm, rather than a change in the eTRF arm.) The elevation in circulating triglyceride levels likely is due to the longer fasting duration preceding testing (18 h vs. 12 h in the control arm) and likely reflects triglyceride re-esterification following lipolysis and possibly also hepatic and intramuscular storage of triglyceride (Browning et al., 2012; Soeters et al., 2012). eTRF also tended to increase morning heart rate by 5 ± 3 bpm (p=0.10) to 74 ± 7 bpm post-intervention, but the effect did not reach statistical significance. This potential increase may reflect a change in sensory nervous system activity due to the longer daily fasting duration and accompanying lipolysis (Patel et al., 2002; Pequignot et al., 1980). The increases in fasting triglycerides and potentially also heart rate merit further study—particularly in a trial that matches the fasting duration prior to testing.
eTRF Reduces Oxidative Stress but Does Not Affect Inflammatory Markers
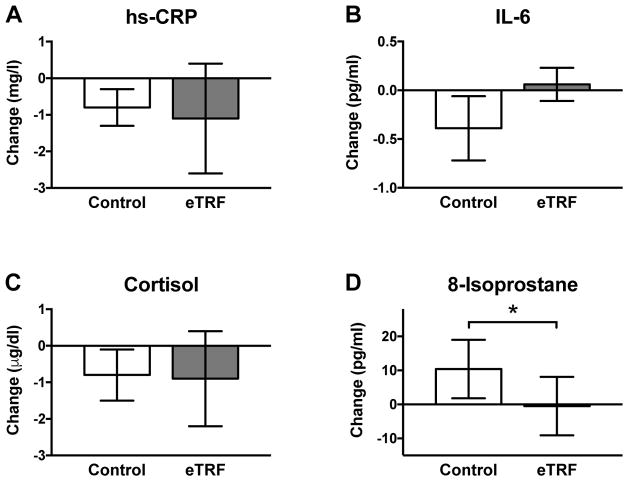
Relative to the control arm, five weeks of eTRF decreased plasma levels of 8-isoprostane, a marker of oxidative stress to lipids, by 11 ± 5 pg/ml (p=0.05) or about 14% (Figure 4). (Both sequence and period effects for 8-isoprostane were statistically significant.) The relative improvement was driven by a worsening in the control arm, suggesting that in our study, eTRF prevented 8-isoprostane levels from becoming worse when participants ate the provided study foods. However, eTRF did not affect any markers of inflammation: morning fasting levels of hs-CRP (Δ=−0.3 ± 1.0 mg/l; p=0.77), cortisol (Δ=−0.1 ± 1.3 μg/dl; p=0.95), and IL-6 (Δ=0.45 ± 0.27 pg/ml; p=0.12) were all unchanged.
Figure 4. Inflammatory and Oxidative Stress Markers.
eTRF did not affect the inflammatory markers (A) hs-CRP, (B) IL-6, or (C) cortisol. However, eTRF reduced levels of (D) 8-isoprostane, a marker of oxidative stress to lipids. All data are paired, with N=8 completers in each arm. Data are presented as least squares mean ± SEM. * p ≤ 0.05. See also Table S2.
Only one prior TRF trial has measured inflammatory markers, and it reported a reduction in IL-1β but not in IL-6 or TNF-α (Moro et al., 2016). In general, most clinical trials report that IF does not affect hs-CRP, TNF-α, or IL-6 (Bhutani et al., 2013; Halberg et al., 2005; Harvie et al., 2013; Harvie et al., 2011; Moro et al., 2016; Trepanowski et al., 2017b; Wei et al., 2017), indicating that IF does not affect most inflammatory markers in humans. By contrast, our finding of an improvement in oxidative stress relative to the control arm is in agreement with an 8-week IF trial that reported dramatic reductions in 8-isoprostane, nitrotyrosine, protein carbonyls, and 4-hydroxynonenal adducts (Johnson et al., 2007). Although fewer trials have examined the effects of IF on oxidative stress markers, both our and Johnson et al.’s (Johnson et al., 2007) data suggest that IF may affect oxidative stress levels more than inflammatory markers. Because eTRF reduces lipid peroxidation, it may, in turn, reduce the risk of atherosclerosis.
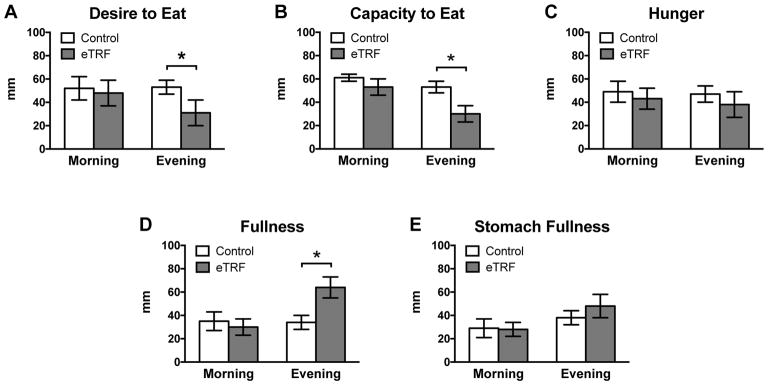
eTRF Reduces Appetite in the Evening
As shown in Figure 5, there were no differences in subjective measures of appetite in the morning (p≥0.20). However, eTRF substantially reduced the desire to eat (Δ=−22 ± 7 mm; p=0.007) and the capacity to eat (Δ=−23 ± 6 mm; p=0.001) in the evening and non-significantly decreased hunger levels (Δ=−9 ± 6 mm; p=0.15). Participants also reported that eTRF dramatically increased sensations of fullness in the evening (Δ=31 ± 6 mm; p<0.0001) and nearly significantly increased sensations of a full stomach (Δ=10 ± 5 mm; p=0.07).
Figure 5. Subjective Appetite.
Participants rated their appetite on a 0–100 mm visual analog scale, ranging from “Not at All” (0 mm) to “Extremely” (100 mm). (A–E) eTRF did not affect appetite in the morning. In the evening, eTRF reduced (A) desire to eat and (B) capacity to eat and increased (D) feelings of fullness. Changes in evening levels of (C) hunger and (E) stomach fullness did not quite reach statistical significance. All data are paired, with N=8 completers in each arm. Data are presented as least squares mean ± SEM. * p ≤ 0.05. See also Table S2 and Figure S3.
As an exploratory analysis to support self-reported appetite ratings, we also measured metabolic hormones in the morning. As shown in Table S2, eTRF decreased morning fasting values of the satiety hormone PYY by 23 ± 7 pg/ml (p=0.003). However, it did not affect morning fasting levels of the hunger hormone ghrelin (Δ=−5.7 ± 6.6 pg/ml; p=0.41), the incretin GLP-1 (Δ=−1.2 ± 1.0 pmol/ml; p=0.26), or the adipokines leptin (Δ=−0.6 ± 1.0 ng/ml; p=0.54) and high-molecular weight adiponectin (Δ=408 ± 765 ng/ml; p=0.61).
Thus, despite the longer daily fasting duration for the eTRF schedule, eTRF does not increase hunger—at least, not when food intake is calorie-matched to the control arm. On the contrary, eTRF decreased the desire and capacity to eat and increased feelings of fullness in the evening. eTRF may therefore help curb food intake in the evening and, in turn, facilitate weight loss. This is consistent with rodent studies, which have reported that both eTRF and other forms of TRF reduce appetite hormones and body weight (Belkacemi et al., 2011; Belkacemi et al., 2010; Chaix et al., 2014; Chung et al., 2016; Duncan et al., 2016; Garcia-Luna et al., 2017; Hatori et al., 2012; Kudo et al., 2004; Manzanero et al., 2014; Olsen et al., 2017; Park et al., 2017; Philippens et al., 1977; Sherman et al., 2011; Sherman et al., 2012; Sundaram and Yan, 2016; Wu et al., 2011; Zarrinpar et al., 2014). In contrast, previous studies on mid-day and late TRF in humans report conflicting results for hunger (Gill and Panda, 2015; Stote et al., 2007), food intake (Gill and Panda, 2015; Tinsley et al., 2017), and body weight (Gill and Panda, 2015; Moro et al., 2016; Stote et al., 2007; Tinsley et al., 2017). It remains to be determined whether these discrepancies were due to limitations or differences in the study design (e.g., no control group or only measuring hunger at one time of day) or the timing of food intake.
Feasibility and Acceptability
Although our study was an efficacy trial, we also collected preliminary data on feasibility and acceptability. As shown in Figure S3, participants reported that it took 12 ± 10 days (range: 2 – 35 days) to adjust to the eTRF schedule, and all but one participant adjusted within about two weeks. Participants also reported that the challenge of eating within 6 hours each day was more difficult than the challenge of fasting for 18 hours per day (difficulty scores: 65 ± 20 vs. 29 ± 18 mm; p=0.009). In fact, all but one participant reported that it was not difficult or only moderately difficult (<50 mm on a 100-mm scale) to fast for 18 hours daily. Based on their experiences in adhering to eTRF, participants thought that eating within a 7.8 ± 1.8-hour daily period (range: 4 – 10 hours) would be feasible for most people. At the end of the study, seven out of eight participants were willing to eat dinner earlier, based on their subjective experiences in the study, while all eight said they were willing to do so if it improved their health. Thus, while fasting for 18 hours per day is well-tolerated and not difficult, the feasting aspect of eTRF is more difficult for participants, so TRF interventions with an 8-hour or longer eating period may be a better target for future effectiveness trials.
Limitations
This study has several limitations. First, our trial included only eight men. Although our sample size is similar to other extremely well-controlled or inpatient circadian trials, our results need to be replicated in a larger trial that also includes women. Second, we did not match the fasting duration prior to testing, which may have underestimated the improvements in insulin sensitivity and also likely explains the increase in triglycerides and total cholesterol. Although we suspect that the elevation in fasting triglycerides is a transient by-product of eTRF’s extended daily fasting, future trials that measure lipid levels across the 24-hour day and/or that image plaque and ectopic fat depots are needed to confirm that this phenomenon is not pathophysiologic. Third, our trial did not measure glucose levels over a 24-hour period, so we were unable to investigate whether eTRF, by virtue of shifting the timing of lunch and dinner to earlier during the day, lowers mean 24-hour glucose levels, as would be expected based on prior research (Poggiogalle et al., 2018b). Along similar lines, since we did not measure blood pressure across the 24-hour day, measuring only morning fasting values may overestimate eTRF’s effects on blood pressure. Finally, since our trial was an efficacy trial designed to isolate and measure the physiologic effects of eTRF, our study does not provide any insight into feasibility. Future trials are needed to determine the optimal length and timing of the feeding period and whether eTRF is feasible and effective in the general population.
Conclusion
In conclusion, five weeks of eTRF improved insulin levels, insulin sensitivity, β cell responsiveness, blood pressure, and oxidative stress levels in men with prediabetes—even though food intake was matched to the control arm and no weight loss occurred. Our trial was the first randomized controlled trial to show that IF has benefits independent of food intake and weight loss in humans. Our study was also first clinical trial to test eTRF in humans and to show that eTRF improves some aspects of cardiometabolic health. Our trial tested eTRF in men with prediabetes—a population at great risk of developing diabetes—and indicates that eTRF is an efficacious strategy for treating both prediabetes and likely also prehypertension. We speculate that eTRF—by virtue of combining daily intermittent fasting and eating in alignment with circadian rhythms in metabolism—will prove to be a particularly efficacious form of IF. In light of these promising results, future research is needed to better elucidate the mechanisms behind both intermittent fasting and meal timing; to determine which forms of IF and meal timing are efficacious; and to translate them into effective interventions for the general population.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Courtney Peterson (cpeterso@uab.edu). For the specific cases of biospecimen and data sharing requests, such requests will require a Material Transfer Agreement and/or a Data Use Agreement and will be managed by the University of Alabama at Birmingham’s Material Transfer Office, which abides by the Uniform Biological Material Transfer Agreement (UBMTA).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
This clinical trial was conducted at Pennington Biomedical Research Center (PBRC; Baton Rouge, LA), approved by the center’s Institutional Review Board, and conducted in accordance with the Helsinki Declaration of 1975. Prior to enrolling participants, the trial was preregistered on clinicaltrials.gov (NCT01895179). Participants were recruited from the Greater Baton Rouge area between October 2013 and January 2016 via emails, flyers, social media, local radio and TV appearances, and website advertisements. The study population comprised overweight and obese (BMI between 25–50 kg/m2) adult males aged 35–70 with prediabetes. To qualify as having prediabetes, participants needed to exhibit both elevated levels of HbA1c (5.5–6.4%) and impaired glucose tolerance (IGT), defined as a glucose level between 140–199 mg/dL at the end of a 2-hour oral glucose tolerance test (OGTT). Potential participants were excluded if they performed overnight shift work more than once a week; regularly fasted (defined as fasting ≥16 hours per day or having completed twelve 24-hour fasts within the past year); regularly consumed more than 2 servings/day of alcohol; regularly performed heavy physical activity; had gastrointestinal surgery or impaired nutrient absorption; took anti-diabetes medications, steroids, beta blockers, adrenergic-stimulating agents, or other medications that could affect the study endpoints; or were afflicted with diabetes or a significant cardiovascular, renal, cardiac, liver, lung, or nervous system disease. All participants provided both verbal and written informed consent prior to enrolling in the study. Because this was an efficacy trial, participants were continuously enrolled until eight individuals completed the trial (see “Statistical Power” in the section “Quantification and Statistical Analysis”).
METHOD DETAILS
Study Design
The trial was conducted as a randomized, crossover, controlled feeding study. Participants were randomized to initially follow either a control schedule (~12-hour eating window with 12 hours of daily fasting) or an eTRF schedule (~6-hour eating period with 18 hours of daily fasting) for five weeks. Thereafter, they completed an approximately 7-week washout period before crossing over to the other arm. The eating schedules were modestly customized by allowing each participant to choose a habitual time to start eating breakfast every day (all started breakfast between 06:30 – 08:30 h). Their two subsequent meals (lunch and dinner) were spaced by 6 hours for the control schedule versus 3 hours for the eTRF schedule. For example, for a breakfast time of 07:00 h, lunch and dinner would be at 10:00 h and 13:00 h in the eTRF arm but at 13:00 h and 19:00 h in the control arm (Figure 1A). Regardless of their chosen breakfast time, all participants were scheduled to finish eating dinner by mid-afternoon (≤15:00 h) while following the eTRF schedule. Participants were instructed to maintain consistent physical activity and sleep patterns throughout the entire 4-month study.
Each interventional arm of the trial lasted 37 days and was structured as follows. On Day 1 (run-in period), all participants ate three meals over a 10-hour period, starting at their chosen breakfast time and with the meals spaced every 5 hours. The purpose of the one-day run-in period was to ensure that all participants ate the same diet at the same meal times on the day prior to baseline testing. On Day 2 (baseline testing), a 3-hour OGTT and applanation tonometry were performed, and blood was collected to measure fasting levels of lipids and of metabolic, hormonal, and oxidative stress markers. On Days 2–36, participants followed their assigned meal timing schedule. On Day 36, participants’ appetite levels were measured using visual analog scales. Finally, all baseline tests were repeated on Day 37. All physiologic tests were performed starting at each participant’s habitual breakfast time.
Diets
Calories, meal frequency (3 meals/day), and food composition were matched on a meal-by-meal basis in both arms of the trial to eliminate any confounding effects from differences in food intake; the only difference between the two arms was the timing of meals. All food was prepared by the PBRC Research Kitchen using a 5-day rotating menu (Figure 1B). Diets were formulated to contain 50% carbohydrate, 35% fat, and 15% protein, and each meal provided approximately one-third of each participants’ daily energy requirements. To determine whether there are intrinsic benefits to eTRF—independent of weight loss—participants were intentionally fed enough food to maintain their weight using the equation (in kcal/day): 2189 + 19.6 × (weight in kg) – 17.6 × (age in years) (Redman et al., 2009). To ensure that participants maintained their weight, each participant was weighed daily during Days 1–14 and weekly thereafter of the first arm of the study, and any changes in weight were counterbalanced by adjusting calorie intake in ±100 kcal increments. Participants were required to eat all provided meals and were not allowed to eat any non-study foods; any rare protocol deviations (such as sick days) were calculated and matched in the second arm of the study.
Compliance Monitoring
To ensure compliance, participants were required to eat all meals at our research clinic or to be supervised in real-time via remote video monitoring by Skype (Peterson et al., 2016). The start and stop time of every meal eaten in the study was logged. Participants were instructed to start eating each meal within ±30 minutes of the scheduled time and to finish eating each meal within 45 minutes. At the end of the trial, dietary compliance was quantified in two ways: (1) compliance with eating the provided foods and (2) compliance with the meal timing schedules. Compliance with eating the provided foods was quantified as the percent of provided meals that were eaten while being monitored, while compliance with the meal timing schedules was quantified as the percent of meals eaten within one hour of the scheduled time. Due to both the nature of the intervention and the monitoring of compliance, neither study participants nor study staff could be blinded.
OGTTs
Intravenous lines were inserted into participants’ arm veins and fasting blood samples were collected. Participants then consumed 75 g loads of glucose (Azer Scientific, Inc.; Morgantown, PA) within 5 minutes. For the 3-hour OGTTs administered at baseline and post-intervention, the ingestion of glucose was timed to start at each participant’s habitual breakfast time. Blood was subsequently collected at 30, 60, 90, 120, and 180 minutes after glucose ingestion to measure both glucose and insulin. The primary outcomes were mean glucose and insulin levels, which were calculated as the 180-minute AUC value divided by the 180-minute duration of the OGTT. β cell responsiveness was estimated using the insulinogenic index, which was calculated as the change in insulin divided by the change in glucose during the first 30 minutes. Insulin resistance was estimated using the incremental AUC ratio (Conn et al., 1956), which was calculated as the ratio of the incremental AUC values for insulin and glucose, or equivalently, as (mean insulin – fasting insulin)/(mean glucose – fasting glucose).
Applanation Tonometry
Applanation tonometry, which measures arterial stiffness, was performed immediately before each 3-hour OGTT. For the test, participants rested in a supine position, while a 3-lead EKG was placed on their wrists, leg, and/or chest to monitor the cardiac cycle. At the end of a 20-minute rest period, the tonometer (AtCor Medical, Inc.; Itasca, IL) was lightly applied at the wrist to sample radial artery pressure waveforms. The pressure waveform data was processed using SphygmoCor software (Version 8.0; AtCor Medical, Inc.; Itasca, IL). The outcome variables were augmentation index and pulse wave velocity (m/s). Peripheral and central augmentation indices were calculated as a percentage based on the difference in the second systolic peak and diastolic pressure, divided by the difference between the first systolic peak and diastolic pressure (Wilkinson et al., 1998).
Serum Chemistry
All serum samples were analyzed in duplicate. Glucose, cholesterol, and triglycerides were measured on a DXC600 instrument (Beckman Coulter, Inc.; Brea, CA) either using standard reagents, or in the case of HDL cholesterol, using an immunoinhibition assay (Trinity Biotech USA, Inc.; Jamestown, NY and WAKO Chemicals USA, Inc.; Richmond, CA). LDL cholesterol was determined using the Friedewald equation. Insulin and hs-CRP were measured using chemiluminescent immunoassays on an Immulite 2000 instrument (Siemens Corporation; Washington, DC). Fasting leptin, active ghrelin, high-molecular weight adiponectin, and peptide YY (PYY) levels were assayed using radioimmunoassay kits (EMD Millipore Corporation; Billerica, MA) on a gamma counter (Wizard 2470; PerkinElmer, Inc.; Waltham, MA). Fasting levels of glucagon-like peptide-1 (GLP-1) and 8-isoprostane were assayed using ELISA kits (EMD Millipore Corporation; Billerica, MA, and Cayman Chemical Company; Ann Arbor, MI, respectively) on a Bio Rad Microplate reader (Bio-Rad Laboratories, Inc.; Hercules, CA). The inflammatory cytokines IL-1β, IL-6, MCP-1, and TNF-α were measured by immunoassay with fluorescent detection on a Luminex instrument (EMD Millipore Corporation; Billerica, MA). Unfortunately, the values for IL-6 were undetectable in most participants, and the coefficients of variation for the within-sample measurements of MCP-1 and TNF-α were excessively high, so these data were not included.
Subjective Appetite
Participants rated their appetite across five dimensions—hunger, fullness, stomach fullness, desire to eat, and capacity to eat—using Visual Analog Scales (VAS; a 0–100 mm scale). VAS surveys were administered immediately before breakfast and 12 hours after breakfast (which was immediately before dinner in the control arm) on the last day of the intervention (Day 36). Participants rated their appetite levels based how they habitually felt at that time of day during the previous week.
Exit Survey
On the last day of the study (Day 37 of arm 2), all participants completed an exit survey that assessed how many days they felt it took to adjust to the eTRF eating schedule; the difficulty of adhering to the eating versus fasting periods of eTRF (using a VAS rating system); whether they would be willing to eat earlier in the day based on their experiences in the study; and how long they thought the eating period should be in order to be feasible for the general public.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical Power
The statistical power analysis indicated that for a crossover trial, eight completers were required to have 80% power (two-sided test, α=0.05) to detect a 12 mg/dl difference in glucose levels during an OGTT (the primary endpoint), assuming r=0.3 and a within-subjects standard deviation of σ=10 mg/dl.
Randomization
The randomization code was generated using an online random number generator based on atmospheric noise (www.random.org). Since we continued to enroll participants until the planned eight individuals completed the intervention, randomization was performed with replacement in a 1:1 allocation and using a block size of 8 to ensure equal numbers of participants completed each of the two possible sequences. Allocations were concealed from study participants until after they enrolled in the trial.
Statistical Analyses
Statistical analyses were performed as two-sided tests in SAS (version 9.4; Cary, NC) using a significance threshold of α=0.05 for the Type I error rate. Since this was an efficacy study—designed to isolate and measure the physiologic effects of eTRF uncontaminated by non-adherence—data were analyzed for completers only. All collected data for the eight completers were included in the analysis; one participant had unusual pulsatile insulin secretion patterns, but no data were excluded. Differences between treatment arms were evaluated at baseline and as change scores using linear mixed models with heterogeneous compound symmetry, where participants served as the random effect; the treatment, sequence, and period were treated as fixed effects; and the Satterthwaite method was used for calculating degrees of freedom. Three endpoints—mean insulin levels, β cell responsiveness, and 8-isoprostane—had statistically significant sequence and/or period effects, which are reported in the main text; all other statistically significant endpoints did not. All data are presented as least squares mean ± SEM, with the exceptions of the baseline data and the exit survey data, which are presented as raw mean ± SD, and the individual time point data for the OGTT (Figures 2A, 2C, S2), which are graphically presented as raw mean ± SEM for visual clarity.
ADDITIONAL RESOURCES
Clinical Trial Registration URL: https://www.clinicaltrials.gov/ct2/show/NCT01895179
Supplementary Material
HIGHLIGHTS.
Early time-restricted feeding (eTRF) increases insulin sensitivity
eTRF also improves β cell function and lowers blood pressure and oxidative stress
eTRF lowers the desire to eat in the evening, which may facilitate weight loss
Intermittent fasting can improve health even in the absence of weight loss
Acknowledgments
This study was supported by U54GM104940 from the National Institute of General Medical Sciences, by KL2TR001419 from the National Center for Advancing Translational Sciences, and by a NORC Center Grant (P30DK072476) entitled “Nutrition and Metabolic Health Through the Lifespan.” E.F.S. was supported by F31HD084199 from the National Institute of Child Health and Human Development. The authors thank the study participants and the staff at Pennington Biomedical Research Center, without whom this study would not have been possible. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Graphical Abstract includes images made by Servier Medical Art, molekuul_be/Shuttlestock.com, and ClipArtMag.com.
Footnotes
AUTHOR CONTRIBUTIONS
C.M.P. conceived of, designed, and lead the clinical trial, with input from E.R. and W.T.C. C.M.P., E.F.S., K.S.E., and W.T.C. conducted the investigation. R.B. performed the statistical analyses, and C.M.P. and E.F.S. drafted the manuscript. All authors helped interpret the data, revised the manuscript for critical content, and approved the final version of the manuscript.
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni R, Johnston KL, Collins AL, Robertson MD. Investigation into the acute effects of total and partial energy restriction on postprandial metabolism among overweight/obese participants. Br J Nutr. 2016;115:951–959. doi: 10.1017/S0007114515005346. [DOI] [PubMed] [Google Scholar]
- Antoni R, Johnston KL, Collins AL, Robertson MD. Effects of intermittent fasting on glucose and lipid metabolism. Proc Nutr Soc. 2017:1–8. doi: 10.1017/S0029665116002986. [DOI] [PubMed] [Google Scholar]
- Belkacemi L, Selselet-Attou G, Bulur N, Louchami K, Sener A, Malaisse WJ. Intermittent fasting modulation of the diabetic syndrome in sand rats. III. Post-mortem investigations. Int J Mol Med. 2011;27:95–102. doi: 10.3892/ijmm.2010.556. [DOI] [PubMed] [Google Scholar]
- Belkacemi L, Selselet-Attou G, Hupkens E, Nguidjoe E, Louchami K, Sener A, Malaisse WJ. Intermittent fasting modulation of the diabetic syndrome in streptozotocin-injected rats. Int J Endocrinol. 2012;2012:962012. doi: 10.1155/2012/962012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkacemi L, Selselet-Attou G, Louchami K, Sener A, Malaisse WJ. Intermittent fasting modulation of the diabetic syndrome in sand rats. II. In vivo investigations. Int J Mol Med. 2010;26:759–765. doi: 10.3892/ijmm_00000523. [DOI] [PubMed] [Google Scholar]
- Bhanot S, McNeill JH. Insulin and hypertension: a causal relationship? Cardiovasc Res. 1996;31:212–221. [PubMed] [Google Scholar]
- Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Varady KA. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity (Silver Spring) 2013;21:1370–1379. doi: 10.1002/oby.20353. [DOI] [PubMed] [Google Scholar]
- Biston P, Van Cauter E, Ofek G, Linkowski P, Polonsky KS, Degaute JP. Diurnal variations in cardiovascular function and glucose regulation in normotensive humans. Hypertension. 1996;28:863–871. doi: 10.1161/01.hyp.28.5.863. [DOI] [PubMed] [Google Scholar]
- Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JD, Baxter J, Satapati S, Burgess SC. The effect of short-term fasting on liver and skeletal muscle lipid, glucose, and energy metabolism in healthy women and men. J Lipid Res. 2012;53:577–586. doi: 10.1194/jlr.P020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos GM, Rabl C, Peeva S, Ciovica R, Rao M, Schwarz JM, Havel P, Schambelan M, Mulligan K. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14:15–23. doi: 10.1007/s11605-009-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson O, Martin B, Stote KS, Golden E, Maudsley S, Najjar SS, Ferrucci L, Ingram DK, Longo DL, Rumpler WV, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism. 2007;56:1729–1734. doi: 10.1016/j.metabol.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S, Clifton PM, Keogh JB. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res Clin Pract. 2016;122:106–112. doi: 10.1016/j.diabres.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, Mattson MP, Martin B, MacLean PS, Melanson EL, Troy Donahoo W. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity (Silver Spring) 2016;24:1874–1883. doi: 10.1002/oby.21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Piccio L, Childress P, Bollman B, Ghosh A, Brandhorst S, Suarez J, Michalsen A, Cross AH, Morgan TE, et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016;15:2136–2146. doi: 10.1016/j.celrep.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Chou W, Sears DD, Patterson RE, Webster NJ, Ellies LG. Time-restricted feeding improves insulin resistance and hepatic steatosis in a mouse model of postmenopausal obesity. Metabolism. 2016;65:1743–1754. doi: 10.1016/j.metabol.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn JW, Fajans SS, Seltzer HS. Spontaneous hypoglycemia as an early manifestation of diabetes mellitus. Diabetes. 1956;5:437–442. doi: 10.2337/diab.5.6.437. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Smith JT, Narbaiza J, Mueez F, Bustle LB, Qureshi S, Fieseler C, Legan SJ. Restricting feeding to the active phase in middle-aged mice attenuates adverse metabolic effects of a high-fat diet. Physiol Behav. 2016;167:1–9. doi: 10.1016/j.physbeh.2016.08.027. [DOI] [PubMed] [Google Scholar]
- Eshghinia S, Mohammadzadeh F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J Diabetes Metab Disord. 2013;12:4. doi: 10.1186/2251-6581-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond) 2013;37:604–611. doi: 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Luna C, Soberanes-Chavez P, de Gortari P. Prepuberal light phase feeding induces neuroendocrine alterations in adult rats. J Endocrinol. 2017;232:15–28. doi: 10.1530/JOE-16-0402. [DOI] [PubMed] [Google Scholar]
- Gill S, Panda S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015;22:789–798. doi: 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg N, Henriksen M, Soderhamn N, Stallknecht B, Ploug T, Schjerling P, Dela F. Effect of intermittent fasting and refeeding on insulin action in healthy men. J Appl Physiol (1985) 2005;99:2128–2136. doi: 10.1152/japplphysiol.00683.2005. [DOI] [PubMed] [Google Scholar]
- Harvie M, Howell A. Potential Benefits and Harms of Intermittent Energy Restriction and Intermittent Fasting Amongst Obese, Overweight and Normal Weight Subjects-A Narrative Review of Human and Animal Evidence. Behav Sci (Basel) 2017;7 doi: 10.3390/bs7010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, Cutler RG, Evans G, Whiteside S, Maudsley S, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110:1534–1547. doi: 10.1017/S0007114513000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie MN, Sims AH, Pegington M, Spence K, Mitchell A, Vaughan AA, Allwood JW, Xu Y, Rattray NJ, Goodacre R, et al. Intermittent energy restriction induces changes in breast gene expression and systemic metabolism. Breast Cancer Res. 2016;18:57. doi: 10.1186/s13058-016-0714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, Civitarese AE, Bogacka I, Smith SR, Hulver M, Ravussin E. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes Res. 2005a;13:574–581. doi: 10.1038/oby.2005.61. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr. 2005b;81:69–73. doi: 10.1093/ajcn/81.1.69. [DOI] [PubMed] [Google Scholar]
- Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database Syst Rev. 2008:CD003823. doi: 10.1002/14651858.CD003823.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddy KK, Bhutani S, Phillips SA, Varady KA. Effects of different degrees of insulin resistance on endothelial function in obese adults undergoing alternate day fasting. Nutr Healthy Aging. 2016;4:63–71. doi: 10.3233/NHA-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky A, Bhutani S, Varady KA. Meal timing during alternate day fasting: Impact on body weight and cardiovascular disease risk in obese adults. Obesity (Silver Spring) 2014;22:2524–2531. doi: 10.1002/oby.20909. [DOI] [PubMed] [Google Scholar]
- Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, Marks-Shulman PA, Abumrad NN. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33:1438–1442. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackness C, Karmally W, Febres G, Conwell IM, Ahmed L, Bessler M, McMahon DJ, Korner J. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and beta-cell Function in type 2 diabetic patients. Diabetes. 2013;62:3027–3032. doi: 10.2337/db12-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowicz D, Barnea M, Wainstein J, Froy O. Effects of caloric intake timing on insulin resistance and hyperandrogenism in lean women with polycystic ovary syndrome. Clin Sci (Lond) 2013a;125:423–432. doi: 10.1042/CS20130071. [DOI] [PubMed] [Google Scholar]
- Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 2013b;21:2504–2512. doi: 10.1002/oby.20460. [DOI] [PubMed] [Google Scholar]
- Jakubowicz D, Wainstein J, Ahren B, Bar-Dayan Y, Landau Z, Rabinovitz HR, Froy O. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: a randomised clinical trial. Diabetologia. 2015;58:912–919. doi: 10.1007/s00125-015-3524-9. [DOI] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JG, Speed JS, Jin C, Pollock DM. Loss of endothelin B receptor function impairs sodium excretion in a time- and sex-dependent manner. Am J Physiol Renal Physiol. 2016;311:F991–F998. doi: 10.1152/ajprenal.00103.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant AK, Graubard BI. Association of self-reported sleep duration with eating behaviors of American adults: NHANES 2005–2010. Am J Clin Nutr. 2014;100:938–947. doi: 10.3945/ajcn.114.085191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keim NL, Van Loan MD, Horn WF, Barbieri TF, Mayclin PL. Weight loss is greater with consumption of large morning meals and fat-free mass is preserved with large evening meals in women on a controlled weight reduction regimen. J Nutr. 1997;127:75–82. doi: 10.1093/jn/127.1.75. [DOI] [PubMed] [Google Scholar]
- Klempel MC, Kroeger CM, Varady KA. Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metabolism. 2013;62:137–143. doi: 10.1016/j.metabol.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Kroeger CM, Trepanowski JF, Klempel MC, Barnosky A, Bhutani S, Gabel K, Varady KA. Eating behavior traits of successful weight losers during 12 months of alternate-day fasting: An exploratory analysis of a randomized controlled trial. Nutr Health. 2018 doi: 10.1177/0260106017753487. 260106017753487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Akiyama M, Kuriyama K, Sudo M, Moriya T, Shibata S. Night-time restricted feeding normalises clock genes and Pai-1 gene expression in the db/db mouse liver. Diabetologia. 2004;47:1425–1436. doi: 10.1007/s00125-004-1461-0. [DOI] [PubMed] [Google Scholar]
- Lingvay I, Guth E, Islam A, Livingston E. Rapid improvement in diabetes after gastric bypass surgery: is it the diet or surgery? Diabetes Care. 2013;36:2741–2747. doi: 10.2337/dc12-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanero S, Erion JR, Santro T, Steyn FJ, Chen C, Arumugam TV, Stranahan AM. Intermittent fasting attenuates increases in neurogenesis after ischemia and reperfusion and improves recovery. J Cereb Blood Flow Metab. 2014;34:897–905. doi: 10.1038/jcbfm.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2016 doi: 10.1016/j.arr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M, Paoli A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14:290. doi: 10.1186/s12967-016-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Garcia JI, Myers S, Yang JN, Trienekens N, Scheer FA. The Human Circadian System Has a Dominating Role in Causing the Morning/Evening Difference in Diet-Induced Thermogenesis. Obesity (Silver Spring) 2015a;23:2053–2058. doi: 10.1002/oby.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, Scheer FA. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A. 2015b;112:E2225–2234. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen MK, Choi MH, Kulseng B, Zhao CM, Chen D. Time-restricted feeding on weekdays restricts weight gain: A study using rat models of high-fat diet-induced obesity. Physiol Behav. 2017;173:298–304. doi: 10.1016/j.physbeh.2017.02.032. [DOI] [PubMed] [Google Scholar]
- Park S, Yoo KM, Hyun JS, Kang S. Intermittent fasting reduces body fat but exacerbates hepatic insulin resistance in young rats regardless of high protein and fat diets. J Nutr Biochem. 2017;40:14–22. doi: 10.1016/j.jnutbio.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Patel JN, Coppack SW, Goldstein DS, Miles JM, Eisenhofer G. Norepinephrine spillover from human adipose tissue before and after a 72-hour fast. J Clin Endocrinol Metab. 2002;87:3373–3377. doi: 10.1210/jcem.87.7.8695. [DOI] [PubMed] [Google Scholar]
- Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017 doi: 10.1146/annurev-nutr-071816-064634. [DOI] [PubMed] [Google Scholar]
- Pequignot JM, Peyrin L, Peres G. Catecholamine-fuel interrelationships during exercise in fasting men. J Appl Physiol Respir Environ Exerc Physiol. 1980;48:109–113. doi: 10.1152/jappl.1980.48.1.109. [DOI] [PubMed] [Google Scholar]
- Persson SU. Blood pressure reactions to insulin treatment in patients with type 2 diabetes. Int J Angiol. 2007;16:135–138. doi: 10.1055/s-0031-1278267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CM, Apolzan JW, Wright C, Martin CK. Video chat technology to remotely quantify dietary, supplement and medication adherence in clinical trials. Br J Nutr. 2016;116:1646–1655. doi: 10.1017/S0007114516003524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippens KM, von Mayersbach H, Scheving LE. Effects of the scheduling of meal-feeding at different phases of the circadian system in rats. J Nutr. 1977;107:176–193. doi: 10.1093/jn/107.2.176. [DOI] [PubMed] [Google Scholar]
- Poggiogalle E, Jamshed H, Peterson CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism. 2018a doi: 10.1016/j.metabol.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggiogalle E, Jamshed H, Peterson CM. Circadian Regulation of Glucose, Lipid, and Energy Metabolism in Humans. Metabolism. 2018b;78 doi: 10.1016/j.metabol.2017.11.017. TBD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, Ravussin E, Pennington CT. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One. 2009;4:e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Lozano T, Vidal J, de Hollanda A, Scheer FA, Garaulet M, Izquierdo-Pulido M. Timing of food intake is associated with weight loss evolution in severe obese patients after bariatric surgery. Clin Nutr. 2016;35:1308–1314. doi: 10.1016/j.clnu.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Salgin B, Marcovecchio ML, Humphreys SM, Hill N, Chassin LJ, Lunn DJ, Hovorka R, Dunger DB. Effects of prolonged fasting and sustained lipolysis on insulin secretion and insulin sensitivity in normal subjects. Am J Physiol Endocrinol Metab. 2009;296:E454–461. doi: 10.1152/ajpendo.90613.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman H, Frumin I, Gutman R, Chapnik N, Lorentz A, Meylan J, le Coutre J, Froy O. Long-term restricted feeding alters circadian expression and reduces the level of inflammatory and disease markers. J Cell Mol Med. 2011;15:2745–2759. doi: 10.1111/j.1582-4934.2010.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26:3493–3502. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- Soeters MR, Lammers NM, Dubbelhuis PF, Ackermans M, Jonkers-Schuitema CF, Fliers E, Sauerwein HP, Aerts JM, Serlie MJ. Intermittent fasting does not affect whole-body glucose, lipid, or protein metabolism. Am J Clin Nutr. 2009;90:1244–1251. doi: 10.3945/ajcn.2008.27327. [DOI] [PubMed] [Google Scholar]
- Soeters MR, Soeters PB, Schooneman MG, Houten SM, Romijn JA. Adaptive reciprocity of lipid and glucose metabolism in human short-term starvation. Am J Physiol Endocrinol Metab. 2012;303:E1397–1407. doi: 10.1152/ajpendo.00397.2012. [DOI] [PubMed] [Google Scholar]
- Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, Strycula P, Najjar SS, Ferrucci L, Ingram DK, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85:981–988. doi: 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram S, Yan L. Time-restricted feeding reduces adiposity in mice fed a high-fat diet. Nutr Res. 2016;36:603–611. doi: 10.1016/j.nutres.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, Morgan GB, Grandjean PW. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur J Sport Sci. 2017;17:200–207. doi: 10.1080/17461391.2016.1223173. [DOI] [PubMed] [Google Scholar]
- Trepanowski JF, Kroeger CM, Barnosky A, Klempel M, Bhutani S, Hoddy KK, Rood J, Ravussin E, Varady KA. Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: Secondary analysis of a randomized controlled trial. Clin Nutr. 2017a doi: 10.1016/j.clnu.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, Gabel K, Freels S, Rigdon J, Rood J, et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern Med. 2017b;177:930–938. doi: 10.1001/jamainternmed.2017.0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009;90:1138–1143. doi: 10.3945/ajcn.2009.28380. [DOI] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Haus JM, Hoddy KK, Calvo Y. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J. 2013;12:146. doi: 10.1186/1475-2891-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegman MP, Guo MH, Bennion DM, Shankar MN, Chrzanowski SM, Goldberg LA, Xu J, Williams TA, Lu X, Hsu SI, et al. Practicality of intermittent fasting in humans and its effect on oxidative stress and genes related to aging and metabolism. Rejuvenation Res. 2015;18:162–172. doi: 10.1089/rej.2014.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Brandhorst S, Shelehchi M, Mirzaei H, Cheng CW, Budniak J, Groshen S, Mack WJ, Guen E, Di Biase S, et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aai8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–2084. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- Williams KV, Mullen ML, Kelley DE, Wing RR. The effect of short periods of caloric restriction on weight loss and glycemic control in type 2 diabetes. Diabetes Care. 1998;21:2–8. doi: 10.2337/diacare.21.1.2. [DOI] [PubMed] [Google Scholar]
- Woodie LN, Luo Y, Wayne MJ, Graff EC, Ahmed B, O’Neill AM, Greene MW. Restricted feeding for 9h in the active period partially abrogates the detrimental metabolic effects of a Western diet with liquid sugar consumption in mice. Metabolism. 2017 doi: 10.1016/j.metabol.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Wu T, Sun L, ZhuGe F, Guo X, Zhao Z, Tang R, Chen Q, Chen L, Kato H, Fu Z. Differential roles of breakfast and supper in rats of a daily three-meal schedule upon circadian regulation and physiology. Chronobiol Int. 2011;28:890–903. doi: 10.3109/07420528.2011.622599. [DOI] [PubMed] [Google Scholar]
- Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014;20:1006–1017. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.